Abstract
An emerging concept is that the mammalian myocardium has the potential to regenerate, but that regeneration might be too inefficient to repair the extensive myocardial injury that is typical of human disease1-8. However, the degree to which stem cells or precursor cells contribute to the renewal of adult mammalian cardiomyocytes remains controversial. Here we report evidence that stem cells or precursor cells contribute to the replacement of adult mammalian cardiomyocytes after injury but do not contribute significantly to cardiomyocyte renewal during normal aging. We generated double-transgenic mice to track the fate of adult cardiomyocytes in a ‘pulse-chase’ fashion: after a 4-OH-tamoxifen pulse, green fluorescent protein (GFP) expression was induced only in cardiomyocytes, with 82.7% of cardiomyocytes expressing GFP. During normal aging up to one year, the percentage of GFP+ cardiomyocytes remained unchanged, indicating that stem or precursor cells did not refresh uninjured cardiomyocytes at a significant rate during this period of time. By contrast, after myocardial infarction or pressure overload, the percentage of GFP+ cardiomyocytes decreased from 82.8% in heart tissue from sham-treated mice to 67.5% in areas bordering a myocardial infarction, 76.6% in areas away from a myocardial infarction, and 75.7% in hearts subjected to pressure overload, indicating that stem cells or precursor cells had refreshed the cardiomyocytes.
Despite recent enthusiasm about the idea of regenerating myocardium by using resident cardiac stem cells, fundamental questions remain unanswered. Are cardiomyocytes constantly replaced by endogenous stem or precursor cells? Does injury lead to replacement with new cardiomyocytes from a stem cell pool? It is essential to answer these questions unambiguously and quantitatively.
Advances in mouse genetic engineering have allowed cells to be tracked using a ‘fate-mapping’ approach. This ‘pulse-chase’ strategy has been used to study pancreatic beta-cell turnover in adult mice using an inducible Cre-loxP system9. Pancreatic beta cells were labeled with a reporter gene, and the mice then underwent normal aging or pancreatectomy to determine the origin of adult beta cells. Because the percentage of genetically labeled beta cells remained unchanged, these experiments showed that new adult beta cells formed by self-duplication rather than by differentiation of stem cells.
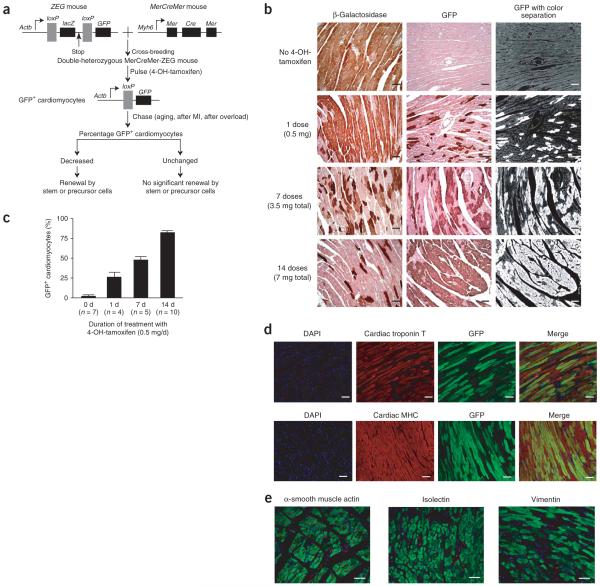
Here we used an inducible cardiomyocyte-specific transgenic mouse fate-mapping approach to determine the frequency with which cardiomyocytes are refreshed from stem or precursor cells. We generated double-transgenic MerCreMer-ZEG mice by crossbreeding transgenic B6129-Tg(Myh6-cre/Esr1)1Jmk/J (hereafter referred to as MerCreMer) mice, in which the cardiomyocyte-specific α-myosin heavy chain (Myh6) promoter drives expression of a 4-OH-tamoxifen-inducible Cre recombinase fusion protein10, with B6.Cg-Tg(ACTB-Bgeo/GFP)21Lbe/J (hereafter referred to as ZEG) reporter mice, in which constitutive β-galactosidase expression is replaced by the expression of GFP upon the removal of a loxP-flanked stop sequence11. Cardiomyocytes in these MerCreMer-ZEG mice are irreversibly marked with GFP following treatment with 4-OH-tamoxifen at a selected time point (the ‘pulse’). During normal aging or after experimental injury (the ‘chase’), the percentage of GFP+ cardiomyocytes can either remain unchanged or decrease. Stem cells, which presumably do not express the gene for α-myosin heavy chain (α-MHC), would not be genetically labeled with GFP. Thus, if stem cells replenish cardiomyocytes, the percentage of GFP+ cardiomyocytes will decrease over time; if stem or precursor cells do not contribute to cardiomyocyte renewal or regeneration, the percentage will remain unchanged (Fig. 1a).
Figure 1.
Generation of MerCreMer-ZEG mice for genetic fate mapping of adult mammalian cardiomyocytes. (a) A dual reporter strain ZEG mouse was crossbred with the MerCreMer strain to create MerCreMer-ZEG double heterozygous mice. Upon the pulse of 4-OH-tamoxifen, the reporter switches from β-galactosidase (encoded by lacZ) to GFP in cardiomyocytes only. A reduction in the percentage of GFP+ cardiomyocytes during aging or following myocardial infarction (MI) or pressure overload might indicate that new cardiomyocytes have been generated by stem or progenitor cells. (b) Dose-dependent effects of the 4-OH-tamoxifen pulse on GFP labeling in MerCreMer-ZEG mouse myocardium. Representative immunohistochemistry images of β-galactosidase (left) or GFP (center) after different durations of daily 4-OH-tamoxifen, counterstained with hematoxylin. A computer-based color separation technique (right) was used to facilitate the counting of GFP-positive cardiomyocytes; the result is shown in c. (d) High-efficiency cardiomyocyte GFP labeling. We used immunofluorescence costaining to visualize nuclei (DAPI; blue), cardiomyocytes (cardiac troponin T or cardiac MHC; red), and GFP (green). The yellowish cells indicate cardiomyocytes labeled with GFP. (e) Labeling was specific for cardiomyocytes. Immunostaining was used to examine GFP expression in other cell types, including vascular smooth muscle cells (α-smooth muscle actin; red), endothelial cells (isolectin-B4, red), and fibroblasts (vimentin; red). Scale bars, 20 μm. Data shown as mean ± s.e.m.
The hearts of MerCreMer-ZEG mice had normal histological structure and echocardiographic function, and the mice were fertile (data not shown). We first examined the labeling efficiency of GFP in cardiomyocytes from 8-week-old MerCreMer-ZEG mice after different 4-OH-tamoxifen pulse regimens, as the experimental strategy depended upon high-efficiency GFP+ labeling of cardiomyocytes with little GFP+ labeling in non-cardiomyocytes. Without 4-OH-tamoxifen, 98.6 ± 2.3% of cardiomyocytes expressed β-galactosidase and only 0.3 ± 0.1% of cardiomyocytes expressed GFP at 10 weeks of age (Fig. 1b,c). Following the 4-OH-tamoxifen pulse, expression of β-galactosidase decreased and expression of GFP increased in a dose-dependent manner (Fig. 1b). Using an automated color separation technique (Fig. 1b), we quantified the percentages of GFP+ cardiomyocytes that were achieved by different total dosages of 4-OH-tamoxifen, showing labeling efficiencies of 26.6 ± 10.1%, 48.1 ± 7.9% and 82.7 ± 5.5% following 1, 7 and 14 d of 4-OH-tamoxifen treatment, respectively (Fig. 1c).
We then used immunostaining for cardiac troponin T and MHC to confirm that GFP labeling occurred only in cardiomyocytes (Fig. 1d), but not in other cell types in the myocardium including vascular smooth muscle cells, endothelial cells, and fibroblasts (Fig. 1e). We detected no GFP expression in other tissues including skeletal muscle, lung, kidney, liver, brain, aorta and skin (data not shown), showing that the inducible MerCreMer-ZEG mouse strain provided highly efficient and specific cardiomyocyte labeling.
If we were to observe a decrease in the percentage of GFP+ cardiomyocytes during normal aging, this would imply that stem cells constantly replace cardiomyocytes. However, we found no significant change in GFP+ cardiomyocytes throughout a one-year aging period; the percentages of GFP+ cardiomyocytes were 82.7 ± 1.7% at 0 months after the 4-OH-tamoxifen pulse, 85.0 ± 1.8% at 3 months, 82.4 ± 1.1% at 6 months and 83.2 ± 1.3% at 12 months (Fig. 2a). The percentage of GFP+ cardiomyocytes was balanced by the percentage of β-galactosidase+ cardiomyocytes: 18.3 ± 2.4% at 0 months, 17.8 ± 3.8% at 3 months, 18.0 ± 2.1% at 6 months and 19.4 ± 2.8% at 12 months (Fig. 2b). Because leakage of the system, defined as spontaneous labeling of cardiomyocytes with GFP+ in the absence of 4-OH-tamoxifen (Fig. 2c), would interfere with the interpretation of results, we studied control animals that had received no 4-OH-tamoxifen. The cumulative leakage rates were very low: 0.2 ± 0.2% at 2 months, 1.5 ± 1.1% at 5 months, 1.4 ± 1.0% at 8 months and 1.7 ± 1.1% at 14 months (Fig. 2c). These data indicate that leakage of GFP expression in the absence of 4-OH-tamoxifen was unlikely to influence the results.
Figure 2.
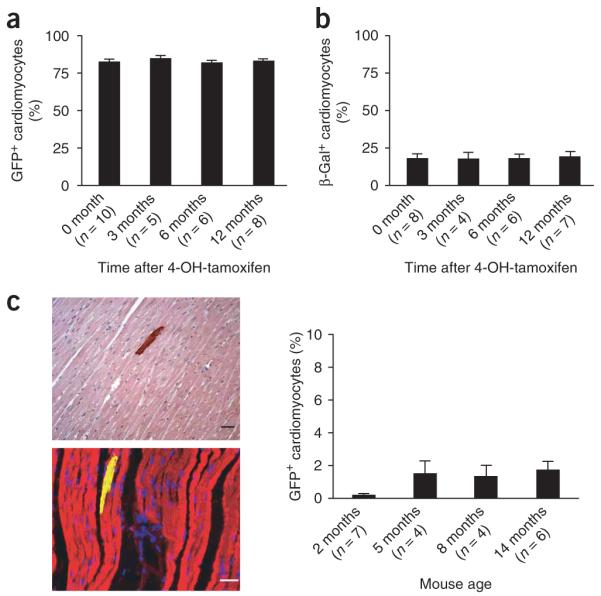
Adult mammalian cardiomyocytes are not detectably replaced by stem or progenitor cells during normal aging. (a) Immunohistochemistry for GFP during aging 0, 3, 6 and 12 months after the 4-OH-tamoxifen pulse. There were no significant differences in the percentage of GFP+ cardiomyocytes. (b) Immunohistochemistry for β-galactosidase (β-gal) during aging 0, 3, 6 and 12 months after the 4-OH-tamoxifen pulse. (c) Leakage of GFP in the absence of 4-OH-tamoxifen pulse was at a very low level, even over 14 months. Shown are representative GFP immunohistochemistry (upper; brown with hematoxylin counterstain) and immunofluorescence (lower; yellow) images showing rare GFP labeling in the absence of 4-OH-tamoxifen (red, troponin T; blue, DAPI). The graph shows leakage of GFP labeling at different mouse ages. Scale bars, 20 μm. Data shown as mean ± s.e.m.
To test whether there was a preferential regional localization of GFP+ myocytes or whether changes in GFP+ myocyte number with aging differed in different parts of the heart, we performed GFP and β-galactosidase staining on atrial and right ventricular tissue. In the right ventricle, we found no significant change in the percentage of GFP+ cardiomyocytes or β-galactosidase+ cardiomyocytes throughout a one-year aging period (Supplementary Fig. 1a,b online). In atrial tissue, the number of GFP+ cardiomyocytes was lower than in ventricular tissue, indicating lower labeling efficiency. We found no significant change in the percentage of GFP+ atrial cardiomyocytes or β-galactosidase+ cardiomyocytes during aging in atrial tissue (Supplementary Fig. 1c,d).
To exclude the possibility that cardiac stem cells were labeled with GFP, we performed immunostaining for the stem-cell markers Kit and Sca-1 (also known as Ly6a) together with GFP2,3. We detected no Kit+GFP+ or Sca-1+GFP+ cells in MerCreMer-ZEG mouse hearts (Supplementary Fig. 2 online). Furthermore, because bone marrow might be involved in cardiomyocyte renewal and regeneration, we also analyzed bone marrow cells from mice used in the aging study. We found no induction of GFP+ in bone marrow cells (data not shown). Therefore, it is unlikely that extracardiac GFP+ cells affected the results. Collectively, these data indicate that stem cells do not replace adult mouse cardiomyocytes during normal aging (up to one year).
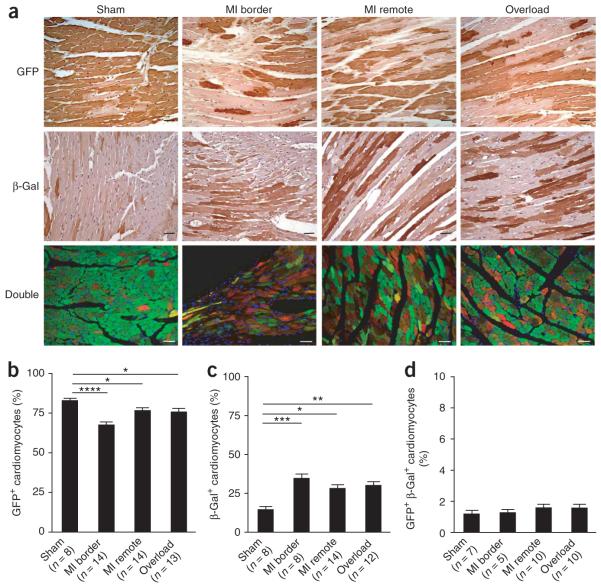
We then tested whether stem cells are activated following injury. After the 4-OH-tamoxifen pulse, we divided the MerCreMer-ZEG mice randomly into three groups, which received different treatments: sham operation, experimental myocardial infarction, and pressure overload by transverse aortic constriction. Three months after surgery, we found a significant decrease in the percentage of GFP+ cardiomyocytes in the border of myocardial infarctions and in remote areas from infarcted hearts, as well as in pressure-overloaded myocardium (Fig. 3a); the percentages of GFP+ cardiomyocytes were 82.8 ± 1.5% in sham-operated mice, 67.5 ± 2.0% in myocardial infarction border areas (P < 0.0001), 76.6 ± 1.8% in myocardial infarction remote areas (P < 0.05) and 75.7 ± 2.2% in pressure-overloaded hearts (P < 0.05) (Fig. 3b, Supplementary Fig. 3 online). The leakage of GFP labeling in the absence of 4-OH-tamoxifen in cardiomyocytes following myocardial injury remained at an insignificant level (data not shown). The decrease in the percentage of GFP+ cardiomyocytes was balanced by an increase in the percentage of β-galactosidase+ cardiomyocytes: 14.5 ± 2.0% in sham-operated mice, 34.6 ± 2.8% in myocardial infarction border areas (P < 0.001), 28.1 ± 2.4% in myocardial infarction remote areas (P < 0.05), and 30.0 ± 2.4% in pressure-overloaded hearts (P < 0.01) (Fig. 3c, Supplementary Fig. 3).
Figure 3.
Stem or progenitor cells replenish adult mammalian cardiomyocytes after myocardial injury. (a) The percentage of GFP+ cardiomyocytes decreased after myocardial injury. MerCreMer-ZEG mice with 4-OH-tamoxifen pulse labeling received experimental myocardial infarction or left ventricular overload. After 3 months, mouse hearts were fixed and stained with antibodies to GFP (green) or to β-galactosidase (red) (double staining in yellow). Shown are representative images of GFP and β-galactosidase staining (brown) in hearts after sham operation, myocardial infarction (border and remote areas), and pressure overload. (b) Percentage of GFP+ cardiomyocytes. (c) Percentage of β-galactosidase+ cardiomyocytes. (d) Percentage of GFP+ β-galactosidase+ cardiomyocytes. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Scale bars, 20 μm. Data shown as mean ± s.e.m.
It is important to recognize that many cells infiltrate the myocardial infarction border zone, including fibroblasts and monocytes, and it can be difficult to assess the cell type of these smaller cells. To deal with this concern, we required a cell to have typical cardiomyocyte morphology with sarcomeres to be counted. Furthermore, cell fusion might allow the expression of both GFP and β-galactosidase in the same cell because, although only one copy of the ZEG transgene is present in the genome, many mature cardiomyocytes have two nuclei. We examined the frequency of mosaicism of Cre-mediated recombination by immunofluorescence microscopy, using antibodies to GFP (green) and to β-galactosidase (red) to identify cells that were positive for both (yellow). The percentage of cardiomyocytes that were positive for both GFP and β-galactosidase was 1.2 ± 0.2% in sham-operated mice, 1.3 ± 0.2% in myocardial infarction border areas, 1.6 ± 0.7% in myocardial infarction remote areas, and 1.6 ± 0.8% in pressure-overloaded hearts (Fig. 3d). These results argue against changes in the percentage of GFP+ cardiomyocytes being caused by changes in the frequency of mosaic cells arising from cell fusion.
To test whether a pool of potential cardiac progenitor cells exists in infarcted hearts, we performed real-time PCR on mRNA isolated from the infarct zone of infarcted hearts and from cardiac tissue of sham-operated mice, 1, 3 and 7 d after surgery. The expression of Kit mRNA was 5.4-fold higher 3 d after myocardial infarction than it was 3 d after sham surgery, consistent with published data12. Other stem cell markers (Nanog, Zpf42 (also known as Rex-1), Dppa3, and Rif1) showed a small but significant increase in mRNA expression 7 d after myocardial infarction (Supplementary Fig. 2c,e,f). On the basis of the PCR data, we performed immunohistochemical staining for Kit on hearts harvested 3 d after myocardial infarction (n = 5) or sham surgery (n = 6); Kit+ cells were extremely rare in normal myocardium (1 cell observed in 25,554 cells counted), but we found 7.5 ± 1.3 Kit+ cells per 1,000 nuclei in infarcted myocardium (Supplementary Fig. 2d).
The decrease in the percentage of GFP+ cardiomyocytes observed 3 months after myocardial injury indicates that the GFP+ cardiomyocytes were diluted by a pool of GFP- precursor cells. However, this reduction could also arise from a decline in the division rate of GFP+ cardiomyocytes. To exclude this possibility, we delivered 5-bromo-2-deoxyuridine (BrdU) by osmotic minipumps to identify cells going into S-phase during the first week after injury. We performed triple staining for Nkx2-5 (a nuclear marker of cardiomyocytes), BrdU and GFP on sections from animals put to death 10 or 30 d after surgery (Supplementary Fig. 4a online). We found no BrdU+Nkx2-5+ nuclei in the hearts of sham-operated mice. There were more BrdU+Nkx2-5+ nuclei per 1,000 Nkx2-5+ nuclei in the myocardial infarction border area than in the myocardial infarction remote area or in pressure-overloaded hearts (Supplementary Fig. 4b). None of the BrdU+Nkx2-5+ cells was positive for GFP. Most of the BrdU+Nkx2-5+ cells did not have cardiomyocyte morphology, although these cells could be myocyte precursors. To explore the hypothesis that new cardiomyocytes were generated, we performed triple staining for cardiac troponin T, BrdU and GFP on myocardial sections from animals put to death 30 d after surgery (Supplementary Fig. 5a online). We found no BrdU+troponin T+ cells in the hearts of sham-operated mice. After myocardial infarction, BrdU+troponin T+GFP+ cells were observed only in the infarction border area. There were significantly more BrdU+troponin T+GFP- cells than BrdU+troponin T+GFP+ cells in the myocardial infarction border area, myocardial infarction remote area and after pressure overload (Supplementary Fig. 5b). These data are consistent with the idea that cardiomyocytes (detected by troponin T) in injured myocardium arise from a precursor pool (GFP- cells) that enters the cell cycle in the first week (detected by BrdU staining) after injury.
To exclude the possibility that the decrease in the percentage of GFP+ cardiomyocytes and the corresponding increase in the percentage of β-galactosidase+ cardiomyocytes after injury are due to GFP-labeled cells being more susceptible to injury, we performed TUNEL staining for apoptosis in combination with GFP staining at different time points after injury: 5 h, 10 h and 1 week after myocardial infarction or pressure overload. The percentage of apoptotic myocytes that were GFP+ was not significantly different in the different groups, and was not different from the percentage of non-apoptotic cardiomyocytes that were GFP+, indicating that GFP+ and GFP- cardiomyocytes are equally susceptible to apoptosis after injury (data not shown).
In summary, our results show that it is unlikely that stem or precursor cells contribute significantly to the formation of cardiomyocytes during normal aging up to one year in the mouse. By contrast, precursor cells might participate in the formation of new cardiomyocytes after injury. This study alone cannot prove that stem cells contribute to cardiomyocyte refreshment, nor can it define which stem cells contribute to the formation of new cardiomyocytes after injury. We do not know why almost 20% of the cardiomyocytes were not labeled with GFP. Possible explanations include differences in α-MHC promoter activity or 4-OH-tamoxifen sensitivity among cardiomyocytes, or other unknown differences. We cannot conclusively state that GFP+ and GFP- cells have exactly the same myocyte phenotype (although they appeared to have the same morphology), raising the possibility that cells might behave differently after injury. For example, injury could preferentially stimulate the proliferation of GFP- cells rather than the formation of new myocytes by progenitor cells. In this study, we assumed that precursor cells were not labeled by the 4-OH-tamoxifen pulse. Although we attempted to exclude this possibility by studying markers for adult stem cells, it is important to recognize that there is no known definitive marker that distinguishes adult cardiac myocytes from precursors. Our results are most easily interpreted in the context of a precursor pool that expresses α-MHC after differentiation to cardiac myocytes, but we cannot exclude the possibility that some progenitors were labeled by the 4-OH-tamoxifen pulse. Furthermore, our results provide only indirect evidence for the involvement of progenitor cells in the increase in GFP- cells. An immature population of cardiomyocytes could be responsible for the formation of new cardiomyocytes. A combined strategy of conditional genetic labeling of progenitor cells together with this fate-mapping approach could provide insight into the source of the GFP- cardiomyocytes.
METHODS
All experiments were conducted in accordance with the Guide for the Use and Care of Laboratory Animals and approved by the Harvard Medical School Standing Committee on Animals.
Generation of MerCreMer-ZEG mice
We generated double transgenic Mer-CreMer-ZEG mice by crossbreeding cardiomyocyte-specific MerCreMer mice10 and ZEG mice (Jackson Laboratory)11. β-galactosidase-GFP is under the control of a cytomegalovirus (CMV) enhancer/chicken actin promoter (Actb); the background strain was C57BL/6J (N7). The background strain of the MerCreMer mice was C57BL/6SV129. Genotyping was performed by PCR on tail DNA using the following primers: MerCreMer forward: 5′-GTCTGAC TAGGTGTCCTTCT-3′; MerCreMer backward: 5′-CGTCCTCCTGCTGGTA TAG-3′; ZEG forward: 5′-AAGTTCATCTGCACCACCG-3′; ZEG backward: 5′-TCCTTGAAGAAGATGGTGCG-3′; ZEG control forward: 5′-CTAGGCCA CAGAATTGAAAGATCT-3′; and ZEG control backward: 5′-GTAGGTG GAAATTCTAGCATCATCC-3′. Double heterozygous MerCreMer-ZEG mice were used for the subsequent experiments.
4-OH-tamoxifen pulse
To induce Cre recombination for GFP labeling in cardiomyocytes, we injected 4-OH-tamoxifen (Sigma), dissolved in sunflower oil (Sigma) at a concentration of 5 mg/ml, intraperitoneally into 8-week-old MerCreMer-ZEG mice daily at a dosage of 0.5 mg/d. To evaluate GFP labeling efficiency and optimize the activation of Cre recombination, we examined mouse hearts 5 d after completion of 1, 7 or 14 injections of 4-OH-tamoxifen.
Experimental myocardial infarction and pressure overload
Five days after the last of the 14 injections (7 mg total) of 4-OH-tamoxifen, we subjected MerCreMer-ZEG mice to experimental myocardial infarction or pressure overload. Myocardial infarction was produced by permanent ligation of the left coronary artery ∼3 mm below the left atrial appendix, as described13. Pressure overload was created by transverse aortic constriction using a 26-gauge needle, as described14. We used echocardiography to confirm successful creation of myocardial infarction and left ventricle hypertrophy 2 weeks after surgery (Supplementary Table 1 online). Three months after surgery, mice were put to death and the hearts were removed for histological examination.
BrdU protocol
MerCreMer-ZEG mice received 14 d of 4-OH-tamoxifen injections, followed by sham operation, myocardial infarction or pressure overload. At the time of surgery, osmotic minipumps (Alzet) were implanted subcutaneously, delivering BrdU (Sigma) at 1 μg per hour per gram body weight for 7 d.
Immunohistochemistry and immunofluorescence microscopy
We prepared paraformaldehyde-fixed paraffin-embedded mouse sections for immunohistochemistry and fluorescence microscopy as described15. The antibodies used were GFP-specific antibody (1:200, Abcam), β-galactosidase-specific antibody (1:200, Invitrogen), cardiac troponin T-specific antibody (1:100, Developmental Studies Hybridoma Bank), cardiac MHC-specific antibody (1-100, Abcam), α-smooth muscle actin-specific antibody (1:100, Sigma), vimentin-specific antibody (1:100, Sigma), Kit-specific antibody (1:50, Santa Cruz Biotechnology), Ly6a-specific antibody (1:100, R&D Systems), Nkx2-5-specific antibody (1:20, R&D Systems) and BrdU-specific antibody (1:50, Abcam). We used different Alexa Fluor secondary antibodies (Invitrogen) to obtain fluorescent colors. TUNEL staining was performed with the ApopTag Red in situ Apoptosis Detection Kit (Chemicon).
Image analysis
Three high-power images per animal were counted by an observer blinded to all experimental information, with an average total myocyte count of 312 ± 11 per animal. The myocardial infarction border was defined as an image not farther away than one high-power field from scar tissue, but did not include scar tissue. Myocardial infarction remote tissue was defined as tissue from the posterior wall. Microscopic images were captured digitally and the green channel was isolated for further analysis. Only cells with clear cardiomyocyte morphology were included, requiring the presence of sarcomeres. The presence or absence of nuclei was not included as a parameter. Non-stained (GFP or β-galactosidase) cells were clearly distinct from the background in all images. The denominator was the sum of negatively and positively stained cells. For analysis of BrdU+ and Nkx2-5+ nuclei, we counted the total number of nuclei in the Nkx2-5 channel first, and then the number of BrdU+Nkx2-5+ cells in the merged channel.
Data analysis
Results are presented as mean ± s.e.m. Statistical comparison was performed with nonparametric two-way analysis of variance. A probability value of <0.05 was considered to be statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
P.C.H. was supported by a fellowship from the American Heart Association. V.F.M.S. was supported by a Ph.D. fellowship of the Research Foundation—Flanders (FWO) and by a Belgian American Educational Foundation research fellowship. M.E.D. was supported by an NRSA fellowship from NIH/NHLBI. This study was supported by NIH/NHLBI grant R01HL081404.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
References
- 1.Jackson KA, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 3.Oh H, et al. Cardiac muscle plasticity in adult and embryo by heart-derived progenitor cells. Ann. NY Acad. Sci. 2004;1015:182–189. doi: 10.1196/annals.1302.015. [DOI] [PubMed] [Google Scholar]
- 4.Yoon YS, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J. Clin. Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winitsky SO, et al. Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro. PLoS Biol. 2005;3:e87. doi: 10.1371/journal.pbio.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laugwitz KL, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker RO, Chapin S, Sherry R. Regeneration of the ventricular myocardium in amphibians. Nature. 1974;248:145–147. doi: 10.1038/248145a0. [DOI] [PubMed] [Google Scholar]
- 8.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 9.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 10.Sohal DS, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ. Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 11.Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 12.Fazel S, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J. Clin. Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducharme A, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J. Clin. Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockman HA, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J. Clin. Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.