Abstract
Background
Endothelial-cardiac myocyte (CM) interactions play a key role in regulating cardiac function, but the role of these interactions in CM survival is unknown. This study tested the hypothesis that endothelial cells (ECs) promote CM survival and enhance spatial organization in a 3-dimensional configuration.
Methods and Results
Microvascular ECs and neonatal CMs were seeded on peptide hydrogels in 1 of 3 experimental configurations: CMs alone, CMs mixed with ECs (coculture), or CMs seeded on preformed EC networks (prevascularized). Capillary-like networks formed by ECs promoted marked CM reorganization along the EC structures, in contrast to limited organization of CMs cultured alone. The presence of ECs markedly inhibited CM apoptosis and necrosis at all time points. In addition, CMs on preformed EC networks resulted in significantly less CM apoptosis and necrosis compared with simultaneous EC-CM seeding (P<0.01, ANOVA). Furthermore, ECs promoted synchronized contraction of CMs as well as connexin 43 expression.
Conclusions
These results provide direct evidence for a novel role of endothelium in survival and organization of nearby CMs. Successful strategies for cardiac regeneration may therefore depend on establishing functional CM-endothelium interactions.
Keywords: endothelium, cardiomyopathy, heart failure, tissue
Recent studies suggest that the mammalian heart possesses some ability to regenerate itself through several potential mechanisms,1 including generation of new cardiomyocytes (CMs) from extracardiac progenitors,2-4 CM proliferation,5-7 or fusion with stem cells with subsequent hybrid cell division.8 These mechanisms are insufficient to regenerate adequate heart tissue in humans, although some vertebrates can regenerate large volumes of injured myocardium.
Several approaches in cell transplantation and cardiac tissue engineering have been investigated as potential treatments to enhance cardiac function after myocardial injury.9,10 Implantation of skeletal muscle cells, bone marrow cells, embryonic stem cell-derived CMs, and myoblasts can enhance cardiac function.11 Cell-seeded grafts have been used instead of isolated cells for in vitro cardiac tissue growth or in vivo transplantation.12-18 These grafts can develop a high degree of myocyte spatial organization, differentiation, and spontaneous and coordinated contractions. On implantation in vivo, cardiac grafts can integrate into the host tissue and neovascularization can develop. However, the presence of scar tissue and the death of cells in the graft can limit the amount of new myocardium formed,15,16,19 most likely due to ischemia.19 Therefore, creating a favorable environment to promote survival of transplanted cells and differentiation of progenitor cells remains one of the most important steps in regeneration of heart tissue.
One of the key factors for myocardial regeneration is revascularization of damaged tissue. In the normal heart, there is a capillary next to almost every CM, and endothelial cells (ECs) outnumber cardiomyocytes by ≈3:1.20 Developmental biology experiments reveal that myocardial cell maturation and function depend on the presence of endocardial endothelium at an early stage.20 Experiments with inactivation or overexpression of vascular endothelial growth factor (VEGF) demonstrated that at later stages, either an excess or a deficit in blood vessel formation results in lethality due to cardiac dysfunction.20-23 Both endocardium and myocardial capillaries have been shown to modulate cardiac performance, rhythmicity, and growth.24 In addition, a recent study showed the critical importance of CM-derived VEGF in paracrine regulation of cardiac morphogenesis.25 These findings and others highlight the significance of interactions between CMs and endothelium for normal cardiac function. However, little is known about the specific mechanisms forthese interactions, as well as the role of a complex, 3-dimensional organization of myocytes, ECs, and fibroblasts in the maintenance of healthy cardiac muscle.
The critical relation of CMs and the microvasculature suggests that successful cardiac regeneration will require a strategy that promotes survival of both ECs and CMs. The present study explored the hypothesis that ECs (both as preexisting capillary-like structures and mixed with myocytes at the time of seeding) promote myocyte survival and enhance spatial reorganization in a 3-dimensional configuration. The results demonstrate that CM interactions with ECs markedly decrease myocyte death and show that endothelium may be important not only for the delivery of blood and oxygen but also for the formation and maintenance of myocardial structure.
Methods
Cell Isolation
ECs were isolated from hearts and lungs of C57BL/6 8-week-old, male mice (Charles River Laboratories, Wilmington, Mass) as previously described by Lim et al.26 In all experiments, cells from both hearts and lungs were mixed and used simultaneously. Mouse CMs were isolated by modifying the protocol for rat neonatal CMs as previously described by Wang et al.27 In brief, primary cultures were obtained from 1- to 2-day-old C57BL/6 mice by incubating mouse hearts in 0.1% trypsin in Hanks’ buffered saline solution (both from Gibco BRL) for 7 hours, followed by digestion in 0.08% collagenase type II (Worthington) in Hanks’ buffered saline solution. Before being seeded on the peptide hydrogel, the cell suspension was preplated for 2 hours on a plastic cell-culture dish to allow nonmyocytes to attach, and the cells remaining in suspension were used in subsequent experiments. Mouse cardiac fibroblasts were isolated by following a previously described protocol.28,29 The Harvard Medical School Standing Committee on Animals approved the animal protocols.
Three-Dimensional Culture
Cells were cultured in 3-dimensional, 1% peptide hydrogel scaffolds (peptide sequence AcN-RARADADARARADADA-CNH2).30 To test whether ECs promote CM survival, 3 experimental groups were established: (1) CMs alone; (2) EC-CM coculture, wherein ECs and CMs were seeded at the same time; and (3) prevascularized, wherein EC networks were preformed by seeding ECs 1 day before CMs were added. To test whether the effect of ECs on myocyte survival is endothelium specific, we also performed experiments with myocyte-fibroblast cocultures. The cell seeding density was 0.7×106 cells/cm2 (CMs alone) or 1.4×106 cells/cm2 (both coculture groups and the prevascularized group in a 1:1 CM-nonmyocyte cell ratio). To exclude the possibility that the increasing cell density of added ECs caused the myocyte spatial reorganization, control experiments were performed with myocytes seeded separately at a density of 1.4×106 cells/cm2. In addition, control cultures with ECs only at seeding densities of 1.4×106 or 0.7×106 cells/cm2 were used to ensure that the presence of myocytes did not affect the EC ability to form capillary-like networks. To test whether the effect of ECs on myocyte survival required cell contact, CMs were cultured with EC-conditioned medium. All experiments were performed in triplicate. Cells were cultured for up to 7 days at 37°C and 5% CO2 in culture medium (10% fetal calf serum), with the medium being changed on days 2, 4, and 6.
Immunohistochemistry and Cell Death Assays
Cells were analyzed with the live/dead viability/cytotoxicity kit (Molecular Probes). For immunohistochemical analyses, the samples were fixed in 2% formaldehyde for 1 hour, rinsed with phosphate-buffered saline, permeabilized with 0.1% Triton X-100 in phosphate-buffered saline for 1 hour, and incubated with blocking buffer (20% fetal calf serum and 0.1% Triton X-100 in phosphate-buffered saline) for 2 hours at room temperature. Cultures were incubated with primary antibodies (mouse monoclonal anti-α-actinin, clone EA-53 [Sigma]), mouse anti-connexin [Cx]43 monoclonal antibody [Sigma], or rabbit anti-human anti-von Willebrand factor [Sigma]) for 30 minutes at room temperature, with subsequent washing in the blocking buffer overnight, followed by incubation with the second-ary antibodies (Alexa Fluor 488 goat anti-mouse IgG1, Alexa Fluor 594 goat anti-mouse IgM [both from Molecular Probes] or goat anti-rabbit IgG-fluorescein isothiocyanate [Sigma]) for 30 minutes. Finally, cultures were incubated with 4′6-diamidino-2-phenylindole (DAPI; Molecular Probes) to visualize cell nuclei. Samples incubated with secondary antibodies served as negative controls only.
Apoptosis was determined using a terminal dUTP nick end-labeling (TUNEL) kit (Roche) according to the manufacturer’s instructions. In brief, samples were fixed and stained by the immunofluorescent staining protocol described earlier, followed by exposure to a reaction mix containing terminal deoxynucleotidyl transferase and nucleotide mixture in equilibration buffer for 60 minutes at 37°C. For both TUNEL and necrosis assays, images of 3 different areas of each sample were taken with a fluorescence microscope, and the ratio of TUNEL-positive or necrotic CMs to the total number of myocytes was calculated for that sample, with myocytes identified as actinin-positive cells.
For high-resolution analysis of cell structures formed in EC-CM cocultures, cells were labeled with CellTracker dyes (green 5-chloromethylfluorescein diacetate [CMFDA] for ECs and orange 5-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine [CMRA] for myocytes) before being seeded on the peptide hydrogel. Cells were then cocultured for 3 days and fixed. Samples were embedded in paraffin, and 5-μm-thick slices were obtained and analyzed by fluorescence microscopy.
Evaluation of Contractile Areas
To compare the areas of spontaneous contractions, 80-frame videos were taken with a videomicroscope (25 ms/frame) of 3 randomly selected regions of each sample containing at least 100 cells. Contracting cells or cell clusters and structures contracting in synchrony were selected, and average areas of contracting regions were calculated for each image with Matlab (The Mathworks, Inc) and custom-written software.
Statistics
ANOVA with post hoc t tests and Bonferroni corrections was used. A probability value <0.05 was considered statistically significant.
Results
EC-CM Interactions Affect Myocyte Reorganization
To explore interactions between CMs and ECs in 3-dimensional culture, we used peptide hydrogels, a tissue engineering scaffold. Cells seeded on the surface of the hydrogel attach and then migrate into the hydrogel. When CMs alone were used, cells attached on day 1 and then formed small clusters of cells at days 3 and 7 (Figure 1). In contrast, when CMs were seeded together with ECs, cells formed interconnected linear networks, as commonly seen with ECs in 3-dimensional culture environments,31 with increasing spatial organization from day 1 to day 7 (Figure 1). To establish whether preformed endothelial networks enhanced the organization of myocytes, we also seeded ECs 1 day before myocytes were added. These ECs formed similar interconnected networks in the absence of myocytes; preforming the vascular network did not lead to significant differences in morphology (data not shown). Furthermore, to exclude the possibility that the increasing cell density of added ECs caused the spatial organization, we also performed control experiments with varying numbers and combinations of cells; there was no effect of doubling or halving cell numbers, indicating that the spatial organization effect was specifically due to ECs.
Figure 1.
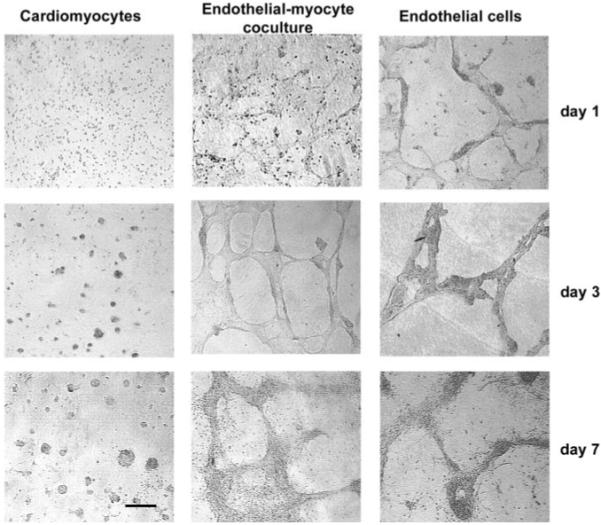
ECs promote CM reorganization. When CMs were cultured alone (left column), they aggregated into sparse clusters. When CMs were cultured with ECs (center), cells organized into capillary-like networks. There was no difference in morphological appearance between coculture or prevascularized cultures (not shown) and ECs alone (right column). Bar=100 μm. Abbreviations are as defined in text.
To establish that both myocytes and ECs were forming networks together, we performed immunofluorescence studies with specific antibodies, as well as analysis of cross sections of CM-EC cocultures, whereby cells were labeled with CellTracker dyes before seeding. Immunofluorescent staining demonstrated that >95% of CMs were present within these networks, suggesting that CMs preferentially migrate to or survive better near ECs (Figure 2). The analysis of cross sections demonstrated the presence of what appeared to be EC-derived, tubelike structures (Figure 3), with myocytes spread on the outer part of the capillary wall. Along with the capillary-like structures, clusters of intermingled cells (both myocytes and ECs) not containing the lumen were also observed (not shown). However, when the lumen was present, ECs were always on the inner side and myocytes on the outer side of the structure.
Figure 2.
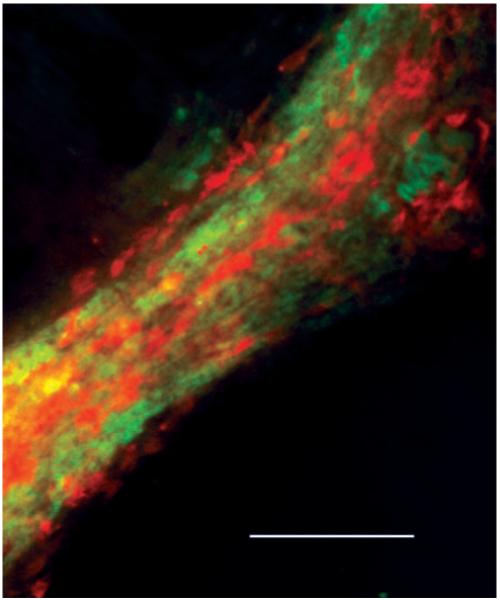
CMs appear on outside of endothelial networks. High-magnification, double-immunofluorescence image of structures formed in EC-CM coculture at day 7 demonstrating CMs (sarcomeric actinin, red) spread on top of ECs (von Willebrand factor, green) with no myocytes present outside structure. Bar=100 μm. Abbreviations are as defined in text.
Figure 3.
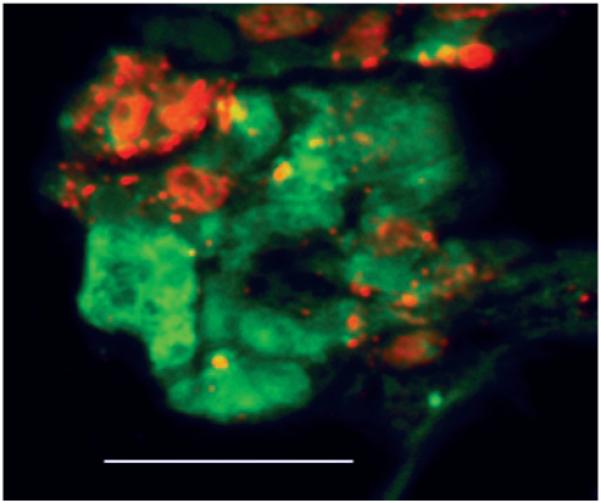
ECs form tubelike structures with myocytes spreading on outer wall. Cross section of paraffin-embedded sample of 3-day coculture of myocytes (red) and ECs (green) incubated in CellTracker dye before seeding on hydrogel. Bar=50 μm. Abbreviations are as defined in text.
In CM-fibroblast cocultures, cells rapidly (within 24 hours) formed large clusters consisting of cells of both types (not shown). At later time points, fibroblast proliferation resulted in their migration outside the clusters and spreading on the hydrogel without any pattern. However, in contrast to EC-CM cocultures, CMs remained in the clusters and demonstrated only limited spreading. Immunofluorescent staining revealed that there was no orientation of myocytes relative to the fibroblasts in the clusters. In cultures with EC-conditioned medium, myocyte morphology and spatial organization remained similar to those of myocyte controls.
ECs Improve Survival of CMs
To test the hypothesis that ECs promote CM survival, we assessed apoptosis and necrosis in the 3-dimensional cultures. Quantitative analyses of CMs positive for TUNEL and necrosis staining demonstrated significantly decreased myocyte apoptosis and necrosis when cultured with ECs, compared with CM-only cultures (Figure 4, P<0.01). This effect was observed at all 3 time points, although the decreased necrosis was most pronounced at day 1. In addition, CMs seeded on the preformed EC networks had a lower rate of apoptosis at day 1 relative to same-time seeding cultures (P<0.05, post hoc test), suggesting that early EC-CM interactions provided by the presence of well-attached and prearranged ECs may further promote CM survival. In contrast to the ECs, cardiac fibroblasts did not affect myocyte survival (P>0.05, Figure 4), with ratios for myocyte apoptosis and necrosis in the myocyte-fibroblast cocultures being similar to those for myocyte-only controls. However, addition of EC-conditioned medium resulted in a significant decrease in apoptosis and necrosis ratios of myocytes (P<0.01). Interestingly, the effect of conditioned medium on myocyte necrosis was similar in magnitude to the effect of ECs, whereas myocyte apoptosis ratios in the conditioned-medium group were only partially decreased compared with those in the presence of ECs. These results suggest that the prosurvival effect of ECs on CMs may not only be merely due to the local interactions between myocytes and ECs during myocyte attachment but may also involve direct signaling between myocytes and ECs.
Figure 4.
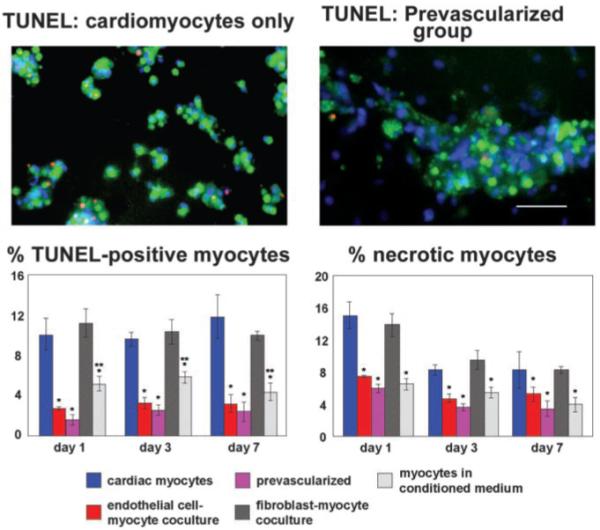
ECs prolong survival of CMs. Top, dual immunostaining of CMs and EC-myocyte prevascularized groups at day 3 in culture, with TUNEL-positive cells in red; green indicates sarcomeric actinin; blue, DAPI. Bottom, presence of ECs decreased CM apoptosis and necrosis, both in coculture conditions and when cultures were prevascularized by seeding with ECs 1 day before CMs (mean±SD, P<0.01). EC-conditioned medium decreased myocyte apoptosis and necrosis (P<0.01), whereas fibroblasts did not have any effect (P>0.05). *Different from myocytes alone; **different from EC-myocyte coculture and pre-vascularized. Bar=100 μm. Abbreviations are as defined in text.
Preformed Endothelial Networks Promote Coordinated, Spontaneous Contractions
In the prevascularized group with preformed vascular structures, synchronized, spontaneous contractions of large areas (Figure 5, top panels) were detected as early as days 2 to 3after seeding, in contrast to the coculture group, wherein such contractions were observed on days 6 to 7. In CM-only cultures, beating of separate cells and small cell clusters was also detected at days 2 to 3, similar to that in the prevascularized group. However, the average area of synchronized beating at day 3 in the myocyte-only group (3.5±0.5×102 μm2) was nearly 3 orders of magnitude smaller than the synchronously contracting area in the prevascularized group (4.3±2.5×105 μm2, mean±SD, n=5). These data suggest that ECs promote synchronized CM contraction, particularly when vascular networks are already formed.
Figure 5.
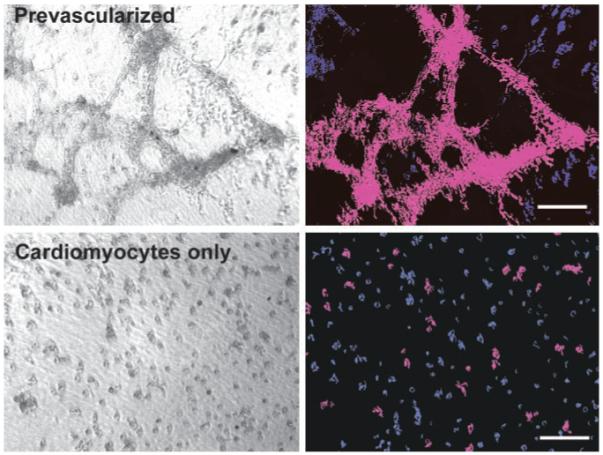
ECs promote large-scale, synchronized contraction of CMs. Left, phase-contrast video of beating areas in CM-only and prevascularized groups (day 3). Right, motion analysis of video showing regions of synchronized contractions (connected areas in purple are contracting synchronously) and nonmoving areas in blue. Bars=100 μm. Abbreviations are as defined in text.
ECs Promote Cx43 Expression
Staining for Cx43 showed striking differences in the distribution pattern of this gap junction protein between EC-CM cocultures and CMs cultured alone. In myocyte-only cultures, Cx43 expression was barely detectable at day 1 (not shown); at days 3 and 7, Cx43 expression was sparse throughout the cell clusters (Figure 6). In the presence of ECs (in both coculture and prevascularized groups), Cx43 staining was evident at day 1, both between ECs and distributed among CMs. As early as day 3 in culture, patches of localized junction-like Cx43, in addition to diffuse staining, were observed for myocytes in the coculture group (Figure 6). In the prevascularized group at day 3, wherein spontaneous contractions were already observed, more junction-like patches of Cx43 were observed compared with the coculture group, indicating electrical connections between myocytes (Figure 6). In addition to junctions between myocytes, there was also evidence of Cx43 localized at the interface between ECs and myocytes (Figure 6) detected in both the coculture group (at day 7) and the preculture group (as early as day 3). When myocytes and myocyte-EC coculture groups were cultured for 3 days with or without addition of 100 ng/mL of neutralizing anti-mouse VEGF antibody (R&D Systems), we observed no differences in either apoptosis or Cx43 staining between VEGF antibody-containing cultures and controls.
Figure 6.
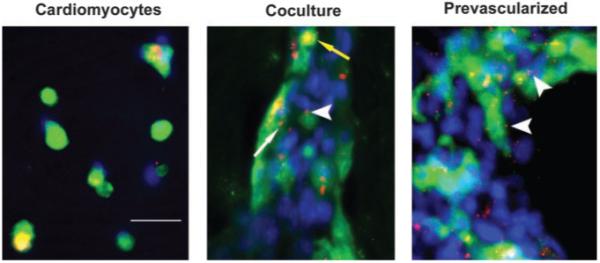
ECs promote Cx43 expression. Cultures at 3 days immunostained for Cx43 (red) and anti-sarcomeric actinin (green); nuclei are stained with DAPI (blue). For CMs alone (left), Cx43 staining is diffuse and sparse, with no evidence of gap junctions; for coculture (center), both diffuse (yellow arrow) and patchlike (thin, white arrow) Cx43 staining is observed; for prevascularized (right), increased patchlike staining indicates presence of gap junctions. Thick arrow-heads indicate junctions between myocytes and ECs. Bar=50 μm. Abbreviations are as defined in text.
Discussion
Currently, many different strategies are being pursued for regeneration of myocardium, including recruitment of endogenous myocyte precursors, injection of skeletal myoblasts, infusion of endothelial progenitor cells, and ex vivo cultivation of myocardium-like tissues. Each strategy holds promise but also faces extraordinary challenges. This study suggests that consideration of geometrically appropriate endothelial-myocyte relations may be a key strategy for myocardial regeneration. Most studies to date have found that a paucity of injected cells will ultimately survive within the myocardium. Our data indicate that the presence of EC networks may greatly promote myocyte survival and that the prosurvival effect is endothelium specific, because myocyte coculturing with cardiac fibroblasts did not affect either myocyte apoptosis or necrosis. These findings suggest that efficiency of cell therapies could be greatly improved by including ECs or progenitors as part of the regenerated myocardial environment. Furthermore, consideration of endothelial effects could provide strategies for reducing proarrhythmias reported in initial studies of skeletal myoblast transplantation.10
Our data suggesting improved synchrony of myocytes in the presence of ECs raise the intriguing hypothesis thatelectrical organization in the heart may be guided by vascular networks. This hypothesis is further supported by a recent study showing that the endothelium in arterioles can support electrical coupling as the mode of signal transmission.32 Previously, functional Cx43 channels have been described for both myocyte-myocyte and endothelial-endothelial cell junctions.33-36 In addition, a possible presence of myoendothelial Cx43 gap junctions between smooth muscle cells and endothelium in arterioles of the hamster cheek pouch has been suggested.32,34 Our results suggest that heterocellular endothelial-CM junctions may exist between CMs and ECs, which has not been reported for adult cardiac tissue.24 Interestingly, a recent study demonstrated that VEGF enhanced Cx43 expression in myocytes,37 suggesting that a VEGF-dependent mechanism for signaling between ECs and myocytes may also play a role in our system. We observed no differences in either apoptosis or Cx43 staining between VEGF antibody-containing cultures and controls; further experiments will be necessary to determine whether other cytokines or growth factors mediate these effects. It is important to note that theobserved difference in Cx43 staining and improved electrical connectivity in the presence of endothelial structures may be merely a consequence of better myocyte spreading and survival, rather than biological interactions between ECs and CMs.
Irrespective of which strategy is used to regenerate myocardium, establishing or maintaining a functional and stable capillary network will be crucial for myocardial performance. In addition to maintaining a minimum intercapillary distance to provide oxygen and nutrients, the endothelium may directly affect myocyte function. For example, cardiac ECs produce nitric oxide, endothelin, and prostacyclin; all of these molecules can affect cardiac growth and contractility. These results are highly consistent with experiments in developmental biology that demonstrate dependence of cardiac development on endothelial signals.20,36
In conclusion, the presence of EC networks profoundly improves CM survival and organization. These data have broad implications for cardiac tissue engineering and suggest that strategies that deliver myocytes or ECs alone may not be sufficient, unless endogenous mechanisms can recruit or maintain the complementary cells. Ultimately, the survival and function of transplanted myocytes may depend on nearby ECs.
Acknowledgments
Daria Narmoneva was supported by the American Heart Association. This project was supported by grants from the Center for Innovative Technology for Medicine and the National Institutes of Health.
References
- 1.Leferovich JM, Heber-Katz E. The scarless heart. Cell Dev Biol. 2002;13:327–333. doi: 10.1016/s1084952102000885. [DOI] [PubMed] [Google Scholar]
- 2.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laflamme MA, Myerson D, Saffitz JE, et al. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 4.Nadal-Ginard B, Kajstura J, Leri A, et al. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;92:139–150. doi: 10.1161/01.res.0000053618.86362.df. [DOI] [PubMed] [Google Scholar]
- 5.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodeling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 6.Anversa P, Leri A, Kajstura J, et al. Myocyte growth and cardiac repair. J Mol Cell Cardiol. 2002;34:91–105. doi: 10.1006/jmcc.2001.1506. [DOI] [PubMed] [Google Scholar]
- 7.Quaini F, Urbanek K, Beltrami AP, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 8.Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 9.Mann BK, West JL. Tissue engineering in the cardiovascular system: progress toward a tissue engineered heart. Anat Rec. 2001;263:367–371. doi: 10.1002/ar.1116. [DOI] [PubMed] [Google Scholar]
- 10.Menache P. Cell transplantation in myocardium. Ann Thorac Surg. 2003;75:S20–S28. doi: 10.1016/s0003-4975(03)00462-4. [DOI] [PubMed] [Google Scholar]
- 11.Dowell JD, Rubart M, Pasumarthi KBS, et al. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 2003;58:336–350. doi: 10.1016/s0008-6363(03)00254-2. [DOI] [PubMed] [Google Scholar]
- 12.Akins RE, Boyce RA, Madonna ML, et al. Cardiac organogenesis in vitro: reestablishment of three-dimensional tissue architecture by dissociated neonatal rat ventricular cells. Tissue Eng. 1999;5:103–118. doi: 10.1089/ten.1999.5.103. [DOI] [PubMed] [Google Scholar]
- 13.Eschenhagen T, Didie M, Munzel F, et al. Basic Res Cardiol. 2002;97:I-146–I-152. doi: 10.1007/s003950200043. [DOI] [PubMed] [Google Scholar]
- 14.Leor J, Aboulafia-Etzion S, Dar A, et al. Bioengineered cardiac grafts: a new approach to repair the infarcted myocardium? Circulation. 2000;102(suppl III):III-56–III-61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 15.Ozawa T, Mickle D, Weisel RD, et al. Optimal biomaterial for creation of autologous cardiac grafts. Circulation. 2002;106(suppl I):I-176–I-182. [PubMed] [Google Scholar]
- 16.Shimizu T, Yamato M, Isoi Y, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40–e48. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 17.Papadaki M, Bursac N, Langer R, et al. Tissue engineering of functional cardiac muscle: molecular, structural and electrophysiological studies. Am J Physiol Heart Circ Physiol. 2001;280:H168–H178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 18.Kellar RS, Landeen LK, Shepherd BR, et al. Scaffold-based three-dimensional human fibroblast culture provides a structural matrix that supports angiogenesis in infarcted heart tissue. Circulation. 2001;104:2063–2068. doi: 10.1161/hc4201.097192. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Methot D, Poppa V, et al. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cel Cardiol. 2001;33:903–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 20.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 21.Carmeliet P, Ng YS, Nuyens D, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF168. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeting inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 23.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127:3941–3946. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- 24.Brutsaert DL, Fransen P, Andries LJ, et al. Cardiac endothelium and myocardial function. Cardiovasc Res. 1998;38:281–290. doi: 10.1016/s0008-6363(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 25.Giordano FJ, Gerber H-P, Williams S-P, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim YC, Garcia-Cardena G, Allport JR, et al. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am J Pathol. 2003;162:1591–1601. doi: 10.1016/S0002-9440(10)64293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, De Keulenaer GW, Lee R. Vitamin D3-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 2003;277:26496–26500. doi: 10.1074/jbc.M202133200. [DOI] [PubMed] [Google Scholar]
- 28.Hao J, Wang B, Jones SC, et al. Interaction between angiotensin II and Smad proteins in fibroblasts in failing heart and in vitro. Am J Physiol (Heart Circ Physiol) 2000;279:H3020–H3030. doi: 10.1152/ajpheart.2000.279.6.H3020. [DOI] [PubMed] [Google Scholar]
- 29.Tsuruda T, Jougasaki M, Boerrigter G, et al. Cardiotrophin-1 stimulation of cardiac fibroblast growth: roles for glycoprotein 130/leukemia inhibitory factor receptor and the endothelin type A receptor. Circ Res. 2002;90:128–134. doi: 10.1161/hh0202.103613. [DOI] [PubMed] [Google Scholar]
- 30.Holmes TC. Novel peptide-based biomaterial scaffolds for tissue engineering. Trends Biotechnol. 2002;20:16–21. doi: 10.1016/s0167-7799(01)01840-6. [DOI] [PubMed] [Google Scholar]
- 31.Vailhé B, Vittet D, Feige J-J. In vitro models of vasculogenesis and angiogenesis. Lab Invest. 2001;81:439–452. doi: 10.1038/labinvest.3780252. [DOI] [PubMed] [Google Scholar]
- 32.Dora K, Xia J, Duling BR. Endothelial cell signaling during conducted vasomotor responses. Am J Physiol Heart Circ Physiol. 2003;285:H119–H126. doi: 10.1152/ajpheart.00643.2002. [DOI] [PubMed] [Google Scholar]
- 33.Van Veen TAB, van Rijen HVM, Opthof T. Cardiac gap junction channels: modulation of expression and channel properties. Cardiovasc Res. 2001;51:217–229. doi: 10.1016/s0008-6363(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 34.Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol (Heart Circ Physiol) 1995;37:H729–H739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- 35.Carter TD, Chen XY, Carlile G, et al. Porcine aortic endothelial gap junctions: identification and permeation by caged InsP3. J Cell Sci. 1996;109:1765–1773. doi: 10.1242/jcs.109.7.1765. [DOI] [PubMed] [Google Scholar]
- 36.Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- 37.Pimentel RC, Yamada KA, Kleber AG, et al. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res. 2002;90:671–677. doi: 10.1161/01.res.0000014823.75393.4d. [DOI] [PubMed] [Google Scholar]


