Abstract
The inositol 1,4,5-trisphosphate (InsP3) receptor is essential for signal Ca2+ release from intracellular stores and for capacitative Ca2+ entry. We have isolated the promoter and proximal DNA segments of the human type I InsP3 receptor gene. Transcription initiation in human G-292 osteosarcoma and HL-60 promyelocytic leukemia cells was shown to occur predominantly from an adenine residue located 39 base pairs downstream of a consensus TATA box element. Upstream DNA including the TATA box promoted directional transcription of a chloramphenicol acetyltransferase reporter gene when transfected into G-292 cells. A negative regulatory element in the distal promoter and a positive element in the proximal region were identified by deletion mapping and transcription assays. The proximal region enhanced transcription in response to 12-O-tetradecanoylphorbol-13-acetate or serum, but conferred transcriptional repression in response to 1,25-dihydroxyvitamin D3 or 17β-estradiol. The repressive effect of 17β-estradiol was mediated by the nuclear estrogen receptor, as estrogen-dependent transcriptional repression was inhibited by the antiestrogen tamoxifen and the estrogen receptor antagonist ICI 182,780. This is the first study of the type I InsP3 receptor gene promoter, and the results suggest a mechanism by which chronic estrogen treatment of osteoblasts affects type I InsP3 receptor gene expression, signal transduction, and secretion.
Inositol 1,4,5-trisphosphate (InsP3)1 mediates the calcium-mobilizing effects of a wide range of hormones, cytokines, and neurotransmitters (1). Activation of G-protein-linked and tyrosine kinase-linked receptors stimulates phosphatidylinositol-specific phospholipase C-mediated hydrolyis of phosphatidylinositol 4,5-bisphosphate with the resultant generation of the second messenger molecules, diacylglycerol, and InsP3. InsP3 binds to specific receptors located on endoplasmic reticulum, causing the release of sequestered Ca2+ into the cytosol and the stimulation of Ca2+-dependent processes including secretion (1–3).
InsP3 receptors have been purified as 250–313-kDa proteins and have been shown to function as tetrameric Ca2+ channel complexes whose conductance is regulated by the binding of InsP3 (4, 5). Three distinct, highly homologous (>60%) cDNAs of 8.8–10.7 kb encoding full-length InsP3 receptors have been cloned from rodent, human, and other sources (6–13). Partial sequences of two additional InsP3 receptor types, which are highly homologous to type II, have also been identified (14). Recent studies have shown that individual InsP3 receptor types are variably expressed in different tissues, and that multiple receptor types can be expressed within a given cell and these may assemble as heterotetramers in a single Ca2+ conductance channel (15, 16). Furthermore, InsP3-induced calcium release and InsP3 receptor-mediated calcium entry may be variably controlled by different InsP3 receptor subtypes (17, 18).
The expression and function of InsP3 receptors are hormonally regulated. Treatment of promyelocytic HL-60 cells with retinoic acid or 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) stimulates InsP3 receptor gene transcription, increases the expression of functional InsP3-regulated intracellular Ca2+ channels, and up-regulates cell secretory capacity (19, 20). In pancreatic islets of fasted and then fed rats and also in insulin-secreting RINm5F cells treated chronically with high glucose, type III InsP3 receptor expression is increased (21). On the other hand, stimulation of neuronal and pancreatoma cells with phosphoinositide-linked hormones causes the rapid down-regulation of the type I InsP3 receptor protein (22, 23). In primary osteoblasts and osteoblastic cell lines, type I InsP3 receptor messenger RNA expression is decreased by 17β-estradiol and 1,25(OH)2D3 but increased by phorbol esters and serum (24). These studies suggest that chronic regulators of cell secretory capacity may affect these changes through regulation of InsP3 receptor levels.
As an initial step to help understand how InsP3 receptor gene expression may be regulated under different physiological or pathological situations, we have isolated genomic clones encoding the promoter and six of the initial exons of the human type I InsP3 receptor gene. The major transcription start sites are identified, and it is shown that the type I InsP3 receptor gene promoter functions in human osteoblastic cell lines to regulate transcription of a reporter gene. Furthermore, it is shown that several chronic regulators of osteoblasts secretory activity affect type I InsP3 receptor promoter activity. Significantly, 17β-estradiol via the estrogen receptor down-regulates promoter activity, consistent with its effects on type I InsP3 mRNA levels in primary osteoblasts and osteoblastic osteosarcoma cells (24). These findings are interpreted in terms of the potential mechanisms by which chronic estrogen treatment may affect osteoblast secretory capacity.
EXPERIMENTAL PROCEDURES
Cell Culture
Cells from the G-292 human osteosarcoma cell line (ATCC CRL 1423) were cultured in McCoy’s 5a medium (Life Technologies, Inc.) supplemented with 10% fetal bovine serum, 2 mm glutamine, 100 units/ml penicillin, and 100 µg/ml streptomycin at 37 °C in a humidified atmosphere of 5% CO2 in air. Experiments with G-292 cells were conducted in phenol red-free Dulbecco’s modified Eagle’s medium (Life Technologies, Inc.) and 2% charcoal-stripped fetal bovine serum depleted of endogenous steroids as described (24, 25). G-292 cells have osteoblastic phenotype in that they express osteocalcin mRNA, bone morphogenic protein (BMP-7) receptor, and secrete interleukin-6 in response to interleukin-1β and other osteoblast secretagogues (26). Cells from the human promyelocytic leukemia HL-60 cell line (ATCC CCL 240) were cultured and maintained in RPMI 1640 medium with 20% fetal bovine serum as described previously (19).
Isolation of Human Genomic DNA Clones Containing the Type I InsP3 Receptor Gene Promoter
A human placental genomic DNA library in bacteriophage λFIXII (Stratagene Inc., La Jolla, CA) was screened by plaque hybridization (27) to obtain type I InsP3 receptor gene clones. Probes for the human type I InsP3 receptor gene were derived by PCR amplification of human HL-60 cell cDNA using primers based upon the mouse and rat type I InsP3 receptor cDNA sequences (7, 8). Primer pairs were numbered according to the mouse type I InsP3 receptor cDNA and included: p489s, 5′-GAG ACT GCC TCT TTA AGC TAT GTC C-3′ (489–513 nt) and p970a, 5′-CAG TTG ACG GAG TTG ACC TCA TTG C-3′ (antisense of 945–969 nt); p85s, 5′-GAG AGA AAG CGC ACG CCG AGA GGA G-3′ (85–109 nt) and p513a, 5′-GGA CAT AGC TTA AAG AGG CAG TCT G-3′ (antisense of 489–513 nt); and p85s, 5′-GAG AGA AAG CGC ACG CCG AGA GGA G-3′ (85–109 nt) and p255a, 5′-GCT CCT CTG TAG TCA GCT CCT TGG T-3′ (antisense of 241–255 nt). The p85s oligonucleotide was selected as the most 5′ primer in the cDNA because the reported mouse and rat type I InsP3 receptor cDNA sequences diverge upstream of this point. PCR products were gel-purified, subcloned into pGEM-11Zf(+) (Promega Corp., Madison, WI), and sequenced to confirm their identities.
Type I InsP3 receptor cDNA probes were labeled with [α-32P]dCTP by the random primer method and column-purified. For library screening, SRB/P2 host bacteria (Stratagene Inc., La Jolla, CA) were infected with the λFIXII phage and plated at 5 × 104 plaque-forming units onto each of 10 150-mm plates. Resultant plaques were transferred in duplicate to Colony/Plaque Screen nylon membranes (NEN Life Science Products). The membranes were prehybridized and hybridized according to manufacturer’s recommendations using 5 × 105 cpm/ml 32P-labeled type I InsP3 receptor cDNA probe (1 × 109 dpm/µg). Positive plaques were identified by autoradiography and selected through secondary and tertiary screenings. Purified phage DNAs were digested with various restriction enzymes for mapping, hybridization, subcloning, and sequencing. For analysis of the type I InsP3 receptor gene promoter region, a 3.2-kb XbaI/HindIII fragment containing 5′-flanking gene sequences, the first exon, and part of the first intron was subcloned into pGEM-11Zf(+). An internal 1.5-kb BglII fragment of the XbaI/HindIII fragment was subcloned and sequenced from both strands in its entirety using M13 and internal primers. DNA sequencing reactions were performed by the dideoxy chain termination method using modified T7 DNA polymerase (Sequenase version 2.0, U. S. Biochemical Corp.) and [α-35S]ATP (NEN Life Science Products). Sequences were analyzed using MatInspector version 2.0 (28).
Primer Extension Analysis
Primer extension was carried out as described (27) with modification. A 25-mer oligonucleotide, p109a, corresponding to the antisense sequence of bases +109 through +85 of the reported mouse type I InsP3 receptor cDNA (7) (+37 through +13 of the later reported human sequence; Ref. 11) was end-labeled using [γ-32P]ATP (7,000 Ci/mmol, ICN Biomedical Inc, Costa Mesa, CA) and T4 polynucleotide kinase and purified using Bio-spin 6 columns (Bio-Rad). The labeled primer (50 fmol, 4 × 105cpm/reaction) was allowed to anneal to 20 µg of total cellular RNA derived from either G-292 osteoblasts or HL-60 promyelocytic cells by incubation for 1 h at 62 °C in Superscript II reverse transcriptase buffer (Life Technologies, Inc.) containing RNasin. The reactions were cooled to 46–48 °C, and then extended for 25 min by addition of dNTPs and Superscript II reverse transcriptase. The mixtures were subjected to RNase A digestion, phenol/chloroform extraction, and ethanol/sodium acetate precipitation. Samples were analyzed by electrophoresis on denaturing 6% polyacrylamide gels alongside DNA sequencing reactions of the 0.5-kb human type I InsP3 receptor SacI genomic subclone using the same p109a type I InsP3 receptor oligonucleotide as used for the extension.
Type I InsP3 Receptor Gene Promoter-CAT Reporter Plasmid Constructs
The eukaryotic expression vector pCAT-Basic (Promega Corp, Madison, WI), which contains the bacterial chloramphenicol acetyltransferase (CAT) gene but lacks promoter and enhancer sequences, was used as the parent vector in constructing reporter plasmids for the analysis of putative InsP3 receptor promoter and transcriptional regulatory sequences. The 1.5-kb BglII restriction fragment, containing bp −1150 through +350 of the human type I InsP3 receptor gene, was filled-in and blunt end-ligated into the filled-in, XbaI-cut pCAT-Basic vector. Clones with the promoter in the forward orientation in front of the CAT gene were isolated, digested with PstI and SalI, and subjected to unidirectional digestion using exonuclease III followed by mung bean nuclease. This produced a series of constructs containing progressively larger deletions starting from the SalI site in the vector multiple cloning site and proceeding into 5′ end of the upstream region of the promoter construct. The resultant DNAs were ligated and used to transform competent DH5α Escherichia coli. Recombinant pCAT constructs were purified by Midi Kit procedures (Qiagen), sequenced to identify the extent of the deletion, and used in transient transfection assays. In some experiments, the 0.5-kb SacI restriction fragment including bp −359 through +153 of the type I InsP3 receptor gene was cloned in front of the CAT gene of the pCAT-Basic vector and used in transient transfections.
Transfection and CAT Expression Assays
G-292 cells were cultured to ca. 50% confluency in 60-mm2 tissue culture dishes, washed twice with Opti-MEM (Life Technologies, Inc.), and then transfected with a DNA-LipofectAMINE (Life Technologies, Inc.) mixture according to manufacturer’s instructions. Equal molar amounts of the various recombinant pCAT-Basic expression vectors (approximately 3 µg) were transfected along with a fixed amount (1.5 µg) of the pSV-β-galactosidase control vector (Promega Corp.). Cells were incubated for 12–14 h with the DNA-LipofectAMINE mixture, washed twice, and then incubated with phenol red-free Dulbecco’s modified Eagle’s medium containing 2% charcoal-stripped fetal bovine serum. Hormones and other test agent were then added. After 48–72 h, the cells were harvested, washed, and soluble extracts prepared by repeated freeze-thawing in 0.15 ml of 0.25 m Tris, pH 8.0, and used for determination of β-galactosidase and CAT activities. β-Galactosidase activity in 20 µl of each sample was determined spectrophotometrically as described (29) using o-nitrophenyl-β-d-galactopyranoside as substrate. After normalization for β-galactosidase activities, CAT activities in the soluble extracts (usually ~50–100 µl) were determined by incubation at 37 °C for 12–18 h with 3 µl of [14C]chloramphenicol (50–60 mCi/mmol, NEN Life Science Products), 5 µl of 5 mg/ml acetyl coenzyme A, and 0.25 m Tris-HCl, pH 8.0, in a volume of 150 µl. The mixtures were phase extracted and subjected to thin layer chromatography. Results were analyzed by autoradiography using Bio-Rad Phosphor Imaging and Molecular Imager Systems, version 1.4.
Reagents and Materials
Water-soluble 17β-estradiol, 17α-estradiol, tamoxifen, retinoic acid, and 12-O-tetradecanoylphorbol-13-acetate (TPA), were purchased from Sigma. Retinoic acid and TPA were dissolved as stock solutions in dimethyl sulfoxide. 1,25(OH)2D3 was generously provided by Dr. J. Napoli (SUNY, Buffalo). The concentration and purity of the 1,25(OH)2D3 were assessed by UV spectroscopy with an absorbance maximum at 265 nm (ϵ265 = 18,200), minimum at 229 nm, and a maximum/minimum ratio of 1.80. ICI 182,780 was generously provided by Dr. A. E. Wakeling of Zeneca Pharmaceuticals, Macclesfield, United Kingdom. Hormones and reagents were added from 2,000-fold stock concentrations. Equivalent volumes of vehicle had no effect on cell viability or CAT activity. Restriction enzymes and other DNA modification reagents were from Promega Corp. Oligonucleotides were obtained from Bio-Synthesis, Inc. (Lewisville, TX).
RESULTS
Cloning and Sequence Analysis of the 5′-Region of the Human Type I InsP3 Receptor Gene
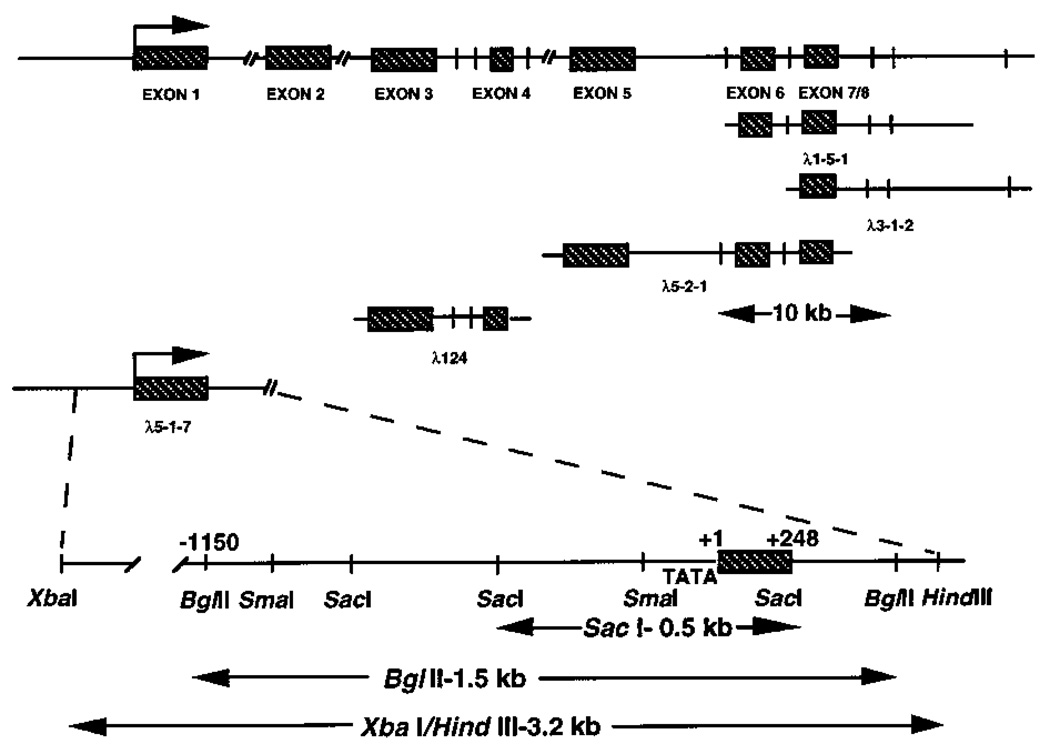
To study the regulatory elements governing the tissue-specific expression of the type I InsP3 receptor, a human placental genomic DNA library in a λFIXII host was screened for clones containing the 5′-untranslated and promoter regions of the type I InsP3 receptor gene. Using a series of PCR-derived probes corresponding to the 5′ region of the human type I InsP3 receptor cDNA, five independent clones were isolated and characterized by restriction enzyme mapping, Southern blotting, DNA subcloning, and DNA sequencing (Fig. 1). Three of these clones (λ1-5-1, λ3-1-2, and λ5-2-1) were selected by their hybridization to a human probe corresponding to bp 489–970 of the type I InsP3 receptor cDNA (numbering is in reference to the homologous mouse sequence) and were shown to overlap as indicated. Three separate exons were found by restriction enzyme digestion and Southern hybridization of these overlapping clones. A fourth independent clone (λ124) encoding two exons was isolated using a human cDNA probe derived from bp 85–513 of the cDNA sequence (mouse numbering). The more 5′ of these exons, denoted exon 3, is at least the third exon in the gene and begins at base 241 of the human cDNA sequence (11). The ATG initiation methionine codon at nucleotides 257–259 is included within this exon. The clone λ5-1-7 was isolated using a human 174-bp probe derived from bp 85–187 (mouse cDNA numbering) of the 5′-untranslated region of the type I InsP3 receptor cDNA. Subclones of this λ5-1-7 DNA, including a 0.5-kb SacI fragment and a 3.2-kb XbaI/HindIII fragment were isolated by their hybridization to the same 174-bp probe. The 1.5-kb BglII internal fragment of the 3.2-kb XbaI/HindIII subclone was sequenced completely (Fig. 2). This BglII fragment contains ~1.2 kb of upstream nontranscribed DNA including a consensus TATA box element, the first exon, and part of the first intron of the human type I InsP3 receptor gene.
FIG. 1. Schematic representation of the 5′ region of the human type I InsP3 receptor gene.
Top, exon-intron organization showing SacI sites (vertical bars) and the λFIXII clones characterized in the study. A 10-kb scale is shown for size comparison. Bottom, restriction endonuclease map of the λ5-2-1 clone showing transcription start site (+1), the first exon, as well as the SacI, BglII, and XbaI/HindIII subclones used for sequencing and promoter analyses. Exons are represented by shaded boxes.
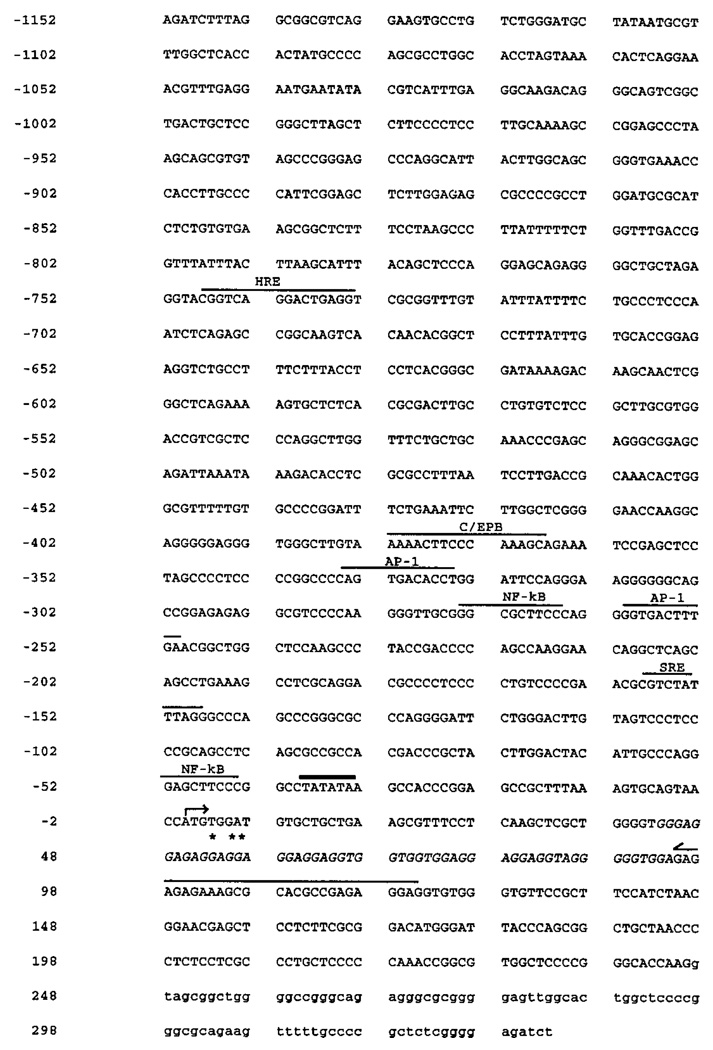
FIG. 2. Nucleotide sequence of the 5′ region of the human type I InsP3 receptor gene.
Nucleotide numbering is referenced to the +1 nucleotide (A) identified as the major transcription start site (arrow) by primer extension. 5′-RACE termination sites are indicated by asterisks and are located within the GGAGGA high homology region, shown in italics. The putative TATA box (beginning at −39 nt) is overlined in bold. Potential transcription regulatory sites are also overlined and include steroid hormone response element (HRE), CAATT enhancer-binding protein element (C/EBP), AP-1 sites, NF-κB sites, and SRE. The antisense primer (p109a) location used for the primer extension is overlined with a half-arrow. The first intron is indicated by lowercase lettering.
Identification of the Transcription Start Site of the Human Type I InsP3 Receptor Gene
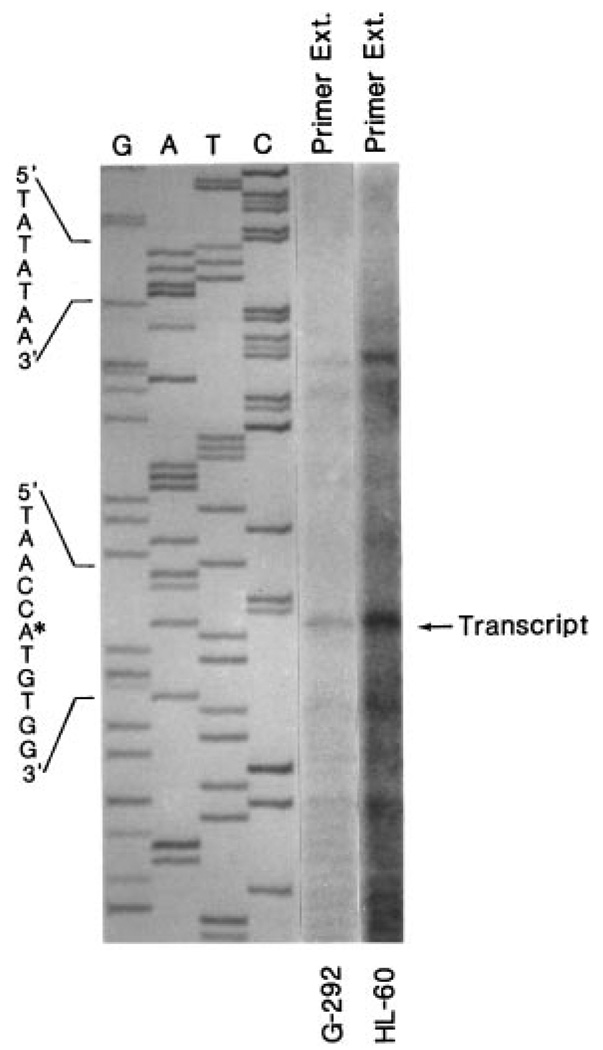
The transcription start site was determined by primer extension and rapid amplification of cDNA ends (5′-RACE). The antisense primer, p109a, corresponding to bases +109 through +85 of the mouse type I InsP3 receptor cDNA (7) (+37 through +13 of the human type I InsP3R cDNA; Ref. 11) was hybridized to and extended by reverse transcription of RNA from human G-292 osteosarcoma cells and human HL-60 promyelocytic cells. The primer extension (Fig. 3) identified an adenine (A) residue as the predominant transcription start site. This adenine is located 39 residues downstream of a consensus TATA box motif (TATATAA) and is referenced in the sequence as position +1 (Fig. 2). Results from three separate experiments using independently isolated RNAs from G-292 and HL-60 cells indicated the same major transcription start site in both cell types. A minor start site (C), also identified in both cell types, was identified at position −26 relative to the major start site and 14 bp downstream of the TATA box. Interestingly, the 5′-RACE analysis performed at 37 °C consistently yielded products shorter (indicated by asterisks in Fig. 2) than expected from the published human and rat cDNAs or from the observed primer extension products. Inspection of the DNA sequence immediately upstream and encompassing the 5′-RACE terminated sites indicates a conserved GGA-rich region, which may impart an unusual secondary structure on the DNA and prevent full-length extension. Successful primer extension through this region required temperatures of 46–48 °C; extensions at 37 °C were terminated prematurely.
FIG. 3. Primer extension analysis on the 5′ end of the human type I InsP3 receptor gene.
Twenty µg of total RNA from human G-292 osteosarcoma cells and human HL-60 promyelocytes were subjected to primer extension using the p109a antisense oligonucleotide corresponding to bases +109 through +85 of the published mouse type I InsP3 receptor cDNA (+118 through +95 of the genomic DNA). The location of the major extended product is shown by the arrow. The corresponding DNA sequence was determined using the p109a primer and the pGEM11/SacI-0.5-kb subclone identified in Fig. 1. The asterisk indicates the adenine identified as the major start site. The putative TATA box sequence is also shown.
The 5′-Flanking Region of the Type I InsP3 Receptor Gene Functions as a Promoter in Transient Transfection Assays
To determine if the 5′-flanking region of the InsP3 receptor gene could function as a promoter and regulate transcription in target cells, the 0.5-kb SacI genomic fragment (−359 bp through +153 bp) was subcloned into the promoterless pCAT-Basic expression vector as described under “Experimental Procedures.” After transient transfection of G-292 osteosarcoma cells with reporter constructs containing the SacI fragment inserted in the forward orientation in front of the CAT gene, CAT activity averaged 4-fold greater (n = 3) than activity in cells transfected with constructs in the reverse orientation (Fig. 4). In G-292 cells transfected with the forward promoter construct, CAT activity was further increased by treatment with 1 nm phorbol ester (TPA) but decreased in cells treated with either 10 nm 17β-estradiol or 10 nm 1,25(OH)2D3. Both 17β-estradiol and 1,25(OH)2D3 decreased CAT expression when added together with TPA when compared with TPA alone.
FIG. 4. The 5′ region of the human type I InsP3 receptor gene functions as a promoter.
The pCAT-Basic/SacI-0.5-kb plasmid (3 µg) containing the −359 bp through +153 bp fragment of the type I InsP3 receptor gene inserted in the forward or reverse orientation in front of the bacterial CAT gene was transfected into G-292 osteosarcoma cells as described. Cells were then treated with hormones or TPA as indicated for 48 h and assayed for soluble CAT activity. At top is the autoradiogram of the CAT assay, and below is the quantified results normalized to the activity of the forward construct. Two separate experiments for the untreated cells are shown. The unlabeled, last lane of the autoradiogram is a positive control for CAT activity. The experiments shown are representative of two additional experiments showing similar results.
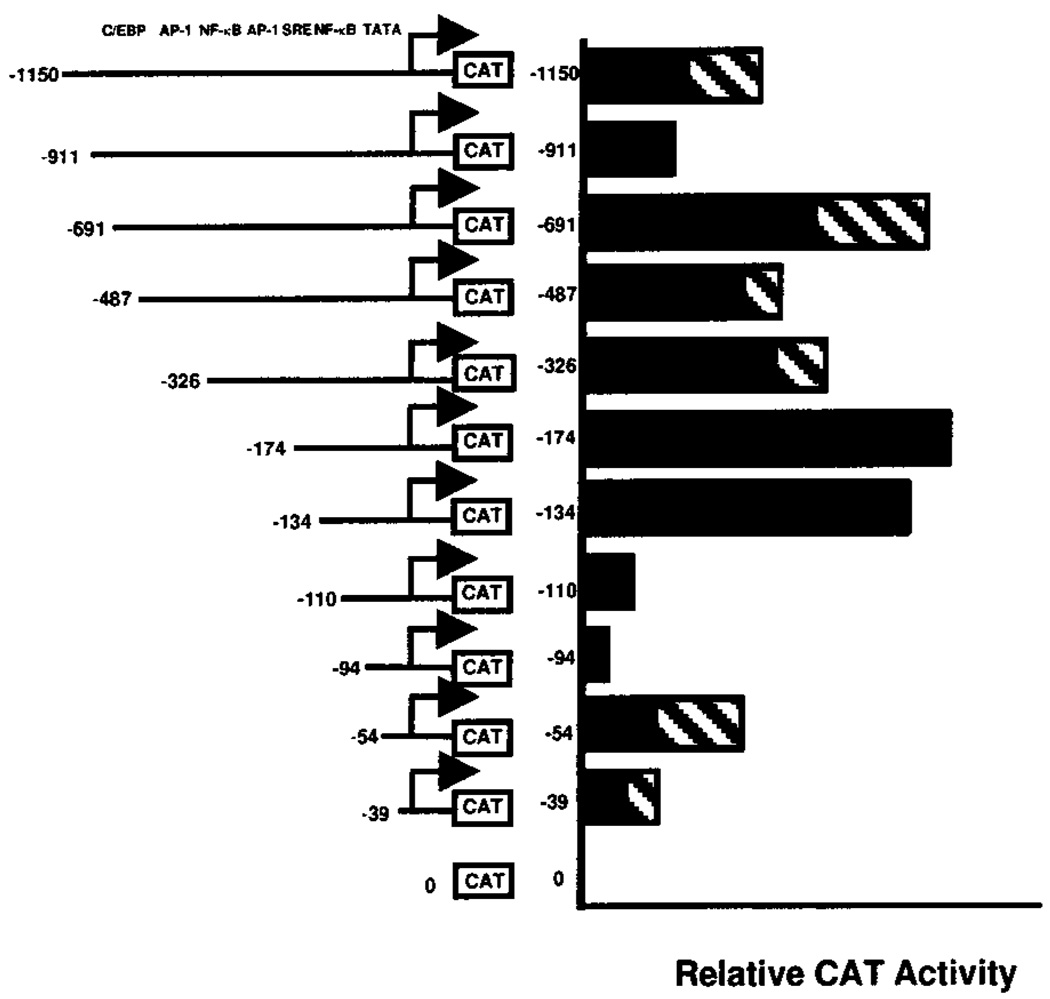
Transcription promoting activity of the 5′-flanking region of the type I InsP3 receptor gene promoter was also determined by transient transfection of G-292 cells with pCAT-Basic constructs containing the 1.5-kb BglII fragment (−1152 through +350 bp) or 5′-deletions of this fragment. These data (Fig. 5) have been normalized and are reported as percent of activity relative to the −174 bp construct, which consistently exhibited the greatest CAT activity. The results summarized in Fig. 5 show that the longer promoter constructs (−1150 bp, −911 bp) had less CAT promoting activity that shorter constructs (−691 bp through −134 bp), and that even shorter constructs (−110 bp through −39 bp) had less CAT promoting activity than the −174 bp promoter construct. Significantly, CAT expression from deletion constructs including just the TATA box, as in the −39 construct, was greater than expression from the pCAT-Basic parental vector, whereas the slightly longer constructs (−54, −134, and −174 bp) that included a consensus NF-κB binding element and a serum response element had greater transcription promoting activity than constructs containing only the TATA box (p < 0.05, paired Student’s t test).
FIG. 5. Effects of 5′ deletions of type I InsP3 receptor promoter function.
Deletion constructs containing different lengths of the human type I InsP3 receptor gene promoter upstream of the CAT reporter gene in the pCAT-Basic plasmid were transiently transfected into G-292 osteosarcoma cells along with the pSV-β-galactosidase vector. CAT activities are reported relative to the −174 bp construct. Numbers indicate the upstream limit of the promoter sequence in each deletion construct. Locations of consensus regulatory sites are shown at the top. Bars represent means ± S.E. (stippled bar) of three to five separate experiments or means of duplicate experiments (−911 bp, −134 bp, −110 bp, −94 bp, and pCAT-Basic alone).
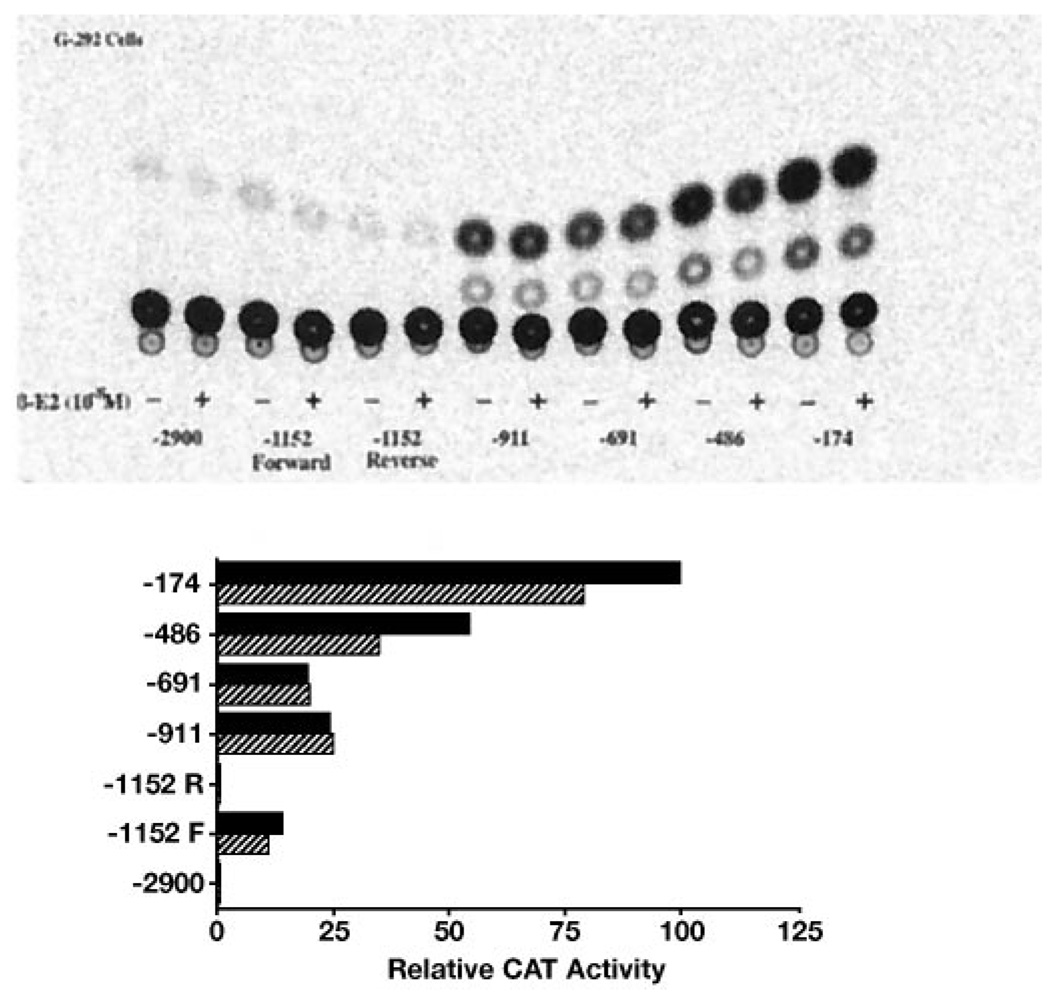
17β-Estradiol Represses Expression of the Type I InsP3 Receptor Promoter in G-292 Cells
We have reported that treatment of human G-292 osteosarcoma cells and rat primary calvarial osteoblasts with estrogen decreases steady state expression of type I InsP3 receptor mRNA (24). That data were consistent with mechanism involving reduced gene transcription. Using the human type I InsP3 receptor promoter CAT reporter constructs, the regions of the promoter that might confer transcription repression by 17β-estradiol were investigated. Following transient transfection of G-292 cells with various deletion constructs of the type I InsP3 receptor gene promoter-pCAT plasmid, cells were incubated with or without 10 nm 17β-estradiol for 48 h and then assayed for CAT activity. Fig. 6 shows that cells transfected with the longer promoter constructs (−2.9 kb and −1152 bp) expressed relatively low CAT activity and 17β-estradiol had little affect on this expression. However, cells transfected with the shorter promoter constructs (−486 bp and −174 bp) expressed greater CAT activity, and this activity was inhibited 20–40% by 10 nm 17β-estradiol (p < 0.05, paired Student’s t test).
FIG. 6. The effects of 17β-estradiol on the function of type I InsP3 receptor promoter deletion constructs.
Deletion constructs containing the indicated lengths of the human type I InsP3 receptor gene promoter upstream of the CAT gene in the pCAT-Basic plasmid were transiently transfected into G-292 osteosarcoma cells along with the pSV-β-galactosidase vector. Cells were treated for 48 h with (stippled bars) or without (solid bars) 10 nm 17β-estradiol as indicated and normalized CAT activities determined as described. At top is the autoradiogram of the CAT assay, and below are the quantified results. The experiment is representative of two experiments showing similar results.
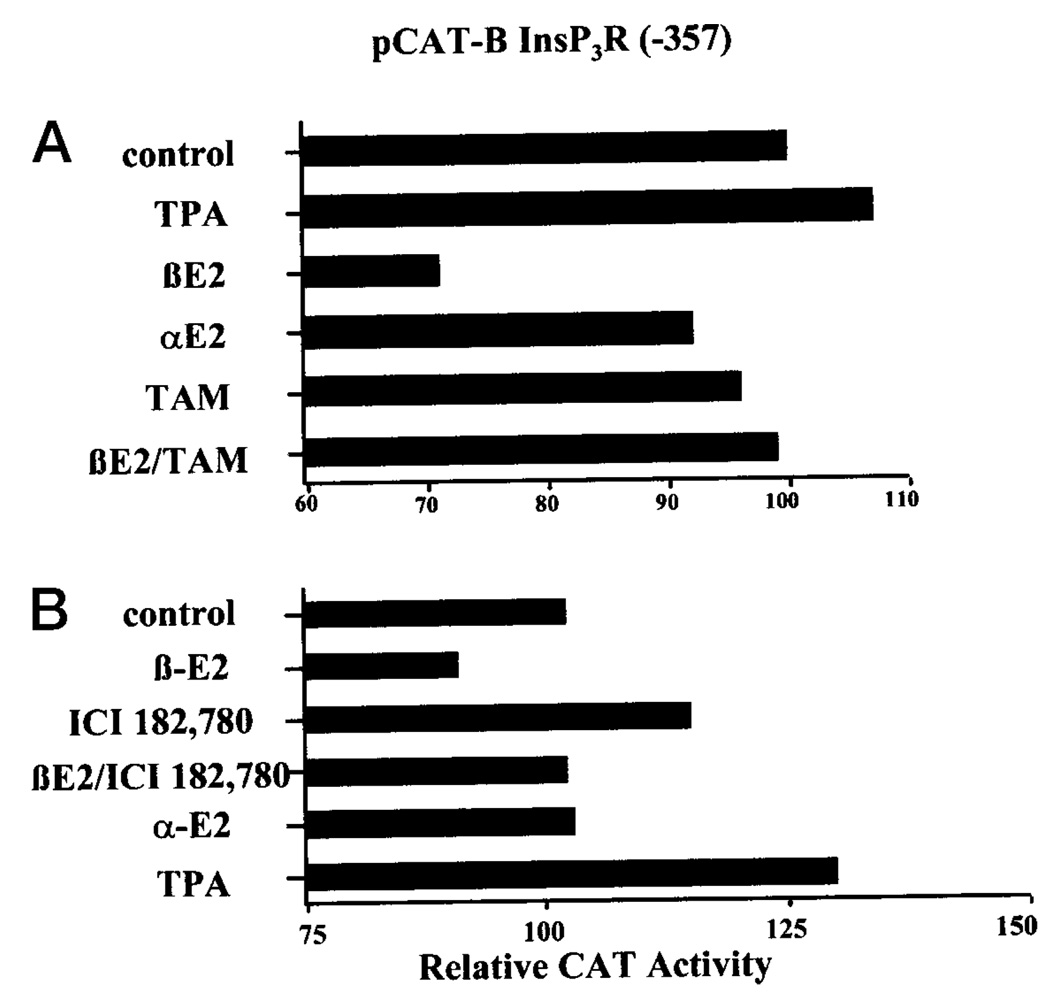
The Effect of 17β-Estradiol is Inhibited by Tamoxifen and ICI 182,780
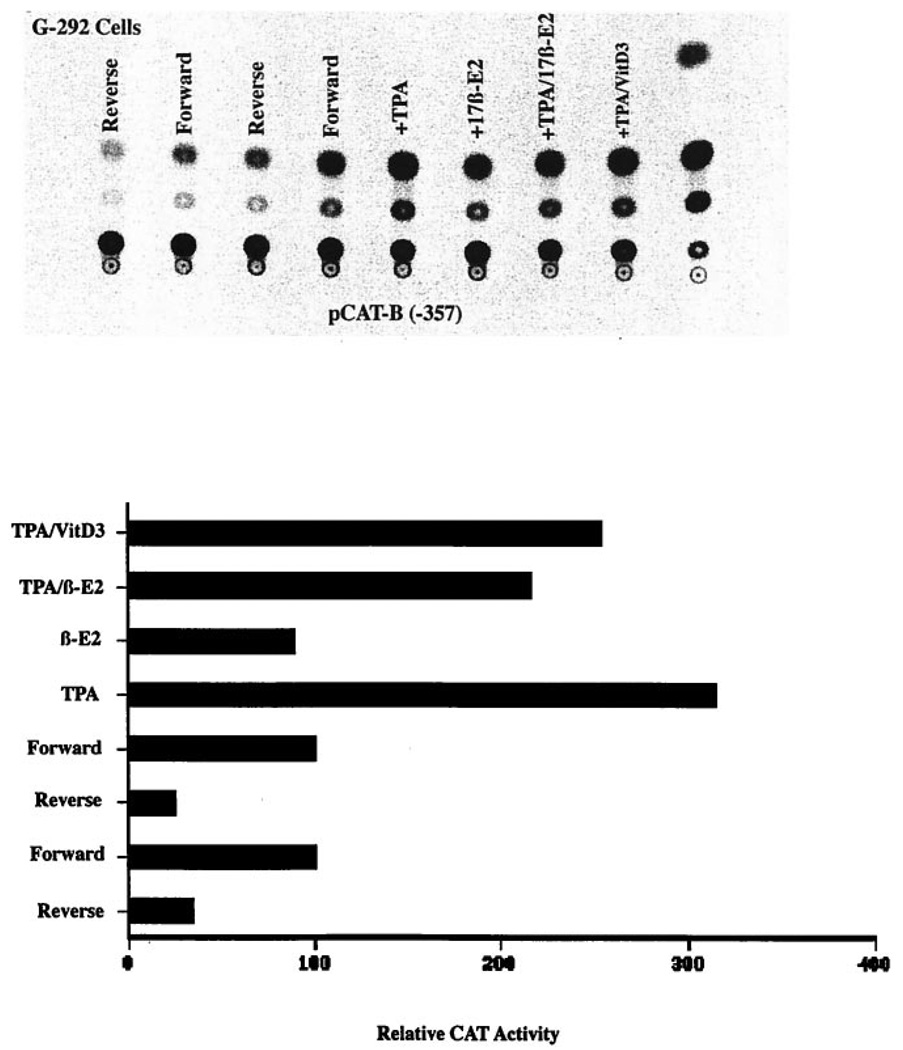
To determine whether the inhibitory effect of 17βestradiol on type I InsP3 receptor promoter activity in G-292 cells might be mediated by the estrogen receptor, CAT expression from the type I InsP3 receptor promoter construct was determined in cells treated with 17β-estradiol plus the antiestrogen tamoxifen, the estrogen receptor antagonist ICI 182,780, or 17α-estradiol, the inactive isomer of 17β-estradiol. The data in Fig. 7A show that 10 nm 17β-estradiol reduced CAT expression in cells transfected with the −357 bp type I InsP3 receptor gene promoter and that 100 nm tamoxifen did not mimic this response. However, tamoxifen did block the effect of 17β-estradiol. In this experiment, a slight inhibitory effect of 17α-estradiol was seen but this was not reproduced in the other experiments. In a separate series of experiments, the pure estrogen receptor antagonist, ICI 182,780, was used. In these experiments (Fig. 7B), 10 nm 17β-estradiol reduced CAT expression from the −357 bp type I InsP3 receptor promoter construct to 87 ± 2% (mean ± S.E., p < 0.01) of control, whereas CAT expression in cells treated with 17β-estradiol plus 20 nm ICI 182,780 was 122 ± 9%. Although cells treated with 17β-estradiol plus ICI 182,780 had elevated CAT activity, this was not significantly different from cells treated with ICI 182,780 alone (124 ± 8%). In these series of experiments, 17α-estradiol was again inactive as an inhibitor.
FIG. 7. The effects of 17β-estradiol, 17α-estradiol, tamoxifen, and ICI 182,780 on type I InsP3 receptor promoter function.
The −357 bp construct of the human type I InsP3 receptor gene promoter upstream of the CAT reporter gene in the pCAT-Basic plasmid was transiently transfected into G-292 osteosarcoma cells along with the pSV-β-galactosidase vector. In A, cells were treated for 48 h with or without 1 nm TPA, 10 nm 17β-estradiol, 10 nm 17α-estradiol, 100 nm tamoxifen, or the indicated combination. In B, cells were treated for 48 h with or without 10 nm 17β-estradiol, 20 nm ICI 182,780, or the combination. Extracts were normalized for β-galactosidase activities, and CAT activities were determined as described. The experiments are representative of two (A) and three (B) such experiments. Means ± S.E. for all experiments are reported under “Results.”
DISCUSSION
The effects of many bone resorptive agents are mediated through their actions on osteoblasts to stimulate the production and secretion of osteoclast-activating factors, including IL-6 (30, 31). Parathyroid hormone, parathyroid hormone-related peptide, bradykinin, prostaglandins, and endothelins induce bone resorption, require osteoblasts for this action, and stimulate IL-6 secretion from osteoblasts (32). These hormones all increase phosphoinositide turnover, InsP3 formation, and calcium mobilization in osteoblasts, suggesting that the InsP3 receptor may be important in osteoblast signal transduction and may be an essential component in the regulation of IL-6 secretion. On the other hand, estrogens have antiosteoporotic effects mediated in part by their effects on osteoblasts to reduce IL-6 secretion (33). The mechanism for this action of estrogen in osteoblasts has been attributed to the repression of IL-6 gene transcription, despite the lack of consensus estrogen response elements in the IL-6 promoter. Indeed, additional experimental evidence suggests that 17β-estradiol affects estrogen receptor-dependent IL-6 gene repression in osteosarcoma cells by direct inhibitory interaction with the activator transcription factors NF-κB and C/EBPβ (34). The present study indicates that an additional mechanism may contribute to the reduced IL-6 secretory capacity of osteoblasts following estrogen treatment, viz. that chronic estrogen treatment reduces the expression of the type I InsP3 receptor gene resulting in diminished osteoblast secretory activity.
In the present investigation, the 5′ region of the human type I InsP3 receptor gene including its putative promoter was cloned and its regulation in osteoblasts by estrogen and other agents is described. Three overlapping genomic clones and two additional neighboring clones of the human type I InsP3 receptor gene were isolated by hybridization with cDNA probes. Approximately 50 kb of genomic DNA was isolated and determined to encode eight of the promoter proximal exons within the first 10% (through bp 880) of the 10-kb human type I InsP3 receptor cDNA. The relative large size of this portion of the gene suggests that the entire human type I InsP3 receptor gene may be as large as 500 kb. An interesting feature of the promoter proximal region of the gene is that the exon containing the translation start site is preceded by at least two noncoding exons. Examination of the exon-intron boundaries, as illustrated for the first such boundary at bp +246 included in the sequence reported in Fig. 2, showed them all to be classical AGgt … agG sequences. Another interesting feature of the first exon is the repetitive GGAGG(A/T) sequence from +45 through +94. This sequence is found with high homology in several other signal transduction genes including those for c-Myb, c-ErbB2, epidermal growth factor receptor P1, IGF II, and snRNA U1 (NCBI). An unusual RNA secondary structure may be imparted by this sequence since the 5′-RACE analyses performed at 37 °C were arrested within this sequence, and the primer extension required temperatures between 46–48 °C to traverse through this region.
The identification of the major transcription start site by primer extension locates the initiation site 40 bp downstream from a TATATAA sequence, suggesting that the latter serves as a functional TATA box. Indeed, the transcription reporter constructs showed that inclusion of the DNA through and including the TATA box conferred significantly greater transcription promoting activity than the promoterless CAT parental vector. This TATATAA stretch is surrounded by GC-rich sequences, also typical of consensus TATA elements. With this orientation, examination of the upstream sequence reveals several consensus transcription regulatory sequences that potentially could control type I InsP3 receptor gene expression in a cell-specific and hormonally regulated fashion. Included among these are recognition sequences for AP-1, serum response element (SRE)-binding factors, NF-κB, C/EBP, and nuclear receptors (see Fig. 2).
Paired AP-1 sequences are identified at −334 and −261 in the upstream region. AP-1 sites can act as positive regulatory elements for phorbol ester-induced transcription through activation of c-Jun/c-Fos signaling (35). In G-292 cells transfected with the −357 bp type I InsP3 receptor promoter pCAT construct, TPA stimulated 3 times more CAT expression compared with untreated cells. An SRE-like sequence is found at −159 and maximal CAT activity was stimulated by 2% charcoal-stripped serum with the −174 construct (Fig. 5). However, the −134 construct, which lacks the consensus SRE, still exhibited CAT activity comparable to the −174 construct. Consensus regulatory sequences for NF-κB at positions −274 and −52, as well as for C/EBP at −382, are also identified, although a role for these elements in regulating transcription in osteoblasts is not immediately apparent.
Paired steroid receptor half-sites separated by a 4-base spacer are found at −746. Previous studies have demonstrated that retinoic acid induces InsP3 receptor mRNA expression and increases the rate of type I InsP3 receptor gene transcription in HL-60 cells (19). The nuclear receptor half-sites found at −746 (CGGTCAggacTGAGGT) have a 4-base spacer and are in an inverted repeat orientation (IR-4); however, the IR-4 configuration of the hormone response half-site is not an optimal arrangement for retinoids or other nuclear hormones (36). Interestingly, consensus vitamin D-responsive elements or estrogen-responsive elements were not identified, even though previous studies have shown that levels of type I InsP3 receptor mRNA are positively regulated by vitamin D in HL-60 cells (20) and negatively regulated by estrogens and vitamin D in primary osteoblasts and osteosarcoma cell lines (24).
The effects of 17β-estradiol on type I InsP3 receptor promoter activity were investigated in greater detail. Our previous studies of human G-292 osteosarcoma cells and rat calvarial osteoblasts have indicated that estrogens decrease type I InsP3 receptor mRNA levels without an effect on mRNA stability, suggesting an inhibitory effect on the rate of gene transcription (24). The data presented here are consistent with this contention. In G-292 cells transfected with InsP3 receptor promoter constructs, exposure to 10 nm 17β-estradiol inhibited CAT activity up to 40%. CAT repression by 17β-estradiol was most prominent with constructs that also had the greatest basal activity in the 2% charcoal-stripped serum, i.e. the −326 and −174 constructs. Of additional interest is the fact that maximal promoter repression by 17β-estradiol averaged 40%, whereas reduction of the type I InsP3 receptor mRNA is typically about 75% in similarly treated cells (24).
The lack of effect of 17α-estradiol and the observed antagonism of the 17β-estradiol response by tamoxifen and ICI 182,780 strongly support the contention that the transcriptional repression observed with 17β-estradiol is mediated by the estrogen receptor. However, the lack of consensus estrogen response elements in the type I InsP3 receptor promoter sequence suggests a non-classical action of the estrogen receptor on gene expression (37, 38). Stein and Yang (34) observed similar estrogen receptor-dependent inhibition of IL-6 promoter activity in human U2-OS osteoblastic cells and presented evidence that the inhibition is not mediated through consensus estrogen response elements in the promoter but instead involves estrogen receptor binding to and repression of the stimulatory transcription factors NF-κB and C/EBPβ. Several observations reported here are consistent with a similar mechanism acting at the InsP3 receptor promoter. First, consensus response elements for the estrogen receptor are not found in the isolated InsP3 receptor promoter DNA. Second, transcription repression by 17β-estradiol was observed with the proximal promoter constructs, the same constructs that show maximal stimulation with serum. Furthermore, consensus binding sites for NF-κB, C/EBPβ, and serum response factor are found in the proximal promoter. Thus, the −350 bp and −174 bp promoter constructs had greatest activity in the presence of the 2% charcoal-stripped serum, and these were the constructs that showed the most significant repression with 17β-estradiol. The same parallel was observed in U2-OS osteoblastic cells between the regions of the IL-6 promoter necessary for transcription enhancement by either TPA or IL-1β and the ability of 17β-estradiol to repress transcription. In the case of the IL-6 promoter, experimental observations supported a mechanism by which the agonist-activated estrogen receptor was not required to be bound to the promoter to depress transcription, but rather the receptor sequestered and transrepressed the activator transcription factors, NF-κB and C/EBPβ. Studies are directed toward determining whether a similar mechanism is operative at the type I InsP3 receptor promoter in osteoblasts and whether this estrogen-dependent down-regulation of the type I InsP3 receptor plays a role in the blunted the secretion of osteoclast activating cytokines from estrogen-treated osteoblasts.
Footnotes
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EBI Data Bank with accession number(s) AF009963.
The abbreviations used are: InsP3, inositol 1,4,5-trisphosphate; kb, kilobase pair(s); bp, base pair(s); 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; PCR, polymerase chain reaction; nt, nucleotide(s); CAT, chloramphenicol acetyltransferase; TPA, 12-O-tetradecanoylphorbol-13-acetate; RACE, rapid amplification of cDNA ends; IL, interleukin; SRE, serum response element.
REFERENCES
- 1.Berridge MJ. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Spat A, Bradford PG, McKinney JS, Rubin RP, Putney JW., Jr Nature. 1986;319:514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- 3.Furuichi T, Mikoshiba K. J. Neurochem. 1995;64:953–960. doi: 10.1046/j.1471-4159.1995.64030953.x. [DOI] [PubMed] [Google Scholar]
- 4.Supattapone S, Worley PF, Baraban JM, Snyder SH. J. Biol. Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- 5.Ferris CD, Huginar RL, Supattapone S, Snyder SH. Nature. 1989;342:87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- 6.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 7.Furuichi T, Yoshikawa S, Mikoshiba K. Nucleic Acids Res. 1989;17:5385–5386. doi: 10.1093/nar/17.13.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mignery GA, Newton CL, Archer BT, III, Sudhof TC. J. Biol. Chem. 1990;265:12679–12685. [PubMed] [Google Scholar]
- 9.Sudhof TC, Newton CL, Archer BT, III, Ushkaryov YA, Mignery GA. EMBO J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blondel O, Takeda J, Janssen H, Seino S, Bell GI. J. Biol. Chem. 1993;268:11356–11363. [PubMed] [Google Scholar]
- 11.Yamada N, Makino Y, Clark RA, Pearson DW, Mattei M, Guenet J, Ohama E, Fujino I, Miyawaki A, Furuichi T, Mikoshiba K. Biochem. J. 1994;302:781–790. doi: 10.1042/bj3020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto-Hino M, Sugiyama T, Hikichi K, Mattei MG, Hasegawa K, Sekine S, Sakurada K, Miyawaki A, Furuichi T, Hasegawa M, Mikoshiba K. Recept. Channels. 1994;2:9–22. [PubMed] [Google Scholar]
- 13.Maranto AR. J. Biol. Chem. 1994;269:1222–1230. [PubMed] [Google Scholar]
- 14.Ross CA, Danoff SK, Schell MJ, Snyder SH, Ullrich A. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa T, Okano H, Furuichi T, Aruga J, Mikoshiba K. Proc. Natl. Acad. Sci. U. S. A. 1991;88:6244–6248. doi: 10.1073/pnas.88.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wojcikiewicz RJH, He Y. Biochem. Biophys. Res. Commun. 1995;213:334–341. doi: 10.1006/bbrc.1995.2134. [DOI] [PubMed] [Google Scholar]
- 17.Jayaraman T, Ondriasova E, Ondrias K, Harnick DJ, Marks AR. Proc. Natl. Acad. Sci. U. S. A. 1995;92:6007–6011. doi: 10.1073/pnas.92.13.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berridge MJ. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford PG, Wang X, Jin Y, Hui P. J. Biol. Chem. 1992;267:20959–20964. [PubMed] [Google Scholar]
- 20.Bradford PG, Jin Y, Hui P. Mol. Pharmacol. 1993;44:292–297. [PubMed] [Google Scholar]
- 21.Blondel O, Moody MM, Depaoli AM, Sharp AH, Ross CA, Swift H, Bell GI. Proc. Natl. Acad. Sci. U. S. A. 1994;91:7777–7781. doi: 10.1073/pnas.91.16.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojcikiewicz RJH, Furuichi T, Nakade S, Mikoshiba K, Nahorski SR. J. Biol. Chem. 1994;269:7963–7969. [PubMed] [Google Scholar]
- 23.Wojcikiewicz RJH. J. Biol. Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood KL, Dziak R, Bradford PG. J. Bone Miner. Res. 1996;11:1889–1896. doi: 10.1002/jbmr.5650111209. [DOI] [PubMed] [Google Scholar]
- 25.Strobl JS, Kirkwood KL, Lantz TK, Lewine MA, Peterson VA, Worley JF., III Cancer Res. 1990;50:5399–5405. [PubMed] [Google Scholar]
- 26.Kirkwood K, Higgins D, Rueger D, Bradford P. J. Dent. Res. 1997;76:342. [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. pp. 2.60–2.81.pp. 7.79–7.83. [Google Scholar]
- 28.Quandt K, Frech K, Karas H, Wingender E, Werner T. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenthal N. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- 30.Rodan GA, Martin TJ. Calcif. Tissue Int. 1981;33:349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- 31.Martin TJ. Osteoporosis Int. 1993;1:S121–S125. [Google Scholar]
- 32.Jones TH. Clin. Endocrinol. 1994;40:703–713. doi: 10.1111/j.1365-2265.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- 33.Girosole G, Jilka RL, Passeri G, Boswell S, Boder G, Williams DC, Manolagas SC. J. Clin. Invest. 1992;89:883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein B, Yang MX. Mol. Cell. Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill CS, Treisman R. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 36.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kaster P, Mark M, Chambon P, Evans RM. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb P, Lopez GN, Uht RM, Kushner PJ. Mol. Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 38.Gaub MP, Bellard M, Scheuer I, Chambon P, Sassone CP. Cell. 1990;63:1267–1276. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]