Abstract
Many strategies for repairing injured myocardium are under active investigation, with some early encouraging results. These strategies include cell therapies, despite little evidence of long-term survival of exogenous cells, and gene or protein therapies, often with incomplete control of locally-delivered dose of the factor. We propose that, ultimately, successful repair and regeneration strategies will require quantitative control of the myocardial microenvironment. This precision control can be engineered through designed biomaterials that provide quantitative adhesion, growth, or migration signals. Quantitative timed release of factors can be regulated by chemical design to direct cellular differentiation pathways such as angiogenesis and vascular maturation. Smart biomaterials respond to the local environment, such as protease activity or mechanical forces, with controlled release or activation. Most of these new biomaterials provide much greater flexibility for regenerating tissues ex vivo, but emerging technologies like self-assembling nanofibers can now establish intramyocardial cellular microenvironments by injection. This may allow percutaneous cardiac regeneration and repair approaches, or injectable-tissue engineering. Finally, materials can be made to multifunction by providing sequential signals with custom design of differential release kinetics for individual factors. Thus, new rationally-designed biomaterials no longer simply coexist with tissues, but can provide precision bioactive control of the microenvironment that may be required for cardiac regeneration and repair.
Keywords: tissue engineering, biomaterials, regeneration, microenvironment
One of the ubiquitous facts of life in cell biology laboratories is that cells are finicky about their growth conditions. Cells grow best in certain media and on certain substrates, and many cells require specific signals to prevent differentiation, or other signals to guide differentiation. In laboratory jargon, we say “these cells like this” or “those cells need that.” This trivial observation that cells require highly specific conditions has enormous implications for regenerative medicine. For example, the majority of cells injected into the myocardium won’t survive1; those cells that do survive don’t necessarily couple physiologically with the native myocardium.2,3 This is not surprising given the drastic change in the local milieu of normal or injured myocardium compared with the idealized conditions of the laboratory incubator. Thus, even if we get cells in the right places at the right time, whether they live and develop into normally functioning mature tissue will be determined by the local cellular microenvironment.
Ultimately, regenerating tissues must have spatially and temporally orchestrated distributions of growth factors. Some proteins like epidermal growth factor (EGF) can confine signaling to short distances or even only to the cell that secreted the EGF ligand, so control of EGF signaling on the length scale of a single cell may be necessary.4 In contrast, other factors like insulin-like growth factor-1 (IGF-1) may act at great distances within a tissue or through the circulation.5 In addition, the local microenvironmental concentration of factors is probably crucial. For example, many studies have focused on injection of large quantities of vascular endothelial growth factor (VEGF) protein or overexpression of VEGF by gene transfer, but the precise VEGF microenvironmental concentration is a critical determinant of functional vessel formation.6 Excessive VEGF can lead to abnormal blood vessel formation, including irregular lumen formation, leaky vessels, and hemangiomas.6
Thus, careful control of the cellular microenvironment may be as important for achieving regeneration or repair as the nature of the cells themselves. One of the critical ways that we can control the local cellular microenvironment is through biomaterials that are designed to direct cellular behavior. The term, biomaterials, has many definitions, and one traditional definition is that a biomaterial is a nonliving substance used in a medical device, like a joint prosthesis. But the technology of biomaterials has evolved, and the expanded definition includes substances that are designed to control the biological environment of cells and tissues. More than being simply compatible with the host and serving a structural role, biomaterials can now instruct cells through microenvironmental cues. Although most bioactive material strategies have been designed for noncardiac tissues like bone, there are many lessons from these studies that apply to future myocardial applications.
Biomaterials for Cells
Many 3-dimensional materials are under investigation for myocardial tissue engineering, including fibrin glue, poly lactic-coglycolic acid (PLGA), self-assembling peptide nanofibers, gelatin hydrogels, and hyaluronic acid (HA) hydrogels.7-9 There are substantial challenges for existing materials for implantation including poor vascularization, stimulation of the host inflammatory response, and toxic degradation.10 Thus far, no material has promoted the myocardial cell survival, maturation, and physiological integration necessary for functional repair. Investigators have now turned to a variety of chemical modifications of the local microenvironment within these matrices.
Controlling Cellular Adhesion
Cellular adhesion was an undesirable property for traditional biomaterials, because encapsulation and fibrosis were detrimental reactions. For myocardial tissues, however, promoting endothelial adhesion and cardiomyocyte adhesion is obviously beneficial. One strategy is to incorporate the common integrin adhesion peptide sequence RGD. Cannizzaro et al used biotin-streptavidin linkages to bind biotin-RGD peptide sequences to biotinylated poly(ethylene glycol) (PEG) and poly-lactic acid copolymers.11 This resulted in increased endothelial cell spreading in the polymer. Wang et al attached RGD moieties to poly(ether urethane) (PEU) surfaces; this modification greatly increased endothelial cell adhesion and viability rates when compared with PEU alone.12 RGD sequences are not cell-specific, however, and RGD peptides bound to silicon membranes result in enhanced fibroblast adhesion as well.13
Simple availability of cellular adhesion ligands is insufficient to maximize adhesion, because the spacing and orientation of RGD ligands are also important. Lee et al varied the nanoscale organization of RGD in alginate gel adhesion of preosteoblasts.14 Regardless of the bulk density of RGD islands, changing the spacing between the RGD islands altered cell spreading and proliferation. In addition to RGD peptide sequences, entire extracellular matrix proteins can be coupled to biomaterials to promote endothelial cell adhesion or to orient other adhesive proteins. For example, osteopontin has an RGD sequence and promotes endothelial cell survival and angiogenesis,15 but binding of osteopontin to a surface in random orientations may limit its adhesive properties. Collagen I can be used to bind osteopontin to polymers in orientations that promote endothelial adhesion.16 Thus, both spacing and orientation of adhesion sequences can regulate cellular adhesion.
Controlling Biodegradation
Optimally, a myocardial biomaterial must degrade without toxicity and with a controlled degradation rate. Unlike a permanently implanted structural prosthesis, myocardial scaffolds should last long enough to guide integration of recruited or applied cells, but not persist so long as to interfere with the eventual cell-cell physiological coupling essential in the myocardium. Various approaches can control biomaterial degradation. Burdick et al studied the effect of hyaluronic acid macromer concentration on the properties of photopolymerizable networks.17 Varying macromer concentration increased degradation time by hyaluronidase from <1 day to ≈38 days. Accelerating degradation can compromise mechanical integrity of a biomaterial, and thus it is desirable to control independently degradation and stiffness. Kong et al showed that modifying alginates could preserve stiffness but accelerate degradation, improving bone formation by bone marrow stromal cells.18 Similarly, Lee et al demonstrated that cross-linking of oxidized guluronate hydrogels could dissociate degradation from mechanical stiffness.19 Thus, degradation of polymers may potentially be designed for the necessary regenerative strategy while at least temporarily maintaining mechanical integrity.
Controlling Porosity
The pore size and pore connectivity of a bioactive material are key factors. Pores on a length scale of microns to millimeters influence trafficking of cells; myocardial cell dimensions are typically 10 μm to >100 μm. Large interconnected pores can be beneficial for promoting colonization of biomaterials,20 but excessively large pores could impair vascularization, because endothelial cells are unable to bridge pores greater than a cell diameter.21 Porosity can affect the integrity of the material and thus cellular effects may need to be balanced with mechanical properties.20,22,23 In contrast, pores <100 nanometers will influence diffusion of nutrients and factors. Poor diffusion of oxygen and nutrients may cause the failure of many implanted grafts and poor survival of implanted cells.10 Diffusion in polymers is dependent on pore size (both length and cross-sectional area) and pore numbers.24 Some hydrogels, like those formed by self-assembling peptides (described below), have very small pore sizes that promote endothelial adhesion and capillary formation, but still allow rapid migration of cells because the hydrogels are very flexible.25
Protein Delivery
Although many biomaterials can provide necessary mechanical support and adhesion sites, they cannot guide cellular phenotype as growth factors can. Superficially, binding growth factors to various biomaterials for delivery may appear to be a relatively simple task. However, the design of protein delivery systems must meet several criteria that are specific to the protein and the application.26 The delivered factor should not only be beneficial in the biological process, but increasing the amount of that factor over the endogenous level must have an additional beneficial effect. Delivery of a protein to receptors that are already optimally activated by the endogenous protein in fact may be detrimental, as described for overexpression of VEGF.6,27-29 In addition, the factor should be targeted to the specific population of cells to reduce the possibility of toxic effects on other cells. Finally, the kinetics of release, degradation, and spatial distribution are critical. For example, a chemotactic factor must be delivered with the gradient that drives chemotaxis, but the gradient should dissipate when the recruited cells are present. Thus, the strategy for protein delivery must be highly tailored.
Many materials implanted into mammals will adsorb large quantities of proteins nonspecifically, a process called fouling, and known to stimulate the foreign body reaction.8 It is important for permanent implants to be nonfouling to minimize giant cell formation and encapsulation. In contrast, biomaterials designed to deliver proteins and foster cell growth use protein binding sites for delivery (Figure 1). Some proteins such as platelet-derived growth factor and fibroblast growth factor-2 have domains that bind extracellular matrix proteins or highly charged materials and limit diffusion,30,31 whereas other proteins like IGF-1 diffuse more rapidly. Kanematsu et al incorporated a variety of different growth factors into collagenous matrices32 and found very different release profiles from the matrices both in vitro and in vivo, with rapid loss of IGF-1 compared with other factors. These data reinforce that growth factor release must be designed specifically for the target indication.
Figure 1.

Protein release by biomaterials. There are several potential mechanisms for protein delivery by biomaterials. (1) Tethered growth factor incorporation. A modification is made to either the polymer or the factor to tether the factor to the biomaterial. (2) The growth factor is incorporated within microspheres or noncovalently coupled to the material to allow quantitative control of release. (3) Proteolytic “smart” release, when the factor is tethered by an enzymatically-cleaveable sequence allowing release only when that protease is present and active. (4) The future of protein delivery by biomaterials is a multifunctional, bioactive material consisting of combinations of delivery methods.
In addition to covalently coupling factors and nonspecific binding to biomaterials, delivery of factors can be achieved through the unusually high affinity of streptavidin for biotin. Because this molecular Velcro interaction is very high affinity, and because streptavidin is a tetramer with 4 binding sites for biotin, biotinylated polymers can be easily coupled to factors via streptavidin.33 As mentioned above, Cannizzaro et al used this technique to link RGD to PLA-PEG polymers.11 Additionally, Hoya et al found that fluorophore-conjugated avidin delivered via tail vein injection bound specifically to the kidney microvasculature surfaces that were biotinylated.34 This demonstrates the potential for delivering a factor to a biotinylated material in vivo. Release of biotin-streptavidin bound factors is expected to be very slow, but biofunctional streptavidin polymer constructs can be engineered to allow release of biotin-coupled factors.35
Delivery of Angiogenic Factors
In myocardial tissue, each myocyte is immediately adjacent to several capillaries. The diffusion of oxygen alone dictates that any successful attempt to regenerate myocardium will be dependent on the proper spatial vascularization.36,37 Endothelial cells also provide growth and survival signals to cardiac myocytes, and endothelial cells may promote electrical coupling of myocytes.38 Adverse ventricular remodeling may be prevented, at least in part, by neovascularization of the infarcted area.39 Therefore, promoting myocardial angiogenesis may have an impact beyond the obvious benefit of oxygen and blood supply. Although clinical trials of myocardial therapeutic angiogenesis have largely been disappointing to date,40 this may be caused by inadequate controls on dose and control of delivery.41 Biomaterial-based angiogenic protein delivery offers promise for better control of local therapeutic angiogenesis.
Most studies of angiogenic protein factors in biomaterial delivery have evaluated single factors like FGF-2 and VEGF. For example, Iwakura et al incorporated FGF-2 into gelatin hydrogels and injected hydrogel microspheres directly into rat myocardium 4 weeks after infarction.42 Injection of the gelatin microspheres increased myocardial perfusion and new vessel formation at the center of the infarct as well as the border zones. Both regional and global systolic function improved after 4 weeks of treatment. This strategy has had preliminary clinical success in patients undergoing coronary bypass surgery.43 When sustained-release heparin-alginate microcapsules with FGF-2 were surgically implanted in ischemic and viable but ungraftable myocardium, there was a trend toward improved perfusion and no apparent adverse effects. FGF-2 in the serum of patients who received myocardial controlled-release FGF-2 was not increased. VEGF can also be delivered by hydrogels, and some data suggest that this is a particularly effective approach for delivering VEGF.44
Because angiogenesis arises from a series of events that include recruitment of cells but must also include further maturation of the new vessels,45 biomaterials are an attractive means of providing multiple signals. Marui et al loaded a combination of FGF-2 and hepatocyte growth factor into collagen microspheres.46 The single growth factors were not able to improve blood flow as measured by laser Doppler imaging; however, the dual growth factor approach resulted in an increase in blood flow, as well as increased vessel formation and maturity. Richardson et al used polymer microspheres to encapsulate VEGF and PDGF and examined angiogenesis after hind-limb ischemia induction.47 The authors were able to vary the release rates of the growth factors by incorporating 1 factor into the polymer directly and the other embedded in microspheres within the polymer. Protein release profiles were determined by changing the degradation rates by altering polymer formulations. When implanted subcutaneously after hind-limb ischemia, the dual growth factor approach improved angiogenesis and increased vessel size and maturity compared with the individual factors alone. Thus, temporally controlled, multiple factor release by biomaterials is a promising approach to therapeutic angiogenesis.
Smart Biomaterials Respond to Their Environment
One of the most challenging problems in tissue engineering and regeneration is to reproduce the serial signals that comprise normal developmental processes and successful regeneration in nature. In relatively few circumstances will we aim to deliver several factors with identical kinetics. Therefore, the future of bioactive materials is in design of smart biomaterials that respond to their environment with predetermined responses such as protein release, allowing not only delayed release but release initiated by microenvironmental conditions.48 Although the design of smart biomaterials is in its infancy, the potential for engineering these materials has been demonstrated by recent studies. Various disease conditions can lead to increases in local temperature, acidity, or metalloproteinase activation, and these microenvironment conditions can be exploited by smart biomaterials (Figure 2).
Figure 2.
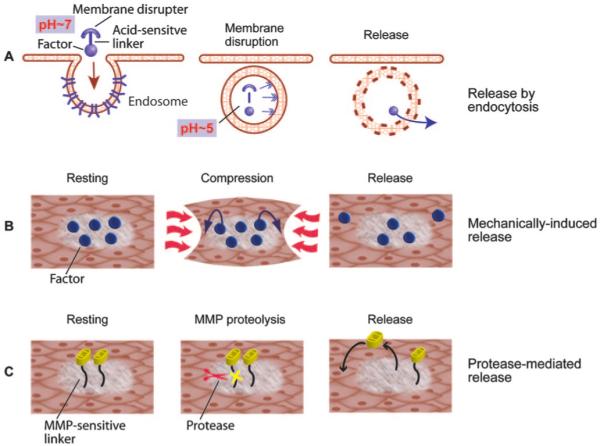
Smart biomaterials react to the local environment. A, An acid-sensitive linker is used to tether a growth factor to a membrane disruptive compound. At neutral pH, the complex is stable; however when the complex enters an endosome and is exposed to acidic pH, the linker is dissolved, leaving the membrane disruptor to destroy the endosome and releasing the factor into the cell.52 B, Biomaterials can respond to the mechanical conditions of the tissue. The release of the factor is controlled by the compression of the tissue.54 C, Biomaterials can be designed to release factors in the presence of matrix metalloproteinases. In disease states where specific MMPs are secreted and activated, the linker customized to contain specific recognition sequences allows protease-mediated release.51
Metalloproteinase-Mediated Release
Matrix metalloproteinases (MMPs) can cleave a variety of substrates including components of the extracellular matrix. MMP activation is easily demonstrated in remodeling myocardium, and both pharmacological and gene deletion studies have demonstrated a role for MMPs in progressive cardiac dilation.49 Because MMPs recognize specific amino acid sequences, act locally in the pericellular environment, and are normally expressed at low levels in quiescent tissues, MMPs are potential triggers for smart biomaterial behavior. Chau et al designed dextran-methotrexate conjugates for potential tumor therapy, fast growing tumors express high levels of MMP-2 and MMP-9.50 The methotrexate was attached to dextran via a peptide that contained a cleavage sequence for MMP-2 and MMP-9. This led to a time-dependent release of methotrexate in vitro by MMP-2 or MMP-9. Furthermore, methotrexate was released by conditioned media from tumor cells that express MMP-2 and MMP-9, but not from tumor cells that did not express these enzymes. The sequence specificity of the peptide was demonstrated by poor release profiles when scrambled peptide linkers or no peptide linkers were used to couple the methotrexate. MMP-sensitive linkages could thus be used to release factors in response to tissue remodeling enzymes. Similarly, Lutolf et al developed hydrogels with MMP-sensitive linkages between polyethylene glycol chains entrapping bone morphogenetic protein-2.51 This strategy allowed rapid bone formation in rats because of proteolytic invasiveness of the gels and subsequent release of bone morphogenetic protein-2. Thus, there is potential to use this smart biomaterial approach to deliver factors to the myocardium in response to MMPs stimulated by cardiac remodeling.
In addition to enzymatic recognition sequences, proteins can be released from biomaterials by pH changes. Many biomaterials are internalized by cells via endocytosis. In the acidic endosome, a biomaterial can be designed to destroy the endosome and release the factor to the cytoplasm. Murthy et al created a polymer with a membrane-disruptive backbone attached to polyethylene glycol via acid-sensitive linkers.52 Drugs and recognition sequences were tethered and then released in hepatocyte endosomes.
Another example of smart biomaterials is the release of proteins in response to mechanical forces, which is highly relevant to the myocardium. Mechanical stimuli induce expression of the VEGF gene and protein in a variety of cells,53 including cardiac myocytes. Lee et al developed an analogous biomaterial approach with VEGF-containing alginate hydrogels. When these hydrogels were exposed to mechanical strain in vitro, the release rate of VEGF increased. After implantation in ischemic hind-limbs of diabetic mice, this mechanically-induced release also increased collateral vessel formation.54 Thus, biomaterials could potentially be designed to respond to mechanical loads to help regenerating myocytes or exogenous cells to adjust to excessive myocardial mechanical load.
Self-Assembly and Injectable Microenvironments
One of the most important barriers to cardiac tissue engineering is that any new tissue must engraft into the already-diseased myocardium with electrophysiological and mechanical integration. This challenge is daunting for integrating large volume of myocardial tissue, which must also appropriately vascularize, but could be addressed by reconstitution of small microenvironments of myocardium in situ. Small microenvironments could conceivably be introduced by injection percutaneously, in the same manner that some cell therapies are currently performed. The concept of injectable tissue engineering has been most vigorously pursued to date in the orthopedic arena.55 However, recent progress in molecular self assembly suggests that in situ cardiac reconstitution may be possible with new biomaterials.
Building new myocardium with injectable microenvironments may be accomplished with emerging nanotechnology approaches. Nanotechnology refers broadly to manipulation and design on the molecular level, generally 1 to 100 nanometers. Nanofibers (≈7 to 20 nanometers in diameter) can be designed to self-assemble under a variety of conditions with specific peptides. Molecular self-assembly occurs naturally (eg in protein folding) and is mediated by weak noncovalent interaction between molecules via hydrogen bonding, van der Waals interactions, ionic bonds, and hydrophobic interactions.56 Nature has created a wide variety of molecules that are amphiphilic, having both hydrophilic and hydrophobic regions. Amphiphilic molecules like phospholipids can undergo self-assembly and can be designed to form nanotubes and nanospheres. Amphiphilic peptide sequences can also be designed with alternating hydrophobic and hydrophilic amino acids, and these peptides are in solution at low pH or low osmolarity. However, when exposed to physiological ionic strength and pH conditions, the peptides interact and self-assemble into stable nanofibers that interweave rapidly to form a hydrated gel.56
Although hydrogels from self-assembling peptides don’t contain typical integrin-binding motifs, they can be outstanding cell culture substrates and 3-D encapsulation culture systems. Chondrocytes cultured within peptide nanofiber hydrogels produce a cartilage-like extracellular matrix rich in glycosaminoglycans and collagen, and the progressive accumulation of matrix leads to cartilage-like mechanical behavior.57 Liver progenitor cells embedded within peptide nanofibers adopt mature hepatocyte properties including binucleation and expression of cytochrome P450s that suggest hepatocyte maturation.58 Endothelial cells cultured within nanofiber scaffolds form typical capillary-like networks in vitro. Interestingly, coculture of cardiac myocytes with endothelial cells leads to rapid assembly of capillary-like channels, with accumulation of cardiac myocytes on the outside of the endothelial-lined channel. The endothelial cells promote connexin 43 expression and electrical synchrony of the cardiac myocytes.38 This suggests that endothelial cells and cardiac myocytes retain the ability to assemble into myocardium-like structures, and that injection of cells in the appropriate self-assembling peptides could allow cells to assemble in situ.
The biophysical properties of self-assembling peptides are well-suited to building myocardial microenvironments by injection, because they can be maintained in solution but form nanofibers at normal pH and osmolarity. When injected into the left ventricular free wall of mice, the nanofibers create hydrogel microenvironments easily discernable by standard histology (Figure 3). Initially, the nanofibers are cell-free, but they are then spontaneously populated by endothelial cells, with clear capillary structures forming over a period of weeks.59 Over time, the vascular structures express maturation markers such as α-smooth muscle actin, and the vascular structures contain red blood cells, suggesting coupling to the host vasculature.
Figure 3.

Self-assembling peptide nanofibers create injectable myocardial micronenvironments. Mouse myocardium was injected with self-assembling peptide nanofibers. The nanofibers assemble into a local microenvironment in vivo (dotted lines) that is easily distinguishable from the native myocardium (NM) under standard light microscopy. This microenvironment contains many nuclei (blue=DAPI) at this stage (14 days). The microenvironment contains endothelial cells (green=isolectin; these cells also stain for CD31) that cluster (arrows) and eventually form functional vessels.59 In addition, cells that stain positively for myocyte markers (red=α-sarcomeric actin) are also present and tend to be located adjacent or nearby endothelial cells (circles show these cells located near endothelial cells).
Self-assembling peptide nanofibers can be modified in a broad variety of manners, allowing cell-specific signals to be delivered. For example, Silva et al synthesized selfassembling peptides containing an IKVAV epitope.60 This sequence is found in laminin and encourages neurite adhesion and growth. When cultured within these microenvironments, neuron progenitors differentiated into neurons. Further modifications of peptide sequences and design of smart peptide nanofibers will allow controlled quantitative delivery of factors to the myocardium.
Self-assembling peptide microenvironments can support the growth and differentiation of embryonic stem cells to cardiac myocytes within the myocardium.59 Because differentiation of embryonic stem cells and other stem and progenitor cells is highly dependent on local microenvironmental factors, the ability to modify bioactive injectable materials like peptide nanofibers may be a crucial component of future cell therapy strategies. For example, long-term, the clinical use of embryonic stem cells would be prohibited by even rare formation of teratomas. Delivery of local factors that ensure differentiation of all of the stem cells in the microenvironment could be a key crucial factor in preventing the development of teratomas.
Although many types of bioactive biomaterials have been developed, relatively few have been used to modify the hostile environment of infarcted myocardium. These include fibrin glue and alginate preparations, with early suggestions of improvement in neovascularization and cardiac remodeling.7 Preliminary studies suggest that self-assembling peptide nanofiber microenvironments are stable within infarcted myocardium and can be engineered to deliver myocardial survival factors efficiently (unpublished observations).
Biomaterials for Gene Delivery
Although many problems have delayed gene transfer approaches as viable therapies, critical issues such as toxicity and quantitative control of delivery may be addressed by new biomaterials. Naked DNA injection into the heart fails because of low transfection efficiencies, nonspecific targeting to multiple cell types, and degradation. Current viral methods for widespread myocardial delivery do not appear clinically viable. Implantation of transfected cells to deliver proteins has interesting potential but this type of genetically-engineered cell therapy will be limited by survival of implanted cells as well as control over the amount of protein secreted. Thus, there is a potential role for the development of biomaterials that quantitatively delivery DNA to target cells or to enhance survival of gene-targeted cells. Polymers can protect DNA from degradation, allowing persistent transfection by endocytosis for months.61 Although very few attempts have been made to deliver DNA to the myocardium, there has been some limited success. Christman et al mixed fibrin glue, a biopolymer formed by mixing fibrinogen with thrombin, with DNA encoding the gene for the angiogenic protein pleitropin. Injection of the DNA within fibrin glue into ischemic rat myocardium led to increased neovasculature formation as compared with fibrin glue or plasmid injection alone.62 Although biomaterials may help solve some problems facing myocardial gene therapy like degradation of DNA and survival of transfected cells, the quantitative problems of gene control make protein delivery by biomaterials a more attractive approach.
Conclusions
The unprecedented excitement over molecular and cell approaches to cardiac repair should be tempered by the complexity of the regeneration response. Regeneration requires a precise series of spatially and temporally controlled events analogous to the remarkably coordinated events of organ development. Although our initial single factor or single cell-type approaches may yield benefit, it is likely that we will have to design multi-factorial strategies for cardiac repair. For this reason, new biomaterials that provide cell instructions in precisely controlled manners may be essential. Advances in bioactive biomaterials allow not only controlled release, but protection of factors from degradation or release of factors in response to environmental cues. The design of these new biomaterials will require substantial fundamental biological insight, because factors such as dose, timing, spatial range of delivery, and the conditions for environmentally-controlled release will be highly specific for the target tissue and disease. Ultimately, though, novel biomaterials may be used to guide the tissue repair response at the pericellular level in the same manner that we guide cells in the laboratory.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute.
References
- 1.Itescu S, Schuster MD, Kocher AA. New directions in strategies using cell therapy for heart disease. J Mol Med. 2003;81:288–296. doi: 10.1007/s00109-003-0432-0. [DOI] [PubMed] [Google Scholar]
- 2.Dowell JD, Rubart M, Pasumarthi KB, Soonpaa MH, Field LJ. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 2003;58:336–350. doi: 10.1016/s0008-6363(03)00254-2. [DOI] [PubMed] [Google Scholar]
- 3.Rubart M, Soonpaa MH, Nakajima H, Field LJ. Spontaneous and evoked intracellular calcium transients in donor-derived myocytes following intracardiac myoblast transplantation. J Clin Invest. 2004;114:775–783. doi: 10.1172/JCI21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeWitt A, Iida T, Lam HY, Hill V, Wiley HS, Lauffenburger DA. Affinity regulates spatial range of EGF receptor autocrine ligand binding. Dev Biol. 2002;250:305–316. [PubMed] [Google Scholar]
- 5.Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT, Benezra R. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science. 2004;306:247–252. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105:151–163. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 9.Silva EA, Mooney DJ. Synthetic extracellular matrices for tissue engineering and regeneration. Curr Top Dev Biol. 2004;64:181–205. doi: 10.1016/S0070-2153(04)64008-7. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann WH, Melnychenko I, Eschenhagen T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials. 2004;25:1639–1647. doi: 10.1016/s0142-9612(03)00521-0. [DOI] [PubMed] [Google Scholar]
- 11.Cannizzaro SM, Padera RF, Langer R, Rogers RA, Black FE, Davies MC, Tendler SJ, Shakesheff KM. A novel biotinylated degradable polymer for cell-interactive applications. Biotechnol Bioeng. 1998;58:529–535. [PubMed] [Google Scholar]
- 12.Wang DA, Ji J, Sun YH, Shen JC, Feng LX, Elisseeff JH. In situ immobilization of proteins and RGD peptide on polyurethane surfaces via poly(ethylene oxide) coupling polymers for human endothelial cell growth. Biomacromolecules. 2002;3:1286–1295. doi: 10.1021/bm0255950. [DOI] [PubMed] [Google Scholar]
- 13.Lateef SS, Boateng S, Hartman TJ, Crot CA, Russell B, Hanley L. GRGDSP peptide-bound silicone membranes withstand mechanical flexing in vitro and display enhanced fibroblast adhesion. Biomaterials. 2002;23:3159–3168. doi: 10.1016/s0142-9612(02)00062-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee KY, Alsberg E, Hsiong S, Comisar W, Linderman J, Ziff R, Mooney DJ. Nanoscale adhesion ligand organization regulates osteoblast proliferation and differentiation. Nano Lett. 2004;4:1501–1506. doi: 10.1021/nl0493592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asou Y, Rittling SR, Yoshitake H, Tsuji K, Shinomiya K, Nifuji A, Denhardt DT, Noda M. Osteopontin facilitates angiogenesis, accumulation of osteoclasts, and resorption in ectopic bone. Endocrinology. 2001;142:1325–1332. doi: 10.1210/endo.142.3.8006. [DOI] [PubMed] [Google Scholar]
- 16.Martin SM, Schwartz JL, Giachelli CM, Ratner BD. Enhancing the biological activity of immobilized osteopontin using a type-1 collagen affinity coating. J Biomed Mater Res A. 2004;70A:10–19. doi: 10.1002/jbm.a.30052. [DOI] [PubMed] [Google Scholar]
- 17.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong HJ, Kaigler D, Kim K, Mooney DJ. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules. 2004;5:1720–1727. doi: 10.1021/bm049879r. [DOI] [PubMed] [Google Scholar]
- 19.Lee KY, Bouhadir KH, Mooney DJ. Controlled degradation of hydrogels using multi-functional cross-linking molecules. Biomaterials. 2004;25:2461–2466. doi: 10.1016/j.biomaterials.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Karande TS, Ong JL, Agrawal CM. Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, architecture, and nutrient mixing. Ann Biomed Eng. 2004;32:1728–1743. doi: 10.1007/s10439-004-7825-2. [DOI] [PubMed] [Google Scholar]
- 21.Salem AK, Stevens R, Pearson RG, Davies MC, Tendler SJ, Roberts CJ, Williams PM, Shakesheff KM. Interactions of 3T3 fibroblasts and endothelial cells with defined pore features. J Biomed Mater Res. 2002;61:212–217. doi: 10.1002/jbm.10195. [DOI] [PubMed] [Google Scholar]
- 22.Guan L, Davies JE. Preparation and characterization of a highly macroporous biodegradable composite tissue engineering scaffold. J Biomed Mater Res A. 2004;71:480–487. doi: 10.1002/jbm.a.30173. [DOI] [PubMed] [Google Scholar]
- 23.Wei HJ, Liang HC, Lee MH, Huang YC, Chang Y, Sung HW. Construction of varying porous structures in acellular bovine pericardia as a tissue-engineering extracellular matrix. Biomaterials. 2005;26:1905–1913. doi: 10.1016/j.biomaterials.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Botchwey EA, Dupree MA, Pollack SR, Levine EM, Laurencin CT. Tissue engineered bone: measurement of nutrient transport in three-dimensional matrices. J Biomed Mater Res A. 2003;67:357–367. doi: 10.1002/jbm.a.10111. [DOI] [PubMed] [Google Scholar]
- 25.Narmoneva DA, Oni O, Sieminski AL, Zhang S, Gertler JP, Kamm RD, Lee RT. Self-assembling short oligopeptides and the promotion of angiogenesis. Biomaterials. 2005;26:4837–4846. doi: 10.1016/j.biomaterials.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Chen RR, Mooney DJ. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 27.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banfi A, von Degenfeld G, Blau HM. Critical role of microenvironmental factors in angiogenesis. Curr Atheroscler Rep. 2005;7:227–234. doi: 10.1007/s11883-005-0011-7. [DOI] [PubMed] [Google Scholar]
- 30.Ohta M, Suzuki Y, Chou H, Ishikawa N, Suzuki S, Tanihara M, Mizushima Y, Dezawa M, Ide C. Novel heparin/alginate gel combined with basic fibroblast growth factor promotes nerve regeneration in rat sciatic nerve. J Biomed Mater Res A. 2004;71A:661–668. doi: 10.1002/jbm.a.30194. [DOI] [PubMed] [Google Scholar]
- 31.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, Tabata Y. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25:4513–4520. doi: 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 33.Stayton PS, Nelson KE, McDevitt TC, Bulmus V, Shimoboji T, Ding Z, Hoffman AS. Smart and biofunctional streptavidin. Biomol Eng. 1999;16:93–99. doi: 10.1016/s1050-3862(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 34.Hoya K, Guterman LR, Miskolczi L, Hopkins LN. A novel intravascular drug delivery method using endothelial biotinylation and avidin-biotin binding. Drug Deliv. 2001;8:215–222. doi: 10.1080/107175401317245895. [DOI] [PubMed] [Google Scholar]
- 35.Salem AK, Cannizzaro SM, Davies MC, Tendler SJ, Roberts CJ, Williams PM, Shakesheff KM. Synthesis and characterisation of a degradable poly(lactic acid)-poly(ethylene glycol) copolymer with biotinylated end groups. Biomacromolecules. 2001;2:575–580. doi: 10.1021/bm010030+. [DOI] [PubMed] [Google Scholar]
- 36.Beard DA, Bassingthwaighte JB. Modeling advection and diffusion of oxygen in complex vascular networks. Ann Biomed Eng. 2001;29:298–310. doi: 10.1114/1.1359450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuurbier CJ, van Iterson M, Ince C. Functional heterogeneity of oxygen supply-consumption ratio in the heart. Cardiovasc Res. 1999;44:488–497. doi: 10.1016/s0008-6363(99)00231-x. [DOI] [PubMed] [Google Scholar]
- 38.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–968. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohtsuka M, Takano H, Zou Y, Toko H, Akazawa H, Qin Y, Suzuki M, Hasegawa H, Nakaya H, Komuro I. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. Faseb J. 2004;18:851–853. doi: 10.1096/fj.03-0637fje. [DOI] [PubMed] [Google Scholar]
- 40.Sivakumar B, Harry LE, Paleolog EM. Modulating angiogenesis: more vs less. J Am Med Assoc. 2004;292:972–977. doi: 10.1001/jama.292.8.972. [DOI] [PubMed] [Google Scholar]
- 41.Yoon YS, Johnson IA, Park JS, Diaz L, Losordo DW. Therapeutic myocardial angiogenesis with vascular endothelial growth factors. Mol Cell Biochem. 2004;264:63–74. doi: 10.1023/b:mcbi.0000044375.33928.62. [DOI] [PubMed] [Google Scholar]
- 42.Iwakura A, Fujita M, Kataoka K, Tambara K, Sakakibara Y, Komeda M, Tabata Y. Intramyocardial sustained delivery of basic fibroblast growth factor improves angiogenesis and ventricular function in a rat infarct model. Heart Vessels. 2003;18:93–99. doi: 10.1007/s10380-002-0686-5. [DOI] [PubMed] [Google Scholar]
- 43.Laham RJ, Sellke FW, Edelman ER, Pearlman JD, Ware JA, Brown DL, Gold JP, Simons M. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled trial. Circulation. 1999;100:1865–1871. doi: 10.1161/01.cir.100.18.1865. [DOI] [PubMed] [Google Scholar]
- 44.Peattie RA, Nayate AP, Firpo MA, Shelby J, Fisher RJ, Prestwich GD. Stimulation of in vivo angiogenesis by cytokine-loaded hyaluronic acid hydrogel implants. Biomaterials. 2004;25:2789–2798. doi: 10.1016/j.biomaterials.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 45.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 46.Marui A, Kanematsu A, Yamahara K, Doi K, Kushibiki T, Yamamoto M, Itoh H, Ikeda T, Tabata Y, Komeda M. Simultaneous application of basic fibroblast growth factor and hepatocyte growth factor to enhance the blood vessels formation. J Vasc Surg. 2005;41:82–90. doi: 10.1016/j.jvs.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 47.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 48.Anderson DG, Burdick JA, Langer R. Materials science. Smart biomaterials. Science. 2004;305:1923–1924. doi: 10.1126/science.1099987. [DOI] [PubMed] [Google Scholar]
- 49.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 50.Chau Y, Tan FE, Langer R. Synthesis and characterization of dextranpeptide-methotrexate conjugates for tumor targeting via mediation by matrix metalloproteinase II and matrix metalloproteinase IX. Bioconjug Chem. 2004;15:931–941. doi: 10.1021/bc0499174. [DOI] [PubMed] [Google Scholar]
- 51.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 52.Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS. Bioinspired pH-responsive polymers for the intracellular delivery of biomolecular drugs. Bioconjug Chem. 2003;14:412–419. doi: 10.1021/bc020056d. [DOI] [PubMed] [Google Scholar]
- 53.Feng Y, Yang JH, Huang H, Kennedy SP, Turi TG, Thompson JF, Libby P, Lee RT. Transcriptional profile of mechanically induced genes in human vascular smooth muscle cells. Circ Res. 1999;85:1118–1123. doi: 10.1161/01.res.85.12.1118. [DOI] [PubMed] [Google Scholar]
- 54.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 55.Elisseeff J. Injectable cartilage tissue engineering. Expert Opin Biol Ther. 2004;4:1849–1859. doi: 10.1517/14712598.4.12.1849. [DOI] [PubMed] [Google Scholar]
- 56.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 57.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semino CE, Merok JR, Crane GG, Panagiotakos G, Zhang S. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation. 2003;71:262–270. doi: 10.1046/j.1432-0436.2003.7104503.x. [DOI] [PubMed] [Google Scholar]
- 59.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by highepitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 61.Yun YH, Goetz DJ, Yellen P, Chen W. Hyaluronan microspheres for sustained gene delivery and site-specific targeting. Biomaterials. 2004;25:147–157. doi: 10.1016/s0142-9612(03)00467-8. [DOI] [PubMed] [Google Scholar]
- 62.Christman KL, Fang Q, Yee MS, Johnson KR, Sievers RE, Lee RJ. Enhanced neovasculature formation in ischemic myocardium following delivery of pleiotrophin plasmid in a biopolymer. Biomaterials. 2005;26:1139–1144. doi: 10.1016/j.biomaterials.2004.04.025. [DOI] [PubMed] [Google Scholar]


