Abstract
α1-Adrenoceptors of the locus coeruleus (LC) have been implicated in behavioral activation in novel surroundings, but the endogenous agonist that activates these receptors has not been established. In addition to the canonical activation of α1-receptors by norepinephrine (NE), there is evidence that dopamine (DA) may also activate certain brain α1-receptors. This study examined the contribution of DA to exploratory activity in a novel cage by determining the effect of infusion of various dopaminergic and adrenergic drugs into the mouse LC. It was found that the D2/D3 agonist, quinpirole, which selectively blocks the release of CNS DA, produced a dose-dependent and virtually complete abolition of exploration and all movement in the novel cage test. The quinpirole-induced inactivity was significantly attenuated by coinfusion of DA but not by the D1 agonist, SKF38390. Furthermore, the DA attenuation of quinpirole inactivity was blocked by coinfusion of the α1-adrenergic receptor antagonist, terazosin, but not by the D1 receptor antagonist, SCH23390. LC infusions of either quinpirole or terazosin also produced profound inactivity in DA-β-hydroxylase knockout (Dbh −/−) mice that lack NE, indicating that their behavioral effects were not due to an alteration of the release or action of LC NE. Measurement of endogenous DA, NE, and 5HT and their metabolites in the LC during exposure to the novel cage indicated an increase in the turnover of DA and NE but not 5HT. These results indicate that DA is a candidate as an endogenous agonist for behaviorally activating LC α1-receptors and may play a role in the activation of this nucleus by novel surroundings.
Keywords: locus coeruleus, α1-adrenoceptor, DA, motor activity, novelty
INTRODUCTION
α1-Adrenoceptors in widely distributed regions of the CNS have been shown to play a significant role in behavioral activation under a variety of conditions (Stone et al., 2007b). Pharmacological blockade of these receptors in the motor and piriform cortex, nucleus accumbens, preoptic area, lateral hypothalamus, vermis cerebellum, locus coeruleus (LC), and dorsal raphe produces immobility in a novel test cage, whereas stimulation leads to behavioral activation in the familiar home cage (Stone et al., 2004a,b). The LC appears to be a key region in this system in that it contains a dense concentration of α1-receptors (Jones et al., 1985), which when blocked with locally injected terazosin produces one of the strongest degrees of immobility and catalepsy in the novel cage test (7 out of 10 min immobile), and when stimulated with catecholamines produces vigorous exploratory behavior in familiar surroundings (Stone et al., 2004a,b). Blockade of LC α1-receptors also impairs lateral hypothalamic self-stimulation behavior in rats (Lin et al., 2007), suggesting a broad role in positively motivated behaviors. Presumably, LC activation via stimulation of local α1-receptors produces increased postsynaptic α1-noradrenergic neurotransmission in one or more of these target areas and is responsible for behavioral activation under these conditions.
The endogenous neurotransmitter that activates LC α1-receptors is either norepinephrine (NE), dopamine (DA), or epinephrine (EPI). EPI-containing nerve terminals emanating from the paragigantocellularis nucleus of the ventrolateral medulla are known to innervate the LC (Astier et al., 1990). Although we have shown that exogenous EPI injected in the LC can stimulate behavioral activity in the home cage, only a portion of this effect is blocked by an α1-receptor antagonist (Stone et al., 2003). Furthermore, preliminary experiments in our laboratory have failed to reveal substantial amounts of EPI in homogenates of the LC or reliable inhibition of behavioral activity after local inhibition of EPI biosynthesis in the LC by the phenylethanolamine-N-methyltransferase (PNMT) inhibitor dichloromethyl-benzylamine (Stone, Quartermain, Lin, unpublished results).
There is more consistent evidence that either or both NE and DA are the endogenous agonists for these receptors. With respect to NE, the high concentrations of extracellular NE found in the LC originates from either recurrent noradrenergic axon collaterals (Nakamura et al., 1988) or somatodendritic release from LC noradrenergic neurons (Fernández-Pastor et al., 2005; Singewald and Philippu, 1998). We and others (De Sarro et al., 1987; Stone et al., 2005) have shown that local reduction of NE release in the LC by injection of α2-adrenoceptor agonists produces marked immobility in the novel cage test in mice and rats.
With respect to DA, although tract tracing and immunohistochemical studies have been inconclusive regarding a dopaminergic innervation of the LC (Deutch et al., 1986; Somerville et al., 2007), substantial amounts of this catecholamine (Dishman et al., 1997; Versteeg et al., 1976) and high concentrations of its metabolite, dihydroxyphenylacetic acid, are found in this nucleus (Lambas-Senas et al., 1990), although its cellular localization is still unclear. Although a microdialysis study of the cat LC found relatively small amounts of extracellular DA (Crochet and Sakai, 1999), a push–pull cannula study in the same species found equal levels of extracellular DA and NE in this nucleus (Kaehler et al., 1999), which is not unexpected given that DA is the immediate precursor of NE. In addition, an early study showed that activation of the DAergic cell bodies in the ventral tegmental area produced activation of LC neurons as evidenced by the accumulation of metabolites of NE in noradrenergic projection areas (Deutch et al., 1986). Although DA possesses only 1/50 the affinity of NE at α1-receptors (Leedham and Pennefather, 1986), endogenous DA has been shown conclusively by electrophysiological methods to activate these receptors in the avian preoptic area (Cornil et al., 2002) and is also known to be equally efficacious as NE at these receptors in cell culture (Rey et al., 2001; Zhang et al., 2004).
In addition, the arousing effect of the stimulant modafinil, which is dependent on α1-receptor activity (Duteil et al., 1990; Stone et al., 2002), has been found to be potently inhibited by selective blockade of DA release with the D2 dopaminergic agonist, quinpirole (Wisor and Eriksson, 2005). The latter authors have suggested that DA is an endogenous agonist at α1-receptors involved in arousal.
A contribution of DA to the activation of the LC would have significant implications for an understanding of the role of this nucleus in behavioral regulation and motivation. Therefore, the present experiments were undertaken to test this hypothesis. This was accomplished by examining the effect of microinjection of various selective dopaminergic and adrenergic agents in the vicinity of the mouse LC on novel cage-induced behavioral activity in both outbred and genetically altered (NE-deficient) mice.
MATERIALS AND METHODS
Subjects
Swiss Webster male mice (Taconic), 8–10 weeks old, female DA-β-hydroxylase knockout (Dbh −/−) mice that completely lack NE, and female and male wild-type control mice, 4–9 months of age, of a mixed C57BL6/J and 129SvEv genetic background (Thomas et al., 1998) were subjects. The animals were housed singly with nesting material for 5 days prior to surgery in standard polycarbonate mouse cages (12.5 cm × 17 cm × 28 cm) at a room temperature of 22°C ± 1°C under a 12 h light/dark cycle (lights on 05:00 h). Food and water were available ad libitum.
Surgery
Mice were anesthetized with pentobarbital (70 mg/kg) and implanted stereotaxically with unilateral 26 ga cannula guide tubes in the dorsal pons in the region of the LC at the following coordinates (−5.5 mm posterior to Bregma, ±0.9 mm lateral to the mid-line, 3.9 mm beneath the surface of the skull). We have shown in a previous study that unilateral blockade of LC α1-adrenoceptors in the mouse is sufficient to produce maximal inactivity in the 10-min novel cage test (Stone et al., 2004a,b). All animals were given 10 days for recovery prior to behavioral testing.
Procedure
All experiments were performed between 1000 and 1400 h. Mice were gently restrained under a double layer of gauze on a corkboard and a 33 ga cannula connected by PE tubing to a syringe pump (Razel) was inserted into the cannula guide tube (protruding 1 mm below the bottom of the guide). A total of 350 nl of solution was infused at 150 nl/min over a 2.3-min period with the cannula remaining in place for 30 s after infusion. The animals received either vehicle (saline), the D2 dopaminergic agonist, quinpirole (Q), DA, the D1/D5 agonist, SKF38393 (SKF), the α1-adrenoceptor antagonist, terazosin (T), or the D1/D5 antagonist, SCH23390 (SCH), singly or in combination, in doses ranging from 0.3 to 30 nmol per mouse. All drugs were prepared fresh each day in saline. Minimum doses were determined empirically in pilot studies. Immediately following the injection, the animal was transferred to a clean unfamiliar mouse cage of identical type and dimensions as the home cage (“novel home cage”) with Bed-O-Cob bedding (0.63 mm) and was videotaped for the next 10 min. The animals received four such injections at 4 days intervals with the first and fourth using Q at 3 nmol, and the second and third being any of the drugs singly or in combination. Thus different groups of animals received two different drug treatments in addition to the Q3 trials. A given drug treatment was represented equally on the second and third injections.
Behavioral ratings
Videotapes were rated either by a trained observer (in blind fashion) for a measure of total behavioral activity [the number of gross movements (GM) made] and time immobile (no observable movements) or by a videotracking system (Smart System, San Diego Inst) for ambulation (the number of times the animal crossed the long and short axes of the cage). GMs were defined as any movement involving at least the head plus forelimbs that was followed by a momentary pause. These include ambulations to a cage wall and other discrete forward movements, rearing responses, stretch and attend responses, turns and large grooming movements. Interrater agreement on this measure has averaged above r = 0.9 in this laboratory. Central α1-adrenoceptor activity has been shown in previous studies to have a general modulating effect on a variety of different active behaviors, and the number of GM made in the novel cage test has been shown to be a function of this activity (Stone et al., 1999, 2001, 2004a,b). All experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (1996), and were approved by the New York University School of Medicine IUCAC.
DA turnover in the vicinity of the LC
Undisturbed mice or mice exposed to a novel home cage for 20 min were sacrificed by rapid decapitation. The LC and adjacent tissue were grossly dissected over ice as a 1.5 mm × 1.5 mm × 1 mm block of tissue centering on the lateral extent of IV. ventricle from a 1-mm brain section between −5 and −6 mm Bregma. NE, 3-methoxy-4-hydroxyphenylglycol (MHPG), DA, 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) were assayed by high-performance liquid chromatography (HPLC) as described by Dunn (1993).
Histology
After the experiment, the implanted animals were deeply anesthetized and injected through the cannula with 350 nl of 2% methyl blue. Brains were fixed in 10% formalin for 3 days and then sectioned for determination of cannula location.
Statistical analysis
The individual data points comprised 2–3 observations per animal taken under different drug conditions. All analyses involved either one- or two-way ANOVAs. Because of the diverse nature of drug treatments used there were no significant correlations between responses to the different drug treatments across the four trials. Therefore, each of the data points was treated as an independent observation. All individual mean comparisons were planned and were Bonferroni-corrected.
RESULTS
Localization of quinpirole effect
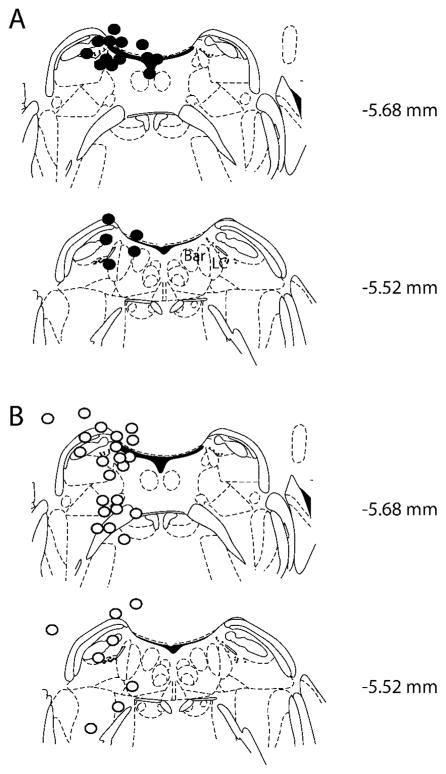
An initial group of 44 animals that were implanted at various sites in the pons and cerebellum were injected with Q at 3 nmol and assessed for behavioral activity in the novel home cage test. Of these, 19 (43.2%) developed immobility of greater than 7.5 min out of the 10 min test and were judged as Q3-positive animals. Histological analysis (Fig. 1) indicated that of the 19, 4 were located in the 4th ventricle and were excluded, whereas 14 of the remaining 15 (93.3%) had cannulas within 0.5 mm of the border of the LC core, and only 9 (31.0%) of the 25 Q3-negative animals had cannulas this close. This difference in percentage was highly significant by Fisher’s exact test (P < 0.0001).
Fig. 1.
Locations of cannula tips for quinpirole-positive (A) and -negative (B) animals in initial experiment. Quinpirole was infused at 3 nmol unilaterally. LC, locus coeruleus; BAR, Barrington’s nucleus. Figures adapted from Franklin and Paxinos (1997).
Alternately, if the group of 40 mice was divided into those having cannulas within 0.5 mm of the LC and those outside this distance, a similar result was obtained, with the “within” group having more than a threefold higher level of immobility than the “outside” group (within, 6.73 ± 0.64 min (23); outside, 2.21 ± 0.56 min (17), t38 = 5.24, P < 0.001).
In subsequent experiments, we therefore adopted the procedure of first screening all implanted animals with an initial Q3 test and only using Q3-positive animals for two subsequent drug challenges at 4 days intervals (the location of the novel home cage in the laboratory was changed for each test). Pilot experiments showed that the majority of animals had reliable responses to repeated administrations of this drug for up to four injections at 4 days intervals. A final Q3 test (fourth injection) was therefore employed to determine if the initial response to the drug was maintained throughout the series of injections. Of a total of 116 initially Q3-positive animals, 62.2% maintained the immobility response to the drug on the 4th challenge, and of these, 75.3% were found to have cannulas within 0.5 mm of the LC and were used in the final analysis. All responses to Q3 were computed as the average of the first and fourth injection, whereas all responses to other Q doses and to all other drugs were based on responses to the second or third injections. There was a high and significant correlation between immobility scores of separate groups of mice given the same drug treatments on different tests (r10 = 0.92, P < 0.001), which suggests that responses to the same treatments were stable and largely independent of order effects.
Dose response to quinpirole and comparison with terazosin and SCH23390
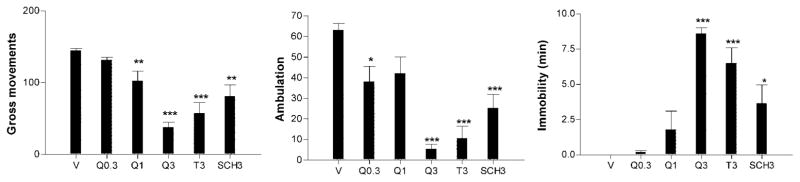
The effect of Q at doses between 0.3 and 3 nmol on activity level in the novel cage is shown in Figure 2. A one-way ANOVA comprising the vehicle and all Q doses indicated that the drug produced significant dose-dependent reductions of both GM (F3,27 = 91.23, P < 0.0001) and ambulation (F3,27 = 25.98, P < 0.0001) and a significant dose-dependent increase in immobility (F3,27 = 73.57, P < 0.0001). Figure 2 also shows the effects of terazosin 3 nmol (T3) and SCH23390 3 nmol (SCH3). Comparison of the vehicle, Q3, T3, and SCH3 conditions by one-way ANOVAs followed by posthoc Bonferroni tests revealed that T3 significantly reduced GM (P < 0.0001) and ambulation (P < 0.0001) and increased immobility (P < 0.0001), and that SCH3 also reduced GM (P < 0.01) and ambulation (P < 0.005), but produced only a borderline increase in immobility (P < 0.06). However, the degree of inactivity produced by Q3 on all behavioral measures was significantly greater than that produced by SCH3 (all Ps < 0.001), whereas there were no significant differences between the Q3 and T3 conditions on any measure.
Fig. 2.
Effect of various doses of quinpirole (Q), terazosin (T), and SCH23390 (SCH) or vehicle (v, saline) in the LC on behavioral activation in novel home cage test. Values are means and SEMs of 7–10 determinations for Q 0.3–3 nmol and 5–7 determinations for T3 and SCH3. *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle condition.
Attenuation of Q3 immobility by DA but not by SKF38393
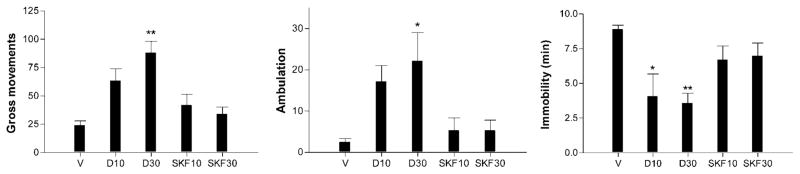
The effect of coinfusion of DA and SKF38393 (SKF) at 10 and 30 nmol on Q3-induced inactivity is shown in Figure 3. A one-way ANOVA comprising only the DA conditions (0, 10, and 30 nmol) showed that the catecholamine produced a dose-dependent attenuation of the reduction in GM (F2,24 = 14.98, P < 0.0001) and ambulation (F2,24 = 3.52, P < 0.05), and of the increase in immobility (F2,24 = 16.21, P < 0.0001) caused by the D2 agonist (Fig. 3). In the absence of Q3, DA had no significant effect on any behavior at either dose (data not shown). The same analysis for the SKF conditions (0, 10, and 30 nmol) failed to reveal a significant action on Q3-induced immobility.
Fig. 3.
Dose-dependent reversal of quinpirole-induced inactivity by coinfusion of DA but not SKF38393. Mice were infused with quinpirole (3 nmol) in the presence of either DA or SKF at 0, 10, and 30 nmol. DA or SKF38393 were dissolved in the Q3-containing saline solution (veh). Means and SEMs of 6–10 determinations. *P < 0.05, **P < 0.001 vs. vehicle.
Blockade of the attenuating effect of DA by an antagonist of α1-adrenergic but not D1 dopaminergic receptors
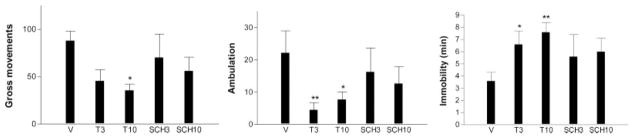
The effects of T and SCH (both at 3 and 10 nmol) on the attenuating effect of DA (30 nmol) of Q3-induced inactivity are shown in Figure 4. In this figure, the Q3-DA30 group is the same as used in the foregoing comparison (3). A one-way ANOVA comprising the Q3-DA30 condition in the presence of 0, 3, and 10 nmol T showed that the α1-antagonist produced a blockade of the DA reversal effect that was significant for GM (F2,27 = 8.63, P < 0.005) and immobility (F2,27 = 6.86, P = 0.005), and was of borderline significance for ambulation (F2,27 = 3.24, P < 0.06). Analyses of linear trend components suggested that these effects were dose-dependent for GM (F1,27 = 11.22, P < 0.005) and for time immobile (F1,27 = 9.08, P < 0.01). The one-way ANOVA for SCH at 0, 3, and 10 nmol did not reveal a significant effect on any measure. Neither T nor SCH at 10 nmol significantly altered any of the behaviors of animals treated with only Q3 (data not shown).
Fig. 4.
Blockade of DA-reversal of quinpirole-inactivity by terazosin but not by SCH23390. Mice were infused with 3 nmol quinpirole + 30 nmol DA in the presence of either 0 (veh), 3 or 10 nmol of terazosin or SCH23390. Terazosin or SCH23390 were dissolved in the Q3-DA30-containing saline solution (veh). Means and SEMs of 6–10 determinations. *P < 0.01 vs. vehicle condition.
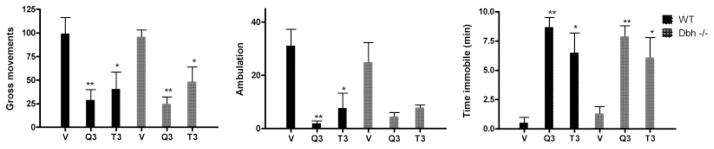
Effect of quinpirole and terazosin in NE-deficient mice
Because of the smaller size and increased fragility of the Dbh −/− mice, the initial screening injection was omitted and the animals were injected only three times with Q3, T3, and vehicle in that order. Eight of the 10 WT and 6 of the 11 Dbh −/− mice were subsequently found to have cannulas located within 0.5 mm of the LC and completed the Q3 and T3 injections. Because of the loss of cannulas, only five of each group completed the vehicle injection. The results are shown in Figure 5. A 2 ×3 (genotype × drug) ANOVA revealed significant main effects of drug for each dependent measure (GM, F2,31 = 13.08, P < 0.001; ambulation, F2,31 = 13.48, P < 0.001; time immobile, F2,31 = 16.19, P < 0.0001), but there was no significant effects of genotype (GM, F1,31 = 0.02, NS; ambulation, F1,31 = 2.15, NS; time immobile, F1,31 = 0.04, NS) or interactions of genotype and drug for any dependent measure (GM, F2,31 = 0.17, NS; ambulation, F2,31 = 1.67, NS; time immobile, F2,31 = 0.16, NS).
Fig. 5.
Induction of inactivity in NE-deficient mice by quinpirole and terazosin. Dbh −/− or wild type mice were infused with vehicle (v, saline), quinpirole or terazosin at 3 nmol. Means and SEMs of 6–8 determinations for Q and T and 5 determinations for vehicle. *P < 0.05, **P < 0.01.
Turnover of LC DA during exposure to novel home cage
The effects of novel home cage exposure on levels and turnover of DA, NE, and 5HT in the vicinity of the LC are shown in Table I. Novel cage exposure produced a highly significant increase in the concentration of DOPAC and in the ratios of DOPAC/DA, and MHPG/NE indicative of increases in the turnover of DA and NE. The concentrations of 5HT and 5HIAA and the 5HIAA/5HT ratio were not significantly altered.
TABLE I.
Effect of novel home cage exposure on turnover of NE, DA, and 5HT in the vicinity of the LC
| Group | DA | DOPAC | DOPAC/DA | NE | MHPG | MHPG/NE | 5HT | 5HIAA | 5HIAA/5HT |
|---|---|---|---|---|---|---|---|---|---|
| Control (9) | 0.019 | 0.015 | 0.786 | 0.349 | 0.016 | 0.047 | 0.398 | 0.485 | 1.109 |
| 0.002 | 0.002 | 0.027 | 0.040 | 0.002 | 0.001 | 0.042 | 0.112 | 0.147 | |
| Novel cage (9) | 0.023 | 0.025 | 1.049 | 0.369 | 0.023 | 0.060 | 0.406 | 0.568 | 1.281 |
| 0.001 | 0.003 | 0.072 | 0.032 | 0.003 | 0.003 | 0.038 | 0.134 | 0.189 | |
| t | −1.76 | −3.35 | 3.43 | −0.40 | 1.92 | 4.40 | −0.13 | −0.47 | −0.72 |
| p | 0.0960 | 0.0004 | 0.0034 | NS | 0.0720 | 0.0004 | NS | NS | NS |
Values are means and SEMs of groups of 9 mice. Values of amines are in ng/mg wet weight.
DISCUSSION
These results provide indirect pharmacological evidence that DA can stimulate the population of α1-adrenoceptors in the vicinity of the LC that functions in behavioral activation in the novel home cage test. Quinpirole, a D2/D3 agonist that selectively reduces the release of CNS DA (Devoto et al., 2002), produced a significant and near total dose-dependent abolition of all movement in this test when injected within 0.5 mm of this nucleus, but was much less effective at greater distances. No loss of the righting reflex (maintenance of an upright position) was observed as would have occurred with a sedative effect. The quinpirole-induced immobility was significantly and dose-dependently reversed by the addition of DA, whereas DA given alone had no effect on nonquinpirole-treated animals who were already maximally active. The DA reversal appeared to be acting via stimulation of α1-adrenoceptors, since (a) it was significantly blocked by the α1-antagonist, terazosin; (b) it was not blocked by the D1 antagonist, SCH23390; and (c) the D1 agonist, SKF38390, failed to reverse the Q-induced inactivity. These data therefore add to the previous direct electrophysiological evidence of the stimulation of CNS α1-adrenoceptors by DA discussed earlier.
Since D2 receptors may be present on noradrenergic as well as dopaminergic neurons (Smiakowska and Legutko, 1992), it could be argued that the behavioral effects of quinpirole were due to an inhibition of NE release and a subsequent failure of NE to activate α1-receptors in the LC. This possibility, however, appears very unlikely because quinpirole and terazosin were equally effective in control and Dbh −/− ice, which completely lack NE. Combining these results suggests that DA is an endogenous ligand for α1-receptors in the LC, although the source of the DA is uncertain. The DA could be coming from dopaminergic terminals that innervate the LC, or from the LC itself, which produces DA instead of NE in Dbh −/− ice (Thomas et al., 1998). DA release from noradrenergic neurons has also been observed in wild-type animals. Because DA possesses only 1/50 the affinity of NE at α1-adrenoceptors, the question arises as to how this catecholamine can stimulate these receptors as suggested by the present and previous experiments. However, α1-receptors, are now known to form heterodimers that possess agonist properties different from individual subtypes (Hague et al., 2006). It is possible therefore that DA has a greater affinity for a receptor heterodimer than for individual α1-receptors. Since the only catecholamine present in the Dbh −/− animals is DA, it is highly likely that the latter compound is an agonist for some behaviorally activating α1-receptors in the NE-deficient mice. This is consistent with activation of α1- and α2-receptors in the avian preoptic area by endogenous DA discussed earlier. Whether this is also obtained in wild type mice is not presently clear, but it is known that both DA and NE are released at many sites in the CNS.
The presumed excitation of LC α1-receptors by DA must, of course, await further direct confirmation by appropriate electrophysiological methods. However, if DA is, in fact, responsible for the stimulation of these receptors during novel home cage exposure, it should be possible to demonstrate an increase in DA release near the LC during this stimulus. This was confirmed in the present experiment from measures of the ratio of DOPAC:DA in the dorsal pons in the vicinity of the LC of mice exposed to the novel home cage. It has been suggested that DOPAC in the LC arises from noradrenergic rather than dopaminergic neurons, because there is a high correlation between levels of this metabolite and the electrical activity of LC neurons (Buda et al., 1994). However, as discussed earlier, significant concentrations of DA have been demonstrated in the extracellular fluid near the LC, which makes it more likely that the metabolite arises from dopaminergic neurons. This is in agreement with the present finding that the level of DOPAC, a measure of turnover, was similar to the level of MHPG despite the low concentration of the parent amine DA. It is possible that the high correlation previously observed between LC activity and DOPAC concentrations may result from correlated activities between dopaminergic afferents and LC neurons. This is supported by the present finding of increased turnover of both DA and NE in the vicinity of the LC during novel cage exposure.
The exact location of the α1-receptors mediating behavioral activation in a novel environment also remains to be determined. Although radioligand binding experiments have detected the presence of α1-receptors in the LC (Chamba et al., 1991; Jones et al., 1985; Stone et al., 2004a,b), in situ hybridization techniques have failed to detect mRNA for α1-receptors in this region (Pieribone et al., 1994), suggesting that these receptors may be located on terminals from distal neurons (e.g., glutamatergic nerve terminals) that innervate the LC (Singewald and Philippu, 1998). One appealing hypothesis is that α1-receptors located on glutamatergic neurons innervating the LC increase glutamate release, which in turn increases the excitability of LC neurons. On the other hand, more recent in situ studies (Day et al., 1997) and more sensitive methods (i.e., RT-PCR) have detected α1-receptor mRNA expression by LC neurons (Osborne et al., 2002), and electrophysiological experiments indicate that activation of α1-receptors on LC dendrites increases excitability of these cells (Finlayson and Marshall, 1986; Ivanov and Aston-Jones, 1995; Nakamura et al., 1988). Interestingly, some excitatory effects of α1-receptor activation on LC neurons are most evident during early development but disappear in adults (Finlayson and Marshall, 1986; Nakamura et al., 1988), while others persist into adulthood (Osborne et al., 2002). We obtained our behavioral results in adult mice, while most of the aforementioned studies examined rats. Further investigation will be needed to determine the relative contribution of α1-receptors present on LC dendrites and those present on nerve terminals in the LC to the novelty-induced motor response.
The functional significance of the presumed dopaminergic excitation of LC α1-receptors is not presently understood. However, it may be related to the different types of behaviors mediated by this nucleus. The LC has long been known to be involved in both negative affect-related [stress (Weiss and Simson, 1985), opiate withdrawal (Taylor et al., 1988)] and positive affect-related behaviors [self stimulation (Lin et al., 2007) as well as reward-based operant behavior (Aston-Jones and Cohen, 2005)]. Novel home cage exploration is predominantly a positively motivated behavior in that it is accompanied by robust Fos expression in brain areas involved in approach behavior and motor/motivational processes but not in stress response regions (Stone et al., 2007a), and that animals will work to gain access to novel chambers (Klebaur and Bardo, 1999). Furthermore, DA is the primary monoamine neurotransmitter of brain circuits mediating reward (Nestler and Carlezon, 2006). Therefore, it is possible that dopaminergic neurotransmission in the LC codes selectively for positive-affect related behaviors mediated by this nucleus.
Acknowledgments
Contract grant sponsor: NIMH; Contract grant numbers: MH45265, MH50947.
References
- Astier B, Van Bockstaele E, Aston-Jones G, Pieribone V. Anatomical evidence for multiple pathways leading from the rostral ventrolateral medulla (nucleus paragigantocellularis) to the locus coeruleus in rat. Neurosci Lett. 1990;118:141–146. doi: 10.1016/0304-3940(90)90612-d. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Buda M, Lachuer J, Devauges V, Barbagli B, Blizard D, Sara SJ. Central noradrenergic reactivity to stress in Maudsley rat strains. Neurosci Lett. 1994;167:33–36. doi: 10.1016/0304-3940(94)91021-9. [DOI] [PubMed] [Google Scholar]
- Chamba G, Weissmann D, Rousset C, Renaud B, Pujol JF. Distribution of α-1 and α-2 binding sites in the rat locus coeruleus. Brain Res Bull. 1991;26:185–193. doi: 10.1016/0361-9230(91)90225-9. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Sakai K. α-2 Adrenoceptor mediated paradoxical (REM) sleep inhibition in the cat. Neuroreport. 1999;10:2199–2204. doi: 10.1097/00001756-199907130-00036. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of α-1a-, α-1b- and α-1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- De Sarro GB, Ascioti C, Froio F, Libri V, Nistico G. Evidence that locus coeruleus is the site where clonidine and drugs acting at α-1 and α-2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol. 1987;90:675–685. doi: 10.1111/j.1476-5381.1987.tb11220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch A, Goldstein M, Roth R. Activation of the locus coeruleus induced by selective stimulation of the ventral tegmental area. Brain Res. 1986;363:307–314. doi: 10.1016/0006-8993(86)91016-4. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Pira L, Diana M, Gessa GL. Co-release of noradrenaline and dopamine in the prefrontal cortex after acute morphine and during morphine withdrawal. Psychopharmacology. 2002;160:220–224. doi: 10.1007/s00213-001-0985-y. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, Yoo HS, Mougey EH, Meyerhof JL. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res Bull. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- Dunn A. Neurochemical methods for evaluating cerebral biogenic amine responses to cytokines and their involvement in the central actions of interleukin-1. In: De Souza E, editor. Neurobiology of cytokines. San Diego: Academic Press; 1993. pp. 209–222. [Google Scholar]
- Duteil J, Rambert FA, Pessonnier J, Hermant J-F, Gombert R, Assous E. Central a1-adrenergic stimulation in relation to the behaviour stimulating effect of modafinil; studies with experimental animals. Eur J Pharmacol. 1990;180:49–58. doi: 10.1016/0014-2999(90)90591-s. [DOI] [PubMed] [Google Scholar]
- Fernández-Pastor B, Mateo Y, Gómez-Urquijo S, Meana JJ. Characterization of noradrenaline release in the locus coeruleus of freely moving awake rats by in vivo microdialysis. Psychopharmacology. 2005;180:570–579. doi: 10.1007/s00213-005-2181-y. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Marshall KC. Locus coeruleus neurons in culture have a developmentally transient α-1 adrenergic response. Brain Res. 1986;390:292–295. doi: 10.1016/s0006-8993(86)80238-4. [DOI] [PubMed] [Google Scholar]
- Franklin KBT, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Hague C, Lee SE, Chen ZJ, Prinster SC, Hall RA, Minneman KP. Heterodimers of α1B- and α1D-adrenergic receptors form a single functional entity. Mol Pharmacol. 2006;69:45–55. doi: 10.1124/mol.105.014985. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Extranuclear dendrites of locus coeruleus neurons: Activation by glutamate and modulation of activity by α-adrenoceptors. J Neurophysiol. 1995;74:2427–2436. doi: 10.1152/jn.1995.74.6.2427. [DOI] [PubMed] [Google Scholar]
- Jones LS, Gauger LL, Davis JN. Anatomy of brain α-1 adrenergic receptors: In vitro autoradiography with [125-I]-heat. J Comp Neurol. 1985;231:190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- Kaehler ST, Singewald N, Philippu A. The release of catecholamines in hypothalamus and locus coeruleus is modulated by peripheral chemoreceptors. Naunyn-Schmied Arch Pharmacol. 1999;360:428–434. doi: 10.1007/s002109900094. [DOI] [PubMed] [Google Scholar]
- Klebaur J, Bardo M. The effects of anxiolytic drugs on novelty-induced place preference. Behav Brain Res. 1999;101:51–57. doi: 10.1016/s0166-4328(98)00145-4. [DOI] [PubMed] [Google Scholar]
- Lambas-Senas L, Gillon J-Y, Bouilloux J-P, Seccia M, Buda M, Renaud B. In vivo monitoring of catecholaminergic metabolism in the C1 region of rat medulla oblongata: A comparative study by voltammetry and intracerebral microdialysis. J Neurochem. 1990;54:2042–2049. doi: 10.1111/j.1471-4159.1990.tb04909.x. [DOI] [PubMed] [Google Scholar]
- Leedham JA, Pennefather JN. Selectivities of some agonists acting at α-1- and α-2-adrenoreceptors in the rat vas deferens. J Auton Pharmacol. 1986;6:39–46. doi: 10.1111/j.1474-8673.1986.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Lin Y, Cabeza de Vaca S, Carr K, Stone E. Role of α1-adrenoceptors of the locus coeruleus in self-stimulation of the medial forebrain bundle. Neuropsychopharmacology. 2007;32:835–841. doi: 10.1038/sj.npp.1301145. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Sakaguchi T, Kimura F, Aoki F. The role of α1-adrenoceptor-mediated collateral excitation in the regulation of the electrical activity of locus coeruleus neurons. Neuroscience. 1988;27:921–929. doi: 10.1016/0306-4522(88)90195-9. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Osborne PB, Vidovic M, Chieng B, Hill CE, Christie MJ. Expression of mRNA and functional α (1)-adrenoceptors that suppress the GIRK conductance in adult rat locus coeruleus neurons. Br J Pharmacol. 2002;135:226–232. doi: 10.1038/sj.bjp.0704453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of α-1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey E, Hernandez-Diaz FJ, Abreu P, Alonso R, Tabares L. Dopamine induces intracellular Ca2+ signals mediated by α1B-adrenoceptors in rat pineal cells. Eur J Pharmacol. 2001;430:9–17. doi: 10.1016/s0014-2999(01)01250-x. [DOI] [PubMed] [Google Scholar]
- Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. Prog Neurobiol. 1998;56:237–267. doi: 10.1016/s0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Smiakowska M, Legutko B. Haloperidol-induced increase in neuropeptide Y immunoreactivity in the locus coeruleus of the rat brain. Neuroscience. 1992;47:351–355. doi: 10.1016/0306-4522(92)90251-v. [DOI] [PubMed] [Google Scholar]
- Somerville EM, Horwood JM, Lee MD, Kennett GA, Clifton PG. 5-HT2C receptor activation inhibits appetitive and consummatory components of feeding and increases brain c-fos immunoreactivity in mice. Eur J Neurosci. 2007;25:3115–3124. doi: 10.1111/j.1460-9568.2007.05567.x. [DOI] [PubMed] [Google Scholar]
- Stone E, Zhang Y, Rosengarten H, Yeretsian J, Quartermain D. Brain a1-adrenergic neurotransmission is necessary for behavioral activation to environmental change in mice. Neuroscience. 1999;94:1245–1252. doi: 10.1016/s0306-4522(99)00394-2. [DOI] [PubMed] [Google Scholar]
- Stone E, Lin Y, Itteera A, Quartermain D. Pharmacological evidence for the role of brain α1B-adrenergic receptors in the motor activity and spontaneous movement of mice. Neuropharmacology. 2001;40:254–261. doi: 10.1016/s0028-3908(00)00151-9. [DOI] [PubMed] [Google Scholar]
- Stone E, Cotecchia S, Lin Y, Quartermain D. Role of brain a1B-adrenoceptors in modafinil-induced behavioral activity. Synapse. 2002;46:269–270. doi: 10.1002/syn.10127. [DOI] [PubMed] [Google Scholar]
- Stone E, Grunewald G, Lin Y, Ahsan R, Rosengarten H, Kramer K, Quartermain D. Role of epinephrine stimulation of CNS a1-adrenoceptors in motor activity in mice. Synapse. 2003;49:67–76. doi: 10.1002/syn.10212. [DOI] [PubMed] [Google Scholar]
- Stone E, Lin Y, Ahsan R, Quartermain D. Role of locus coeruleus a1-adrenoceptors in motor activity in rats. Synapse. 2004a;54:164–172. doi: 10.1002/syn.20074. [DOI] [PubMed] [Google Scholar]
- Stone E, Lin Y, Ahsan R, Quartermain D. Gross mapping of α1-adrenoceptors that regulate behavioral activation in the mouse brain. Behav Brain Res. 2004b;152:167–175. doi: 10.1016/j.bbr.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Stone E, Lin Y, Ahsan M, Quartermain D. α1 and α2-adrenergic balance in the dorsal pons and gross behavioral activity of mice in a novel environment. Psychopharmacology. 2005;183:127–132. doi: 10.1007/s00213-005-0171-8. [DOI] [PubMed] [Google Scholar]
- Stone E, Lehmann M, Lin Y, Quartermain D. Reduced evoked fos expression in activity-related brain regions in animal models of depression. Prog Neuropsychopharmacol Biol Psychiat. 2007a;31:1196–1207. doi: 10.1016/j.pnpbp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Stone E, Quartermain D, Lin Y, Lehmann M. Central α1-adrenergic system in behavioral activity and depression. Biochem Pharmacol. 2007b;73:1063–1075. doi: 10.1016/j.bcp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Garcia EJ, Grant SJ, Roth RH, Redmond DE., Jr Clonidine infusions into the locus coeruleus attenuate behavioral and neurochemical changes associated with naloxone-precipitated withdrawal. Psychopharmacology. 1988;96:121–134. doi: 10.1007/BF02431544. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine β-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Versteeg DH, Van Der GJ, De Jong W, Palkovits M. Regional concentrations of noradrenaline and dopamine in rat brain. Brain Res. 1976;113:563–574. doi: 10.1016/0006-8993(76)90057-3. [DOI] [PubMed] [Google Scholar]
- Weiss J, Simson PG. Neurochemical basis of stress-induced depression. Psychopharmacol Bull. 1985;21:447–457. [PubMed] [Google Scholar]
- Wisor JP, Eriksson KS. Dopaminergic-adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience. 2005;132:1027–1034. doi: 10.1016/j.neuroscience.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang WP, Ming OY, Thomas SA. Potency of catecholamines and other L-tyrosine derivatives at the cloned mouse adrenergic receptors. Neuropharmacology. 2004;47:438–449. doi: 10.1016/j.neuropharm.2004.04.017. [DOI] [PubMed] [Google Scholar]