Abstract
Callosal morphology is thought to reflect the capacity for inter-hemispheric communication and thus, in addition to other cerebral characteristics, may serve as a neuroanatomical substrate of general intellectual capacity. We applied novel computational mesh-based methods to establish the presence and direction of correlations between intelligence and callosal thickness at high spatial resolution while removing the variance associated with overall brain size. Within healthy subjects (n=62), and within males (n=28) and females (n=34) separately, we observed significant positive correlations between callosal morphology and intelligence measures (full scale, performance, and verbal). These relationships were pronounced in posterior callosal sections and were confirmed by permutation testing. Significant negative correlations were absent. Positive associations between intelligence and posterior callosal thickness may reflect a more efficient inter-hemispheric information transfer, positively affecting information processing and integration, and thus intellectual performance. At the same time, regional variations in callosal size might also partly reflect the underlying architecture of topographically connected cortical regions relevant for processing higher-order cognitive information. Our findings emphasize the importance of incorporating posterior (callosal) regions into the theories and models proposed to explain the anatomical substrates of intelligence.
Keywords: Fibers, Gender, IQ, Lateralization, MRI, Sex
Introduction
The corpus callosum (CC) is the largest white matter structure in the human brain, connecting the two hemispheres through more than 200 million fibers. Midsagittal callosal area is an indicator of the total number of small diameter fibers within the CC (Aboitiz et al., 1992). Since small diameter fibers are particularly involved in transferring higher-order cognitive information (Aboitiz, 1992), callosal morphology may reflect the capacity for inter-hemispheric processes which modulate intellectual abilities.
Indeed, several prior studies suggest that the structural integrity of the CC is associated with intellectual abilities. For example, callosal morphology and cognitive functioning are correlated in certain neurological conditions (e.g., epilepsy and hydrocephalus), developmental disabilities (e.g., mental retardation), or diseases (e.g., sickle cell disease) (Fletcher et al., 1992; Strauss et al., 1994; Atkinson, Jr. et al., 1996; Spencer et al., 2005; Schatz and Buzan, 2006). Some studies examining the relationships between intelligence and gray matter/white matter in healthy subjects using voxel-based morphometry (Haier et al., 2004; Haier et al., 2005), however, have failed to detect significant correlations between intelligence and white matter sections in the CC. Nevertheless, some data from healthy twins and siblings suggest a common genetic origin for callosal white matter and intelligence (Hulshoff Pol et al., 2006).
To further explore possible relationships between callosal morphology and cognitive measures, we investigated the presence and direction of correlations between callosal morphology and full-scale intelligence in a large sample of healthy subjects (n=62) with a wide range of intelligence quotients (IQ range: 74–139). In addition, we examined correlations between callosal morphology and performance and verbal IQ scores separately. Anatomical mesh-based modeling methods were employed to conduct correlation analyses at 100 equidistant points that reflect callosal thickness with an extremely high spatial resolution across the entire CC in the midsagittal plane (Luders et al., 2006a). Importantly, our approach does not require a priori definitions of callosal segments (Witelson, 1989; Aboitiz et al., 1992; Clarke and Zaidel, 1994), circumventing limitations associated with previously employed parcellation schemes (Tomaiuolo et al., 2006; Hofer and Frahm, 2006; Zarei et al., 2006; Luders et al., 2007).
Prior studies have reported relationships between brain size and intelligence (McDaniel, 2005) as well as between brain size and callosal size (Rauch and Jinkins, 1994; Jancke et al., 1997). Therefore, regional relationships between callosal thickness and intelligence were investigated while removing effects explained by total brain volume (TBV). Previous studies also revealed gender-specific relationships between brain anatomy and intelligence (Gur et al., 1999; McDaniel, 2005; Witelson et al., 2006; Narr et al., 2006b) as well as gender effects on callosal morphology, although the presence of gender differences in callosal size and/or shape remains somewhat controversial (Bishop and Wahlsten, 1997; Luders et al., 2006b). Thus, we also assessed gender effects on the relationship between callosal thickness and intelligence.
Methods
Subjects
We analyzed the brains of 28 males (mean age: 28.1±7.3) and 34 females (mean age: 28.8±7.4) from an overlapping sample of healthy control subjects for other studies examining alterations in brain structure in schizophrenia (Narr et al., 2005a; 2005b) and structure-function relationships in a healthy sub-sample (Narr et al., 2006b). However, in the present study we reduced our study group to exclude three left-handed subjects (2 males, 1 female) to ensure analyses were performed in a homogeneous group of exclusively right-handed subjects. Handedness was determined using a modified 20-item Edinburgh Handedness Inventory (Oldfield, 1971), as described previously (Narr et al., 2006a)1. Participants were recruited through local newspaper advertisements and community word of mouth and were determined to have no history of psychiatric illness as assessed by clinical interview using the SCID-NP (Spitzer et al., 1992). Study exclusion criteria included serious neurological or endocrine disorders, and any medical condition or treatment known to affect the brain. Moreover, all subjects were carefully screened with respect to mental deficits and excluded when meeting DSM-IV criteria for mental retardation and/or when their IQ was below 70. The North Shore – Long Island Jewish Health System IRB approved all procedures and informed written consent was obtained from all subjects. Additional approval for image processing and analysis was received from the UCLA IRB.
Intelligence assessments
Using the Wechsler Adult Intelligence Scale (WAIS-R, Wechsler D., 1981) we measured the full-scale IQ – a composite score obtained from 11 subtests in verbal and performance categories – which is standardized in a United States population sample to have a mean of approximately 100 and standard deviation of approximately 15. In addition, we measured the performance IQ (from 5 subtests), as well as the verbal IQ (from 6 subtests), separately. In the present investigation, full-scale IQ scores ranged between 74 and 139, while performance IQ scores ranged between 70 and 124, and verbal IQ scores ranged between 71 and 147.5. There were no significant differences in intelligence measurements between males and females. The significance values, as well as group-specific means and standard deviations of all intelligence measures are shown in Table 1.
Table 1.
Group-specific means, standard deviations (SD), and significance values of gender differences in intelligence measures.
| Full-Scale IQ | Performance IQ | Verbal IQ | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Combined Sample (n=62) | 99.81 ± 12.53 | 99.44 ± 12.26 | 100.65 ± 13.41 |
| Males (n=28) | 100.25 ± 11.57 | 97.79 ± 12.68 | 102.2 ± 10.93 |
| Females (n=34) | 99.44 ± 13.42 | 100.79 ± 11.91 | 99.37 ± 15.20 |
| Gender Difference | p ≤ 0.80 | p ≤ 0.34 | p ≤ 0.41 |
Image acquisition and preprocessing
High-resolution 3D SPGR MR images were obtained on a 1.5 Tesla scanner (General Electric, Milwaukee, WI) as a series of 124 contiguous 1.5 mm coronal brain slices (256 × 256 matrix, 0.86 mm × 0.86 mm in-plane resolution). All images were corrected for intensity inhomogeneities (Zijdenbos and Dawant, 1994; Sled et al., 1998) and for head tilt and alignment by reorienting each volume into the standard position of the ICBM-305 average brain (Mazziotta et al., 1995) using rigid-body transformations (Woods et al., 1998).
Measurement of TBV
TBV was determined in cm3 as the sum of voxels representing gray matter, white matter, and intracranial CSF but excluding the cerebellum (inter-rater reliability for scalp editing procedures, rI= .99 (Narr et al., 2006b)). Tissue classification procedures as well as group-specific means and standard deviations for TBV are summarized elsewhere (Narr et al., 2006b).
Measurement of callosal thickness
Regional callosal thickness was estimated in a three-step approach as detailed elsewhere (Luders et al., 2006a; Luders et al., 2006b; Luders et al., 2007). Briefly, one rater (E.L.) manually outlined upper and lower callosal boundaries (top and bottom) in the midsagittal section of each brain (Step I). Subsequently, the spatial average from 100 equidistant surface points representing the top and bottom traces was calculated by creating a new midline segment, also consisting of 100 equidistant points (Step II). The distances between 100 corresponding surface points from this new midline to callosal top and to callosal bottom were then quantified (Step III). These regional distances indicate callosal thickness with a high spatial resolution (that is, at 100 locations distributed evenly over the callosal surface).
Correlation analyses
For descriptive purposes we investigated the correlation between (a) TBV and full-scale IQ, as well as between (b) TBV and callosal thickness within the combined sample. Since relationships between TBV and both these measures were expected, for the assessment of the correlation between callosal thickness and IQ (described below), we controlled for the variance associated with differences between individual brain sizes.
We calculated the partial correlation coefficients between individual full-scale IQ measures (performance and verbal IQ measures, respectively) and callosal distance measures at 100 equidistant points for the combined sample (n=62), as well as within males (n=28) and females separately (n=34), while removing the variance associated with TBV. Moreover, we tested for significant differences in the slopes of the gender-specific relationships using an interaction design. Correlation coefficients (r) and associated significance values (p) were coded in color and projected onto the 3D group-averaged callosal surface models. While an uncorrected two-tailed alpha level of p≤0.05 was determined as the threshold for mapping significance values, permutation testing was employed to confirm the overall significance (controlling for TBV). That is, for each group (combined sample, males, and females) but also with respect to gender interaction effects, the number of significant results from 10.000 randomizations was compared to the number of significant results in the true assignment of covariates to the data, resulting in an overall corrected p-value that is adjusted for the multiple statistical tests on the callosal surface.
Results
TBV correlations
TBV and full-scale IQ within the combined sample were significantly correlated (r = .284; p ≤ 0.025). Similarly, TBV was significantly associated with callosal thickness at numerous locations (Figure 1). More specifically, we observed significant positive correlations in the callosal posterior body and also in most extreme anterior and posterior callosal sections, located within the anterior third and splenium. Significant negative correlations were completely absent. Altogether, these findings are in close agreement with results from previous studies where relationships between brain size and intelligence (McDaniel, 2005) as well as between brain size and callosal size (Rauch and Jinkins, 1994; Jancke et al., 1997) have been reported. Thus to ensure that callosal thickness/IQ associations are not driven by overall brain size effects, all subsequent analyses were performed while removing variance explained by TBV.
Figure 1.
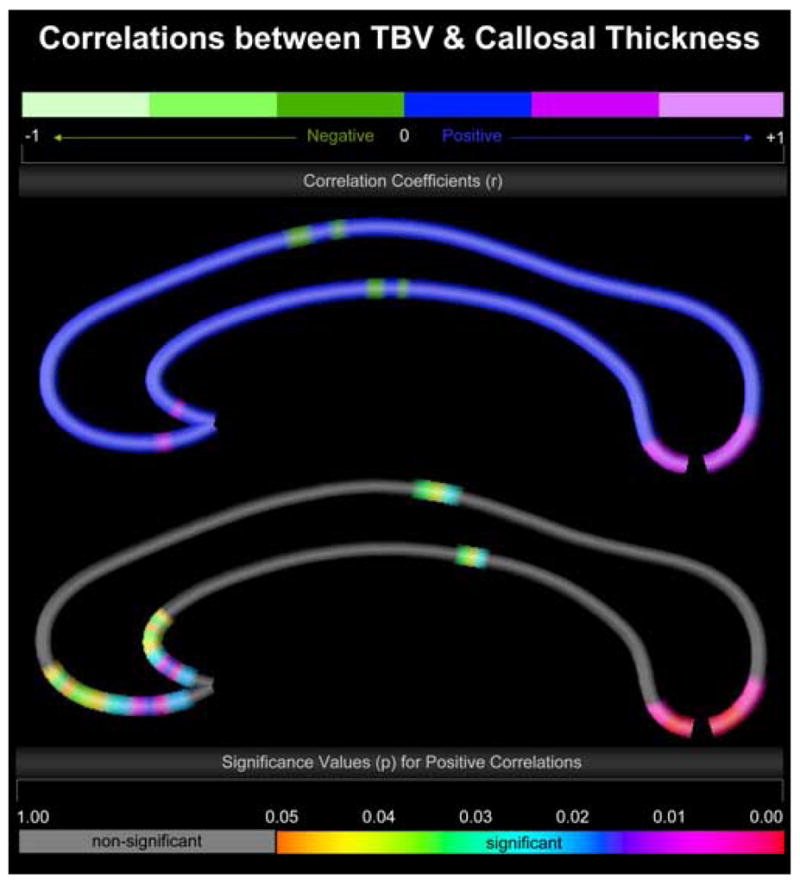
Correlations between TBV and callosal thickness. Illustrated are correlation coefficients (top map) and associated significance values (bottom map). The upper color bar encodes the r-values that depict the magnitude and direction of correlations between TBV and callosal distance measures. The lower color bar encodes the p-values, with gray color indicating regions where no significant correlations between TBV and callosal distance measures were detected. The callosal anterior section is located on the left; the callosal posterior section points to the right.
Full-scale IQ correlations within the combined sample
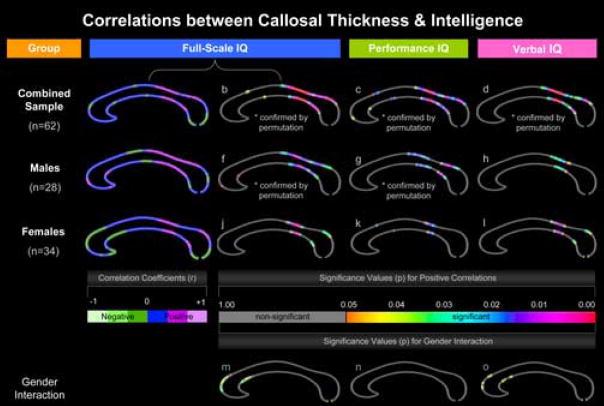
We detected significant positive correlations between callosal thickness and full-scale IQ across the posterior surface of the CC, corresponding to posterior body, isthmus, and anterior sections of the splenium (regions defined according to the traditional Witelson CC classification scheme). In addition, another smaller region at the border between anterior third and anterior body exhibited positive correlations (Figure 2-b). Permutation testing confirmed positive correlations for the combined sample (Table 2). Negative correlations, as depicted in the r-map (Figure 2-a), did not reach the threshold of significance. To further illustrate relationships between callosal thickness and full-scale IQ, we sampled the callosal point that showed the highest p-value (point #65). The correlations between full-scale IQ and callosal thickness measures obtained from this same surface point in each subject and after partialling out TBV are shown in Figure 3 (r = .476, p ≤ 0.00009).
Figure 2.
Correlations between callosal thickness and intelligence. Partial correlation coefficients and associated significance values for full-scale IQ measurements are shown in the first and second columns of callosal maps, while significance values for performance and verbal IQ correlations are illustrated in the third and fourth column. Measurements are shown for the combined sample (first row), males (second row), and females (third row) separately. The fourth row depicts significant differences in the slopes of the gender-specific relationships (gender interaction). The left color bar encodes the r-values that depict the magnitude and direction of correlations between full-scale IQ measures and callosal distance measures. The right color bar encodes the significance associated with the partial correlations and also gender interactions associated with full-scale IQ, performance IQ, and verbal IQ.
Table 2.
Correlations between callosal thickness and intelligence measures (corrected p-values computed by permutation testing for p<0.05.). The asterisk * denotes significant findings.
| Full-Scale IQ | Performance IQ | Verbal IQ | |
|---|---|---|---|
| Combined Sample (n=62) | 0.01* | 0.01* | 0.03* |
| Males (n=28) | 0.03* | 0.05* | 0.17 |
| Females (n=34) | 0.09 | 0.28 | 0.11 |
| Gender Interaction | 0.15 | 1.00 | 0.27 |
Figure 3.
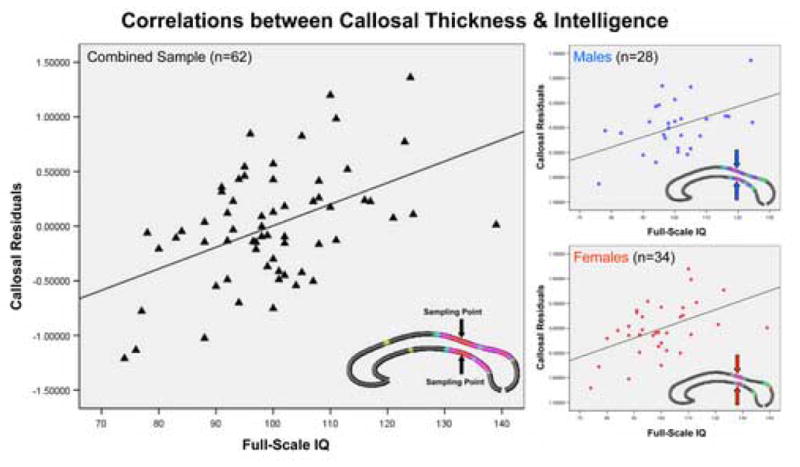
Correlations between callosal thickness and intelligence. Partial regression plots showing relationships between full-scale IQ and callosal thickness (callosal residuals) at the most significant callosal surface point (point # 65), within the combined sample (left), and within males (top right) and females (bottom right). The most significant callosal surface point is located in the callosal posterior body, as marked by arrows.
Full-scale IQ correlations within males
The spatial location and extent of significant positive correlations between callosal thickness and full-scale IQ in males resembled the outcomes of the combined-sample analysis in posterior callosal regions (posterior body, isthmus, and splenium). In addition, the thickness of a small region located close to the anterior end of the CC was significantly positively correlated with intelligence (Figure 2-f). Permutation testing confirmed positive correlations within males (Table 2). Partial regression plots showing positive relationships between full-scale IQ and callosal thickness in males at the most significant callosal surface point are shown in Figure 3 (r = .457, p ≤0.015). Negative correlations, as visualized in the r-map (Figure 2-e), were only present in two regions within the anterior body and splenium but again did not reach the threshold of significance.
Full-scale IQ correlations within females
Significant relationships in females were less pronounced and more spatially restricted than in males. Callosal thickness was positively correlated with full-scale IQ in regions of the posterior body and anterior splenium (but omitting the isthmus; Figure 2-j). Partial regression plots showing positive relationships between full-scale IQ and callosal thickness in females at the most significant callosal surface point are shown in Figure 3 (r = .490, p ≤0.003). Positive correlations, however, were not confirmed by permutation testing (Table 2). Negative correlations, as shown in the r-map (Figure 2-i), were present in extended regions towards the anterior and posterior end (anterior third and splenium) but did not reach the threshold of significance.
Full-scale IQ gender interaction
The different directions observed for the correlation between callosal thickness and full-scale IQ in the anterior third in males (positive correlation) and females (negative correlation) appear to provide a basis for the spatially discrete significant gender interaction detected in anterior callosal regions (Figure 2-m). These interaction effects, however, were not confirmed by permutation testing (Table 2).
Performance IQ and verbal IQ correlations
As further demonstrated, significance profiles for performance and verbal IQ correlations, within the combined sample (Figure 2-c,d), but also in males (Figure 2-g,h) and females (Figure 1-k,l), resemble the results observed for full-scale IQ correlations. That is, significant negative correlations were completely absent, while significant positive correlations were detected predominantly in posterior callosal regions (posterior body, isthmus, and anterior splenium). These findings were confirmed by permutation testing within the combined sample and in males, but not in females (for details see Table 2). Significant gender interactions with respect to performance IQ were completely absent (Figure 2-n), while significant gender interaction effects on verbal IQ correlations were revealed in anterior regions (Figure 2-o), but not confirmed by permutation testing (Table 2).
Discussion
Within the combined sample, and also within males and females examined as separate groups, we observed significant positive correlations between callosal morphology and intelligence measures mainly in posterior callosal sections. These relationships were confirmed by permutation testing within the combined sample and in males, although permutations were at sub-threshold significance in females. Significant negative correlations were absent for all tests performed.
Direction of the correlations
Our observations support prior reports of predominantly positive associations between intelligence and callosal measures in non-normative samples (Fletcher et al., 1992; Strauss et al., 1994; Atkinson, Jr. et al., 1996; Spencer et al., 2005; Schatz and Buzan, 2006). Midsagittal callosal area may reflect the total number of small diameter fibers within the CC (Aboitiz et al., 1992). Increased midsagittal callosal thickness, which provides more spatially detailed information than parcellated area measures, presumably also reflects increases in the number of fibers connecting both hemispheres and/or an increased degree of fiber myelination. This may facilitate more efficient inter-hemispheric information transfer that would likely benefit the integration and processing of information, thus enhancing intellectual performance. More specifically, it was proposed, that failure to activate the appropriate hemisphere in regions directly involved in a task may explain declines in performance (Gur et al., 2002). Moreover, bilateral activation (in contrast to the involvement of only a single hemisphere) can be beneficial for performance in certain tasks (Shaywitz et al., 1995; Gur et al., 2002). That is, increased numbers of callosal fibers and/or greater myelination might thus benefit cognitive performance by involving the appropriate hemisphere and/or increasing the number of processing units by involving both hemispheres, and also by allowing for a rapid and coordinated engagement of processing units between hemispheres according to task-specific requirements.
Alternatively, variations in callosal size might merely reflect the number of cortical neurons or interconnections between brain regions relevant for processing information of the kind that is measured on intelligence tests (Strauss et al., 1994). Previous findings relating intelligence to other aspects of brain morphology in an overlapping sample are consistent with this hypothesis (Narr et al., 2006b). That is, intelligence was positively correlated with cortical thickness in cortical sections that are likely to be connected through the callosal regions that showed significant relationships with IQ here. Consequently, cognitive abilities might be fostered, not only by efficient inter-hemispheric information transfer but also through advantageous architectonic constellations in cortical regions. Regardless of the biological mechanisms driving correlations between CC thickness and intelligence, our data indicate that variations in callosal sections strongly relate to general intellectual capacity.
Localization of the correlations
The spatial localization of relationships between CC thickness and IQ measures in male and female sub-samples resembled the correlation profile observed in the combined sample. This lends support to the assumption that, regardless of gender, posterior sections of the CC in particular are associated with intelligence. Interestingly, similar pronounced relationships in exclusively posterior callosal sections (e.g., in the splenium) were revealed in a non-normative sample of 47 epilepsy patients (Strauss et al., 1994).
Callosal tracts have been shown to be organized topographically (Witelson, 1989; Hofer and Frahm, 2006; Zarei et al., 2006). Callosal fibers traveling through the posterior body of the CC are suggested to connect primary motor (as well as pre-motor), primary sensory and parietal association cortices. Fibers of the isthmus are believed to connect primary sensory (even primary motor), posterior parietal, and (superior) temporal cortices, while fibers of the anterior splenium have been shown to connect posterior parietal and (inferior) temporal cortices.2 As summarized recently, cognitive functions consist of neural transactions within distributed, interactive, and overlapping networks of neurons in posterior and anterior association cortices (Fuster, 2006). Although the precise anatomical boundaries are not well characterized, the posterior association cortex is roughly described as spanning “post-rolandic” regions (as opposed to “pre-rolandic” regions – the substrate for the anterior association cortex). Our observation of significant positive correlations in posterior body, isthmus, and anterior splenium, may thus reflect the relevance of inter-hemispheric connectivity between the posterior association cortices for intellectual outcomes. As outlined previously (Strauss et al., 1994; Haier et al., 2003; Narr et al., 2006b), a number of studies have provided evidence that posterior brain regions are involved in aspects of problem-solving that are typically measured in standard intelligence tests – either via modulating pathways to (pre)frontal regions or by serving as a key location for the convergence of information. This assumption is supported by other findings of significant positive correlations between intelligence measures and a variety of cerebral measurements in posterior brain regions, including cortical thickness, gray matter concentration, and neurometabolites (Jung et al., 1999a; Jung et al., 1999b; Haier et al., 2003; Haier et al., 2004; Narr et al., 2006b).
Interestingly, a number of research findings also suggest the (pre)frontal cortex to be strongly involved in cognitive processes (Duncan and Owen, 2000; Duncan et al., 2000; Fuster, 2006). Connections between the prefrontal cortices are mainly attributed to the callosal anterior third and anterior body, so it is surprising that significant correlations in these regions are only minor (for full-scale and performance IQ) or absent (for verbal IQ). However, the involvement of non-frontal cerebral regions (and thus also non-anterior inter-hemispheric pathways) in intellectual processes may have been considerably underestimated in previous studies and possibly biased outcomes – for instance, if analyses were based on a priori defined regions of interest in exclusively anterior brain sections. In support of these assumptions, a recently proposed theory – the Parieto-Frontal Integration Theory (P-FIT) of Intelligence – highlights the integration of not only frontal lobes but also posterior brain regions (e.g., the parietal lobe) in most intelligence research and especially emphasizes the importance of the CC in the “distributed” nature of the intelligence network (Jung and Haier, 2007).
Gender differences in correlation profiles
Although several previous studies revealed sexually dimorphic associations between brain morphology or metabolism and cognitive measurements (Gur et al., 1999; Pfleiderer et al., 2004; Jung et al., 2005; Witelson et al., 2006; Narr et al., 2006b), significant gender differences in the slopes of correlations between callosal morphology and intelligence measures were spatially discrete and were not confirmed by permutation testing. Relationships between callosal thickness and IQ may therefore be similar in males and females.
When further comparing correlations across intelligence categories (full-scale, performance, verbal), both males and females show larger correlation coefficients for full-scale IQ measures. Significant correlations in males (and in the combined sample) are less pronounced for performance IQ, and least pronounced for verbal IQ. The findings within the entire sample and in males corroborate previous reports from a sample of hydrocephalic children (albeit males and females combined), where verbal and nonverbal measures correlated positively with the size of the CC, but the correlation was higher for nonverbal measures (Fletcher et al., 1992).
In females, on the other hand, verbal IQ shares an almost identical correlation profile with full-scale IQ, which is intriguing. Stronger verbal skills in women, as often reported in the literature (Halpern, 1992; Kimura, 1999), may mediate these outcomes. Nevertheless, gender-specific cognitive abilities are not likely to provide a sufficient explanation for our findings because men and women in the present analysis did not differ in verbal IQ (nor full-scale and performance IQ). It is possible, however, that a sexually dimorphic organization of male and female brains also involves information transfer via inter-hemispheric pathways that modulate particular intellectual outcomes, which might account for the observed correlation profiles in men and women.
Implications for future studies
We have calculated correlations between callosal thickness and intelligence across subjects based on computing correspondences between points lying at equal proportions of the distance along the callosal boundary. Although this is reasonable given the approximate topographic ordering of callosal fiber projections to the cortex (Witelson, 1989; Hofer and Frahm, 2006; Zarei et al., 2006), the simple matching of callosal boundaries is likely to be slightly conservative in detecting systematic effects on callosal structure, albeit unlikely to detect spurious associations. New and complementary imaging techniques, such as diffusion tensor imaging tractography, may be able to provide additional, higher-order constraints that favor the matching of anatomical tracts within the CC, across subjects, to the maximum possible extent. Such landmarks, if available, could readily be used as constraints – in the same way as gyral landmarks can constrain cortical surface matching (Thompson et al., 2004). Moreover, future studies in which callosal morphology is investigated across imaging modalities including high-resolution structural magnetic resonance imaging, diffusion tensor imaging, and spectroscopy, may help to elucidate the mechanisms by which the CC forms networks critical to higher cognitive functioning through biochemical, micro-, and macro-structural mechanisms. Finally, investigations in groups of patients with callosal congenital malformations or after partial or complete callosotomy might provide helpful insights to further characterize relationships between callosal features, hemisphere-specific alterations in cortical morphology, and intellectual outcomes (Chiarello, 1980; Campbell, Jr. et al., 1981).
Acknowledgments
This work was supported by the National Institutes of Health through the NIH Roadmap for Medical Research, grant U54 RR021813 entitled Center for Computational Biology (CCB). Additional support was provided by the NIH/NCRR resource grant P41 RR013642. Paul M. Thompson was supported by the National Institute on Aging, AG016570; the National Institute for Biomedical Imaging and Bioengineering, NS049194; and the National Institute of Child Health and Human Development, HD050735. Katherine L. Narr was supported a by a Career Development Award (K01 MH073990). Robert M. Bilder, Philip R. Szeszko, and the acquisition of scans and characterization of the participants, were supported by the National Institute of Mental Health (MH60374).
Footnotes
Handedness information was not available for three male subjects. However, excluding these three subjects did not affect statistical outcomes when analyzing the combined sample of 62 subjects compared to 59 subjects. Thus, all final analyses were conducted with these three subjects included (n=62).
Of note, anatomical boundaries vary to some extent from one proposed scheme to another. Recent anatomical maps based on diffusion tensor imaging (Hofer and Frahm, 2006; Zarei et al., 2006) complement, refine and somewhat revise traditional assumptions of trans-callosal fiber distributions (Witelson, 1989), but also slightly differ from each other.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aboitiz F. Brain connections: interhemispheric fiber systems and anatomical brain asymmetries in humans. Biol Res. 1992;25:51–61. [PubMed] [Google Scholar]
- 2.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson DS, Jr, bou-Khalil B, Charles PD, Welch L. Midsagittal corpus callosum area, intelligence, and language dominance in epilepsy. J Neuroimaging. 1996;6:235–239. doi: 10.1111/jon199664235. [DOI] [PubMed] [Google Scholar]
- 4.Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: myth or reality? Neurosci Biobehav Rev. 1997;21:581–601. doi: 10.1016/s0149-7634(96)00049-8. [DOI] [PubMed] [Google Scholar]
- 5.Campbell AL, Jr, Bogen JE, Smith A. Disorganization and reorganization of cognitive and sensorimotor functions in cerebral commissurotomy. Compensatory roles of the forebrain commissures and cerebral hemispheres in man. Brain. 1981;104:493–511. doi: 10.1093/brain/104.3.493. [DOI] [PubMed] [Google Scholar]
- 6.Chiarello C. A house divided? Cognitive functioning with callosal agenesis. Brain Lang. 1980;11:128–158. doi: 10.1016/0093-934x(80)90116-9. [DOI] [PubMed] [Google Scholar]
- 7.Clarke JM, Zaidel E. Anatomical-behavioral relationships: corpus callosum morphometry and hemispheric specialization. Behav Brain Res. 1994;64:185–202. doi: 10.1016/0166-4328(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 8.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 9.Duncan J, Seltz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Am J Ophthalmol. 2000;130:687. doi: 10.1016/s0002-9394(00)00752-2. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher JM, Bohan TP, Brandt ME, Brookshire BL, Beaver SR, Francis DJ, Davidson KC, Thompson NM, Miner ME. Cerebral white matter and cognition in hydrocephalic children. Arch Neurol. 1992;49:818–824. doi: 10.1001/archneur.1992.00530320042010. [DOI] [PubMed] [Google Scholar]
- 11.Fuster JM. The cognit: a network model of cortical representation. Int J Psychophysiol. 2006;60:125–132. doi: 10.1016/j.ijpsycho.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb Cortex. 2002;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- 13.Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. Neuroimage. 2004;23:425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. The neuroanatomy of general intelligence: sex matters. Neuroimage. 2005;25:320–327. doi: 10.1016/j.neuroimage.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Haier RJ, White NS, Alkire MT. Individual differences in general intelligence correlate with brain function during nonreasoning tasks. Intelligence. 2003:429–441. [Google Scholar]
- 17.Halpern DF. Sex differences in cognitive abilities. New York: Erlbaum; 1992. [Google Scholar]
- 18.Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 19.Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van OC, van Haren NE, Collins DL, Evans AC, Amunts K, Burgel U, Zilles K, de GE, Boomsma DI, Kahn RS. Genetic contributions to human brain morphology and intelligence. J Neurosci. 2006;26:10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cereb Cortex. 1997;7:48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- 21.Jung RE, Brooks WM, Yeo RA, Chiulli SJ, Weers DC, Sibbitt WL., Jr Biochemical markers of intelligence: a proton MR spectroscopy study of normal human brain. Proc Biol Sci. 1999a;266:1375–1379. doi: 10.1098/rspb.1999.0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of Intelligence: Converging Neuroimaging Evidence. Behavioral and Brain Sciences. 2007;30 doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- 23.Jung RE, Haier RJ, Yeo RA, Rowland LM, Petropoulos H, Levine AS, Sibbitt WL, Brooks WM. Sex differences in N-acetylaspartate correlates of general intelligence: an 1H-MRS study of normal human brain. Neuroimage. 2005;26:965–972. doi: 10.1016/j.neuroimage.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 24.Jung RE, Yeo RA, Chiulli SJ, Sibbitt WL, Jr, Weers DC, Hart BL, Brooks WM. Biochemical markers of cognition: a proton MR spectroscopy study of normal human brain. Neuroreport. 1999b;10:3327–3331. doi: 10.1097/00001756-199911080-00014. [DOI] [PubMed] [Google Scholar]
- 25.Kimura D. Sex and Cognition. Cambridge: MIT Press; 1999. [Google Scholar]
- 26.Luders E, Di Paola M, Tomaiuolo F, Thompson PM, Toga AW, Vicari S, Petrides M, Caltagirone C. Callosal morphology in Williams syndrome: a new evaluation of shape and thickness. Neuroreport. 2007;18:203–207. doi: 10.1097/WNR.0b013e3280115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006a;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- 28.Luders E, Narr KL, Zaidel E, Thompson PM, Toga AW. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006b;17:1103–1106. doi: 10.1097/01.wnr.0000227987.77304.cc. [DOI] [PubMed] [Google Scholar]
- 29.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- 30.McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005:337–346. [Google Scholar]
- 31.Narr KL, Bilder RM, Luders E, Thompson P, Woods RP, Robinson D, Szeszko P, Dimtcheva T, Gurbani M, Toga A. Asymmetries of Cortical Shape: Effects of Handedness, Sex and Schizophrenia. 2006a doi: 10.1016/j.neuroimage.2006.08.052. Accepted for publication in NeuroImage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, DeLuca H, Thompson PM. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005a;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- 33.Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, Sevy S, Wang Y, Schrock K, Bilder RM. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005b;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 34.Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. Relationships between IQ and Regional Cortical Gray Matter Thickness in Healthy Adults. Cereb Cortex. 2006b doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- 35.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 36.Pfleiderer B, Ohrmann P, Suslow T, Wolgast M, Gerlach AL, Heindel W, Michael N. N-acetylaspartate levels of left frontal cortex are associated with verbal intelligence in women but not in men: a proton magnetic resonance spectroscopy study. Neuroscience. 2004;123:1053–1058. doi: 10.1016/j.neuroscience.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Rauch RA, Jinkins JR. Analysis of cross-sectional area measurements of the corpus callosum adjusted for brain size in male and female subjects from childhood to adulthood. Behav Brain Res. 1994;64:65–78. doi: 10.1016/0166-4328(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 38.Schatz J, Buzan R. Decreased corpus callosum size in sickle cell disease: relationship with cerebral infarcts and cognitive functioning. J Int Neuropsychol Soc. 2006;12:24–33. doi: 10.1017/S1355617706060085. [DOI] [PubMed] [Google Scholar]
- 39.Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- 40.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 41.Spencer MD, Gibson RJ, Moorhead TW, Keston PM, Hoare P, Best JJ, Lawrie SM, Johnstone EC. Qualitative assessment of brain anomalies in adolescents with mental retardation. AJNR Am J Neuroradiol. 2005;26:2691–2697. [PMC free article] [PubMed] [Google Scholar]
- 42.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 43.Strauss E, Wada J, Hunter M. Callosal morphology and performance on intelligence tests. J Clin Exp Neuropsychol. 1994;16:79–83. doi: 10.1080/01688639408402618. [DOI] [PubMed] [Google Scholar]
- 44.Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang Y, van Erp TG, Cannon TD, Toga AW. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23(Suppl 1):S2–18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 45.Tomaiuolo F, Scapin M, Di Paola M, Le Nezet P, Fadda L, Caltagirone C, Collins DL. Gross Anatomy of the corpus callosum in Alzheimer’s Disease: regions of degeneration and their neuropsychological correlates. Dementia and Geriatric Cognitive Disorders. 2006 doi: 10.1159/000097371. (in press) [DOI] [PubMed] [Google Scholar]
- 46.Wechsler D. Wechsler Adult Intelligence Scale. 1981 Revised: Manual. [Google Scholar]
- 47.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112 (Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 48.Witelson SF, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain. 2006;129:386–398. doi: 10.1093/brain/awh696. [DOI] [PubMed] [Google Scholar]
- 49.Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 50.Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zijdenbos AP, Dawant BM. Brain segmentation and white matter lesion detection in MR images. Crit Rev Biomed Eng. 1994;22:401–465. [PubMed] [Google Scholar]