Abstract
Gender identity—one's sense of being a man or a woman—is a fundamental perception experienced by all individuals that extends beyond biological sex. Yet, what contributes to our sense of gender remains uncertain. Since individuals who identify as transsexual report strong feelings of being the opposite sex and a belief that their sexual characteristics do not reflect their true gender, they constitute an invaluable model to understand the biological underpinnings of gender identity. We analyzed MRI data of 24 male-to-female (MTF) transsexuals not yet treated with cross-sex hormones in order to determine whether gray matter volumes in MTF transsexuals more closely resemble people who share their biological sex (30 control men), or people who share their gender identity (30 control women). Results revealed that regional gray matter variation in MTF transsexuals is more similar to the pattern found in men than in women. However, MTF transsexuals show a significantly larger volume of regional gray matter in the right putamen compared to men. These findings provide new evidence that transsexualism is associated with distinct cerebral pattern, which supports the assumption that brain anatomy plays a role in gender identity.
Keywords: Brain, Gender Identity, MRI, Transgender, VBM
Introduction
Individuals who identify as transsexual report a history of persistent discomfort with the sex they were assigned at birth and a strong identification with the opposite sex. Many describe significant symptoms of psychological distress (Sánchez and Vilain, 2009) and take steps to alter features of their bodies (e.g., through the use of sex hormones and plastic surgery) to make them congruent with their sense of gender. While no formal epidemiological study has been conducted in the United States, reports from European and Asian countries estimate that the prevalence of transsexualism ranges from 1:100,000 to 1:2,900 (DeCuypere G. et al., 2007).
Despite increased public awareness of transsexualism, our scientific understanding of the development of gender identity is limited. Both environmental events (Eagly and Wood, 1999; Wood and Eagly, 2002) and innate differences (Breedlove, 1994; Dorner, 1985; Gooren, 2006) have been implicated as influencing this fundamental human characteristic. Regarding transsexualism, it has been suggested that sexual differentiation of the brain during embryonic development deviates from the sexual differentiation of the rest of the body (Zhou et al., 1995). This hypothesis implies that neuroanatomy plays a critical role in determining gender identity. Thus, the study of the underlying correlates of transsexualism may help to further identify the mechanisms that contribute to the development of gender identity.
To explore this hypothesis, several studies examined brain structures in male-to-female (MTF) transsexuals. One early in vivo study did not detect any associations between transsexualism and the anatomy of the corpus callosum (Emory et al., 1991). However, two subsequent post mortem brain analyses revealed that MTF transsexuals had a female-like central subdivision of the bed nucleus of the stria terminalis (BSTc) with respect to its size (Zhou et al., 1995) and number of neurons (Kruijver et al., 2000). Another post mortem study published recently reported female-like volumes and neuronal densities of the interstitial nucleus of the anterior hypothalamus (INAH3) in MTF transsexuals (Garcia-Falgueras and Swaab, 2008). These three post mortem studies seem to support the hypothesis that brain anatomy is associated with transsexualism. Yet, the generalization of these findings is limited by the inherent pitfalls of post mortem studies, the relatively small number of MTF transsexuals examined (n1=6; n2=6; n3=11), as well as the subjects' long-term treatment with estrogen. Granted, some argue that estrogen treatment does not alter certain brain structures (Garcia-Falgueras and Swaab, 2008). Nevertheless, other studies have shown that treatment with anti-androgen and estrogen decreases brain volumes of MTF transsexuals subjects towards female proportions (Hulshoff Pol et al., 2006).
To extend these prior findings while overcoming some of their limitations, we investigated variations in brain structure in 60 control subjects (30 males, 30 females) and 24 male-to-female (MTF) transsexuals who had not been treated with female hormones. More specifically, we used magnetic resonance imaging (MRI) to investigate neuroanatomy at high-resolution in vivo, and applied a sophisticated computational image analysis approach to compare regional volumes of gray matter throughout the brain.
Materials and Methods
Subjects
Twenty-four MTF transsexuals were recruited through fliers provided to local transsexual community organizations and to professionals who offer services to the transsexual community. Thirty male and thirty female control subjects were selected from the International Consortium for Brain Mapping (ICBM) database of normal adults (http://www.loni.ucla.edu/ICBM/Databases/). The mean age (SD) of the MTF transsexuals was 46.73 (13.18) years with an age range between 23 and 72 years. Male and female control subjects were closely age-matched (males 46.57±12.45, 23-69 years; females 46.77±12.88, 23-73 years). Transsexual subjects were 76% dextral, control males were 93% dextral, and control females were 90% dextral, where handedness was determined based on self-reports of hand preference for selected activities. For study inclusion, transsexual subjects needed to self-identify as a MTF transsexual, report no history of hormonal treatment, and declare the intention of undergoing estrogen replacement therapy. MTF transsexuals were evaluated to be free of psychosis according to a standardized diagnostic interview (Robins et al., 1989) and confirmed to be genetic males as defined by the presence of the SRY gene in their genome (Jordan et al., 2002). All control subjects had to pass a physical and neurological screening examination performed by a neurologist. All subjects gave informed consent according to guidelines from the Institutional Review Board of the University of Los Angeles, California (UCLA), and were compensated for their participation in this study.
Image acquisition
Brain images were acquired on a 1.5-T MRI system (Siemens Sonata, Erlangen, Germany) using a 3D T1-weighted sequence (MPRAGE) with the following parameters: TR = 1900 ms; TE = 4.38 ms; flip angle = 15°; 160 contiguous 1 mm sagittal slices; FOV = 256 mm × 256 mm2; matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm.
Image Analysis
Data were preprocessed and examined using SPM5 software (Wellcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Analyses were performed on gray matter segments (Ashburner and Friston, 2005) that were multiplied by the non-linear components derived from the normalization matrix (modulated gray matter volumes) and smoothed with a Gaussian kernel of 12 mm full width at half maximum. These smoothed modulated gray matter volumes are hereafter referred to as gray matter to simplify matters.
We used the general linear model (GLM) and applied analyses of covariance (ANCOVA) to examine voxel-wise gray matter differences between MTF transsexuals, males, and females, while removing the variance associated with age. Statistical outcomes were corrected for multiple comparisons, using false discovery rate (FDR) (Benjamini and Hochberg, 1995) at p<0.001. Significant findings were mapped onto the average 3D cortical model and restricted to clusters exceeding a minimum of 123 voxels (the expected number of voxels per cluster), calculated according to the theory of Gaussian random fields (Fig. 1). Finally, to gain a better understanding of how significance profiles from different sets of comparisons are spatially related to each other, we performed additional post hoc t-tests and overlaid significant group differences as maximum intensity projections on the SPM standard glass brain template in three orthogonal planes. These exploratory outcomes are also presented at p<0.001, corrected for multiple comparisons using FDR (Fig. 2).
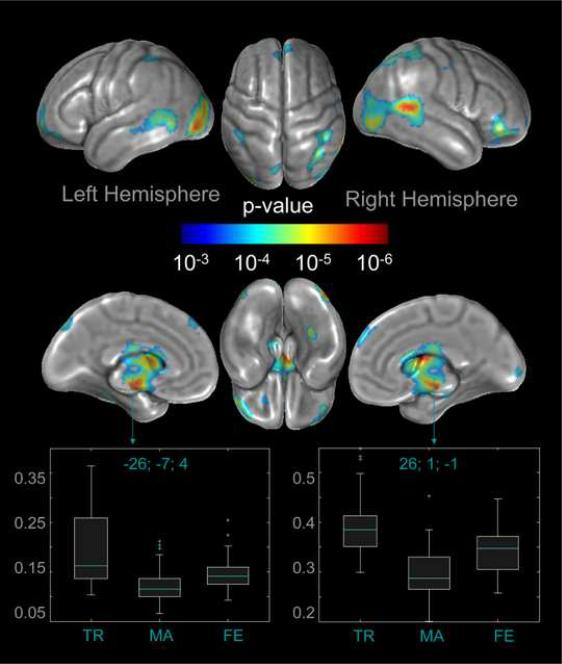
Fig. 1.
Analysis of variance (ANOVA) between samples. The color-coded brain maps illustrate the brain regions where gray matter volumes differ significantly between the three groups (MTF transsexuals, males, females), after removing the variance associated with age. Statistical outcomes are corrected for multiple comparisons, using FDR at p<0.001. Shown are clusters exceeding a spatial extent threshold of 123 voxels. The two box plots display the estimated parameters for clusters located in the region of the left and right putamen, where MTF transsexuals (TR) had more gray matter than males (MA) and females (FE).
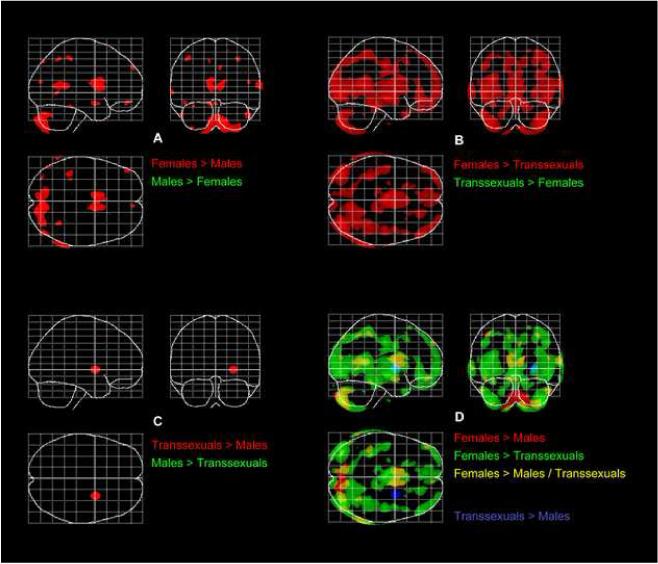
Fig. 2.
T-tests between samples. The overlay maps on the SPM standard glass brain template (sagittal, axial, and coronal view) illustrate where independent-sample group comparisons revealed significant gray matter volume differences between females and males (Panel A), between females and MTF transsexuals (Panel B), as well as between males and MTF transsexuals (Panel C), after removing the variance associated with age. Panel D depicts the overlay of significance profiles from different sets of comparisons. Findings are significant at p<0.001, FDR-corrected.
Results
As demonstrated in Fig. 1, we detected significant differences between MTF transsexuals, males, and females in a large number of regions across the brain. More specifically, within the frontal lobe, we observed gray matter volume differences bilaterally in the superior frontal gyrus, close to midline and also at the frontal pole, as well as within the right orbital gyrus. Furthermore, we noticed pronounced gray matter volume differences bilaterally across the occipital and posterior temporal lobes, as well as in the parietal lobe, near the intraparietal sulcus, and closer to midline (left). Additional group effects on regional gray matter volume were detected subcallosally in both hemispheres at the brain midline. These regions constitute part of the basal ganglia (i.e., the caudate nucleus and putamen) and limbic system (i.e., the subcallosal gyrus, mammillary body, amygdala, thalamus and hypothalamus). Moreover, we identified two clusters indicating group differences on the basal surface of the right temporal lobe and left frontal lobe.
For each of 22 significantly different regions (twelve within the right hemisphere and ten within the left hemisphere), cluster-specific box plots were generated to illustrate the magnitude and direction of gray matter volume differences between groups (see supplement 1 and 2). Altogether, females had the largest gray matter volumes in all but two significant clusters, which were located in the left and right putamen. Here, MTF transsexuals had the largest gray matter volumes (see Fig. 1). For the remaining clusters, MTF transsexuals had the smallest gray matter volumes, but their data spectrum largely overlapped with that of males.
As illustrated in the spatial profiles of significant group differences (Fig. 2), females had more gray matter than males in large portions of the brain (Females > Males; red clusters in Panel A). Similarly, females had more gray matter than MTF transsexuals (Females > Transsexuals; red clusters in Panel B). Although the differences between females and MTF transsexuals did partly overlap with the difference between females and males (Females > Males / Transsexuals; yellow clusters in Panel D), they were spatially more extended, and also evident in a few regions where females and males did not differ (Females > Transsexuals; green clusters in Panel D). There was no region where females had significantly less gray matter than males (Panel A) or MTF transsexuals (Panel B). Similarly, there was no region where MTF transsexuals had significantly less gray matter than males (Panel C). MTF transsexuals, however, showed significantly more gray matter than males in the right putamen (Transsexuals > Males; red clusters in Panel C; blue clusters in Panel D). MTF transsexuals also showed significantly more gray matter than males in the left putamen when findings were not corrected for multiple comparisons (p<0.001, maps not shown).
Discussion
Overall, our study provides evidence that MTF transsexuals possess regional gray matter volumes mostly consistent with control males. However, the putamen was found to be “feminized” in MTF transsexuals. That is, the gray matter volume of this particular structure in the MTF transsexual group was both larger than in males and within the average range of females. Interestingly, in a positron emission tomography (PET) study, it was demonstrated that the left putamen in a sample of MTF transsexuals (n=12), who had no history of estrogen treatment, activated differently to odorous steroids when compared to control males (Berglund et al., 2008). Taken together, these findings lend support to the hypothesis that specific neuroanatomical features are associated with transsexual identity, where the particular role of the putamen requires investigation in future studies.
Further research needs to resolve whether the observed distinct features in the brains of transsexuals influence their gender identity or possibly are a consequence of being transsexual. Alternatively, other variables may be independently affecting both the expression of a transsexual identity and the neuroanatomy in transsexuals that led to the observed association between both. Some possible candidates include genetic predisposition, psychosocial and environmental influences, hormonal exposures, or most likely an interplay between these variables. In support of the influence of genetics and environment, multiple cases of transsexualism occurring within families have been reported (Green, 2000) as well as studies on heritability in twins (Coolidge et al., 2002) and preliminary findings on specific genetic variations in MTF transsexuals (Hare et al., 2009; Henningsson et al., 2005). Furthermore, both genes and environmental demands have been demonstrated to determine brain anatomy (e.g., regional gray matter) (Draganski et al., 2004; Thompson et al., 2001). Finally, hormones have been shown to affect brain development (Arnold and Gorski, 1984), and neuroanatomical alterations in MTF transsexuals (Kruijver et al., 2000; Zhou et al., 1995) have been detected in cerebral structures shown to significantly change in response to hormonal exposure (Del et al., 1987; Guillamon et al., 1988). The MTF transsexuals of the current study had no history of hormonal treatment. Thus, we can exclude the potential effects of administered female hormones as a confounding factor for our findings. Moreover, it has been demonstrated that naturally circulating hormones in adult MTF transsexuals at baseline do not differ significantly from hormonal levels in male control subjects (Goodman et al., 1985; Meyer, III et al., 1986; Spijkstra et al., 1988). However, it remains to be established whether pre-, peri-, or postnatal hormonal effects in early childhood could foster transsexualism. Further studies will need to resolve the degree to which genetic variability and environmental factors influence the development of gender identity (Schweizer et al., 2009), possibly (but not necessarily) via affecting brain structures.
A limitation of the current study is that MTF transsexuals are considered as one homogeneous group rather than dividing them into MTF transsexuals who are sexually attracted (a) to males, (b) to females, (c) to both sexes, or (d) to neither sex. Based on self-report, a common yet limited method of assessing sexual orientation (Moradi et al., 2009), our transsexual sample (n=24) consisted of 6 male-oriented and 18 female-oriented subjects. Given this unequal distribution and given that information on sexual orientation was unavailable for control subjects, we abstained from conducting analyses for different subtypes. However, a number of previous findings appear to indicate brain-structural alterations associated with sexual orientation (Allen and Gorski, 1992; Byne et al., 2001; LeVay, 1991; Ponseti et al., 2007; Savic and Lindstrom, 2008; Swaab and Hofman, 1990; Witelson et al., 2008). Moreover, a highly controversial line of research has suggested that homosexual and non-homosexual MTF transsexualism are etiologically heterogeneous (Blanchard, 1989a; Blanchard, 1989b), which may be associated with differences in neuroanatomy. Therefore, future studies that take into consideration sexual orientation will not only further reveal the underlying determinants of gender identity in general, but also possibly advance our understanding of different transsexual subtypes.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health through the NIH Roadmap for Medical Research, grant U54 RR021813 entitled Center for Computational Biology (CCB). Information on the National Centers for Biomedical Computing can be obtained from <http://nihroadmap.nih.gov/bioinformatics>. Additional support was provided by the NIH/NCRR resource grant P41 RR013642, Dr. Sánchez's NIH training grant 5 T32 HD07228: 26, Dr. Gaser's BMBF grant 01EV0709, and Dr. Narr's NIH K-award MH073990.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Allen LS, Gorski RA. Sexual orientation and the size of the anterior commissure in the human brain. Proc.Natl.Acad.Sci.U.S.A. 1992;89:7199–7202. doi: 10.1073/pnas.89.15.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu.Rev.Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Pratical and Powerful Approach to Multiple Testing. J.R.Statist.Soc. 1995;57:289–300. [Google Scholar]
- Berglund H, Lindstrom P, Dhejne-Helmy C, Savic I. Male-to-female transsexuals show sex-atypical hypothalamus activation when smelling odorous steroids. Cereb.Cortex. 2008;18:1900–1908. doi: 10.1093/cercor/bhm216. [DOI] [PubMed] [Google Scholar]
- Blanchard R. The classification and labeling of nonhomosexual gender dysphorias. Arch.Sex Behav. 1989a;18:315–334. doi: 10.1007/BF01541951. [DOI] [PubMed] [Google Scholar]
- Blanchard R. The concept of autogynephilia and the typology of male gender dysphoria. J.Nerv.Ment.Dis. 1989b;177:616–623. doi: 10.1097/00005053-198910000-00004. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Sexual differentiation of the human nervous system. Annu.Rev.Psychol. 1994;45:389–418. doi: 10.1146/annurev.ps.45.020194.002133. [DOI] [PubMed] [Google Scholar]
- Byne W, Tobet S, Mattiace LA, Lasco MS, Kemether E, Edgar MA, Morgello S, Buchsbaum MS, Jones LB. The interstitial nuclei of the human anterior hypothalamus: an investigation of variation with sex, sexual orientation, and HIV status. Horm.Behav. 2001;40:86–92. doi: 10.1006/hbeh.2001.1680. [DOI] [PubMed] [Google Scholar]
- Coolidge FL, Thede LL, Young SE. The heritability of gender identity disorder in a child and adolescent twin sample. Behav.Genet. 2002;32:251–257. doi: 10.1023/a:1019724712983. [DOI] [PubMed] [Google Scholar]
- DeCuypere G, Van HM, Michel A, Carael B, Heylens G, Rubens R, Hoebeke P, Monstrey S. Prevalence and demography of transsexualism in Belgium. Eur.Psychiatry. 2007;22:137–141. doi: 10.1016/j.eurpsy.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Del AA, Segovia S, Guillamon A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res. 1987;429:295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- Dorner G. Sex-specific gonadotrophin secretion, sexual orientation and gender role behaviour. Exp.Clin.Endocrinol. 1985;86:1–6. doi: 10.1055/s-0029-1210466. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Eagly AH, Wood W. The origins of sex differences in human behavior: Evolved dispositions versus social roles. American Psychologist. 1999;54:408–423. [Google Scholar]
- Emory LE, Williams DH, Cole CM, Amparo EG, Meyer WJ. Anatomic variation of the corpus callosum in persons with gender dysphoria. Arch.Sex Behav. 1991;20:409–417. doi: 10.1007/BF01542620. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Swaab DF. A sex difference in the hypothalamic uncinate nucleus: relationship to gender identity. Brain. 2008;131:3132–3146. doi: 10.1093/brain/awn276. [DOI] [PubMed] [Google Scholar]
- Goodman RE, Anderson DC, Bu'lock DE, Sheffield B, Lynch SS, Butt WR. Study of the effect of estradiol on gonadotrophin levels in untreated male-to-female transsexuals. Arch.Sex Behav. 1985;14:141–146. doi: 10.1007/BF01541659. [DOI] [PubMed] [Google Scholar]
- Gooren L. The biology of human psychosexual differentiation. Horm.Behav. 2006;50:589–601. doi: 10.1016/j.yhbeh.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Green R. Family cooccurrence of “gender dysphoria”: ten sibling or parent-child pairs. Arch.Sex Behav. 2000;29:499–507. doi: 10.1023/a:1001947920872. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Segovia S, Del AA. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res.Dev.Brain Res. 1988;44:281–290. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- Hare L, Bernard P, Sanchez FJ, Baird PN, Vilain E, Kennedy T, Harley VR. Androgen receptor repeat length polymorphism associated with male-to-female transsexualism. Biol.Psychiatry. 2009;65:93–96. doi: 10.1016/j.biopsych.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsson S, Westberg L, Nilsson S, Lundstrom B, Ekselius L, Bodlund O, Lindstrom E, Hellstrand M, Rosmond R, Eriksson E, Landen M. Sex steroid-related genes and male-to-female transsexualism. Psychoneuroendocrinology. 2005;30:657–664. doi: 10.1016/j.psyneuen.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Cohen-Kettenis PT, Van Haren NEM, Peper JS, Brans RGH, Cahn W, Schnack HG, Gooren LJG, Kahn RS. Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure. European Journal of Endocrinology. 2006;155:S107–S114. [Google Scholar]
- Jordan BK, Jain M, Natarajan S, Frasier SD, Vilain E. Familial mutation in the testis-determining gene SRY shared by an XY female and her normal father. J Clin Endocrinol Metab. 2002;87:3428–3432. doi: 10.1210/jcem.87.7.8646. [DOI] [PubMed] [Google Scholar]
- Kruijver FP, Zhou JN, Pool CW, Hofman MA, Gooren LJ, Swaab DF. Male-to-female transsexuals have female neuron numbers in a limbic nucleus. J Clin.Endocrinol Metab. 2000;85:2034–2041. doi: 10.1210/jcem.85.5.6564. [DOI] [PubMed] [Google Scholar]
- LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- Meyer WJ, III, Webb A, Stuart CA, Finkelstein JW, Lawrence B, Walker PA. Physical and hormonal evaluation of transsexual patients: a longitudinal study. Arch.Sex Behav. 1986;15:121–138. doi: 10.1007/BF01542220. [DOI] [PubMed] [Google Scholar]
- Moradi B, Mohr JJ, Worthington RL, Fassinger R. Research on Sexual (Orientation) minority issues: Conceptual and methodological challenges and opportunities. 56 ed 2009. pp. 5–22. [Google Scholar]
- Ponseti J, Siebner HR, Kloppel S, Wolff S, Granert O, Jansen O, Mehdorn HM, Bosinski HA. Homosexual women have less grey matter in perirhinal cortex than heterosexual women. PLoS.ONE. 2007;2:e762. doi: 10.1371/journal.pone.0000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablensky A, Pickens R, Regier DA, Sartorius N, Towle LH. The Composite International Diagnostic Interview: An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of GeneralPsychiatry. 1989;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Sánchez FJ, Vilain E. Collective self-esteem as a coping resource in male-to-female transsexulas. 56 ed. 2009. pp. 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Lindstrom P. PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proc.Natl.Acad Sci.U.S.A. 2008;105:9403–9408. doi: 10.1073/pnas.0801566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer K, Burner F, Schützmann K, Schönbucher V, Richter-Appelt H. Gender identity and coping in female 46, XY adults with androgen biosynthesis deficiency (intersexuality/DSD) 56 ed 2009. pp. 189–201. [Google Scholar]
- Spijkstra JJ, Spinder T, Gooren LJ. Short-term patterns of pulsatile luteinizing hormone secretion do not differ between male-to-female transsexuals and heterosexual men. Psychoneuroendocrinology. 1988;13:279–283. doi: 10.1016/0306-4530(88)90026-1. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Hofman MA. An enlarged suprachiasmatic nucleus in homosexual men. Brain Res. 1990;537:141–148. doi: 10.1016/0006-8993(90)90350-k. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nat.Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL, Scamvougeras A, Kideckel DM, Buck B, Stanchev PL, Bronskill M, Black S. Corpus callosum anatomy in right-handed homosexual and heterosexual men. Arch.Sex Behav. 2008;37:857–863. doi: 10.1007/s10508-007-9276-y. [DOI] [PubMed] [Google Scholar]
- Wood W, Eagly AH. A cross-cultural analysis of the behavior of women and men: Implications for the origins of sex differences. Psychological Bulletin. 2002;128:699–727. doi: 10.1037/0033-2909.128.5.699. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Hofman MA, Gooren LJ, Swaab DF. A sex difference in the human brain and its relation to transsexuality. Nature. 1995;378:68–70. doi: 10.1038/378068a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.