Abstract
Psychiatric illnesses are disabling disorders with poorly understood underlying pathophysiologies. However, it is becoming increasingly evident that these illnesses result from disruptions across whole cellular networks rather than any particular monoamine system. Recent evidence continues to support the hypothesis that these illnesses arise from impairments in cellular plasticity cascades, which lead to aberrant information processing in the circuits that regulate mood, cognition, and neurovegetative functions (sleep, appetite, energy, etc). As a result, many have begun to consider future therapies that would be capable of affecting global changes in cellular plasticity to restore appropriate synaptic function and neuronal connectivity. MicroRNAs (miRNAs) are non-coding RNAs that can repress the gene translation of hundreds of their targets and are therefore well-positioned to target a multitude of cellular mechanisms. Here, we review some properties of microRNAs and show they are altered by stress, glucocorticoids, mood stabilizers, and in a particular psychiatric disorder, schizophrenia. While this field is still in its infancy, we consider their potential for regulating behavioral phenotypes and targeting key predicted signaling cascades that are implicated in psychiatric illness. Clearly, considerable research is required to better determine any therapeutic potential of targeting microRNAs; however, these agents may provide the next generation of effective therapies for psychiatric illnesses.
Keywords: microRNA (miRNA), psychiatric disorders, schizophrenia, bipolar disorder, depression, stress, glucocorticoids, mood stabilizers
Introduction
Psychiatric disorders such as schizophrenia, bipolar disorder (BPD), and major depressive disorder (MDD) are debilitating afflictions, and present a substantial burden to society. These diseases frequently prevent individuals from leading independent lives, consume considerable resources, and are chronic, lifelong, and potentially life threatening. Collectively, they are heterogeneous with variable phenotypes, which make them challenging to diagnose prior to the onset of severe symptoms, and even more difficult to treat effectively. Furthermore, these disorders are associated with a high degree of comorbidity with other diseases, such as cardiovascular disease, obesity, thyroid problems, and diabetes (Kupfer, 2005).
Researchers have long struggled to understand the molecular basis of psychiatric disorders (Figure 1). In the last half-century, these diseases were predominantly thought to be due to altered levels of certain monoamines. Current research, however, supports the hypothesis that these disorders are driven by disruptions in prominent biological pathways, particularly those contributing to synaptic plasticity. While research has made substantial progress in understanding the biological underpinnings of these disorders, here we will only provide a brief overview of some key findings and general themes (for more complete overviews of BPD, schizophrenia, and MDD, respectively, see (Belmaker & Agam, 2008; Goodwin & Jamison, 2007; Lang, Puls, Muller, Strutz-Seebohm, & Gallinat, 2007)).
Figure 1. miRNAs influence the pathophysiology of psychiatric disorders.

Genetic and environmental factors can influence behavior via many possible mechanisms. Modifiable changes in epigenetic or miRNA expression patterns, along with more permanent genetic polymorphisms contribute to varying degrees of resiliency or vulnerability. It is hypothesized that these “predisposing” elements couple with environmental conditions (e.g. early life stress) and lead to either healthy or dysregulated mood that persists throughout an individual’s lifetime.
Genetic, cytoarchitectural, and molecular findings all suggest that a multitude of factors influence the onset and progression of these illnesses. No particular disorder or behaviroal phenotype has been shown to correlate conclusively with any individual gene variant across multiple studies. Rather, it is thought that a multitude of genetic variants contribute to a predisposed state in which certain individuals are sensitive to certain environmental conditions. In recent years, the field of epigenetics has shown that certain environmental influences can produce lasting changes in DNA that alter gene expression and can ultimately influence long-term behavior (Weaver et al., 2004). In addition, familial, twin, and whole genome-wide association studies have firmly established that psychiatric illnesses show some degree of genetic heritability, and many genetic risk factors are thought to confer vulnerability to the various disorders (Belmaker & Agam, 2008; Sklar et al., 2008). Structural and functional MRI studies in individuals with psychiatric disorders have identified ventricular alterations and volumetric changes in regulatory regions such as the limbic system and prefrontal cortex, which are thought to be vital to proper mood regulation (Drevets, 2000; Drevets, Ongur, & Price, 1998; Drevets et al., 1997; Drevets, Savitz, & Trimble, 2008; Neumeister et al., 2005). Finally, molecular findings support several emerging themes associated with the biological underpinnings of psychiatric illnesses, including fundamental impairments in neuroplasticity (Pittenger & Duman, 2008), increased neuronal vulnerability to aberrant stimuli (Kato, 2008), and dysregulation in neurotrophic factor signaling (Duman, 2004; Shaltiel, Chen, & Manji, 2007).
Notably, although researchers have used genetic, in vivo neuroimaging, and molecular approaches to address questions surrounding the biological underpinnings of psychiatric illnesses, these findings have yet to yield a coherent overall image of the molecular basis of associated behaviors. One emerging area of particular interest is the study of microRNAs (miRNAs). miRNAs are small (about 21–23 nucleotides in length), non-peptide-coding RNAs. These molecules are partially complementary to messenger RNAs (mRNAs); thus they bind to targeted mRNAs and either suppress translation of these mRNAs to proteins, or promote mRNA degradation. Accumulating evidence indicates that miRNAs may act as key regulators in a large number of different cellular processes, including early development, proliferation, apoptosis, metabolism, and cell differentiation. A variety of miRNAs have been found in the central nervous system (CNS), and are believed to play critical roles in brain development and structural plasticity. Evidence from a handful of recent studies suggests that miRNAs may prove to be key to our understanding of the pathophysiology and, ultimately, the therapeutics of psychiatric disorders, due to their crucial role in development and broad genetic regulatory influence. MiRNAs could also serve as a unifying link between many diverse groups of findings; for instance, they influence development of prominent brain structures, and are highly responsive to medications used to treat individuals with psychiatric disorders (Zhou et al., 2008). It is possible that they could be differentially expressed in patients with psychiatric illnesses due to genetic predisposition. It should be cautioned, however, that this field is still relatively new and additional research is warranted to elucidate their possible role in psychiatric illnesses and potential targeting for treatment.
Here, we present an overview of recent studies that have examined the biological role of miRNAs in psychiatric disorders or biological processes that are known to influence behavior. We consider the potential for targeting miRNAs for treatments by examining their response to prominent mood stabilizers, as well as their ability to regulate behavioral behaviroal phenotypes and target predicted signaling cascades affecting processes such as survival and proliferation.
MiRNAs are regulated by stress and glucocorticoids
Chronic psychosocial stress is known to have adverse physiological effects that contribute to cardiovascular disease, impaired immune function, inflammatory diseases, and impaired neural function and behavior (Grippo & Johnson, 2009; Sapolsky, Romero, & Munck, 2000). Glucocorticoids are one of the prominent mediators of cellular stress effects on neural function and behavior, and are known to structurally alter brain cytoarchitecture in regions that contribute to cognition, memory, and emotion. Glucocorticoids have also been shown to modify dendritic pyramidal cells and suppress gliogenesis in the prefrontal cortex (Cerqueira et al., 2005; Czeh, Perez-Cruz, Fuchs, & Flugge, 2008; Diorio, Viau, & Meaney, 1993; Wellman, 2001), impair LTP and inhibit neurogenesis in the hippocampus, and increase synaptic excitability as well as lead to neuronal hippocampal atrophy in the amygdala (Bremner, Elzinga, Schmahl, & Vermetten, 2008; Miller & McEwen, 2006). These changes may account for the impaired executive function, weakened conscious memories, and exaggerated autonomic memories that ultimately contribute to maladaptive behavioral responses to chronic psychosocial stress.
Chronic psychosocial stress produces many changes at the cellular level mediated by glucocorticoids, including compromising cellular defenses and making them more susceptible to insults (ie. free radicals, seizures, etc), reducing cellular energy stores, and decreasing neurogenesis. Notably, however, miRNAs are hypothesized to play a specialized role in cellular responses to stress. During the cellular stress response, miRNAs have the capacity to change from translation suppressors to activators by forming new interactions between miRNA/Argonaute complexes and RNA-binding proteins that alter their subcellular localization (Leung & Sharp, 2007). Because of this modified activity, miRNAs could provide a pivotal role in mediating cellular adaptation to stress. In particular, miRNAs have been implicated in the way cells respond to oxidative stress (Leung, Calabrese, & Sharp, 2006; Marsit, Eddy, & Kelsey, 2006), nutrient deprivation (Bhattacharyya, Habermacher, Martine, Closs, & Filipowicz, 2006), and DNA damage (Crosby, Kulshreshtha, Ivan, & Glazer, 2009; Pulkkinen, Malm, Turunen, Koistinaho, & Yla-Herttuala, 2008).
Recent data investigating the manner in which miRNAs are regulated by chronic restraint stress in Wistar-Kyoto rats found that 13 miRNAs were upregulated and three miRNAs were downregulated in the hippocampus following chronic restraint stress for 21 days (Zhou R, et al 2009 in preparation). Interestingly, one of these miRNAs—miR-221—shares a common downstream target: protein phosphatase 3, regulatory subunit B, alpha isoforms (PPP3R1 or calcineurin B). Calcineurin B is a regulatory subunit of the calcium/calmodulin-regulated protein phosphatase calcineurin. Calcineurin, in turn, comprises a 19 kDa calcium-binding protein, calcineurin B, and a 61 kDa calmodulin-binding catalytic subunit, calcineurin A. PPP3R1 was further confirmed as a target of miR-221 in hippocampal cultures treated with mimics or inhibitors of miR-221 to either suppress or enhance translation. Calcineurin B was also shown to be downregulated by this chronic stress paradigm (Zhou R, et al 2009 in preparation). Interestingly, there is much support in the literature for calcineurin’s role in neuroplasticity (Malleret et al., 2001; Mulkey, Endo, Shenolikar, & Malenka, 1994; Wang & Kelly, 1997; Winder, Mansuy, Osman, Moallem, & Kandel, 1998).
Another recent study found that miR-18a downregulated glucocorticoid receptor (GR) protein in the paraventricular nucleus (PVN) in Fischer 344 (F344) but not Sprague Dawley (SD) rats undergoing repeated restraint stress (Uchida et al., 2008). Cell culture studies also demonstrated that miR-18a blocked translation of GR mRNA, and that miR-18a was upregulated in the PVN in F344 rats, but not in SD rats. Interestingly, the authors claimed that F344 rats did not show hypothalamic-pituitary-adrenal (HPA) axis habituation following 14 days of this stressor. Furthermore, the F344 rats had decreased hippocampal cell proliferation and increased anxiety-like behaviors compared with SD rats. Strain differences in GR protein levels were specific for the PVN and were not detected in the hippocampus or prefrontal cortex. The authors suggest that F344 rats may provide a good model for investigating the effects of chronic stress in situations where miR-18a confers some vulnerability to stress.
Another area where the interplay between stress and miRNAs has been explored is that of maternal care in early life, which has been shown to prime the brain to be either more or less sensitive to stress and, ultimately, to influence adult ability to adapt to stress. Two potential mechanisms that may underlie this phenomenon are (1) miRNA regulation and (2) epigenetics (Figure 1). Both of these processes, in turn, have been reported to modulate GR function. Epigenetic studies have noted that maternal behavior where mothers engage in a high degree of licking/grooming and arched-back nursing of their pups produces adult offspring with a more tolerant HPA axis stress response. These effects can be reversed by cross-fostering or by application of a histone deacetylase (HDAC) inhibitor (Weaver et al., 2004), and are due to altered GR function by acetylating histones H3-K9 and the methylation of the NGFI-A consensus sequence on the exon 1(7) promoter of the GR gene (Fish et al., 2004; Weaver et al., 2004). In humans, a very recent study found epigenetic differences in a neuron-specific glucocorticoid receptor (NR3C1) promoter between human postmortem hippocampus samples from suicide victims with a history of childhood abuse and those from either suicide victims with no history of childhood abuse or controls (McGowan et al., 2009).
In the context of stress research, there is some evidence that microRNAs can modulate glucocorticoids (Rainer et al., 2009) and GR function. One recent study found that miR-18 and miR-124a reduced GR-mediated events and decreased GR protein levels. They also inhibited the GR-response gene GILZ following overexpression of either miRNA (Vreugdenhil et al., 2009). Further work is needed to determine whether miR-18 or miR-124a can be modulated by early life stress or maternal care. If this were found, it would provide an example of a miRNA-influenced regulatory mechanism where early life events can have profound effects on adult behavior.
MicroRNAs dysregulated in psychiatric illnesses
Any suggested role that miRNAs may play in mental health is still very much in its infancy of being demonstrated. Currently, there are no studies confirming the role of miRNAs in MDD and BPD, and just a handful of studies implicating them in schizophrenia (Table 1). For instance, Hansen and colleagues investigated the potential regulation of miRNA genes in schizophrenia by studying the association between schizophrenia and genetic variants of miRNA genes associated with brain-expression. By analyzing eighteen known SNPs within close proximity to brain-expressed miRNAs, they found two SNPs (rs17578796 and rs1700) in mir-206 and mir-198 that exhibited significant allelic association with schizophrenia (Figure 1). Interestingly, a follow-up study discovered that both mir-206 and mir-198 had 15 miRNA predicted targets in common (Hansen et al., 2007).
Table 1. miRNAs implicated in schizophrenia.
The list of miRNAs shown are implicated in schizophrenia; the fold change (if available), chromosome location, study, and brain region of each miRNA are presented.
| mi RNA | Fold change | Chromosome location(s) | Study | Brain Region |
|---|---|---|---|---|
| miR-207 | NA | SNP rs 17578796 | Hansen et al 2007 | NA |
| miR-198 | NA | SNP rs1700 | Hansen et al 2007 | NA |
| hsa-miR-26b | 0.63 | 2q35 | Perkins et al 2007 | PFC |
| hsa-miR-30b | 0.68 | 8q24.22 | Perkins et al 2007 | PFC |
| hsa-miR-29b | 0.69 | 1q32.2, 7132.3 | Perkins et al 2007 | PFC |
| hsa-miR-195 | 0.73 | 17p13.1 | Perkins et al 2007 | PFC |
| hsa-miR-92 | 0.76 | 13q31.3, Xq26.2 | Perkins et al 2007 | PFC |
| hsa-miR-30a-5p | 0.79 | 6q13 | Perkins et al 2007 | PFC |
| hsa-miR-30d | 0.8 | 8q24.22 | Perkins et al 2007 | PFC |
| hsa-miR-20b | 0.81 | Xq26.2 | Perkins et al 2007 | PFC |
| hsa-miR-29c | 0.82 | 1q32.2 | Perkins et al 2007 | PFC |
| hsa-miR-29a | 0.82 | 7q32.2 | Perkins et al 2007 | PFC |
| hsa-miR-212 | 0.82 | 17p13.3 | Perkins et al 2007 | PFC |
| hsa-miR-106b | 1.77 | 7q22.1 | Perkins et al 2007 | PFC |
| hsa-miR-7 | 0.7 | 9q21.32, 15q26.1, 19p13.2 | Perkins et al 2007 | PFC |
| hsa-miR-24 | 0.79 | 9q22.32, 19p13.12 | Perkins et al 2007 | PFC |
| hsa-miR-30e | 0.89 | 1p34.2 | Perkins et al 2007 | PFC |
| hsa-miR-9-3p | 0.77 | 1q22, 5q14.3, 15q26.1 | Perkins et al 2007 | PFC |
| let-7g | 1.8 | 3 | Beveridge et al 2008 | STG |
| miR-181b | 2.8 | 1 | Beveridge et al 2008 | STG |
| miR-185 | 0.42 P/0.41H | 16 | Stark et al 2008 | NA |
| miR-134 | 0.62P/0.57H | 12 | Stark et al 2008 | NA |
| miR-674 | 0.65P/0.75H | 2 | Stark et al 2008 | NA |
| miR-532 | 0.66P/0.61H | X | Stark et al 2008 | NA |
| miR-673 | 0.55P/0.64H | 12 | Stark et al 2008 | NA |
| miR-674 | 0.68P/0.69H | 2 | Stark et al 2008 | NA |
| miR-224 | 0.27P/0.29H | X | Stark et al 2008 | NA |
| miR-491 | 0.6P/0.69H | 4 | Stark et al 2008 | NA |
| miR-93 | 0.83P/0.73H | 5 | Stark et al 2008 | NA |
| miR-383 | 0.75P/0.75H | 8 | Stark et al 2008 | NA |
| miR-212 | 0.77P/0.73H | 11 | Stark et al 2008 | NA |
| miR-422b | 0.59P/0.54H | 18 | Stark et al 2008 | NA |
| miR-708 | 0.58P/0.67H | 12 | Stark et al 2008 | NA |
| miR-540 | 0.41P/0.53H | 7 | Stark et al 2008 | NA |
| miR-106b | 0.8P/0.73H | 5 | Stark et al 2008 | NA |
| miR-140 | 0.59P/0.67H | 8 | Stark et al 2008 | NA |
| miR-194 | 0.74P/0.74H | 1 & 19 | Stark et al 2008 | NA |
| miR-325 | 0.66P/0.73H | X | Stark et al 2008 | NA |
| miR-494 | 0.55P/0.58H | 12 | Stark et al 2008 | NA |
| miR-362 | 0.53P/0.67H | X | Stark et al 2008 | NA |
| miR-409 | 0.78P/0.78H | 12 | Stark et al 2008 | NA |
| miR-323 | 0.77P/0.81H | 12 | Stark et al 2008 | NA |
| miR-669a | 0.67P/0.75H | 2 | Stark et al 2008 | NA |
| miR-151 | 0.78P/0.78H | 15 | Stark et al 2008 | NA |
| miR-18 | 0.7P/0.64H | 14 | Stark et al 2008 | NA |
| miR-219 | NA | 6 | Kocerha et al 2009 | NA |
| miR-596 | NA | 8p | Tabares-Seisdedos et al 2009 | NA |
| miR-597 | NA | 8p | Tabares-Seisdedos et al 2009 | NA |
| miR-124-1 | NA | 8p | Tabares-Seisdedos et al 2009 | NA |
| miR-598 | NA | 8p | Tabares-Seisdedos et al 2009 | NA |
| miR-383 | NA | 8p | Tabares-Seisdedos et al 2009 | NA |
| miR-320 | NA | 8p | Tabares-Seisdedos et al 2009 | NA |
| miR-486 | NA | 8p | Tabares-Seisdedos et al 2009 | NA |
| miR-219 | NA | 3 | Kocerha et al 2009 | NA |
| miR-346 | 0.57P | 10 | Zhu et al 2009 | PFC |
NA= not applicable; P/H = fold change in PFC/Hippocampus; PFC= prefrontal cortex; STG = superior temporal gyrus
In a different study, Perkins and colleagues analyzed 264 human miRNAs from postmortem prefrontal cortex of individuals with schizophrenia or schizoaffective disorder using a custom miRNA microarray. They found that 16 miRNAs were significantly regulated in the prefrontal cortex of schizophrenic patients (15 downregulated, one upregulated) compared to healthy controls (Perkins et al., 2007). Another analysis of miRNA expression in postmortem cortical grey matter from the superior temporal gyrus noted significant upregulation of let-7g and miR-181b expression in schizophrenia (Beveridge et al., 2008). Others have examined 22q11.2 microdeletions, which have previously been shown to place individuals at high risk for schizophrenia. Using mouse genetics to mimic the human 22q11.2 microdeletion, they found biogenic changes in brain miRNAs influenced by this microdeletion (Stark et al., 2008).
Schizophrenia has been associated with N-methyl-d-aspartate receptor (NMDAR)-mediated glutamate signaling dysfunction. Notably, a very recent finding implicates miR-219 in NMDA receptor hypofunction; NMDAR signaling blockade decreased miR-219 in the prefrontal cortex of mice. This work went on to elegantly show that in vivo inhibition of miR-219 altered behavioral responses implicated in dysfunctional NMDA receptor signaling. Antipychotics (haloperidol and clozapine) prevented the pharmacological knock down of miR-219 by the NMDA antagonist dizocilpine (Kocerha et al., 2009). Another recent report investigated the chromosome 8p region, which has been implicated in susceptibility to neuropsychiatric disorders such as schizophrenia. 484 annotated genes are located on 8p; 21 of these are implicated as candidate genes that may contribute to neuropsychiatric disorders. Seven miRNAs are also located at 8p (Tabares-Seisdedos & Rubenstein, 2009) (listed in Table 1). Further studies are warranted to determine the precise role that these implicated miRNAs may have in the development, progression and treatment of schizophrenia and what common predicted targets and mechanisms may confer susceptibility.
MicroRNAs regulate behavioral behaviroal phenotypes
miRNA modulation has recently been shown to influence circadian rhythm in drosophila and rodents. This is particularly relevant to the interplay between miRNAs and mood disorders because circadian rhythm is well known to be dysregulated in individuals with BPD, and may be an appropriate behavioral behaviroal phenotype for that disorder (Hasler, Drevets, Gould, Gottesman, & Manji, 2006). In drosophila, a microarray platform was used to mine miRNAs differentially regulated in the adult heads of wildtype flies compared to arrhythmic clock mutant cyc01 flies. Two candidate miRNAs—miR-263a and miR263b—had a rhythmic expression pattern (low during the day and peak at night) in wild type flies, and were constitutively expressed in the cyc01 mutant (Yang, Lee, Padgett, & Edery, 2008).
In rodents, Cheng and Obrietan found that two miRNAs—miR-219 and miR-132—were regulated in the suprachiasmatic nucleus (SCN) by the light-inducible transcription factor cAMP response element binding protein (CREB). miR-219 was also regulated by the circadian genes CLOCK and BMAL1. Furthermore, in the mouse SCN, miR-132 and miR-219 showed a circadian expression pattern (Cheng et al., 2007). The functional roles of these two miRNAs were explored by manipulating their expression using antagomirs (a small, synthetic cholesterol-modified RNA that is perfectly complementary to the specific miRNA target), and determining their effect on behavioral rhythms measured via wheel running. MiR-219 knockdown lengthened the circadian period, while miR-132 knockdown negatively regulated light-induced clock resetting. These data in addition to Kocerha’s work are among the first to show that miRNA modulation can influence behavior. Collectively, these data also expand our understanding of the complex regulation of circadian rhythms, which appear to occur not only through transcriptional feedback mechanisms, but also via miRNA-mediated translational control.
MicroRNAs and their targets as potential therapies to treat mental illness
Because of the sparse data investigating the putative role of miRNAs in the development of novel therapeutics for psychiatric disorders, we will focus here predominantly on mood disorders. Novel, effective treatments for BPD have been lacking over the past few decades. One of the most reliable and frequently prescribed mood stabilizers, lithium, is also among the oldest in the field. While lithium is considered a “first-line” medication, it is only effective for approximately half of patients, has many undesirable side effects (especially at the higher doses required by many patients), and has a delayed onset of action (Keck et al., 1996).
In conjunction with these clinical observations, many researchers have attempted to elucidate the molecular alterations associated with lithium’s delayed onset of action. Over the past decade, studies have identified a plethora of proteins targeted by lithium treatment, with changes reported in canonical proteins such as B-cell lymphoma 2 (Bcl-2), extracellular signal regulated kinase (ERK), brain derived neurotrophic factor (BDNF), and glycogen synthase kinase 3 (GSK-3β). Broadly, lithium treatment has also been associated with enhanced neuroplasticity, improved neuroprotection, increased rates of neurogenesis, and several other changes in biological function (Chen & Manji, 2006; Coyle & Duman, 2003). Overall, lithium alters a multitude of intracellular cascades, has a large number of direct and indirect targets, and induces system-wide changes (Chen & Manji, 2006; Coyle & Duman, 2003; Shaltiel et al., 2007). Thus, predicting its targets has proven challenging and, at times, almost random (though some major cascades have shown a coherent response).
In this context, it is noteworthy that miRNAs have recently emerged as key regulators of complex temporal and spatial patterns of gene/protein expression changes and, thereby, synaptic and neural plasticity. Researchers have therefore recently begun to investigate whether miRNA expression is altered by treatment with mood stabilizers. Zhou and colleagues identified eight miRNAs whose expression levels decreased in the hippocampus of rats following chronic treatment with lithium or valproate (two structurally dissimilar mood stabilizers); one miRNA increased in response to both drugs (see Table 2) (Zhou et al., 2008). This study went on to validate that decreases in miRNA levels can elicit a concurrent increase in a predicted protein target in cell culture following exogenous application of lithium or VPA. While this work highlights that miRNAs are responsive to mood stabilizer treatment, subsequent studies are needed to validate whether targeting any of these miRNAs individually can elicit mood stabilizer-like effects in rodent models of mania or depression.
Table 2. Predicted targets of miRNAs altered by chronic mood stabilizer treatment.
miRNAs regulated by the mood stabilizers lithium (Li) and valproate (VPA). Predicted miRNA targets were grouped into functional groups: (1) Diseases and Disorders, (2) Molecular & Cellular Functions, and (3) Top Canonical Pathways.
| miRNA | AVG Fold Change to Li/VPA | miRNA Predicted Targets: Diseases & Disorders | miRNA Predicted Targets: Molecular & Cellular Functions | miRNA Predicted Targets: Top Canonical Pathways |
|---|---|---|---|---|
| let-7b | 0.56/0.55 | Genetic Disorders (**4); Neurological Disease (**8); Developmental Disorder (**1); | Cell Morphology (**3); Cell Death (**4); Cellular Development (**3); Cell-To-Cell Signaling and Interaction (**2); Amino Acid Metabolism (**1) | PPARalpha/RXRalpha Activation (***13/140); IL-6 Signaling (**8/82); CD27 Signaling in Lymphocytes (*5/41) |
| let-7c | 0.58/0.57 | Genetic Disorders (**4); Neurological Disease (**8); Developmental Disorder (**1); | Cell Morphology (**3); Cell Death (**4); Cellular Development (**3); Cell-To-Cell Signaling and Interaction (**2); Amino Acid Metabolism (**1) | PPARalpha/RXRalpha Activation (***13/140); IL-6 Signaling (**8/82); CD27 Signaling in Lymphocytes (*5/41) |
| miR-105 | 0.65/0.67 | Developmental Disorder (*2); Genetic Disorder (*6); Neurological Diseases (*8); Psychological Disorders (*6) | Cellular Function & Maintenance (**3); Cellular Growth and Proliferation (**2); Cellular Movement (*4); Cell Cycle (*1); Cell Death (*2) | fMLP Signaling in Neutrophils (***7/95); Axonal Guidance Signaling (***13/325); Ephrin Receptor Signaling (**7/158); GM-CSF Signaling (**4/56); Role of NFAT in Regulation of the Immune Response (**6/130) |
| miR-128a | 0.69/0.69 | Neurological Disease (***18); Genetic Disorder (**10); Psychological Disorders (**10); | Cell Morphology (**7); Cellular Compromise (**4); Cellular Growth and Proliferation (*3); Cellular Development (*2); | RAR Activation (***13/141); Reelin Signaling in Neurons (**8/67); Hepatic Cholestasis (**10/102); PPARalpha/RXRalpha Activation (**12/140); PPAR Signaling (**8/76) |
| miR-24 | 0.68/0.72 | Neurological Disease (*3); Genetic Disorder (*1) | Cell-To-Cell Signaling & Interaction (**3); Cellular Assembly and Organization (***2); Cell Cycle (**1); Cellular Development (**1); Cellular Growth and Proliferation (**3) | PTEN Signaling (**6/84); Integrin Signaling (**9/174); Circadian Rhythm Signaling (*3/28); Actin Cytoskeleton Signaling (*8/176); Notch Signaling (*3/33) |
| miR-30c | 0.67/0.72 | Genetic Disorder (**9); Neurological Disease (**10); Psychological Disorders (**8) | Cellular Development (***2); Cell-To-Cell Signaling and Interaction (**4); Cell Morphology (**10); Cellular Assembly & Organization (**7); | Axonal Guidance Signaling (***19/325); Role of NFAT in Regultion of Immune Response (***10/130); Reelin Signaling in Neurons (**6/67); Integrin Signaling (**10/174) |
| miR-34a | 0.67/0.60 | Developmental Disorder (**1); Genetic Disorder (**1); Neurological Disorder (**3); Psychological Disorders (**1) | Cell Death (**5); Cellular Assembly and Organization (**7); Cell Morphology (**5); Amino Acid Metabolism (**1); Cell-To-Cell Signaling and Interaction (**3) | CDK5 Signaling (***9/83); Synaptic Long Term Potentiation (***9/99); Melatonin Signaling (***7/64); Notch Signaling (***5/33); Inositol Phosphate Metabolism (***9/114) |
| miR-221 | 0.65/0.69 | Neurological Disease (***15); Genetic Disorder (**12); Psychological Disorder (**18); Cancer (**1); Hematological Disease (**1) | Cellular Compromise (***4); Cell Morphology (**4); Cell Death (**6); Cell Cycle (**1) | CCR5 Signaling in Macrophages (***6/55); ERK/MAPK Signaling (**8/162); Axonal Guidance Signaling (**12/325); CXCR4 Signaling (**7/135); CD28 Signaling in T Helper Cells (**5/82) |
| miR-144 | 1.70/1.58 | Developmental Disorder (**2); Neurological Disease (**5); Genetic Disorder (**2); | Cellular Movement (**6); Cell Morphology (**7); Cellular Assembly and Organization (**9); Cell Cycle (**1); Cell Death (**1) | RAR Activation (***9/141); Wnt/Beta-catenin Signaling (***9/144); Aminosugars Metabolism (**4/45); ERK/MAPK Signaling (**8/162); Tight Junction Signaling (**7/135) |
Target prediction for miRNA targets performed using PicTar on human miRNA sequences.
Pathway & functional analysis of predicted targets was performed using Ingenuity Pathway Analysis.
Filter: Consider only molecules and/or relationships where tissues/cell lines = CNS Cell Lines OR Nervous System
(# of predicted miRNA targets in functional group or pathway)
(# of predicted miRNA targets/out of entire # of molecules in pathway)
p < 0.05
p < 0.01
p < 0.001
The p values for the functional analysis of predicted miRNA targets are a measure of the likelihood that the association between a set of Functional Analysis genes in our experiment and a given process or pathway is due to random chance.
More broadly, regulated alteration of miRNAs could prove to have a high therapeutic potential, or at least help to explain the therapeutic effects of current mood stabilizers. Any given miRNA can have hundreds of possible messenger RNA targets, so that altering one miRNA may lead to a diverse set of alterations in downstream protein expression. Given that lithium treatment alters some miRNA levels, it is possible that these molecules serve as upstream regulators of already identified protein targets. The accuracy of bioinformatic prediction software continues to improve as models incorporate new findings surrounding the targeting mechanisms of miRNAs (Chaudhuri & Chatterjee, 2007; Rajewsky, 2006; Sethupathy, Megraw, & Hatzigeorgiou, 2006). This improvement will help researchers to efficiently determine whether the multitude of reported effects from lithium could be due to the alteration of only a handful of miRNAs.
Many mechanisms have been hypothesized to underlie current therapies for psychiatric disorders. These mechanisms include but are not limited to: cell survival, neurogenesis, enhanced neuroplasticity, calcium regulation, and enhanced mitochondrial and endoplasmic reticulum (ER) function. From a therapeutic perspective, however, the great advantage of miRNAs is their ability to target hundreds of genes that could potentially be involved in many of these processes. Ultimately this miRNA may be custom-tailored, or show similarity to one of those already implicated in psychiatric disorders (Table 1), regulated by mood stabilizers (Table 2), and/or regulated by chronic restraint stress (Zhou et al. in preparation). Some of the predicted targets of these miRNAs are diverse genes, including mitochondrial interaction constituents (BMF), intracellular calcium signaling (calcineurin), neuronal migration in development and differentiation (DCX), and those whose influence over neural function occurs at the receptor level (GRM7. GRM7, for example, has already been implicated in anxiety-like behaviors in mouse GRM7−/− and in pharmacological studies (Cryan et al., 2003; Mitsukawa et al., 2006). One could imagine a therapy encompassing the “perfect” miRNA: capable of inducing a diverse set of responses that include many of the therapeutic mechanisms discussed above and mentioned in other reviews.
Conclusion
Accumulating evidence indicates that miRNAs act as key regulators in a large number of different cellular processes such as early development, apoptosis, and metabolism, as well as cell proliferation, differentiation, and death. Several types of miRNAs have been found in the CNS, and these respond to stress and regulate morphogenesis in different cells, including neurons. Because miRNAs act on multiple plasticity cascades, targeting them may prove to be effective in the development of novel therapeutics for psychiatric disorders. While this field is still in its infancy, studies are already demonstrating that miRNAs are altered by glucocorticoids, stress, treatment with mood stabilizers, and in schizophrenia. These findings provide a preliminary foundation and support the hypothesis of their relevance in the development, progression, and even treatment of psychiatric disorders. Continuing to collect data on miRNAs implicated in mental health disorders (Table 1) and regulated by current therapies (Table 2), in conjunction with animal studies that evaluate the perturbation of microRNA expression, may eventually elucidate vital mechanisms underlying the etiology of psychiatric illnesses. In this manner, future therapies may be directed at particular miRNAs of interest that can affect a multitude of targets involved in modulating mechanisms of plasticity and resiliency.
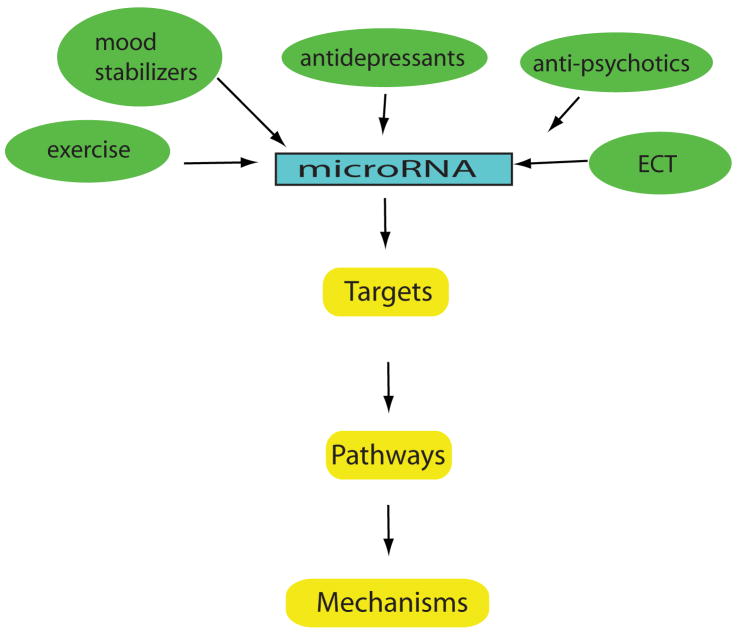
Figure 2. Therapeutic potential of miRNAs in mental health disorders.
The following diagram highlights current treatments known to regulate miRNAs (e.g. mood stabilizers and antipsychotics) and proposes other treatments that may be identified in the future (e.g. electroconvulsive therapy, (ECT)). Targeting miRNAs may provide insight into the common and unique pathways and mechanisms of these treatments. Elucidating more miRNAs and predicted targets may reveal novel therapies that modify plasticity cascades to restore synaptic function, neuronal circuitry, and mood regulation.
References
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17(8):1156–1168. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125(6):1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25(34):7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri K, Chatterjee R. MicroRNA detection and target prediction: integration of computational and experimental approaches. DNA Cell Biol. 2007;26(5):321–337. doi: 10.1089/dna.2006.0549. [DOI] [PubMed] [Google Scholar]
- Chen G, Manji HK. The extracellular signal-regulated kinase pathway: an emerging promising target for mood stabilizers. Curr Opin Psychiatry. 2006;19(3):313–323. doi: 10.1097/01.yco.0000218604.63463.cd. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, et al. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54(5):813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38(2):157–160. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69(3):1221–1229. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der Putten H. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci. 2003;17(11):2409–2417. doi: 10.1046/j.1460-9568.2003.02667.x. [DOI] [PubMed] [Google Scholar]
- Czeh B, Perez-Cruz C, Fuchs E, Flugge G. Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: does hemisphere location matter? Behav Brain Res. 2008;190(1):1–13. doi: 10.1016/j.bbr.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3(3):220–226. 190–221. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5(1):11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, et al. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2. Oxford; New York: Oxford University Press; 2007. [Google Scholar]
- Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12(1):1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T, Olsen L, Lindow M, Jakobsen KD, Ullum H, Jonsson E, et al. Brain expressed microRNAs implicated in schizophrenia etiology. PLoS ONE. 2007;2(9):e873. doi: 10.1371/journal.pone.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an behaviroal phenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kato T. Molecular neurobiology of bipolar disorder: a disease of ‘mood-stabilizing neurons’? Trends Neurosci. 2008;31(10):495–503. doi: 10.1016/j.tins.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Keck PE, Jr, McElroy SL, Strakowski SM, Stanton SP, Kizer DL, Balistreri TM, et al. Factors associated with pharmacologic noncompliance in patients with mania. J Clin Psychiatry. 1996;57(7):292–297. [PubMed] [Google Scholar]
- Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ. The increasing medical burden in bipolar disorder. Jama. 2005;293(20):2528– 2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- Lang UE, Puls I, Muller DJ, Strutz-Seebohm N, Gallinat J. Molecular mechanisms of schizophrenia. Cell Physiol Biochem. 2007;20(6):687–702. doi: 10.1159/000110430. [DOI] [PubMed] [Google Scholar]
- Leung AK, Calabrese JM, Sharp PA. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc Natl Acad Sci USA. 2006;103(48):18125–18130. doi: 10.1073/pnas.0608845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Sharp PA. microRNAs: a safeguard against turmoil? Cell. 2007;130(4):581–585. doi: 10.1016/j.cell.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, et al. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104(5):675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Marsit CJ, Eddy K, Kelsey KT. MicroRNA responses to cellular stress. Cancer Res. 2006;66(22):10843–10848. doi: 10.1158/0008-5472.CAN-06-1894. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MM, McEwen BS. Establishing an agenda for translational research on PTSD. Ann N Y Acad Sci. 2006;1071:294–312. doi: 10.1196/annals.1364.023. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Mombereau C, Lotscher E, Uzunov DP, van der Putten H, Flor PJ, et al. Metabotropic glutamate receptor subtype 7 ablation causes dysregulation of the HPA axis and increases hippocampal BDNF protein levels: implications for stress-related psychiatric disorders. Neuropsychopharmacology. 2006;31(6):1112–1122. doi: 10.1038/sj.npp.1300926. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369(6480):486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57(8):935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8(2):R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Pulkkinen K, Malm T, Turunen M, Koistinaho J, Yla-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett. 2008;582(16):2397–2401. doi: 10.1016/j.febslet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- Rainer J, Ploner C, Jesacher S, Ploner A, Eduardoff M, Mansha M, et al. Glucocorticoid-regulated microRNAs and mirtrons in acute lymphoblastic leukemia. Leukemia. 2009 doi: 10.1038/leu.2008.370. [DOI] [PubMed] [Google Scholar]
- Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006;38(Suppl):S8–13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sethupathy P, Megraw M, Hatzigeorgiou AG. A guide through present computational approaches for the identification of mammalian microRNA targets. Nat Methods. 2006;3(11):881–886. doi: 10.1038/nmeth954. [DOI] [PubMed] [Google Scholar]
- Shaltiel G, Chen G, Manji HK. Neurotrophic signaling cascades in the pathophysiology and treatment of bipolar disorder. Curr Opin Pharmacol. 2007;7(1):22–26. doi: 10.1016/j.coph.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13(6):558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40(6):751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Tabares-Seisdedos R, Rubenstein JL. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.2. [DOI] [PubMed] [Google Scholar]
- Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, et al. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur J Neurosci. 2008;27(9):2250–2261. doi: 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil E, Verissimo CS, Mariman R, Kamphorst JT, Barbosa JS, Zweers T, et al. MicroRNAs miR-18 and miR-124a Downregulate the Glucocorticoid Receptor: Implications for Glucocorticoid Responsiveness in the Brain. Endocrinology. 2009 doi: 10.1210/en.2008-1335. [DOI] [PubMed] [Google Scholar]
- Wang JH, Kelly PT. Postsynaptic calcineurin activity downregulates synaptic transmission by weakening intracellular Ca2+ signaling mechanisms in hippocampal CA1 neurons. J Neurosci. 1997;17(12):4600–4611. doi: 10.1523/JNEUROSCI.17-12-04600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49(3):245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Winder DG, Mansuy IM, Osman M, Moallem TM, Kandel ER. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell. 1998;92(1):25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Yang M, Lee JE, Padgett RW, Edery I. Circadian regulation of a limited set of conserved microRNAs in Drosophila. BMC Genomics. 2008;9:83. doi: 10.1186/1471-2164-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, et al. Evidence for Selective microRNAs and their Effectors as Common Long-Term Targets for the Actions of Mood Stabilizers. Neuropsychopharmacology. 2008 Aug 13; doi: 10.1038/npp.2008.131. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Kalbfleisch T, Brennan MD, Li Y. A MicroRNA gene is hosted in an intron of a schizophrenia-susceptibility gene. Schizophrenia Research. 2009;109:86–89. doi: 10.1016/j.schres.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]