Abstract
Background
Polymorphisms in CYP2B6 are known to predict increased steady-state plasma concentrations of efavirenz. We characterized relationships between genetic polymorphisms and plasma efavirenz concentrations among 45 Haitians who initiated antiretroviral therapy in Port-au-Prince.
Methods
An observational study characterized relationships between clinical factors, pharmacokinetics, and treatment response among antiretroviral-naïve patients initiating once-daily efavirenz plus twice-daily AZT/3TC. Plasma drug concentrations were determined at weeks 2 and 4. Drug doses were directly observed by field workers or designated family members. We retrospectively characterized relationships between efavirenz concentrations and 50 single nucleotide polymorphisms in CYP2B6, and several polymorphisms in CYP2A6, CYP3A4, CYP3A5 and ABCB1.
Results
Plasma for efavirenz assay was obtained 13.9 ±1.6 hours (mean ± SD) post-dose. As expected, CYP2B6 516G→T was associated with increased plasma efavirenz concentrations (Spearman’s rho=0.71, P<0.0001), as were 10 polymorphisms in linkage disequilibrium with 516G→T. Distinct CYP2B6 polymorphisms were associated with decreased plasma efavirenz concentrations (greatest absolute rho=0.48, P=0.0008). Associations were replicated by results from a recent pharmacokinetic study involving 34 healthy, HIV-negative African Americans.
Conclusions
Relatively frequent CYP2B6 polymorphisms may predict decreased plasma efavirenz exposure in patients of African descent. If replicated in other cohorts, the implications of these novel associations for treatment response warrant further study.
Keywords: efavirenz, CYP2B6, pharmacokinetics, HIV-1
INTRODUCTION
Initial therapy for HIV-1 infection with the non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz plus two nucleoside reverse transcriptase inhibitors (NRTIs) typically provides sustained virologic and immunologic benefits, but some patients experience virologic failure [1–6]. Efavirenz is metabolized primarily by cytochrome P450 (CYP) 2B6 [7]. A frequent non-synonymous polymorphism in CYP2B6 exon 4 (516G→T, rs3745274) predicts decreased plasma efavirenz clearance and increased plasma efavirenz exposure at steady state [8–10], as does a less frequent non-synonymous CYP2B6 exon 9 polymorphism, 983T→C (rs28399499) [11–13]. Increased frequencies of both CYP2B6 516T and 983C among individuals of African ancestry [8–14] largely explain their greater mean plasma efavirenz concentrations as compared to Caucasians [15–17]. Additional CYP2B6 polymorphisms have been suggested to affect CYP2B6 activity [18], but have either been extremely infrequent or have not predicted plasma efavirenz exposure in vivo. In two studies involving 169 and 489 individuals, respectively, and in which 15 non-synonymous exonic CYP2B6 polymorphisms were assayed, only 516G→T and 983T→C appeared to predict substantial differences in efavirenz exposure [12, 13].
Most previous pharmacogenomics studies of efavirenz have focused on coding, non-synonymous CYP2B6 polymorphisms [8, 13]. However, many additional CYP2B6 polymorphisms in 5’ and 3’ untranslated regions (UTR) and introns could potentially affect CYP2B6 expression or activity [19, 20]. In this regard, a recent study explored associations between 50 CYP2B6 polymorphisms and pharmacokinetics of single-dose efavirenz among 34 healthy, HIV-negative African Americans [21]. In addition to the expected pharmacokinetic association with the so-called composite CYP2B6 516/983 genotype, associations were suggested between an additional 13 CYP2B6 polymorphisms and efavirenz pharmacokinetics, pending replication in other studies. These polymorphisms were frequent, were in non-coding regions of CYP2B6, and some were not in strong linkage disequilibrium (LD) with either CYP2B6 516G→T or 983T→C.
The present study was designed to characterize relationships between CYP2B6 polymorphisms and steady-state plasma efavirenz concentrations among 45 HIV-positive, antiretroviral-naive Haitians who initiated an efavirenz-containing regimen in Port-au-Prince. Our findings replicate novel associations previously suggested between relatively frequent non-coding CYP2B6 polymorphisms and interindividual variability in plasma efavirenz exposure, and suggest that at least one CYP2B6 variant may be associated with an increased likelihood of subtherapeutic plasma efavirenz concentrations.
MATERIAL AND METHODS
Study Subjects and Design
This pharmacogenomic study involved 45 Haitians of African descent. (An additional individual with undetectable plasma efavirenz, AZT and 3TC concentrations was excluded from analyses for presumed non-adherence). Eligible participants were HIV-1 seropositive, had CD4+ T cells <200 cells/mm3, hemoglobin >7.5 mg/dL, and normal kidney and liver function tests. Patients with prior antiretroviral exposure or requiring medications known or predicted to interact with antiretrovirals were excluded. All participants initiated therapy with efavirenz (600 mg every 24 hours in the evening) plus fixed-dose zidovudine/lamivudine (AZT/3TC, 300/100 mg every 12 hours). To enhance medication adherence, during the four weeks of therapy the study medications were administered under direct observation (morning doses by study personnel [field workers] at the participant’s residence, evening doses by a designated family member [accompagnateur].) All doses were documented in a medication diary.
Plasma Drug Assays
Plasma samples for drug assays were obtained at week 2 and week 4 of therapy, before the morning dose of AZT/3TC. Times since the prior doses of efavirenz, AZT and 3TC were documented. Plasma was separated by centrifugation at 4°C and stored at −70°C. Efavirenz, AZT and 3TC were quantified using two validated methods in the UNC Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Core [22, 23]. The dynamic range for efavirenz was 25 to 10,000 ng/mL, with intra- and interday precision of 4.8–5.5% and accuracy of 100.4–101.7%. The dynamic range for AZT and 3TC was 10–10,000 ng/mL, with intra- and interday precision of <7%, and accuracy of ≥90% for all concentrations.
Statistical Methods
Pharmacokinetic parameters are presented as median and interquartile ranges [IQR]. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. For each participant a single plasma efavirenz concentration value derived based on the geometric mean of week 2 and week 4 values was used for statistical analyses. In one individual without week 2 efavirenz data the week 4 value was used. The geometric mean concentration value for each individual was then used to determine medians, interquartile ranges, and correlations among groups of individuals. Spearman correlation coefficient (rho) was used to assess for dose-response trends in bivariate relationships between continuous variables and genotype, and to determine the directionality of such relationships. The same analyses repeated using Jonckheere-Terpstra test for trend if three genotypes, or Wilcoxon rank sum test if two genotypes, yielded remarkably similar P values (data not shown). For CYP2B6 516/983, genotype was coded as an ordered continuous variable with 1 denoting extensive, 2 intermediate, and 3 slow metabolizer. For other exploratory polymorphism analyses, 1 denoted homozygous, 2 denoted heterozygous, and 3 denoted the other homozygous genotype, ordered as A < C < G < T. (e.g. for 983T→C, C/C = 1, C/T = 2, T/T = 3). For univariate correlations between plasma efavirenz concentrations and genetic polymorphisms, P values that remained significant after Bonferroni correcting for multiple tests are indicated in the Tables. All analyses used a 5% two-sided significance level and were performed with STATA/IC version 10.0 (College Station, TX). Linkage disequilibrium plots (LD) and values r2 were generated using Haploview [24]. Estimated haplotypes for each individual were generated using Powermarker [25], excluding two individuals with less than 85% haplotype probability. Hardy-Weinberg equilibrium was assessed using exact tests [26].
Characterization of Human Genetic Variants
Genomic DNA was extracted from saliva collected with Oragene kits (DNA Genotek Inc, Ottawa, Ontario, Canada). A total of 56 single nucleotide polymorphisms (50 in CYP2B6, 1 in CYP2A6, 1 in CYP3A4, 1 in CYP3A5, and 3 in ABCB1) were assayed in the Vanderbilt DNA Resources Core using MassARRAY® iPLEX Gold (Sequenom, Inc.). Our strategy for CYP2B6 Sequenom assay design was a follows. We tagged the entire CYP2B6 gene using SeattleSNPs [20], including 5 kB in each 5’ and 3’ untranslated regions (UTR), using a cosmopolitan strategy across populations (Yoruba, Asian, African-American, European-American, and Hispanic) with a 5% allelic frequency cut-off, a 0.80 threshold for r2, 85% data convergence for tagging polymorphisms, and 70% data convergence for clustering. Additional polymorphisms of interest (but that were not extremely infrequent) were added based on previous reports [12, 13]. We also added polymorphisms with at least 5% allelic frequency in 20 kB of the 5’ UTR identified using Ensembl Genome Browser [27], as well as upstream polymorphisms possibly associated with CYP2B6 expression based on a previous report [28]. (Final Sequenom assay design available upon request.) Genotypes were confirmed by visual inspection of plots. Laboratory personnel with no knowledge of clinical data performed genotyping. All assays were run in duplicate, and were in complete agreement.
Composite CYP2B6 516/983 genotypes, based on reported associations between CYP2B6 516G→T and 983T→C and steady-state efavirenz pharmacokinetics [11–13], were assigned as described elsewhere [21]: Extensive metabolizer, no variant allele at either position 516 or 983; intermediate metabolizer, a single variant allele at either position 516 or 983, but not both; slow metabolizer, two variant alleles (i.e. either 516 T/T, 983 C/C, or 516 G/T with 983 T/C). Relative CYP2B6 gene copy number was assessed by allelotyping at 17 polymorphic sites using the Sequenom platform.
Protection of Human Subjects
Study volunteers were enrolled and followed at the GHESKIO CENTERS clinic in Port-au-Prince, Haiti. Human experimentation guidelines of the US Department of Health and Human Services were followed in the conduct of this research. All work was conducted in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board of GHESKIO, Cornell University, University of Virginia, and Vanderbilt University, and all participants provided written informed consent.
RESULTS
Participant Characteristics
Forty-five Haitians of African descent were included in this pharmacogenomic association study. Median baseline age was 36 years [IQR 32 – 43 years], median weight was 56 kg [IQR 48 – 59 kg], median BMI was 20.8 kg/m2 [IQR 19.0 – 22.9 kg/m2] and 27 (60%) were female. At baseline, median plasma HIV-1 RNA was 5.3 log10 copies/mL [IQR 4.9 – 5.6 log10 copies/mL] and CD4+ T cell count was 54 cells/mm3 [IQR 25 – 138 cells/mm3].
Efavirenz plasma concentrations
The 45 participants initiated three-drug therapy with efavirenz, AZT and 3TC. Plasma for efavirenz assay at week 2 was obtained 14.1 ± 1.8 hours (mean ± S.D.) post-dose, and at week 4 was obtained 13.8 ± 1.4 hours post-dose. Intraindividual differences between week 2 and week 4 efavirenz concentration values were 18.6% ± 15.5% (mean ± SD). One individual had extremely low plasma efavirenz concentration of 241 ng/mL and 126 ng/mL. Paired efavirenz concentration values were used to calculate geometric mean concentrations for each individual (used in all analyses hereafter). There was an approximately 100-fold interindividual range in plasma efavirenz concentrations. The median plasma efavirenz concentration was 3,295 ng/mL (IQR 2,435 – 6,473 ng/mL, range 174 – 15,380 ng/mL). There was no apparent association between either BMI or sex and plasma efavirenz concentrations (P > 0.05 for each comparison).
Genetic polymorphisms
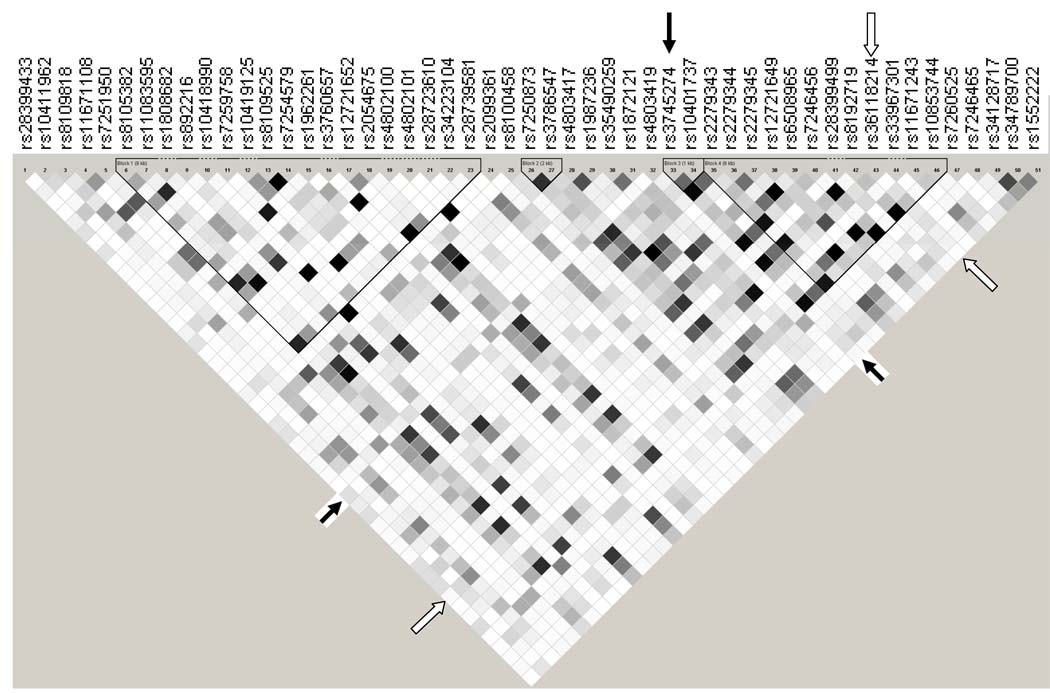
Minor allele frequencies for all 56 polymorphisms are presented in Supplemental Table. All were in Hardy-Weinberg equilibrium (P > 0.05). Based on composite CYP2B6 516/983 genotype (as defined in Materials and Methods), 10 individuals were predicted to be slow metabolizers, 21 intermediate metabolizers, and 14 extensive metabolizers. An LD plot for CYP2B6 polymorphisms is provided in Figure 1.
Figure 1.
Linkage disequilibrium (LD) plot of CYP2B6 polymorphisms. Data from the 45 participants are included. Black, r2 = 1; shades of grey 0 < r2 < 1; white, r2 = 0. The plot was generated using Haploview [24]. Solid arrows indicate the CYP2B6 516G→T (rs3745274) polymorphism. Open arrows indicate rs36118214. The CYP2A6 promoter polymorphism (rs28399433) is also included at far left. Four LD blocks are shown.
Genetic predictors of plasma efavirenz concentrations
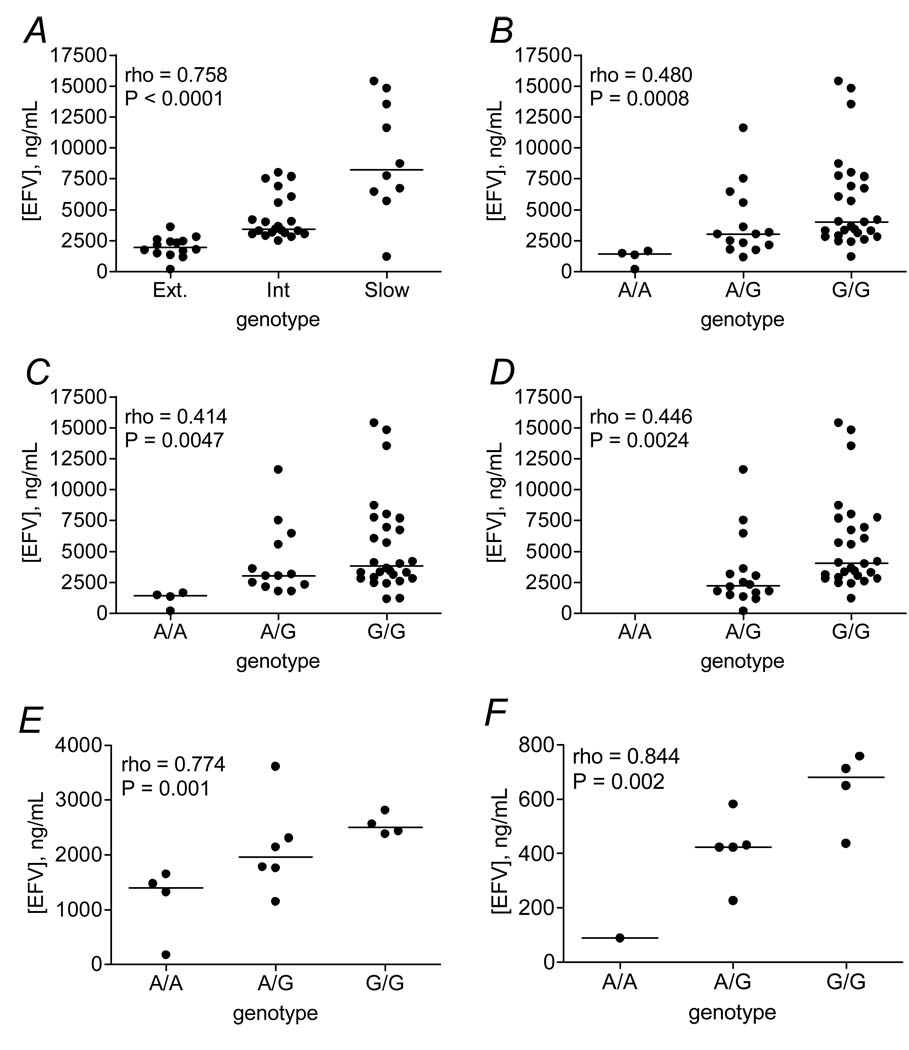
Of the 56 polymorphisms assayed, 20 were associated with efavirenz plasma concentrations at rho > 0.3 or < −0.3 (without correcting for multiple comparisons) as shown in Table 1. Associations for all 56 polymorphisms are provided in Supplemental Table and Supplemental Figure. There was, as expected, a strong correlation between composite CYP2B6 516/983 genotype and higher plasma efavirenz concentrations (rho=0.76, P < 0.0001, Figure 2A). In analyses involving CYP2B6 516G→T and 983T→C separately there was also a significant association between CYP2B6 516G→T and efavirenz plasma concentrations (rho= 0.72, P < 0.0001). Of the other 19 polymorphisms associated with plasma efavirenz concentrations, 10 were in LD with CYP2B6 516G→T at r2 > 0.5 (Figure 1 and Table 1). These included 3 5’ UTR, 6 intronic and 1 exonic polymorphisms. Based on rho values, no polymorphism was more strongly associated with plasma efavirenz concentrations than CYP2B6 516G→T. Among 10 individuals with slow metabolizer composite CYP2B6 516/983 genotypes, plasma efavirenz concentrations did not differ in 2 individuals heterozygous for the CYP2A6 promoter polymorphism (rs28399433) as compared to the 8 individuals lacking this polymorphism (data not shown).
Table 1.
Relationships between genetic polymorphisms and efavirenz plasma concentrations.
| SNP | LD with 516G→Ta (r2) |
LD with rs36118214a (r2) |
Efavirenz (ng/mL), median (IQR) |
n | Efavirenz (ng/mL), median (IQR) |
n | Efavirenz (ng/mL), median (IQR) |
n | rhob | P- VALUE |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CYP2A6 | |||||||||||||
| rs28399433 | 0.124 | 0.02 | GG | - | 0 | GT | 6073 [4170–7993] | 7 | TT | 3066 [2307–5714] | 38 | −0.3494 | 0.0187 |
| CYP2B6 | |||||||||||||
| rs8109818 | 0.266 | 0.443 | AA | 4170 [3294–7738] | 21 | AG | 2963 [1772–4779] | 20 | GG | 2725 [1893–3379] | 4 | −0.4218 | 0.0039 |
| rs8105382 | 0.32 | 0.115 | CC | 2435 [1762–2813] | 3 | CT | 3041 [2307–4067] | 21 | TT | 4170 [3092–7681] | 21 | 0.3578 | 0.0158 |
| rs11083595 | 0.786 | 0.183 | CC | 8228 [6715–14829] | 6 | CG | 3652 [3034–6473] | 27 | GG | 2345 [1402–2691] | 12 | −0.6041 | <0.0001d |
| rs892216 | 0.71 | 0.276 | CC | 1762 [1327–2435] | 11 | CT | 3652 [3034–6473] | 27 | TT | 7738 [3092–14829] | 7 | 0.6065 | <0.0001d |
| rs12721652 | 0.372 | 0.24 | CC | 3911 [3063–7709] | 20 | CT | 3218 [2440–6016] | 20 | TT | 1762 [1326–2435] | 5 | −0.3917 | 0.0078 |
| rs2054675 | 0.831 | 0.194 | CC | 8718 [6715–14829] | 7 | CT | 3533 [3034–6073] | 26 | TT | 2345 [1402–2691] | 12 | −0.6338 | <0.0001d |
| rs28739581 | 0.38 | 0.257 | AA | 1762 [1327–2435] | 5 | AT | 3294 [2497–6473] | 19 | TT | 3911 [3063–7709] | 20 | 0.3807 | 0.0108 |
| rs7250873 | 0.656 | 0.086 | AA | 2409 [1762–2813] | 10 | AG | 3652 [2891–6073] | 25 | GG | 7227 [3034–13528] | 10 | 0.4389 | 0.0026 |
| rs3786547 | 0.831 | 0.194 | CC | 8718 [6715–14829] | 7 | CT | 3533 [3034–6073] | 26 | TT | 2345 [1402–2691] | 12 | −0.6338 | <0.0001d |
| rs35490259 | 0.174 | 0.5 | CC | - | 0 | CT | 2497 [1762–3614] | 17 | TT | 3859 [2963–7298] | 28 | 0.3706 | 0.0122 |
| rs1872121 | 0.228 | 0.941 | AA | 1402 [750–1565] | 4 | AG | 3041 [2307–5558] | 13 | GG | 3826 [2853–7298] | 28 | 0.4144 | 0.0047 |
| rs3745274 | - | 0.242 | GG | 2142 [1478–2569] | 15 | GT | 3826 [3092–6473] | 22 | TT | 8228 [6214–14178] | 8 | 0.7242 | <0.0001d |
| rs10401737 | 0.592 | 0.264 | CC | 7198 [3911–11123] | 12 | CT | 3118 [2569–5558] | 26 | TT | 1762 [1478–2307] | 7 | −0.5913 | <0.0001d |
| rs2279343 | 0.957 | 0.231 | AA | 2225 [1565–2691] | 16 | AG | 4000 [3092–6473] | 21 | GG | 8228 [6214–14178] | 8 | 0.7141 | <0.0001d |
| rs12721649 | 0.165 | 0.668 | AA | - | 0 | AG | 2225 [1565–3379] | 16 | GG | 4033 [2963–7298] | 28 | 0.4461 | 0.0024 |
| rs7246456 | 0.957 | 0.231 | CC | 2142 [1478–2569] | 15 | CT | 4000 [3092–6914] | 23 | TT | 7738 [5714–14829] | 7 | 0.7012 | <0.0001d |
| rs8192719 | 0.957 | 0.231 | CC | 2142 [1478–2569] | 15 | CT | 4000 [3092–6914] | 23 | TT | 7738 [5714–14829] | 7 | 0.7012 | <0.0001d |
| rs36118214 | 0.242 | - | AA | 1402 [750–1565] | 4 | AG | 3037 [2142–5558] | 14 | GG | 4000 [2892–7681] | 27 | 0.4803 | 0.0008d |
| rs10853744 | 0.956 | 0.236 | GG | 2142 [1478–2569] | 15 | GT | 4033 [3092–6914] | 22 | TT | 7738 [5714–14829] | 7 | 0.7019 | <0.0001d |
| Composite 516/983c |
EXT | 1962 [1478–2435] | 14 | INT | 3415 [3092–5558] | 21 | SLO | 8228 [6473–13528] | 10 | 0.7578 | <0.0001d |
The r2 measure of LD between each chromosome 19 polymorphism, rs3745274 (516G→T) and rs36118214 are shown.
Spearman rank correlation coefficient assessing monotonically increasing or decreasing trend by genotype as an ordered continuous variable. The signs (+ or −) for rho value are determined by assigning numbers to each base as follows: A = 1, C = 2, G = 3, T = 4. P value corresponding to the Spearman rank coefficient test. Only polymorphisms with rho >0.3 or < −0.3 are shown.
Composite CYP2B6 genotypes were as follows: extensive metabolizer, no variant allele at either position 516 or 983; intermediate metabolizer, a single variant allele at either position 516 or 983, but not both; slow metabolizer, two variant alleles (i.e. either 516 T/T, 983 C/C, or 516 G/T with 983 T/C).
These P values are significant after Bonferroni correction for multiple comparisons.
Figure 2.
Relationships between selected polymorphisms and plasma efavirenz concentrations. Panel A: Relationship between composite CYP2B6 516/983 genotype and plasma efavirenz concentrations among all study participants. Panel B: Relationship between rs36118214 and plasma efavirenz concentrations among all study participants. Panel C: Relationship between rs1872121 and plasma efavirenz concentrations among all study participants. Panel D: Relationship between rs12721649 and plasma efavirenz concentrations among all study participants. Panel E: Relationship between rs36118214 and plasma efavirenz concentrations among 14 participants with composite CYP2B6 516/983 extensive metabolizer genotypes. Panel F: From a previous study in which 34 healthy, HIV-negative African Americans received a single 600 mg dose of efavirenz [21], relationship between rs36118214 and plasma efavirenz concentrations 24 hours post-dose among 10 participants with composite CYP2B6 516/983 extensive metabolizer genotypes. Horizontal lines represent medians. Each marker in panels A through E represents the geometric mean of paired efavirenz concentrations from each participant.
Nine polymorphisms that were not in strong LD with CYP2B6 516G→T (r2 < 0.5) were also associated with plasma efavirenz concentrations at rho > 0.3 or < −0.3 (Figure 1 and Table 1). These included 3 5’ UTR and 5 intronic polymorphisms in CYP2B6, and the CYP2A6 promoter polymorphism. Of these 9 polymorphisms, an intron 8 polymorphism (rs36118214) was most strongly associated with plasma efavirenz concentrations (rho = 0.48, P = 0.0008, Figure 2B). In contrast to both CYP2B6 516G→T and 983T→C, the less frequent rs36118214 A allele appeared to predict lower plasma efavirenz concentrations. Two of the other polymorphisms associated with plasma efavirenz concentrations were in LD with rs36118214 at r2 > 0.5 (rs1872121 at r2 = 0.941, rs12721649 at r2 = 0.668, Figure 2C and 2D).
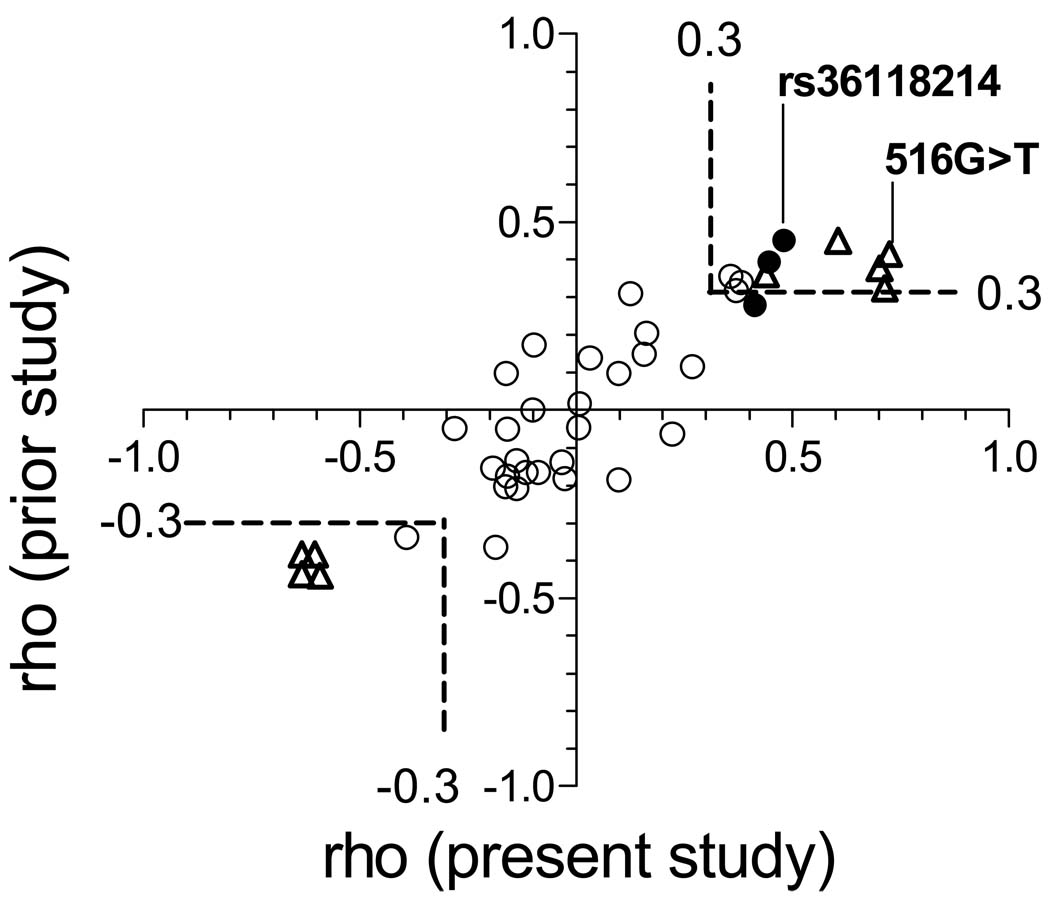
To assess whether associations were spurious, we compared rho values from the present study with those from a recent study which characterized genetic variants and plasma efavirenz pharmacokinetics among 34 healthy, HIV-negative African Americans who received a single 200-mg dose of nevirapine, followed several weeks later by a single 600-mg dose of efavirenz [21]. Forty nine polymorphisms assayed in the present study were also in the previous study. Three polymorphisms associated with plasma efavirenz concentrations in the present study (rs28399433, rs8109818, and rs10853744) were not assayed in the previous study. Associations replicated remarkably well between studies, including polymorphisms in LD with CYP2B6 516G→T, and those in LD with rs36118214 (Figure 3). This supports the validity of these associations.
Figure 3.
Relationships between Spearman’s rho values for each polymorphism from the present and previous studies. Open triangles indentify polymorphisms in LD with CYP2B6 516G→T (rs3745274) at r2 > 0.5. Closed circles identify polymorphisms in LD with rs36118214 at r2 > 0.5. Open circles identify other polymorphisms. The previous study characterized associations between polymorphisms and efavirenz pharmacokinetic following a single 600-mg dose of efavirenz among 34 healthy, HIV-negative African Americans [21]. From that study rho values for 24-hour post-dose efavirenz concentrations are shown. Dashed lines bound rho values > 0.3 or < −0.3. The sign (+ or −) of the rho value signifies ordering of genotypes bases (A < C < G < T) as explained in Materials and Methods, not whether the minor allele predicts higher or lower plasma efavirenz concentrations.
As shown in Figure 2B, four individuals were homozygous for rs36118214, of whom one had extremely low plasma efavirenz concentrations (241 ng/mL and 126 ng/mL at weeks 2 and 4, respectively). This individual was also homozygous for rs1872121 and heterozygous for rs12721649. To further assess associations with lower plasma efavirenz concentrations, subgroup analyses were performed on the 14 individuals with extensive metabolizer composite 516/983 genotypes. In this subgroup analysis, of the 20 polymorphisms associated with plasma efavirenz concentrations noted above only rs36118214 (Figure 2E) and rs1872121 (not shown) were significantly associated with plasma efavirenz concentrations (rho = 0.774, P = 0.0012 and rho = 0.601, P = 0.023, respectively). In sensitivity analyses which excluded the one individual with very low plasma efavirenz concentrations, the association with rs36118214 persisted in the remaining 13 individuals (rho = 0.7325, P = 0.0044), whereas the association with rs1872121 did not (rho = 0.5258, P = 0.0649). Although clinical relevance is uncertain with so few individuals, the patient in the present study with extremely low plasma efavirenz concentrations experienced virologic failure at week 24. No study participant discontinued efavirenz because of medication side effects.
In the previous study of 34 HIV-negative African Americans [21], the only individual homozygous for rs36118214 was also the only individual with extremely rapid plasma clearance of efavirenz. That individual had a 24-hour efavirenz concentration of 89 ng/mL, considerably less than the median value of 582 ng/mL among other participants, and was heterozygous for rs1872121 and homozygous for rs12721649. The association with rs36118214 in that study was also apparent in the subgroup of 10 individuals with extensive metabolizer composite 516/983 genotype (rho = 0.844, P = 0.0021, Figure 2F), and in sensitivity analyses which excluded the individual with extremely low plasma efavirenz concentrations (n = 9, rho = 0.782, P = 0.0076).
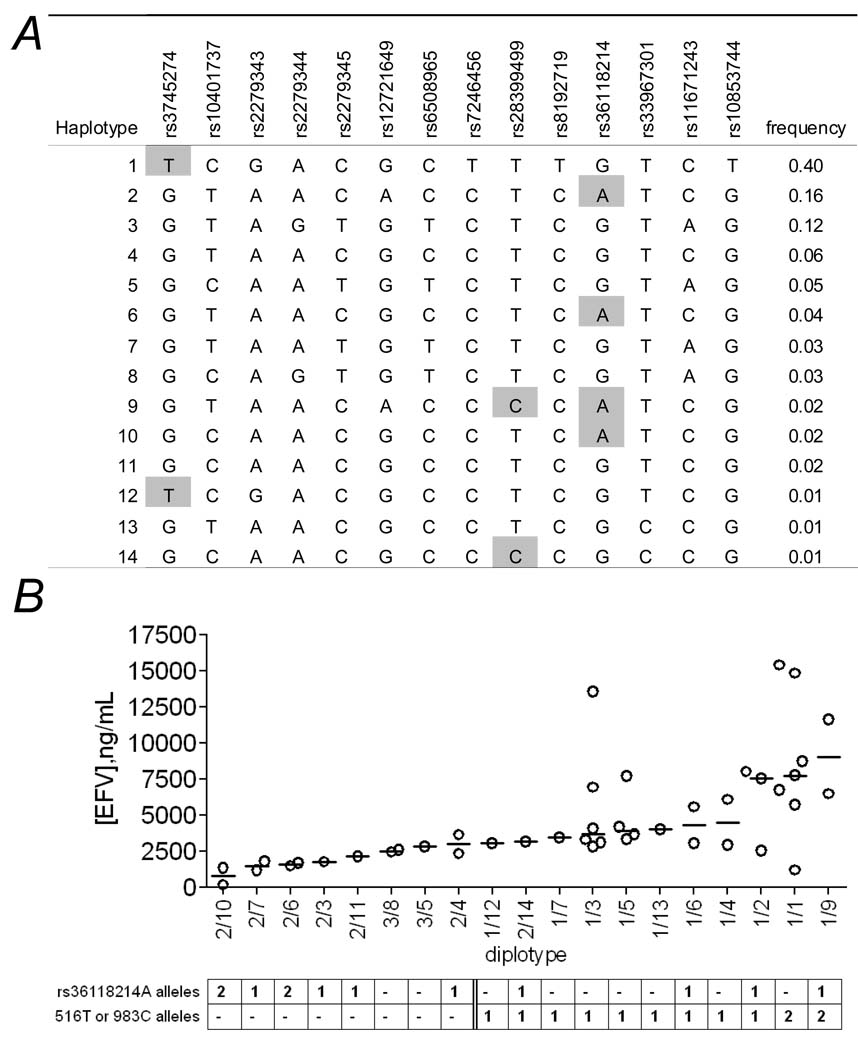
To further describe relationships between rs36118214 and plasma efavirenz concentrations estimated haplotypes were generated which span 14 polymorphisms from CYP2B6 516G→T to rs10853744. This encompasses two adjacent LD blocks (Figure 1) and includes rs36118214, rs12721649, and 983T→C. The 14 predicted haplotypes (numbered based on frequency, not based on “star” nomenclature [18]) and relationships between diplotypes and plasma efavirenz concentrations are shown in Figure 4. Diplotypes with at least one rs36118214 A allele (haplotypes 2, 6 and 10) but no slow metabolizer allele (haplotypes 1, 9 and 14) were associated with lower plasma efavirenz concentrations. In contrast, diplotypes with at least one slow metabolizer allele at position 516G→T or 983T→C were associated with higher plasma efavirenz concentrations regardless of whether an rs36118214 A allele was present. Of the 4 individuals homozygous for rs36118214, two were haplotype 2/10 (including the individual with very low plasma efavirenz concentrations) and two were haplotype 2/6. Thus, no haplotype was unique to the individual with very low efavirenz concentrations.
Figure 4.
Relationships between CYP2B6 diplotypes and plasma efavirenz concentrations. Panel A: Estimated haplotypes in region spanning CYP2B6 rs3745274 (516G→T) to rs36118214. Haplotypes are numbered from 1 to 14 in order of decreasing frequency. Shaded positions represent minor alleles for rs3745274 (516G→T), rs28399499 (983T→C), and rs36118214. Estimated haplotypes were generated using Powermarker [25] for the region spanning rs3745274 to rs36118214. Panel B: Relationships between CYP2B6 diplotypes and plasma efavirenz concentrations. Horizontal lines represent medians. Diplotypes are ordered from lowest to highest median efavirenz concentrations. Each marker represents the geometric mean of paired efavirenz concentrations from each participant.
Lower plasma efavirenz concentrations could be a consequence of increased CYP2B6 gene copy number. By allelotyping at 17 single nucleotide polymorphisms across CYP2B6, 37 participants (including the individual with very low plasma efavirenz concentrations) could be studied for gene copy number by allelotyping. None had more than two copies of CYP2B6 (data not shown).
Although the present study included a directly observed strategy to enhance adherence, and medication diaries documented complete adherence at weeks 2 and 4, we cannot exclude nonadherence. (In fact, one individual with no detectable efavirenz, AZT or 3TC in plasma was excluded from analyses for presumed non-adherence, despite medication diaries indicating adherence). If nonadherence covaried with genotype, one might expect this to also affect AZT and 3TC. However, there was no correlation between plasma concentrations of efavirenz and either AZT or 3TC (rho = 0.0187, P = 0.9 and rho = −0.0057, P = 0.97, respectively), and there was no association between any of the 56 polymorphisms and plasma concentrations of either AZT or 3TC (data not shown). This suggests that associations between polymorphisms and efavirenz concentrations were not explained by gross differences in adherence.
DISCUSSION
Efavirenz is a preferred component of first-line regimens for HIV-1 infection [1, 2], based on its superior performance in randomized clinical trials [3–6]. It is one of the most widely prescribed antiretroviral drugs in both affluent and resource-limited countries. The most important finding from the present study is that at least one CYP2B6 genetic polymorphism appears to be associated with decreased plasma efavirenz exposure. Replication of associations between the present study, involving HIV-infected Haitians who initiated antiretroviral therapy in Port-au-Prince, and a previous study involving healthy African Americans in Nashville, Tennessee [21], support the validity of this finding. This does not, however, preclude the need for further replication in larger cohorts.
Many genetic polymorphisms vary in frequency among populations [29]. The polymorphism most consistently associated with lower plasma efavirenz concentrations in the present report, rs36118214, was relatively frequent in both this study and in the previous study (minor allele frequency 0.24 and 0.22, respectively)[21]. This polymorphism appears to vary markedly in frequency depending on geographic region of ancestry. According to the NCBI web-based resource, dbSNP [14], the frequency of the A allele (based on relatively few genotypes) was 0.42 among Sub-Saharan Africans and 0.21 among African American, but was absent among Europeans and Asians. Genetic polymorphisms that differ in frequency among populations might help explain, at least in part, disparate responses to therapy. In this regard it is noteworthy that in AIDS Clinical Trials Group protocol A5095, in which most participants received efavirenz-containing regimens, virologic failure was considerably more frequent among blacks than among whites, particularly in analyses that adjusted for self-reported nonadherence [30, 31].
Efavirenz is primarily metabolized by CYP2B6 [7]. A number of previous studies have examined associations between genetic polymorphisms and efavirenz pharmacokinetics and/or treatment response [8–13, 32]. Associations between increased plasma efavirenz exposure, CYP2B6 516G→T [8–13], and 983T→C [11–13] have been consistent across multiple studies and populations. Although the 516G→T polymorphism alters amino acid sequence, its effect appears to be through aberrant splicing which reduces functional mRNA and protein [33]. Additional CYP2B6 polymorphisms have been suggested to affect CYP2B6 activity [18], but are either extremely infrequent or have not predicted plasma efavirenz exposure in vivo [12, 13]. In analyses designed to identify CYP2B6 copy number variants among 226 individuals, including 138 whites and 77 blacks, only one individual heterozygous for a copy number variant was identified [34]. This was a crossover between CYP2B6 and the pseudogene CYP2B7 which was associated with increased plasma efavirenz concentrations. CYP2A6, which is located approximately 141 kilobases upstream of CYP2B6 on chromosome 19, catalyzes a secondary metabolism pathway for efavirenz. A recent study suggests that loss of function polymorphisms in CYP2A6 (including the promoter polymorphism studied herein) when combined with CYP2B6 slow metabolism genotypes may predict particularly high efavirenz concentrations [35]. Our inability to show this association with a CYP2A6 promoter polymorphism (rs28399433) among composite 516/983 slow metabolizers may reflect the absence of individuals homozygous for the G allele.
The mechanism(s) by which the polymorphisms identified herein would be associated with decreased efavirenz exposure are unknown. We found no evidence of increased CYP2B6 copy number. Furthermore, the three implicated CYP2B6 polymorphisms are non-coding (rs36118214 and rs12721649 in intron 8, rs1872121 in intron 3). There are several possibilities. These polymorphisms may tag as yet unidentified chromosome 19 variants that are directly causative. Alternatively, at least one of these polymorphisms may in some way be involved in regulating CYP2B6 transcription. Or these may cause aberrant splicing of CYP2B6 that in some way increases enzymatic activity. Extensive genotyping of large cohorts may identify more precise genetic predictors of low plasma efavirenz concentrations.
There were limitations to the present study. Because the sample size was relatively small, only four individuals were homozygous for the polymorphism most strongly associated with decreased plasma efavirenz concentrations. Larger cohorts would also strengthen haplotype analyses. Despite associations being consistent between two studies [21], genomic association studies are plagued by false discovery [36]. It is therefore critical that these putative predictors of decreased plasma efavirenz exposure be replicated in other cohorts. In addition, because genetic predictors of efavirenz pharmacokinetics may differ depending on geographic region of ancestry, associations may differ in other populations. Associations with virologic response in large cohorts should be assessed to understand potential implications for clinical care. Despite the use of directly observed drug administration to enhance adherence, we cannot absolutely exclude nonadherence. We used self-identified race rather than population-informative genetic markers, which could identify unrecognized population stratification.
These findings, if replicated in additional cohorts, may have implications for the HIV-1 pandemic. A lower therapeutic cut-off of 1,000 ng/mL has been suggested for efavirenz [37]. Virologic failure with efavirenz plus two NRTIs typically leads to emergence of resistance to NNRTIs and NRTIs [38]. With continued selective pressure, progressive cross-resistance to multiple drugs including newer NNTRIs such as etravirine can develop [39]. This may result in HIV-1 disease progression, fewer future treatment options, and transmission of multidrug-resistant virus to others. Antiretroviral prescribing strategies could be improved by understanding whether certain individuals are genetically predisposed to virologic failure with efavirenz.
Supplementary Material
Acknowledgments
The authors are grateful to the persons who volunteered for this study. This study was supported in part by NIH grants AI007046 and AI077339 (RAD), AI50410 (ADMK and NLR), AI064021 (DWF), TW006901 and TW00018 (JWP), AI071205, AI54999 and Vanderbilt CTSA grant RR024975 (DWH). Guyrlaine Pierre-Louis and Guerline Antoine performed research study visits at GHESKIO. Hailing Jin, Danielle Richardson, Cara Sutcliffe and Ping Mayo of the Vanderbilt University DNA Resources Core provided technical assistance for genetic assays. We acknowledge Tebeb Gebretsadik, Gail Mayo, Usha Menon, Edward P. Acosta, Ayumi Shintani, Michael Floyd, C. Michael Stein, and Grant R. Wilkinson for performing the previous pharmacogenomics study [21].
Funding:
This study was supported in part by NIH grants AI007046 (RAD), AI50410 (ADMK and NLR), AI064021 (DWF), TW006901 and TW00018 (JWP), AI071205, AI54999 and Vanderbilt CTSA grant RR024975 (DWH)
Footnotes
Potential conflicts of interest:
David W. Haas has received research grants from Bavarian Nordic, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck, Tanox, and Tibotec. He is on scientific advisory boards for Glaxo Smith Kline and Tibotec.
Angela D. M. Kashuba has received research grants from Abbott, Boehringer-Ingelheim, Gilead Sciences, Merck, Pfizer, and Tibotec. She is on scientific advisory boards for Boehringer-Ingelheim and Bristol Meyers Squibb.
No potential conflicts of interest: Paul Leger, Rebecca Dillingham, Carole Anne Beauharnais, Naser L. Rez, Daniel W. Fitzgerald, and Jean William Pape
Reference List
- 1.The Panel on Clinical Practices for Treatment of HIV Infection, Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. [Accessed 27 January 2009]; Available at: http://aidsinfo.nih.gov.
- 2.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach (2006 revision) [Accessed 27 January 2009]; Available at: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- 3.Staszewski S, Morales-Ramirez J, Tashima KT, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. New Engl J Med. 1999;341:1865–1873. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 4.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–1861. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 5.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 6.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward BA, Gorski JC, Jones DR, et al. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 8.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–2400. [PubMed] [Google Scholar]
- 9.Haas DW, Smeaton LM, Shafer RW, et al. Pharmacogenetics of Long-Term Responses to Antiretroviral Regimens Containing Efavirenz and/or Nelfinavir: An Adult AIDS Clinical Trials Group Study. J Infect Dis. 2005;192:1931–1942. doi: 10.1086/497610. [DOI] [PubMed] [Google Scholar]
- 10.Rotger M, Colombo S, Furrer H, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Sonnerborg A, Rane A, et al. Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet Genomics. 2006;16:191–198. doi: 10.1097/01.fpc.0000189797.03845.90. [DOI] [PubMed] [Google Scholar]
- 12.Rotger M, Tegude H, Colombo S, et al. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin Pharmacol Ther. 2007;81:557–566. doi: 10.1038/sj.clpt.6100072. [DOI] [PubMed] [Google Scholar]
- 15.Barrett JS, Joshi AS, Chai M, Ludden TM, Fiske WD, Pieniaszek HJ., Jr Population pharmacokinetic meta-analysis with efavirenz. Int J Clin Pharmacol Ther. 2002;40:507–519. doi: 10.5414/cpp40507. [DOI] [PubMed] [Google Scholar]
- 16.Pfister M, Labbe L, Hammer SM, et al. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trial Group Study 398. Antimicrob Ag Chemother. 2003;47:130–137. doi: 10.1128/AAC.47.1.130-137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribaudo H, Clifford D, Gulick R, et al. Relationships between efavirenz pharmacokinetics, side effects, drug discontinuation, virologic response, and race: results from ACTG A5095/A5097s [abstract 132]. Program and abstracts of the 11th Conference on Retroviruses and Opportunistic Infections (San Francisco); Alexandria, VA; Foundation for Retrovirology and Human Health. 2004. [Google Scholar]
- 18. [Accessed 27 January 2009];Human Cytochrome P450 (CYP) Allele Nomenclature Committee. Available at: http://www.cypalleles.ki.se/
- 13.Haas DW, Ribaudo H, Motsinger A, et al. Pharmacogenetics of plasma drug exposure and treatment outcomes with efavirenz (EFV)- containing regimens: an AIDS Clinical Trials Group (ACTG) Study [abstract L-139]. Program and abstracts of the 15th Conference on Retroviruses and Opportunistic Infections (Boston); Alexandria, VA; Foundation for Retrovirology and Human Health. 2008. [Google Scholar]
- 19.Lamba J, Lamba V, Strom S, et al. Novel single nucleotide polymorphisms in the promoter and intron 1 of human pregnane X receptor/NR1I2 and their association with CYP3A4 expression. Drug Metab Dispos. 2008;36:169–181. doi: 10.1124/dmd.107.016600. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed 27 January 2009];SeattleSNPs Variation Discovery Resource. Available at: http://pga.gs.washington.edu/
- 21.Haas DW, Gebretsadik T, Mayo G, et al. Relationships between CYP2B6 Polymorphisms and Pharmacokinetics Following a Single Dose of Nevirapine or Efavirenz in African Americans. J Infect Dis. 2009;199:872–880. doi: 10.1086/597125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezk NL, Tidwell RR, Kashuba AD. Simultaneous determination of six HIV nucleoside analogue reverse transcriptase inhibitors and nevirapine by liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:137–147. doi: 10.1016/s1570-0232(03)00224-1. [DOI] [PubMed] [Google Scholar]
- 23.Rezk NL, Crutchley RD, Yeh RF, Kashuba AD. Full validation of an analytical method for the HIV-protease inhibitor atazanavir in combination with 8 other antiretroviral agents and its applicability to therapeutic drug monitoring. Ther Drug Monit. 2006;28:517–525. doi: 10.1097/00007691-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Muse SV. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- 26.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [Accessed 27 January 2009];Ensembl Genome Browser. Available at: http://www.ensembl.org/index.html.
- 28.Lamba V, Lamba J, Yasuda K, et al. Hepatic CYP2B6 Expression: Gender and Ethnic Differences and Relationship to CYP2B6 Genotype and CAR Expression. J Pharmacol Exp Ther. 2003;307:906–922. doi: 10.1124/jpet.103.054866. [DOI] [PubMed] [Google Scholar]
- 29.Sachidanandam R, Weissman D, Schmidt SC, et al. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001;409:928–933. doi: 10.1038/35057149. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed 27 January 2009];National Center for Bioinformatics, dbSNP homepage. Available at: http://www.ncbi.nlm.nih.gov/SNP/index.html.
- 30.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA. 2006;296:769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 31.Schackman BR, Ribaudo HJ, Krambrink A, Hughes V, Kuritzkes DR, Gulick RM. Racial differences in virologic failure associated with adherence and quality of life on efavirenz-containing regimens for initial HIV therapy: results of ACTG A5095. J Acquir Immune Defic Syndr. 2007;46:547–554. doi: 10.1097/qai.0b013e31815ac499. [DOI] [PubMed] [Google Scholar]
- 32.Fellay J, Marzolini C, Meaden ER, et al. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359:30–36. doi: 10.1016/S0140-6736(02)07276-8. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann MH, Blievernicht JK, Klein K, et al. Aberrant Splicing Caused by Single Nucleotide Polymorphism c.516G>T [Q172H], a Marker of CYP2B6*6, Is Responsible for Decreased Expression and Activity of CYP2B6 in Liver. J Pharmacol Exp Ther. 2008;325:284–292. doi: 10.1124/jpet.107.133306. [DOI] [PubMed] [Google Scholar]
- 34.Rotger M, Saumoy M, Zhang K, et al. Partial deletion of CYP2B6 owing to unequal crossover with CYP2B7. Pharmacogenet Genomics. 2007;17:885–890. doi: 10.1097/FPC.0b013e3282ef5cd1. [DOI] [PubMed] [Google Scholar]
- 35.di Iulio J, Fayet A, Arab-Alameddine M, et al. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics. 2009 doi: 10.1097/FPC.0b013e328328d577. (Published Ahead of Print) [DOI] [PubMed] [Google Scholar]
- 36.Ioannidis JP, Ntzani EE, Trikalinos TA. Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 37.Marzolini C, Telenti A, Decosterd LA, et al. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 38.Kuritzkes DR, Lalama CM, Ribaudo HJ, et al. Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J Infect Dis. 2008;197:867–870. doi: 10.1086/528802. [DOI] [PubMed] [Google Scholar]
- 39.Woodfall B, Vingerhoets J, Peeters M, et al. Impact of NNRTI and NRTI resistance on the response to the regimen of TMC125 plus two NRTIs in Study TMC125-C227 [Abstract PL5 6] Program and abstracts of the 8th International Congress on Drug Therapy in HIV Infection (Glasgow) 2006 November 12–16;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.