Abstract
Crystal structures of lactose permease from Escherichia coli (LacY) exhibit two six-helix bundles with two-fold pseudo-symmetry separated by a large hydrophilic cavity. The cavity is open only on the cytoplasmic side and contains the side chains important for both sugar and H+ binding at the apex in the middle of protein; the periplasmic side is tightly closed. A plethora of biochemical and biophysical data strongly support an alternating access mechanism in which both the sugar- and H+-binding sites are exposed alternatively to either side of the membrane by reciprocal opening and closing of cytoplasmic and periplasmic cavities. Here we describe a unique mutation that results in an increase in sugar efflux. Asp240 (helix VII), which interacts with Lys319 (helix X), also comprises part of a salt-bridge/H-bond network that is critically involved in the mechanism of sugar/H+ symport. The mutant, which contains Glu in place of Asp240, exhibits a marked decrease in active lactose transport and an enhanced rate of downhill lactose/H+ efflux. Transport is increased to normal levels when the sugar concentration is increased 10-fold, consistent with the decrease in sugar affinity observed for this mutant. Taken as a whole, the results suggest that the primary defect induced by the mutation may involve a decrease in affinity for H+.
Keywords: permease, transport, membranes, symporters, mechanism
LacY1, a paradigm for the Major Facilitator Superfamily (MFS), carries out the tightly coupled symport of a galactoside with an H+, utilizing the free energy released from downhill translocation of H+ in the presence of a H+ electrochemical gradient (Δμ̃H+, interior negative and/or alkaline) to drive accumulation of galactopyranosides against a concentration gradient (reviewed in refs 1, 2). Notably, in the absence of Δμ̃H+, LacY catalyzes the converse reaction, utilizing free energy released from downhill translocation of sugar to drive uphill translocation of H+ with the generation of Δμ̃H+, the polarity of which depends upon the direction of the substrate concentration gradient (3–5). LacY also catalyzes exchange or counterflow of sugar without translocation of H+, and these reactions are unaffected by Δμ̃H+. Therefore, it is likely that the primary driving force for the global conformational change is concurrent occupancy of co-substrate binding sites.
X-Ray crystal structures of a conformationally-constrained mutant (6, 7), as well as wild-type LacY (8), reveal two six-helix bundles with two-fold pseudo-symmetry surrounding a large hydrophilic cavity open only to the cytoplasmic side. The side chains important for both sugar and H+ binding are at the apex of the cavity, which is in the middle of protein, and inaccessible from the periplasmic side of LacY (Fig. 1A). The sugar-binding site is located primarily in the N-terminal bundle on helices IV (Glu126), V (Arg144, Cys148 and Trp151) and VIII (Glu269). In contrast, the residues involved in H+ translocation/coupling are in the C-terminal bundle and include helices VII (Tyr236, Asp240), VIII (Glu269), X (Lys319, His322 and Glu325) and IX (Arg302). These side chains do not represent a pathway through the membrane for either sugar or H+, but form two distinct binding sites located at the approximate middle of the LacY molecule across the cavity near the apex (2).
Fig. 1.
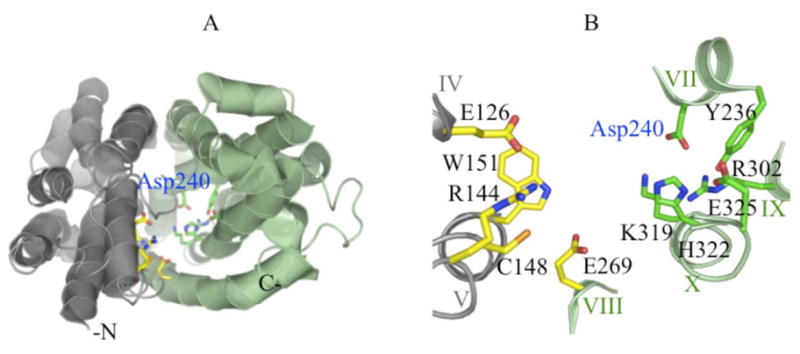
Crystal structure of LacY viewed from the cytoplasm. A) A cartoon presentation of the crystal structure of wild-type LacY (PDB ID 2v8n) in an inward-facing conformation. B) Co-substrate binding sites. The N- or C-terminal six helices are colored as gray or green, respectively. The side chains important in the sugar binding or H+ translocation are shown as yellow or green sticks, respectively.
A mechanism has been proposed for LacY in which both sugar- and H+- binding sites become alternatively accessible to either side of the membrane as a result of reciprocal opening and closing of periplasmic and cytoplasmic cavities (the alternating access model) (2, 6, 8). Several independent lines of evidence, which include site-directed alkylation (9–13), single-molecule Förster resonance energy transfer (14), double electron-electron resonance (15) and site-directed thiol crosslinking (16) indicate that sugar binding or to a lesser extent Δμ̃H+ (interior negative) (10) increases the open probability of a hydrophilic cavity on the periplasmic side of LacY. Moreover, site-directed thiol crosslinking (16) also shows that the periplasmic cavity must close, as well as open, for transport to occur. Although the physiological role of LacY is to take up sugar from periplasmic space, the probability of opening the periplasmic cavity is very low, and the great majority of LacY molecules in the membrane appear to be in an inward-facing conformation, as observed in the crystal structures (6–8).
Mutational analysis indicates that Asp 240 (helix VII) and Lys 319 (helix X) are salt-bridged (17, 18). Thus, single neutral replacement of Asp240 or Lys319 with neutral side chains leads to inactivation of transport, while LacY mutants with double neutral replacement of both side-chains exhibit decreased, but significant transport activity. In addition, the polarity of the interaction is critical, as mutant D240K/K319D is inactive, unlike the Asp237 (helix VII)-Lys358 (helix XI) pair, which can be interchanged with no loss in activity (17, 19, 20). Although replacement of Lys319 with Arg maintains high transport activity, replacement of Asp240 with Glu abolishes active transport (20).
Crosslinking studies with homobifunctional, thiol crosslinking agents indicate that Asp240 is in close proximity to the Lys319 (21). In the crystal structures (6–8), Asp240 is ~5 Å from Lys319, and both residues participate in a complex salt-bridge/H-bond network that plays a central role in H+ translocation/coupling. Although interaction between Asp240 and Lys319 is clearly important for lactose/H+ symport and it has been shown recently that Asp240 mutants have decreased affinity for sugar (I. Smirnova, V. Kasho, J. Sugihara, J-Y. Choe & HRK, manuscript submitted for publication), the role of Asp240 in the transport mechanism is unknown. In this study, we show that the defect in active transport by the D240E mutant is likely due to a decrease in affinity for sugar, as well as an increase in the rate of lactose/H+ efflux down a concentration gradient. Both defects can be explained by a decrease in affinity for H+.
Experimental Procedures
Materials
[14C]lactose was obtained from Amersham Pharmacia Biotech (Piscataway, NJ). Integrated DNA Technologies synthesized oligodeoxynucleotides. Restriction endonucleases, T4 DNA ligase and Vent polymerase were from New England Biolabs (Beverly, MA). All other materials were reagent grade and obtained from commercial sources.
Mutant construction
Oligonucleotide-mediated, site-directed mutagenesis with pT7-5/WTLacY/CH10 (8) as a template and two-step PCR was used to construct plasmid pT7-5/D240E/CH10, which encodes LacY D240E with a 10-His tag at the C-terminus. DNA sequencing of the entire lacY gene confirmed the sequence of the construct.
Growth of cells
E coli T184 (ΔlacZY) or HB101 (lacZ+Y−) containing a given plasmid was grown in Luria-Bertani broth with 100 mg/L of ampicillin. Overnight cultures were diluted 10-fold and allowed to grow for 2 h at 37 °C before induction with 1 mM isopropyl 1-thio-β-D-galactopyranoside. After additional growth for 2–3 h at 37 °C, cells were harvested by centrifugation.
Preparation of right-side-out (RSO) membrane vesicles
RSO membrane vesicles were prepared from E. coli T184 (ΔlacZY) by osmotic lysis as described (22, 23), suspended in 100 mM potassium phosphate KPi (pH 7.2)/10 mM MgSO4 at a protein concentration of about 12–20 mg/ml, frozen in liquid N2, and stored at −80 °C until use.
Transport assays
E. coli T184 (ΔlacZY) containing given LacY were washed with 100 mM KPi (pH 7.2)/10 mM MgSO4 and adjusted to an OD420 of 10.0 (0.7 mg protein/ml). Transport was carried out with [14C]lactose (10 mCi/mmol) at a final concentration of 0.4 mM, unless described otherwise. Lactose transport in RSO membrane vesicles was assayed at a protein concentration of 3.5 mg/ml in the presence of 20 mM ascorbate/0.2 mM phenazine methosulfate under oxygen with 0.4 mM or 4.0 mM [14C]lactose at a specific activity of 10 or 2.5 mCi/mmol, respectively (24).
Efflux and exchange
RSO membrane vesicles containing a given LacY at a protein concentration of ca. 30 mg/ml in 100 mM KPi (pH 7.5) were pre-equilibrated overnight on ice with 10 mM [14C]lactose (10 mCi/mmol) in the presence of 55 μM valinomycin and 1 μM nigericin. Aliquots (2-μl) were diluted 200-fold into the same solution in the absence (efflux) or presence (exchange) of 10 mM unlabeled lactose, and reactions were terminated by dilution and rapid filtration at given times, as described (3).
Δμ̃H+ effect on efflux
RSO membrane vesicles containing D240E LacY at a protein concentration of ca. 29 mg/ml were washed with 100 mM KPi (pH 7.5), pre-equilibrated with 10 mM [14C]lactose (10 mCi/mmol) in the presence of 55 μM valinomycin and diluted 200-fold into 100 mM NaPi at pH 5.5. At given times, reactions were terminated by dilution and rapid filtration (3).
Results
Crystal structures of LacY (6–8) exhibit a complicated salt-bridge/H-bond network in the C-terminal helix bundle composed of residues Tyr236 and Asp240 (helix VII), Lys319, His322 and Glu325 (helix X), and Arg302 (helix XI) (Fig. 1B). These residues are involved primarily in coupling and/or H+ translocation (25), but they also participate in the affinity of the sugar-binding site (I. Smirnova, V. Kasho, J. Sugihara, J.-Y. Choe & HRK, manuscript submitted for publication).
Growth on indicator plates
E. coli HB101 (lacZ+Y−) expressing D240E LacY grows as red colonies on McConkey indicator plates indistinguishable from cells expressing wild-type LacY (data not shown). In contrast, cells transformed with empty vector grow as white or light pink colonies. Thus, cells expressing wild-type or D240E LacY are able to catalyze downhill lactose transport and metabolism of the incoming lactose in a similar manner.
Active transport
The rate of Δμ̃H+-driven uphill lactose accumulation by E. coli T184 (lacZ−Y−), a lacZY deletion strain, transformed with a plasmid encoding wild-type LacY is linear for at least 15 s (Fig. 2A), and reaches a steady state in ~10 min (Fig. 2C). E. coli T184 expressing the D240E mutant transports at about a third of the rate (Fig. 2A), but accumulates lactose very poorly, almost as poorly as cells transformed with empty vector (Fig. 2C). The initial rate of downhill lactose influx was also assayed in E. coli HB101 (lacZ+Y−) containing β-galactosidase, which hydrolyzes lactose to glucose and galactose as soon as it enters the cell (Fig. 2B). Correcting for transport by cells transformed with empty vector, uphill and downhill initial rates of transport are similar for cells expressing wild type or mutant D240E (compare Fig. 2A and B).
Fig. 2.
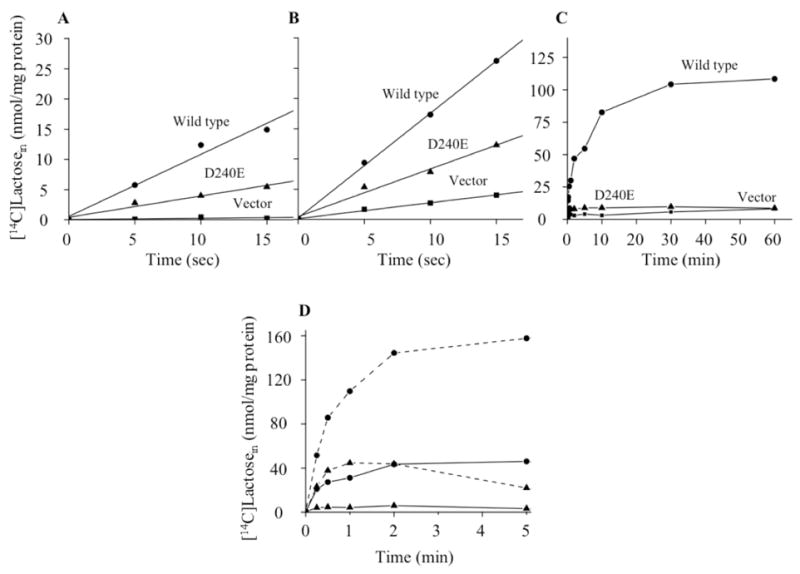
Lactose active transport. A & B). Initial rates of lactose transport for E. coli T184 (ΔlacZY) or HB101 (lacZ+Y−) intact cells, respectively. C). Time course of lactose accumulation for E. coli T184 intact cells. D). Active transport by RSO membrane vesicles prepared from E. coli T184 expressing wild-type or D240E LacY. E. coli T184 or HB101 intact cells containing wild type(λ), mutant D240E (σ) or empty vector (ν) were adjusted to an OD420 of 10.0 (0.7 mg protein/ml). Transport was carried out with [14C]lactose (10 mCi/mmol) at a final concentration of 0.4 mM (solid line). The intracellular lactose concentration is plotted as a function of time. Lactose transport in RSO membrane vesicles in Panel D was assayed at a protein concentration of 3.5 mg/ml in the presence of 20 mM ascorbate/0.2 mM phenazine methosulfate under oxygen with [14C]lactose at a final concentration of 0.4 mM (10 mCi/mmol; solid line) or 4.0 mM (2.5 mCi/mmol; dashed line) as described in Experimental Procedures. The intravesicular lactose concentration after correction for RSO vesicles transformed with empty vector is plotted as a function of time.
RSO vesicles containing D240E LacY also exhibit poor transport activity relative to vesicles with wild-type LacY when incubated with a relatively low lactose concentration under conditions that generate Δμ̃H+ (interior negative) (Fig. 2D). However, when the sugar concentration is increased 10-fold, the mutant transports at a rate and to a steady-state level of accumulation similar to RSO vesicles containing the wild type at the lower lactose concentration (Fig. 2D). The finding is consistent with the observation that the affinity of mutant D240E for β-D-galactopyranosyl-1-thio-β-D-galactopyranoside is ~one-tenth of that obtained for wild-type LacY (I. Smirnova, V. Kasho, J. Sugihara, J.-Y. Choe & HRK, manuscript submitted for publication).
Efflux and equilibrium exchange
Concentrated RSO vesicles were pre-equilibrated with 10 mM [14C]lactose in the presence of valinomycin and nigericin as described in the Experimental Procedures. Then efflux or equilibrium exchange was carried out by rapid 200-fold dilution into buffer without (efflux) or with 10 mM unlabeled lactose (equilibrium exchange), respectively (3). The rate of efflux for vesicles with wild-type LacY is first order, with a t1/2 of ~11 s (Fig. 3A). Efflux with RSO vesicles containing D240E LacY is faster than vesicles containing the wild type, with a t1/2 of ~4 s (Fig. 3A). The reason for the apparent initial rapid loss of lactose is unknown and varies from one experiment to another. However, it is clear that the phenomenon is unrelated to LacY-mediated efflux since it is also observed with vesicles treated with N-ethylmaleimide (Fig. 3A) or with vesicles devoid of LacY (3). The rate of exchange is too fast to measure accurately in vesicles with the wild type (Fig. 3B). However, it is apparent that exchange in vesicles with D240E LacY is significantly reduced, which is consistent with decreased affinity of the D240E mutant for sugar.
Fig. 3.
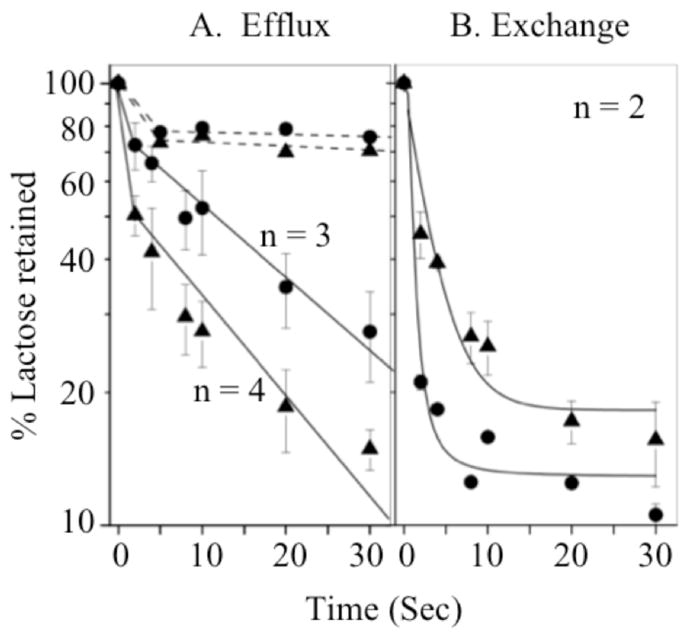
Efflux and equilibrium exchange. RSO membrane vesicles containing wild type (λ) or D240E LacY (σ) in 100 mM KPi (pH 7.5) were pre-equilibrated with 10 mM [14C]lactose (10 mCi/mmol) in the presence of 55 μM valinomycin and 1 μM nigericin and diluted 200-fold into the same buffer in the absence (efflux) or presence (exchange) of 10 mM unlabeled lactose, and reactions were terminated by quenching and rapid filtration at given times, as described. Efflux with N-ethylmaleimide-treated RSO vesicles (10 mM, final concentration for 20 min) is indicated by broken lines. The average percentage of lactose retained is plotted as a function of time.
Sugars were also assayed by nuclear magnetic resonance after incubation of intact E. coli HB101 (lacZ+Y−) with lactose. No lactose is found in the filtrate (extracellular solution) with either the wild type or the D240E mutant after a 1-h incubation; however galactose is detected (Fig. 4). Moreover, a much higher concentration of galactose is found in the filtrate from D240E LacY than from the wild type. Thus, external lactose is transported into E. coli HB 101 (lacZ+Y−) expressing wild type LacY where it is hydrolyzed into galactose and glucose, and the galactose effluxes from the cells via LacY. With cells containing the D240E mutant, considerably more galactose is found in the external medium, which is consistent with the argument that efflux occurs more rapidly.
Fig. 4.
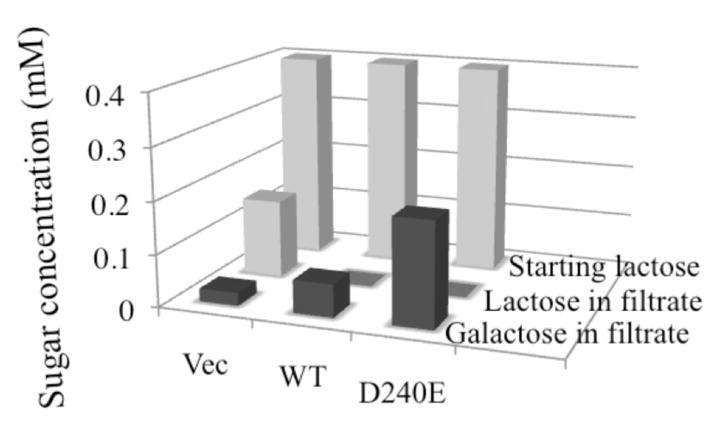
Galactose efflux by intact E. coli HB101. Intact E. coli 101 (lacZ+Y−) expressing wild type, mutant D240E or empty vector were adjusted to an OD420 of 10.0 (0.7 mg protein/ml). Cells were incubated with 0.4 mM lactose (light grey column) for 1 h. After filtration, the filtrates were assayed for galactose and lactose (light gray column) by nuclear magnetic resonance.
Previous studies with RSO vesicles (4) and proteoliposomes (5) demonstrate that the rate of lactose efflux in the wild-type LacY is inhibited by imposition of a pH gradient (ΔpH; interior alkaline) or a membrane potential (Δψ; interior negative), indicating that the lactose efflux down a concentration gradient is coupled with efflux of H+. In order to generate a Δμ̃H+ (interior negative and alkaline), RSO vesicles with D240E LacY prepared in KPi (pH 7.5) were pre-equilibrated with 10 mM [14C]lactose in the presence of valinomycin and rapidly diluted into NaPi (pH 5.5). A Δμ̃H+ generated in this manner also decreases the rate of efflux, increasing t1/2 in vesicles with wild-type LacY from ~13 to >30 s and in vesicles with the D240E mutant from ~5 s to ~13 s (Fig. 5). Therefore efflux of sugar is coupled with release of H+ in the D240E mutant, as with wild-type LacY.
Fig. 5.
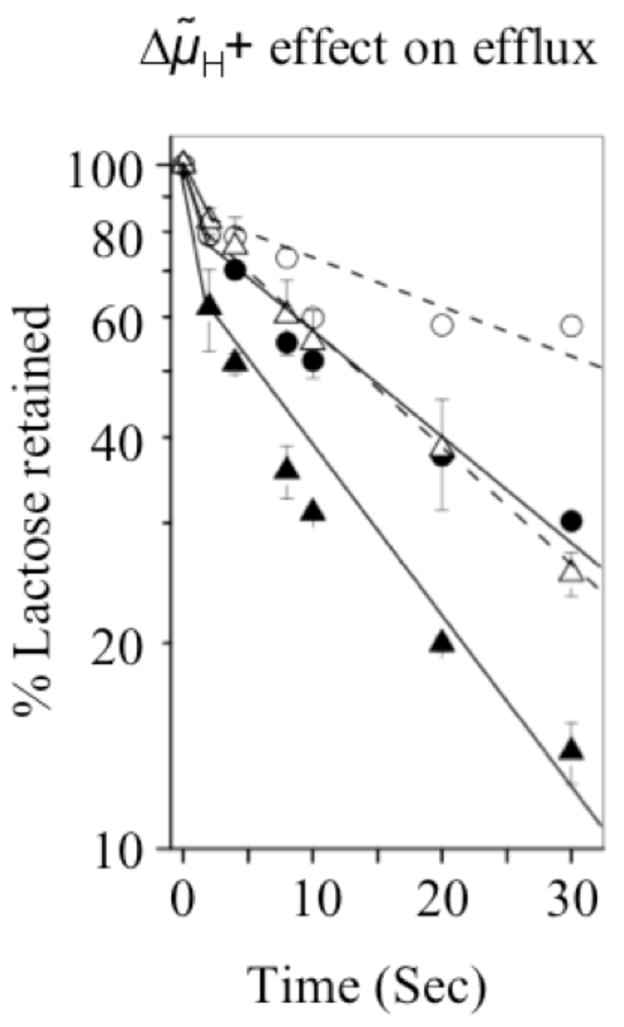
Effect Δμ̃H+ (interior negative and alkaline) on the lactose efflux. RSO membrane vesicles in 100 mM KPi (pH 7.5) were pre-equilibrated with 10 mM [14C]lactose (10 mCi/mmol) in the presence of 55 μM valinomycin and diluted 200-fold into 100 mM KPi at pH 7.5 [wild type LacY (λ, n = 1) or D240E mutant (σ, n = 2)] or 100 mM NaPi at pH 5.5 [wild type LacY (μ, n = 1) or D240E mutant (Δ, n = 3)]. Reactions were terminated by quenching and rapid filtration at given times. The average percentage of lactose retained is plotted as a function of time.
Discussion
A six-step kinetic model for lactose transport has been proposed for lactose/H+ symport (2–4, 25, 26). Efflux and exchange by wild-type LacY are explained by this simple kinetic scheme (Fig. 6). Thus, H+-coupled efflux consists of a minimum of six steps: [1] binding of H+ and [2] binding of lactose to LacY at the inner surface of the membrane; [3] a conformational change to the outward-facing conformation; [4] release of sugar; [5] release of H+; and [6] a conformational change(s) corresponding to return of unloaded LacY to the inner surface of the membrane. Alternatively, during exchange and counterflow, LacY does not deprotonate and only steps 2, 3 and 4 are involved.
Fig. 6.
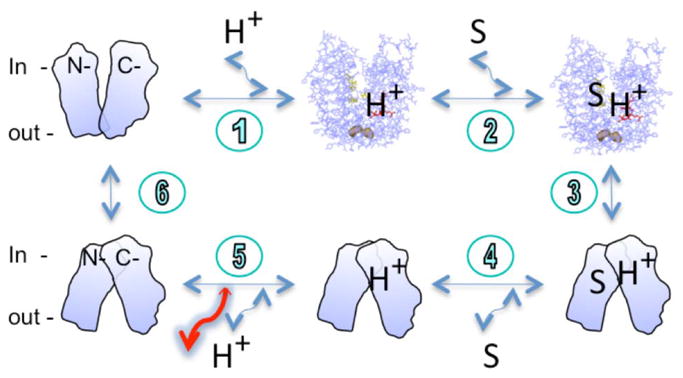
A kinetic model for lactose efflux. Two X-ray crystal structures without (PDB 2v8n) or with bound sugar (PDB 1pv7) represent the inward-facing conformation. The sugar substrate is shown as the capital letter S. Both N- and C-termini are indicated. The orientation of the membrane is marked as in or out. The closest residues, Ile32 (helix I) and Asn245 (helix VII), between two helices bundles are shown in surface model and light brown color. The larger red arrow indicates that step 5 may be enhanced in the D240E mutant.
Recently, a pKa of ~10.5 was determined for sugar binding to purified LacY (27), which supports the contention that at physiological values of pH, LacY is protonated before sugar binds during lactose/H+ symport. But notably, affinity for sugar binding on either side of the membrane is unaffected by Δμ̃H+ (28). However, acidic ambient pH or imposition of Δψ (inside negative), ΔpH (interior alkaline) or Δμ̃H+ (inside negative and alkaline) across the membrane decreases the rate of efflux (3, 4). In addition, deuterium oxide inhibits the rate of downhill efflux or influx at neutral pH, while equilibrium exchange, counterflow and Δμ̃H+-driven active transport are unaffected (29). These data imply strongly that deprotonation (Fig. 6, step 5) is the rate-limiting step for downhill lactose/H+symport and that this step is no longer rate limiting in the presence of Δμ̃H+--i.e., when there is a driving force on the H+ (2, 25). Mutants E325A (26), K319L (30) and R302A (31), which are also part of the salt bridge/H-bond network (Fig. 1B), bind sugar with high affinity and catalyze equilibrium exchange as well as the wild type. However, the mutants are unable to catalyze any mode of lactose translocation involving H+ transduction (i.e., active transport, efflux and influx down a concentration gradient), and they are clearly defective in H+ release (Fig. 6, step 5)
Remarkably, as opposed to mutants E325A, K319L and R302A, the D240E mutant exhibits a faster rate of efflux than the wild type with a reduced rate of equilibrium exchange. Moreover, it has been shown (32) that mutant E240A exhibits enhanced H+ influx in the presence of melibiose. The results are consistent with the conclusion that Asp240 mutations cause a faster release of H+ than the wild type in response to a downhill sugar gradient. Notably, each of these mutants--E325A, R319L, R302A and D240E—is located in the salt bridge/H-bond network, and they apparently perturb the same step in a different manner.
Although the D240E mutant catalyzes lactose transport at an initial rate of ~one third of the wild type, little if any accumulation is observed, which is likely due to the fast rate of lactose efflux. Furthermore, by using a 10-fold higher concentration of lactose, the initial rate of transport, as well as the steady state level of accumulation, approximates the wild type at the lower lactose concentration. Since affinity for sugar is dependent upon the protonation state of LacY, both the decrease in affinity for sugar, as well as a rapid rate of efflux, in the D240E mutant may be explained by a decrease in affinity for H+.
Acknowledgments
The authors thank Roland Reiz for NMR studies, Jon Vinea and Mariae Choi for their assistance and Vladimir Kasho for helpful editing of the manuscript. This work was supported by NIH Grants DK51131, DK069463, GM073210 and GM074929, as well as NSF grant 0450970 to H.R.K.
Footnotes
Abbreviations: LacY, the lactose permease; Δμ̃H+, H+ electrochemical gradient; Δψ, membrane potential; KPi, potassium phosphate; NaPi, sodium phosphate; RSO, right-side-out; NEM, N-ethlymalamide.
References
- 1.Kaback HR. Structure and mechanism of the lactose permease. C R Biol. 2005;328:557–567. doi: 10.1016/j.crvi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Guan L, Kaback HR. Lessons from Lactose Permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaczorowski GJ, Kaback HR. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 1. Effect of pH on efflux, exchange, and counterflow. Biochemistry. 1979;18:3691–3697. doi: 10.1021/bi00584a009. [DOI] [PubMed] [Google Scholar]
- 4.Kaczorowski GJ, Robertson DE, Kaback HR. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 2. Effect of imposed delta psi, delta pH, and delta mu H+ Biochemistry. 1979;18:3697–3704. doi: 10.1021/bi00584a010. [DOI] [PubMed] [Google Scholar]
- 5.Garcia ML, Viitanen P, Foster DL, Kaback HR. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 1. Effect of pH and imposed membrane potential on efflux, exchange, and counterflow. Biochemistry. 1983;22:2524–2531. doi: 10.1021/bi00279a033. [DOI] [PubMed] [Google Scholar]
- 6.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 7.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H(+) symport in LacY. Embo J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci U S A. 2007;104:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ermolova N, Madhvani RV, Kaback HR. Site-directed alkylation of cysteine replacements in the lactose permease of Escherichia coli: helices I, III, VI, and XI. Biochemistry. 2006;45:4182–4189. doi: 10.1021/bi052631h. [DOI] [PubMed] [Google Scholar]
- 10.Nie Y, Ermolova N, Kaback HR. Site-directed Alkylation of LacY: Effect of the Proton Electrochemical Gradient. J Mol Biol. 2007;374:356–364. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie Y, Sabetfard FE, Kaback HR. The Cys154-->Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379:695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie Y, Zhou Y, Kaback HR. Clogging the periplasmic pathway in LacY. Biochemistry. 2009;48:738–743. doi: 10.1021/bi801976r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaback HR, Dunten R, Frillingos S, Venkatesan P, Kwaw I, Zhang W, Ermolova N. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci U S A. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majumdar DS, Smirnova I, Kasho V, Nir E, Kong X, Weiss S, Kaback HR. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci U S A. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smirnova I, Kasho V, Choe JY, Altenbach C, Hubbell WL, Kaback HR. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci U S A. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc Natl Acad Sci U S A. 2008;105:3774–3778. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahin-Toth M, Dunten RL, Gonzalez A, Kaback HR. Functional interactions between putative intramembrane charged residues in the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1992;89:10547–10551. doi: 10.1073/pnas.89.21.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JL, Hwang PP, Hansen C, Wilson TH. Possible salt bridges between transmembrane a-helices of the lactose carrier of Escherichia coli. J Biol Chem. 1992;267:20758–20764. [PubMed] [Google Scholar]
- 19.Dunten RL, Sahin-Tóth M, Kaback HR. Role of the charge pair formed by aspartic acid 237 and lysine 358 in the lactose permease of Escherichia coli. Biochemistry. 1993;32:3139–3145. doi: 10.1021/bi00063a028. [DOI] [PubMed] [Google Scholar]
- 20.Sahin-Tóth M, Kaback HR. Properties of interacting aspartic acid and lysine residues in the lactose permease of Escherichia coli. Biochemistry. 1993;32:10027–10035. doi: 10.1021/bi00089a019. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Guan L, Kaback HR. Helices VII and X in the lactose permease of Escherichia coli: proximity and ligand-induced distance changes. J Mol Biol. 2002;315:53–62. doi: 10.1006/jmbi.2001.5206. [DOI] [PubMed] [Google Scholar]
- 22.Kaback HR. Bacterial Membranes. In: Kaplan NP, Jakoby WB, Colowick NP, editors. Methods in Enzymol. Elsevier: New York; 1971. pp. 99–120. [Google Scholar]
- 23.Short SA, Kaback HR, Kohn LD. D-lactate dehydrogenase binding in Escherichia coli dld- membrane vesicles reconstituted for active transport. Proc Natl Acad Sci USA. 1974;71:1461–1465. doi: 10.1073/pnas.71.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaback HR. Transport in isolated bacterial membrane vesicles. Methods Enzymol. 1974;31:698–709. doi: 10.1016/0076-6879(74)31075-0. [DOI] [PubMed] [Google Scholar]
- 25.Kaback HR, Sahin-Toth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2:610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 26.Carrasco N, Puttner IB, Antes LM, Lee JA, Larigan JD, Lolkema JS, Roepe PD, Kaback HR. Characterization of site-directed mutants in the lac permease of Escherichia coli. 2. Glutamate-325 replacements. Biochemistry. 1989;28:2533–2539. doi: 10.1021/bi00432a028. [DOI] [PubMed] [Google Scholar]
- 27.Smirnova IN, Kasho V, Kaback HR. Protonation and sugar binding to LacY. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0803577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan L, Kaback HR. Binding affinity of lactose permease is not altered by the H+ electrochemical gradient. Proc Natl Acad Sci U S A. 2004;101:12148–12152. doi: 10.1073/pnas.0404936101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viitanen P, Garcia ML, Foster DL, Kaczorowski GJ, Kaback HR. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 2. Deuterium solvent isotope effects. Biochemistry. 1983;22:2531–2536. doi: 10.1021/bi00279a034. [DOI] [PubMed] [Google Scholar]
- 30.Persson B, Roepe PD, Patel L, Lee J, Kaback HR. Site-directed mutagenesis of lysine 319 in the lactose permease of Escherichia coli. Biochemistry. 1992;31:8892–8897. doi: 10.1021/bi00152a028. [DOI] [PubMed] [Google Scholar]
- 31.Sahin-Tóth M, Kaback HR. Arg-302 facilitates deprotonation of Glu-325 in the transport mechanism of the lactose permease from Escherichia coli. Proc Natl Acad Sci U S A. 2001;98:6068–6073. doi: 10.1073/pnas.111139698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JI, Hwang PP, Hansen C, Wilson TH. Possible salt bridges between transmembrane alpha-helices of the lactose carrier of Escherichia coli. J Biol Chem. 1992;267:20758–20764. [PubMed] [Google Scholar]


