Abstract
In mammals, the neuropeptide vasopressin is a key molecule for complex emotional and social behaviours. Two microsatellite polymorphisms, RS1 and RS3, near the promoter of AVPR1A, encoding the receptor subtype most heavily implicated in behaviour regulation, have been linked to autism and behavioural traits. However, the impact of these variants on human brain function is unknown. Here we show that human amygdala function is strongly associated with genetic variation in AVPR1A. Using an imaging genetics approach in a sample of 121 volunteers studied with an emotional face-matching paradigm, we found that differential activation of amygdala is observed in carriers of risk alleles for RS3 and RS1. Alleles in RS1 previously reported to be significantly over- and undertransmitted to autistic probands showed opposing effects on amygdala activation. Furthermore, we show functional difference in human brain between short and long repeat lengths that mirror findings recently obtained in a corresponding variant in voles. Our results indicate a neural mechanism mediating genetic risk for autism through an impact on amygdala signalling and provide a rationale for exploring therapeutic strategies aimed at abnormal amygdala function in this disorder.
Keywords: vasopressin, AVPR1A, fMRI, neuroimaging, autism
Introduction
In non-human mammals, the evolutionarily highly conserved neuropeptide vasopressin is a key modulator of complex emotional and social behaviours, such as attachment,1 social exploration, recognition2 and aggression3 as well as anxiety,4–6 fear conditioning7 and extinction.8 Vasopressin cell and fibre distribution patterns are highly conserved across species;9 vasopressin production occurs in the hypothalamus, the bed nucleus of the stria terminalis and the medial amygdala, which in turn project to the lateral septum and ventral pallidum. Centrally projecting vasopressin systems are sexually dimorphic, with higher levels in men, and a multitude of studies have shown a male-predominant effect of vasopressin for behaviours, such as pair-bonding, pup rearing and aggression. In marked contrast to vasopressin production, the distribution pattern of the major receptor for vasopressin, V1aR, differs considerably between and even within species, and has been linked to social behaviour, for example monogamy.9 In human brain, vasopressin-binding sites were detected in the lateral septum, thalamus, basal amygdaloid nucleus and brainstem,10 but not in cortex.
From the initial description of the disorder by Kanner11 to the currently used diagnostic definition, impairments in social interaction have been regarded as a central feature of autism, suggesting a potential role of prosocial neuropeptides in the pathogenesis and treatment of this common and devastating psychiatric disorder.9,12 Given the high heritability of autism, this implies that genetic variation related to the vasopressin system could contribute to disease risk. Supportive evidence for this assumption comes from genetic studies of the human AVPR1A gene, encoding V1aR, which is located on chromosome 12q and contains two exons that are separated by a 2.2 kb intron. The 5′-flanking region of the gene contains three polymorphic microsatellite repeats.13 Of these, RS3, a complex repeat of (CT)4-TT-(CT)8-(GT)n 3625 bp (base pairs) upstream of the transcription start site, with 16 different alleles in the population, and RS1, a (GATA)n repeat with 9 alleles located 553 bp from the start site, have shown nominally significant transmission disequilibrium in autism.14–16 Specifically, Kim et al.16 found overtransmission of the 334 and 340 bp alleles of RS3 to probands with autism, and Wassink et al.15 found undertransmission of the 312 bp allele of RS1, whereas the 320 bp allele was overtransmitted in a subgroup of autistic probands with preserved language. Finally, Yirmiya et al.14 found no association with specific alleles, but reported significant associations between haplotypes formed from RS1, RS3 and an intronic microsatellite and autism. Taken together, these studies provide convergent, if not conclusive, evidence for a contribution of genetic variation in AVPR1A to risk for autism, which is further supported by linkage to the region of the gene15 and social dysfunction reminiscent of autism found in avpr1a knockout mice.17
In addition to these data on autism, the 5′ microsatellite variants of V1aR encoding genes also open up a fascinating transspecies perspective on the genetics of social behaviour. Hammock and Young18 recently demonstrated that long and short repeat length of a 5′ microsatellite variant that is highly expanded in monogamous, but not promiscuous vole species, predicted individual social behaviour, as well as V1aR distribution, in prairie voles. They showed that long and short variants differed in avpr1a mRNA expression in transfected cells. These authors also noted that highly homologous microsatellite variants at this locus are found in humans and bonobos (pygmy chimpanzees), both known for high levels of social reciprocity, empathy and sociosexual bonding, whereas a 360 bp deletion in the 5′ region of AVPR1A is found in the common chimpanzee, which displays considerably lower levels of social attachment among adults, providing evidence for homologous genetic differences in related highly social and asocial species from different parts of the mammalian lineage. Importantly, a recent study showed that a similar cellular mechanism might operate in humans by demonstrating differences in mRNA expression in human hippocampus between long and short alleles of RS3.19 A further link to social behaviour in general was provided by the same group that found association of RS1–RS3 haplotypes with a personality trait, reward dependence20 and an impact of long and short alleles of RS3 on altruistic behaviour during an economic game, the dictator game.19 Finally, Prichard et al.21 found an association of AVPR1A polymorphisms with another phenotype related to social behaviour, age of first intercourse.
The neural circuits mediating these genetic associations are unknown. However, it is clear that complex social behaviours and mechanistically better-understood functions such as anxiety, social recognition and fear extinction mediated by vasopressin critically depend on the function of the amygdala. The lateral nucleus of the amygdala receives and integrates sensory and prefrontal/limbic inputs and then excites, possibly indirectly, neurons in the central nucleus that evoke fear responses via their projections to brain stem regions, including periaqueductal grey and reticular formation.22 In humans, vasopressin binds to amygdala,10 fear stimuli potently activate amygdala,23 lesions of the amygdala impair recognition of fearful faces and lead to social disinhibition.24 Using functional imaging, abnormal amygdala function has been found in autism,25 decreased amygdala activation has been linked to genetic hypersociability26 and increased aggression, whereas increased activation is observed in social avoidance and phobia.27 We have previously found an impact of the prosocial neuropeptide oxytocin on amygdala activation and connectivity.28
On the basis of these data, we hypothesized that genetic variation linked to autism would modulate amygdala activation. We used an imaging genetics approach in a large sample of healthy control subjects genotyped for the RS1 and RS3 variants of AVPR1A. To probe circuits of emotional arousal, we employed affectively salient social stimuli (angry and fearful faces) previously shown to reliably activate amygdala and be sensitive to neuropeptide effects28 and genetic variation.26,29–31
Materials and methods
Subjects
Subjects were culled from a larger population after careful screening32 to ensure they were free of any lifetime history of psychiatric or neurological illness, psychiatric treatment or drug or alcohol abuse. Previous results from this ongoing study have been reported in subjects that partially overlap with the groups reported here.31 Only Caucasians of European ancestry were studied to avoid stratification artefacts. Although stratification has been shown in Caucasians, previous examinations of these subjects using ancestral marker panels had not uncovered evidence for occult stratification.33 A total of 258 subjects were genotyped. Of these personality variables were evaluated by the Triphasic Personality Questionnaire (TPQ),34 with data available for 228 genotyped subjects. Subject demographics are shown in Table 1. Of this sample, all available scans of subjects meeting the clinical and quality inclusion criteria were used, resulting in an imaging subsample of 121 subjects. Subjects gave written informed consent and participated in the study according to the guidelines of the National Institute of Mental Health Institutional Review Board.
Table 1.
Subject demographics
| Age | 31.5 ± 9.6 |
| Male:female | 109:149 |
| SLC6A4 genotype (n = 201) | S carriers 135 (67.2%), l homozygotes 66 (32.8%) |
| MAO-A 30 bp variable number of tandem repeat (n = 248) | MAO-A-L 126 (50.8%), MAO-A-H 122 (49.2%) |
| TPQ novelty seeking (n = 228) | 15.1 ± 4.5 |
| TPQ harm avoidance (n = 226) | 10.2 ± 5.1 |
| TPQ reward dependence (n = 228) | 19.5 ± 4.1 |
| Performance (n = 121, % correct) | 97.0 ± 7.26 |
| Reaction time (n = 121, sec) | 1.3 ± 0.23 |
Abbreviation: TPQ, triphasic personality questionnaire. Mean ± s.d. shown.
DNA collection and statistical analysis
We used standard methods to extract DNA from white blood cells with the Puregene DNA purification kit (Gentra Systems). We measured linkage disequilibrium between markers as indexed by the D′ and R2 statistics by use of the program LDMAX within the GOLD software package.35 Association with performance and personality scores were assessed using linear regression models, controlling for sex and age.
Microsatellite genotyping
DNA was isolated from whole blood or lymphoblastoid cell lines using standard procedures (Puregene, Gentra Systems, Minneapolis, MN, USA). PCR reaction mixes consisted of 50 ng of research subject DNA, 9 μl True Allele Mix (Applied Biosystems, Foster City, CA, USA) and 1 μl of a 10 uM solution of each of forward and reverse PCR primers (delineated below) in 16 μl reactions. These were amplified in an ABI 9700 thermocycler set for an initial denaturation of 10 min. At 95 °C, 16 cycles of touchdown PCR that included denaturations for 30 s at 95 °C, touchdown annealing in half-degree steps, for 45 s between 63 and 55 °C and an elongation step of 55 s at 72 °C. The thermal ramp rate was an ABI 9600 equivalent. This was followed by 25 cycles of conventional PCR consisting of 30 s at 94 °C, 30 s at 55 °C and 30 s at 72 °C. A final hold was set at 5 min for 72 °C. Samples were then routinely cooled to 4 °C overnight. RS1 microsatellite samples were diluted 1:10 in neat formamide, whereas RS3 microsatellite samples were diluted 1:7, and loaded onto an ABI 3730 × l capillary sequencer for sizing. Sizing was performed using GeneScan 550 standards (Applied Biosystems) and using GeneMarker Software (Softgenetics, State College, PA, USA).
| Microsat | 5′-labelled primer | Reverse primer |
| RS1 | VIC-AGGGA | GTTTCTTACC |
| CTGGTTCT | TCTCAAGTT | |
| ACAATCTGC | ATGTTGGTGG | |
| RS3 | 6FAM-TCCT | GTTTCTTTTT |
| GTAGAGAT | GGAAGAGAC | |
| GTAAGTGC | TTAGATGG |
DNA sequence verification of microsatellite genotypes
RS3 microsatellite PCR products were subjected to agarose gel electrophoresis and then extracted from the gel using Glassmilk (Bio 101). A TOPO TA cloning kit procedure (Invitrogen, Carlsbad, CA, USA) was then followed as described using the manufacturers instructions. Kanamycin-selected white colonies resulting from X-gal medium were picked, grown separately in Luria-Bertani broth and then used for alkaline minipreps. The DNA was subsequently purified using Sephacryl-500 HR, and subjected to bidirectional, Big Dye cycle sequencing on an ABI 3730 × l using Dye version 1.1. Finally, sequence traces were analysed using Mutation Surveyor software (Softgenetics, State College, PA, USA) and binned in 2 bp increments. Sequences were manually compared with size determinations made by microsatellite. This sequencing procedure allowed unambiguous ascertainment of allele size for more than 95% of the samples.
Testing for occult stratification
To examine a potential confound with other genetic variants previously shown to affect amygdala function,30,31 we examined the distribution of the SLC6A4 variant of the serotonin transporter gene and a common 30 bp variable number of tandem repeats polymorphism of the MAO-A gene that impacts transcriptional efficiency.36 Overall genotype frequencies are reported in Table 1. Genotyping was performed as previously described.30,31 Following common practice and our own previous work,30,31 genotypes were grouped for analysis as follows. For SLC6A4, short allele carriers (S homozygotes and heterozygotes) were compared to homozygotes for the L allele.31 For MAO-A, men are hemizygous carriers of either one MAOA-L (2, 3 or 5 repeats) or MAOA-H (3.5 or 4 repeats) allele, whereas women carry two alleles. Therefore, only women can be heterozygote. The female MAOA-L group included all carriers of three or five repeats, so that two genotype groups were analysed for each sex.30 Stratification was assessed by a general linear model with RS1 and RS3 alleles as independent factors, SLC6A4, MAO-H/L genotype, age and sex as dependent variables, with case-wise exclusion of missing data.
Functional imaging task
The face-matching task is a simple perceptual task previously described to robustly engage the amygdala.29 During two blocks of an emotion task, subjects viewed a trio of faces, selecting one of the two faces (bottom) that was identical to the target face (top). Per block, six images were presented sequentially for 5 s, three of each sex and target affect (angry or afraid) derived from a standard set of pictures of facial affect. Emotion tasks alternated with three blocks of a sensorimotor control task where faces were replaced with simple geometric shapes.
Functional image processing
Blood oxygen level-dependent functional magnetic resonance imaging (fMRI) was performed on a GE Signa 3T (Milwaukee, WI, USA) using gradient echo EPI (24 axial slices, 4 mm thickness, 1 mm gap, TR/TE = 2000/28 ms, field of view = 24 cm, matrix = 64 × 64). Images were processed as previously described29–31 using SPM99 (http://www.fil.ion.ucl.ac. uk/spm). Briefly, images were realigned to the first image of the scan run, spatially normalized into a standard stereotactic space (MNI template) using affine and nonlinear (4 × 5 × 4 basis functions) transformations, smoothed with a 8 mm FWHM Gaussian filter and ratio normalized to the whole-brain global mean. A statistical image for the contrast of the emotion task versus the sensorimotor control was then obtained for each subject and analysed in a second-level random effects model (analysis of variance and one-tailed t-test) to identify significant activations within- and between-genotype groups. Genotype effects were formulated as an allele regression model as previously described,37 modelling each allele as a separate regressor. To ensure that we had an adequate number of scans per allele, only alleles with frequencies > 4% in our sample were included, which meant that the 334 bp allele for RS3 and the 312 and 320 bp alleles for RS1, but not the rare 340 bp allele for RS3, could be tested. In addition, based on the findings by Hammock and Young,18 analyses comparing long and short forms of the RS1 and RS3 repeat were run by encoding variants with a combined length (both alleles) of more than 1.5 s.d. above the mean as + 1 and variants less than 1.5 s.d. below the mean as −1, and using multiple regression in the framework of the general linear model. For all analyses, age and sex were included as potentially confounding covariates. Separate post hoc analyses were performed to investigate interactions with sex using the general linear model with sex by genotype interaction covariates.
Statistical inference
Effects on activation of alleles previously associated with autism were tested through appropriate contrasts in a second-level imaging analysis (that is a general linear model analysis in which one scan per condition (‘first level’) is entered for each subject, therefore correctly accounting for between- and within-subject variance). For all imaging data, the significance threshold was set to P < 0.05, corrected for multiple comparisons on the voxel level in a bilateral amygdala region of interest defined by using the WFU pickatlas (http://www.fmri.wfubmc.edu) as previously described.31 We have recently provided empirical evidence that this statistical procedure provides good protection against false positives.38 We did not perform additional corrections for the number of tested alleles, as we only examined a priori hypothesized alleles based on association with autism, as well as on our own data showing association with personality scores, where a global F test was used for multiple comparison correction.
For figures, uncorrected (P < 0.05) functional data were overlaid on a representative MRI using the software package MRIcro.39
Results
Frequencies of the observed alleles of RS1 and RS3 are shown in Table 2. These were comparable to previously published findings in Caucasians.15,16 There was significant linkage disequilibrium between the loci (P < 0.0001 using a permutation test with 10 000 iterations). No significant differences were observed across AVPR1A RS1 and RS3 genotype groups with regard to age (F(29,115) = 0.93, P > 0.57), sex (F(29,115) = 1.1, P > 0.33), SLC6A4 (F(29,115) = 0.75, P > 0.81) or MAO-A genotypes (F(29,115) = 0.93, P > 0.57).
Table 2.
Frequencies for RS1 and RS3 genotype
| RS1 bp | % Carriers | RS3 bp | % Carriers |
|---|---|---|---|
| 308 | 1.5 | 318 | 1.3 |
| 312 | 15.1 | 328 | 6.1 |
| 316 | 36.6 | 330 | 9.3 |
| 320 | 21.3 | 332 | 22.2 |
| 324 | 8.6 | 334 | 21.3 |
| 328 | 12.5 | 336 | 12.0 |
| 332 | 0.9 | 338 | 10.2 |
| 336 | 2.6 | 340 | 1.7 |
| 340 | 0.7 | 342 | 2.8 |
| 344 | 8.3 | ||
| 346 | 3.5 | ||
| 352 | 0.4 |
Sample of 258 subjects. 5 alleles were measured in only one copy in one subject each and not analysed further (RS1: 304 bp, RS3: 326, 348, 350 and 354 bp).
Using linear regression, there was no association of the RS1 or RS3 AVPR1A microsatellite variants with behavioural parameters (correct responses and reaction time) of the fMRI task. In the domain of personality, measured with the Triphasic Personality Questionnaire, we observed significant (F(9,178) = 2.1, P < 0.04) association of the RS1 microsatellite variant with increased novelty seeking and significant (F(9,178) = 2.2, P < 0.03) association with decreased harm avoidance. After these global F tests showed overall significance across all alleles, testing of individual alleles showed that in both of these personality dimensions, the one allele showing association, taking sex and age into account, was bp320 (P < 0.02, positive association with novelty seeking; P = 0.056, negative association with harm avoidance). No effect was observed for reward dependence, or for the RS3 variant.
In neuroimaging, confirming previous results,29 the employed face-matching task paradigm showed strong activation of the amygdala when the task condition (angry and fearful faces) was compared to the control condition (data not shown). For all imaging results, coordinates and statistical information is summarized in Table 3. The 334 bp risk allele of RS3 (present in 21.3% of subjects) showed differential overactivation of the left and right amygdala (significant on the left) (Figure 1). The general linear model (GLM) ‘effects of haplotypes’ plot showed that 334 bp had the highest overall activation of all alleles. For RS1, when compared against all other alleles, no significant effect was observed for the 312 bp allele (present in 15.1% of subjects). However, in a direct comparison of the 312 bp allele, reported to be undertransmitted to autistic probands, and the 320 bp allele (present in 21.3% of subjects), which showed association with personality in our data and was overtransmitted in a subgroup of language-normal subjects with autism, a differential effect (P = 0.057, corrected for multiple comparisons across voxels) was observed in left amygdala activation (activity for 320 bp < 312 bp, Figure 1). The 320 bp allele, relative to all other alleles, also showed significantly less activity in left amygdala (P < 0.04, corrected; figure not shown).
Table 3.
Significant differences in amygdala activation by genotype
| Area | Talairach coordinates | T-value |
|---|---|---|
| RS3 334 bp > all other alleles | ||
| Left amygdala | −22, −4, −25 | 2.97 |
| RS1 312 bp > bp 320 | ||
| Left amygdala* | − 30, 0, −20 | 2.78 |
| RS1 all other alleles > 320 bp | ||
| Left amygdala | − 30, 0, −20 | 2.99 |
| RS1 short > long alleles | ||
| Left amygdala | − 26, 4, −20 | 2.90 |
| RS3 long > short alleles | ||
| Left amygdala | −22, −8, −15 | 3.03 |
All reported voxels are significant at P < 0.05 corrected for multiple comparisons within amygdala ROI except for *P = 0.057, corrected. Coordinates are in mm relative to the anterior commissure in the space defined by the Montreal Neurological Institute template.
Figure 1.
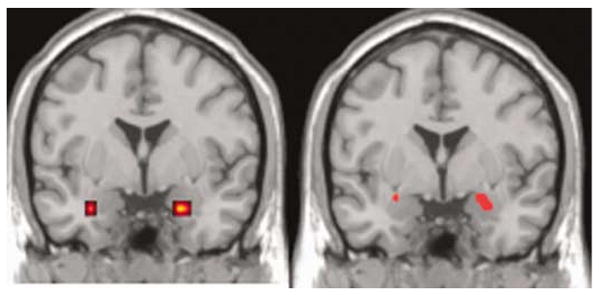
Effect of specific alleles linked to autism on amygdala activation is shown on a representative magnetic resonance imaging (MRI) slice through amygdala (y = 0). Left: significant difference in left amygdala activation in carriers of 334 bp allele of RS3 vs all other alleles. Right: significant difference in directionality in amygdala activation of 312 and 320 bp alleles of RS1. Images thresholded at P < 0.05, uncorrected for multiple comparisons across voxels, no extent threshold, for display. See Table 3 for statistical information of locales significant after multiple comparison correction, which was left amygdala only.
Finally, we compared long and short variants of RS1 and RS3. This showed significant effects on amygdala activation with opposite directionality (Figure 2). For RS3, longer variants were associated with significantly stronger activation of amygdala, whereas for RS1, shorter variants showed stronger activity. In post hoc analyses, no interactions with sex were observed for these results. Outside of amygdala, no differential activation was observed on the P < 0.05, corrected for whole-brain level.
Figure 2.

Effect of length variants in RS1 and RS3 on amygdala activation is shown on a representative magnetic resonance imaging (MRI) slice through amygdala (y = −3). Left: carriers of short allele length of RS1 have significantly higher amygdale activation than carriers of long alleles. Right: carriers of long allele lengths of RS3 have significantly higher amygdala activation than carriers of short alleles. Images thresholded at P < 0.05, uncorrected for multiple comparisons across voxels, no extent threshold, for display. See Table 3 for statistical information of locales significant after multiple comparison correction, which was left amygdala only.
Discussion
Using an imaging genetics approach, we have found that alleles for polymorphic microsatellite repeats linked to autism are associated with differential amygdala activation and personality traits in humans. Furthermore, we provide evidence that long and short forms of the RS1 and RS3 microsatellite repeats differ in their impact on amygdala activity, reminiscent of findings in voles.
We found significant genetic association in AVPR1A with human personality traits. While a previous study20 found association with reward dependence, we report association with harm avoidance and novelty seeking that was accounted for by a variant (RS1 320 bp) also associated with autism. The discrepancy between the previous study and our present data may be due to different ethnicity and size of the evaluated samples and should prompt further study. However, it is well established that amygdala signalling is central for the fear response,24 and a link between genetic variation impacting on harm avoidance and amygdala activation and regulatory circuitry has been demonstrated by our group30,31 and others.40 On the basis of our present findings, we therefore propose that the observed genotype-related variation in amygdala activation may mediate, at least in part, the finding of association of AVPR1A genetic variation with personality traits. As in a sample overlapping with the one studied here, we found no evidence for a direct correlation between amygdala activation and harm avoidance scores,41 it is likely that this mediation occurs through a network of brain regions that is centred on amygdala, but also includes prefrontal regulatory regions such as cingulate31 and ventromedial prefrontal cortex.42 Taken together, these findings support the proposal that microsatellites in the cis-regulatory regions of AVPR1A have functional relevance for brain function related to emotional arousal and social behaviour. However, it should be noted that using the approach taken here, a contribution of more distant polymorphisms that contribute through linkage disequilibrium cannot be excluded.
As we show that specific alleles associated with risk for autism impact on amygdala reactivity, and that over- and undertransmitted alleles reported by studies of autism have opposite effects on amygdala reactivity in RS1, our data provided a new line of independent evidence for the relevance of abnormal amygdala signalling in the pathophysiology of autism. Both under- and overactivation25 of the amygdala have indeed been described in subjects with autism studied using fMRI. Recent studies indicate that amygdala hyperreactivity is observed if participants with autism maintain gaze fixation,25 suggesting overactivation of amygdala, and possibly concomitant social avoidance, as a potential mechanism in at least a subgroup of subjects with autism. Furthermore, recent observations of similar findings in siblings of patients with autism suggest that this mechanism may be related to genetic risk.43 In agreement with this interpretation, the strongest genetic finding in the literature, the overtransmission of the 334 bp allele of RS3 was associated with the highest amygdala activation of all variants across our group of participants. It should be emphasized, however, that knowledge of the effects of the studied polymorphisms on gene expression as well as on more downstream aspects of cellular and systems neurophysiology related to AVPR1A is too limited to draw definitive conclusion with regard to directionality of the observed effects. However, the localization of the effect in brain in amygdala is in good agreement with our regional a priori hypothesis, which was based on the central role of amygdala function for social behaviour and fear processing44 and data showing abundant vasopressin-binding sites in amygdala in humans.10 The present results confirm this hypothesis and are consistent with preclinical data on the localization and function of vasopressin9,45 and with the observed link to personality features that have been shown to be neurally mediated, at least in part, by amygdala. Therefore, our findings strengthen the evidence for a functional impact of vasopressin on neural mechanisms for social behaviour and fear. Amygdala activation could also contribute to the observed impact of intranasally administered vasopressin on social signalling in humans.46,47 It should be noted that while we report association of AVPR1A variation with both personality traits and amygdala activity for alleles previously associated with autism, we do not propose that genetic risk for autism associated with these alleles is via an impact on personality. Few data are available on personality in autism,48 and parsing specifics of temperament in this severe neurodevelopmental disorder is just beginning. Rather, we propose that our data show an impact of AVPR1A variation on a centrepiece of limbic circuitry for emotion and fear response, that is the amygdala, which is linked to both personality traits and autism, identifying altered neural processing of fear related visual stimuli as a mechanism underlying both phenotypes.
Although we cannot exclude that neuropeptide effects in humans are physiologically lateralized, we believe that the left-lateralization of imaging findings is a consequence of more robust activation on the left side, which has been observed frequently in visual stimulation block designs,49 subjected to stringent statistical thresholding, as bilateral effects were observed at uncorrected thresholds (Figures 1 and 2). This corresponds to our previous experience with this paradigm both with regard to some genetic variants30 and under neuropeptide stimulation.28
In addition to differences in amygdala activation related to specific alleles, we also found significant differences between long and short repeat lengths. This mirrors findings in prairie voles by Hammock and Young18 and supports these authors' hypothesis that length variation in this locus is related to sociability, providing evidence for a functional role of length variation in AVPR1A gene expression in humans as well. However, our data suggest a more complex situation in humans than in voles: in the animal model, only one polymorphic repeat is found 5′ of the AVPR1A gene, whereas here we show that length variation in both the RS1 and RS3 loci impact on amygdala activation, but with opposing directionality. Although this finding could partially reflect LD between these loci, which was significant in our data, direct studies of gene expression in cellular and tissue models in humans are necessary to define the precise mechanism behind the observed effect on brain activation. The increase in genetic and functional complexity at this locus could also support the proposal by Hammock and Young18 that microsatellite variation at the 5′ locus of avpr1a confers this locus with high levels of evolvability, because they indicate that this has in fact happened in the lineage leading up to homo sapiens. Although these observed genetic and functional similarities across species are intriguing, it is also necessary to remember that human social behaviour is considerably more complex and dependent on multimodal, especially visual, input (as opposed to the primary olfactory modality observed in rodents), suggesting differences in neural systems impacted by vasopressin. For example, while monogamy is mediated by vasopressin in voles, genetic variation in AVPR1A has not been found to impact on fidelity in women.50 However, significant differences in cortical activity were not observed in the present study, in agreement with reports of a lack of vasopressin-binding sites in cortex in humans.10 As our data show an impact of the studied AVPR1A variants on brain activity and personality, our data provide a systems level correlate of the genetic effects of length variation in the promoter region of AVPR1A identified by Knafo et al.19 and mirror the results from the vole variant obtain in transfected cells.18
Given the strong evidence for male-predominant effects of vasopressin in many species,9 data showing differing responses to vasopressin in men and women in some46,47 studies, and the strongly increased risk for autism in men, we were surprised not to see an interaction of sex and AVPR1A genotype in our data. While this finding indicated that AVPR1A in isolation is not a major contributor to sex effects in amygdala signalling, such effects could still emerge through an interaction with cortical sites differentially sensitive to gonadal steroids that regulate amygdala, for example orbitofrontal cortex. In this view, the present findings mirror recent results from our laboratory on a genetic variant in the X-linked gene MAOA mediating a strongly male-predominant behaviour (violence), where abnormal amygdala activation was also found in both sexes.30 It should also be noted that not all AVPR1A genetic associations are sexually dimorphic, as an effect on sexual behaviour has also been recently observed in men and women.21
The use of a low-level baseline (that is comparing emotional faces to a condition judging geometrc shapes, as opposed to one in which faces of neutral emotion are viewed) was chosen in this study as it has been shown to be necessary for reliable and reproducible amygdala activation.51 Future work could now explore differential activation to other socially relevant stimuli, such as neutral or happy faces, to explore effects of neuropeptides and genetic variation in their receptors on social cognition. In addition to the study of amygdalar activation presented here, it will also be informative to investigate the neural circuits of which amygdala is a part; for example, vasopressin and oxytocin are reported to have opposing effects on fear extinction,8 which depends on medial prefrontal–amygdalar interactions52 that were previously shown to relate to harm avoidance using the task employed here.31 Regarding the latter mechanism, the study of interactions with the 5-HTTLPR polymorphism would also be of interest, as it has been found to impact on amygdala–medial prefrontal interactions31) and interactions between AVPR1A and 5-HTTLPR in their association with behavioural phenotypes have been recently observed.20
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, NIMH and NCI. We thank Dr Andrew Singleton for his technical advice resolving the microsatellites.
References
- 1.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 2.Winslow JT, Insel TR. Neuroendocrine basis of social recognition. Curr Opin Neurobiol. 2004;14:248–253. doi: 10.1016/j.conb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- 4.Appenrodt E, Schnabel R, Schwarzberg H. Vasopressin administration modulates anxiety-related behavior in rats. Physiol Behav. 1998;64:543–547. doi: 10.1016/s0031-9384(98)00119-x. [DOI] [PubMed] [Google Scholar]
- 5.Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, et al. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liebsch G, Wotjak CT, Landgraf R, Engelmann M. Septal vasopressin modulates anxiety-related behaviour in rats. Neurosci Lett. 1996;217:101–104. [PubMed] [Google Scholar]
- 7.Stoehr JD, Cramer CP, North WG. Oxytocin and vasopressin hexapeptide fragments have opposing influences on conditioned freezing behavior. Psychoneuroendocrinology. 1992;17:267–271. doi: 10.1016/0306-4530(92)90067-h. [DOI] [PubMed] [Google Scholar]
- 8.Ibragimov R. Influence of neurohypophyseal peptides on the formation of active avoidance conditioned reflex behavior. Neurosci Behav Physiol. 1990;20:189–193. doi: 10.1007/BF01195453. [DOI] [PubMed] [Google Scholar]
- 9.Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- 11.Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–250. Reprint. [PubMed] [Google Scholar]; Acta Paedosychiatr. 1968;35:100–136. [Google Scholar]
- 12.Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 13.Thibonnier M, Graves MK, Wagner MS, Chatelain N, Soubrier F, Corvol P, et al. Study of V(1)-vascular vasopressin receptor gene microsatellite polymorphisms in human essential hypertension. J Mol Cell cardiol. 2000;32:557–564. doi: 10.1006/jmcc.2000.1108. [DOI] [PubMed] [Google Scholar]
- 14.Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, et al. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol Psychiatry. 2006;11:488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- 15.Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, et al. Examination of AVPR1a as an autism susceptibility gene. Mol Psychiatry. 2004;9:968–972. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, et al. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. 2002;7:503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- 17.Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 18.Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science (New York, NY) 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- 19.Knafo A, Israel S, Darvasi A, Bachner-Melman R, Uzefovsky F, Cohen L, et al. Individual differences in allocation of funds in the dictator game associated with length of the arginine vasopressin 1a receptor RS3 promoter region and correlation between RS3 length and hippocampal mRNA. Genes Brain Behav. 2008;7:266–275. doi: 10.1111/j.1601-183X.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- 20.Bachner-Melman R, Dina C, Zohar AH, Constantini N, Lerer E, Hoch S, et al. AVPR1a and SLC6A4 gene polymorphisms are associated with creative dance performance. PLoS Genet. 2005;1:e42. doi: 10.1371/journal.pgen.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prichard ZM, Mackinnon AJ, Jorm AF, Easteal S. AVPR1A and OXTR polymorphisms are associated with sexual and reproductive behavioral phenotypes in humans. Mutation in brief no. 981. Online. Hum Mutat. 2007;28:1150. doi: 10.1002/humu.9510. [DOI] [PubMed] [Google Scholar]
- 22.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- 23.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 25.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- 27.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 28.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer-Lindenberg A, Buckholtz JW, Kolachana B, A RH, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 32.Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF, et al. The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biol Psychiatry. 2001;49:39–46. doi: 10.1016/s0006-3223(00)00997-5. [DOI] [PubMed] [Google Scholar]
- 33.Devlin B, Roeder K, Wasserman L. Genomic control, a new approach to genetic-based association studies. Theor Popul Biol. 2001;60:155–166. doi: 10.1006/tpbi.2001.1542. [DOI] [PubMed] [Google Scholar]
- 34.Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- 35.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin–rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 36.Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- 37.Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, et al. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11:867–877. 797. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- 38.Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. Neuroimage. 2008;40:655–661. doi: 10.1016/j.neuroimage.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 39.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 40.Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 41.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 42.Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, et al. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry. 2008;13:313–324. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- 43.Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry. 2007;61:512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 45.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science (New York, NY) 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 46.Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci USA. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson R, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 48.Anckarsater H, Stahlberg O, Larson T, Hakansson C, Jutblad SB, Niklasson L, et al. The impact of ADHD and autism spectrum disorders on temperament, character, and personality development. Am J Psychiatry. 2006;163:1239–1244. doi: 10.1176/ajp.2006.163.7.1239. [DOI] [PubMed] [Google Scholar]
- 49.Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Cherkas LF, Oelsner EC, Mak YT, Valdes A, Spector TD. Genetic influences on female infidelity and number of sexual partners in humans: a linkage and association study of the role of the vasopressin receptor gene (AVPR1A) Twin Res. 2004;7:649–658. doi: 10.1375/1369052042663922. [DOI] [PubMed] [Google Scholar]
- 51.Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- 52.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]


