Abstract
Natural rubber is synthesized in specialized articulated cells (laticifers) located in the inner liber of Hevea brasiliensis. Upon bark tapping, the laticifer cytoplasm (latex) is expelled due to liber tissue turgor pressure. In mature virgin (untapped) trees, short-term kinetic studies confirmed that ethylene, the rubber yield stimulant used worldwide, increased latex yield, with a concomitant decrease in latex total solid content, probably through water influx in the laticifers. As the mature laticifers are devoid of plasmodesmata, the rapid water exchanges with surrounding liber cells probably occur via the aquaporin pathway. Two full-length aquaporin cDNAs (HbPIP2;1 and HbTIP1;1, for plasma membrane intrinsic protein and tonoplast intrinsic protein, respectively) were cloned and characterized. The higher efficiency of HbPIP2;1 than HbTIP1;1 in increasing plasmalemma water conductance was verified in Xenopus laevis oocytes. HbPIP2;1 was insensitive to HgCl2. In situ hybridization demonstrated that HbPIP2;1 was expressed in all liber tissues in the young stem, including the laticifers. HbPIP2;1 was up-regulated in both liber tissues and laticifers, whereas HbTIP1;1 was down-regulated in liber tissues but up-regulated in laticifers in response to bark Ethrel treatment. Ethylene-induced HbPIP2;1 up-regulation was confirmed by western-blot analysis. The promoter sequences of both genes were cloned and found to harbor, among many others, ethylene-responsive and other chemical-responsive (auxin, copper, and sulfur) elements known to increase latex yield. Increase in latex yield in response to ethylene was emphasized to be linked with water circulation between the laticifers and their surrounding tissues as well as with the probable maintenance of liber tissue turgor, which together favor prolongation of latex flow.
Hevea brasiliensis (rubber tree) is the only commercial source of natural rubber. Rubber (cis-1,4-polyisoprene) is synthesized as rubber particles in highly specialized cells, which, when mature, form concentric mantels of articulated “laticifer” networks in the inner liber of the rubber tree (Hébant and de Faÿ, 1980; Hébant, 1981). Upon bark tapping, the laticifers are severed and their fluid cytoplasm (“latex”) flows out until coagulation processes lead to the plugging of their extremity (d'Auzac, 1989a; Yeang, 2005). Gooding (1952) was the first to show that tapping induced, during the latex flow, progressive dilution of the latex coming from the remote areas to the tapping cut. Thus, tapping the rubber tree induces not only latex flow but also complex water circulation at the bottom of the trunk from the inner liber tissues (Lustinec et al., 1968; d'Auzac, 1989b) and from the xylem through the vascular rays, which have been reported to be numerous in H. brasiliensis (Hébant and de Faÿ, 1980).
The rubber particles account for up to 55% to less than 30% of the collected latex volume depending on the season, the tapping hour, the tree age, the rubber clone, and the exploitation system. The latex flow rate and duration are the first intrinsic factors known to limit rubber yield: the faster and the longer the latex flow, the higher the yield (d'Auzac, 1989a). These two factors are under the control of the turgor pressure in the liber tissues (Gooding, 1952; Buttery and Boatman, 1964), of the latex dry rubber content (DRC) or total solid content (TSC), and finally of the latex coagulation efficiency (Kongsawadworakul and Chrestin, 2003). In addition, DRC is positively linked to latex viscosity and thus inversely linked to latex fluidity and yield (Van Gils, 1951).
Treatment of the rubber tree bark with Ethrel, an ethylene releaser, markedly increases the production of latex (d'Auzac and Ribaillier, 1969) owing to transient partial removing of the yield-limiting factors. In particular, stimulation of rubber tree yield, either as previously with synthetic auxins or as now with Ethrel, has so far been reported to (1) decrease latex DRC, TSC, and osmolarity (Baptist and de Jonge, 1955; Tjasadihardja and Kardjono, 1974; Coupé and Chrestin, 1989), indicating latex dilution; (2) extend the bark drainage area (Boatman, 1966; Lustinec et al., 1967); and (3) decrease the laticifer-plugging index (Yip and Gomez, 1980). Furthermore, it has been reported that the rubber clones with the lowest latex DRC corresponded to those yielding the highest latex volume and dry rubber production but displayed only slight response to stimulation, and inversely (Tjasadihardja and Kardjono, 1974; Lee and Tan, 1979; Gohet et al., 2003). For example, without stimulation, PB217, a rubber clone with a high yield potential, is characterized by relatively high TSC, short latex flow, and low metabolic activity (Obouayeba et al., 1996). However, with stimulation, PB217 fully expresses its yield potential, due to prolonged latex flow and higher metabolic activity in response to ethylene. Therefore, the PB217 rubber clone is a good model to study and understand the effects of ethylene stimulation on latex yield.
The circulation of water between the different liber tissues, as well as the latex water content, are of major importance in the processes of latex flow. Water coming from the phloem and the xylem can use two complementary routes to circulate between and within tissues: (1) symplastic pathways (Varney et al., 1993), where water and solutes can move from cell to cell through the plasmodesmata (Blackman and Overall, 2001); and (2) intercellular spaces, or apoplastic pathways (Canny, 1995). The apoplastic water cannot easily cross biological membranes, but this process can be facilitated by water channels called “aquaporins.” Unlike the other cells that surround them (parenchyma cells, vascular ray cells, sieve tubes companion cells, etc.), mature latex vessels are devoid of plasmodesmata (de Faÿ et al., 1989). Thus, water fluxes across the laticifer plasmalemma are probably mainly controlled by aquaporins.
Aquaporins belong to a ubiquitous large family of major intrinsic proteins (MIPs) known to facilitate water and/or small neutral solute fluxes across cell membranes (Chrispeels and Maurel, 1994; Maurel et al., 2008). Plant aquaporins have been classified in four subfamilies, including the two major ones: PIPs (for plasma membrane intrinsic proteins) and TIPs (for tonoplast intrinsic proteins). The PIP family has been clustered into two major groups as PIP1 and PIP2. PIP2s have been shown to be far more efficient than PIP1 in mediating water transport (Baiges et al., 2002). In addition, although phosphorylation, pH, Ca2+, and osmotic gradients can affect water channel activity (Johansson et al., 1998; Chaumont et al., 2005; Maurel et al., 2008), the MIP gene expression level has been shown to play a major role in controlling the membrane water permeability. Expression of MIP genes is regulated during development and by different environmental factors, such as light (Cochard et al., 2007) and various stresses. They have been reported to be either down-regulated (Aharon et al., 2003; Quist et al., 2004; Alexandersson et al., 2005) or up-regulated (Guerrero et al., 1990) in response to water stress or to freezing-thawing events (Sakr et al., 2003).
As mentioned above, yield stimulation with Ethrel was reported by several authors to induce dilution of latex. To address the possible role of aquaporins in this process, we constructed a full-length cDNA library from Hevea inner bark RNA of control and Ethrel-stimulated trees. Among about 4,000 ESTs sequenced, three aquaporins encoding PIP1, PIP2, and TIP isoforms were found. The full-length HbPIP2;1 and HbTIP1;1 cDNAs were cloned. They were further characterized with respect to the kinetics of ethylene effects on their expression, in relation to latex dilution and production, of mature virgin rubber trees of the PB217 clone. The function of both HbPIP2;1 and HbTIP1;1 proteins was verified, and their respective promoters were analyzed in silico. Aquaporin gene expression and function in response to ethylene treatments are proposed to favor water circulation within the inner bark tissues of rubber trees, thereby helping ease and prolong latex flow, hence increasing rubber yield.
RESULTS
Kinetic Effects of Ethrel Bark Treatment on Latex Yield and TSC
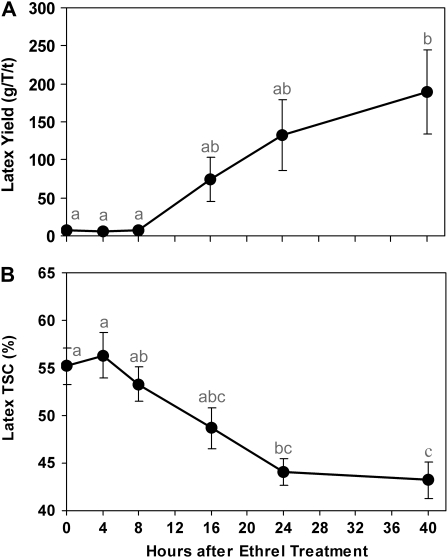
It was shown that the control PB217 mature virgin trees produced very little latex upon bark opening and that ethylene induced a huge increase in latex yield, starting between 8 and 16 h after the treatment (Fig. 1A). This increase in yield was about 10-, 20-, and 27-fold higher than the control at 16, 24, and 40 h after treatment, respectively.
Figure 1.
Kinetic effects of Ethrel on fresh latex yield (A) and latex TSC (B) of mature virgin trees of the PB217 clone. Fresh latex production and TSC were measured at 0, 4, 8, 16, 24, and 40 h after an Ethrel treatment. The error bars represent se. Rather high se values were observed, indicating relatively high differences in the intensity of the response to ethylene between trees. Different letters indicate values that are significantly different (Tukey's test, one-way ANOVA; P < 0.05).
Figure 1B shows that the latex TSC from the control and the early (4 and 8 h) stimulated virgin trees was very high (around 55%) and did not significantly differ. TSC started to decrease significantly at 16 h after the treatment to reach its lowest level (43%) at 40 h. The time course of the decrease in TSC was correlated with the increase in latex yield.
Thus, these results confirmed that ethylene induced a decrease in TSC, which corresponds to latex dilution, probably due to a water influx in the laticifers.
EST Sequences Encoding pHbPIP1;1, pHbPIP2;1, and pHbTIP1;1 Aquaporins
Among about 4,000 ESTs sequenced from our inner bark cDNA library, three aquaporin EST isoform contigs were identified: pHbPIP1;1 (505 bp), pHbPIP2;1 (521 bp), and pHbTIP1;1 (283 bp). The deduced amino acid sequences of pHbPIP1;1, pHbPIP2;1, and pHbTIP1;1 showed 94%, 89.3%, and 91.5% similarity to Gossypium hirsutum (GhPIP1;1), Juglans regia (JrPIP2;1), and Mimosa pudica (MpTIP1), respectively (Fig. 2). The sequences were submitted to the public dbEST database under the accession numbers FJ851076, FJ851077, and FJ851078, respectively.
Figure 2.
Phylogenetic tree of the three rubber aquaporin ESTs with other plant species. The deduced amino acid sequences of three rubber aquaporin ESTs, pHbPIP1;1, pHbPIP2;1, and pHbTIP1;1, were compared with those from G. hirsutum (GhPIP1;1 [ABK60194] and GhTIP [ABR68795]), Medicago truncatula (MtPIP1;1 [AAK66766]), J. regia (JrPIP2;1 [AAO39007] and JrPIP2;2 [AAO39008]), and M. pudica (MpTIP1 [BAD90702]). Multiple sequence alignment was constructed by the VectorNTI Suite 6 software.
Differential Regulation of the pHbPIP1;1, pHbPIP2;1, and pHbTIP1;1 Genes by Ethylene
As the effects of Ethrel on the increase in latex yield and on different parameters in the latex were shown to occur within the first 24 h after the treatment (Coupé et al., 1977; Pujade-Renaud et al., 1994), preliminary studies of the expression of HbPIP1;1, HbPIP2;1, and HbTIP1;1 genes were conducted in the latex and inner bark tissues of the mature virgin trees at 24 h after Ethrel stimulation.
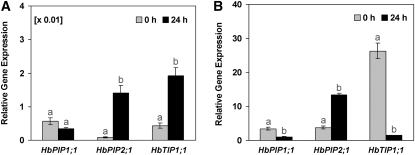
With a 40S ribosomal protein gene (40S) as internal reference, the results showed that these three genes were apparently far less expressed in the latex cells (Fig. 3A) than in the inner bark tissues (Fig. 3B) in control trees. It should be noted that 40S was about 100-fold more expressed in the latex than in the inner bark tissues (Supplemental Fig. S1).
Figure 3.
Relative transcript abundance of pHbPIP1;1, pHbPIP2;1, and pHbTIP1;1 in the latex and inner bark of control or Ethrel-stimulated virgin trees at 24 h after bark treatment. The relative expression corresponds to the ratio of the transcript abundance of each aquaporin gene/transcript abundance of the 40S ribosomal protein endogenous control gene in the latex (A) or the inner bark tissues (B) of the PB217 clone control (0 h) mature virgin rubber trees or trees that had been treated with 2.5% Ethrel 24 h before sampling (24 h). Data are means of three independent experiments and se (n = 9). Different letters indicate values that are significantly different between the transcripts of untreated control and Ethrel-treated cDNA samples (t test; P < 0.05).
While 40S reference gene expression was not modified by ethylene (Supplemental Fig. S1), the three aquaporin isoforms were differentially regulated after bark Ethrel treatments (Fig. 3). HbPIP2;1 was up-regulated in both the inner bark tissues and especially in the laticifers, while HbTIP1;1 was up-regulated in the latex cells but markedly down-regulated in the inner bark tissues. In contrast, HbPIP1;1 was down-regulated in both tissues.
Cloning and Characterization of the Full-Length cDNAs Encoding HbPIP2;1 and HbTIP1;1
Since HbPIP2;1 and HbTIP1;1 were clearly regulated by ethylene, their expression was possibly linked to the increase in latex dilution and yield after Ethrel treatment. Thus, the full-length cDNAs of these two genes were cloned. Both sequences exhibited 100% sequence identity with their corresponding ESTs (data not shown).
The 1,198-bp full-length cDNA HbPIP2;1 clone harbored an open reading frame encoding a 288-amino acid polypeptide with a predicted molecular mass of 30.7 kD. Its deduced amino acid sequence exhibited 91% sequence identity with PIP2 subfamily members from J. regia (JrPIP2;2 and JrPIP2;1; Fig. 4).
Figure 4.
HbPIP2;1 and HbTIP1;1 deduced amino acid sequence alignment with PIP2s and TIPs from other plant species. Alignment of the deduced amino acid sequence of HbPIP2;1 and HbTIP1;1 with the sequences of J. regia (JrPIP2;1 [AAO39007] and JrPIP2;2 [AAO39008]), R. communis (RcTIP1;1 [CAE53881]), and N. tabacum (NtTIP1;1 [BAF95576]). Multiple sequence alignment was constructed by the BioEdit program. Identical amino acids are indicated by asterisks. TM 1 to TM 6, Transmembrane domains of HbPIP2;1 are framed. Two conserved NPA functional motifs are indicated by three dots.
The 1,016-bp-long HbTIP1;1 full-length cDNA clone encoded a putative γ-TIP, exhibiting an open reading frame encoding a 252-amino acid polypeptide with a predicted molecular mass of 25.9 kD. The deduced amino acid sequence of the HbTIP1;1 cDNA shared 91% identity with γ-TIP from Ricinus communis (RcTIP1;1) and 80% with Nicotiana tabacum (NtTIP1;1).
The alignment of their deduced amino acid sequences (Fig. 4) showed that both HbPIP2;1 and HbTIP1;1 had the highly conserved transmembrane domains, separated by loops, especially the functionally important B and E loops harboring the Asn-Pro-Ala (NPA) motif, which has been reported to play a crucial role in selective water conduction (Maurel and Chrispeels, 2001). In addition, HbPIP2;1 exhibited several His residues, especially the one at position 200 in loop D, which are known to confer pH sensitivity to aquaporins (Fischer and Kaldenhoff, 2008). HbPIP2;1 harbored six, whereas HbTIP1;1 showed only one consensus sequence for phosphorylation sites (Ser residues). However, HbPIP2;1 did not contain the Cys residue that confers mercury sensitivity to most aquaporins (Preston et al., 1993).
The full-length cDNA sequences of HbPIP2;1 and HbTIP1;1 were submitted to the GenBank database under accession numbers FJ851079 and FJ851080, respectively.
Functional Analysis of HbPIP2;1- and HbTIP1;1-Encoded Aquaporins in Xenopus Oocytes
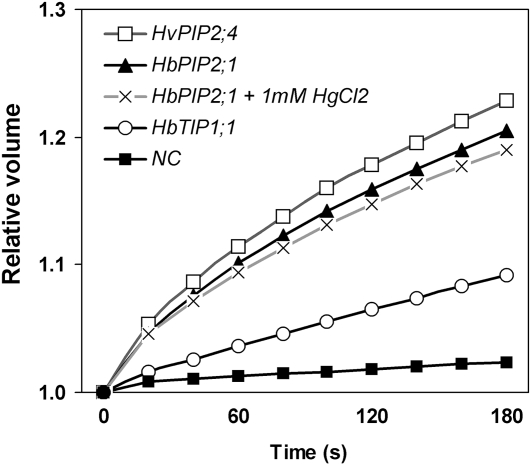
Water transport activity of HbPIP2;1 and HbTIP1;1 was tested in Xenopus laevis oocytes. The change in volume of oocytes when exposed to a hypotonic solution was used to calculate for osmotic water permeability (Pf). The results of the experiments are combined data from at least two individual experiments and are presented in Figure 5. The Pf of oocytes expressing HbTIP1;1 was 5-fold greater than that of the negative control (Table I). The highest water transport activity was observed upon expression of HbPIP2;1, whose oocytes swelled very rapidly, bursting within 3 min, in a similar way to the positive control (the barley [Hordeum vulgare] HvPIP2;4 aquaporin; accession no. AB219525; Fig. 5). The Pf of oocytes expressing HbPIP2;1 showed more than 15-fold higher water permeability than that of the water-injected oocytes (Table I). The water permeability of HbPIP2;1 was not affected by micromolar concentrations (10–100 μm) of HgCl2 (data not shown) and was reduced only by 8% in the presence of 1 mm HgCl2 (Table I), which is already toxic to most cells.
Figure 5.
Kinetics of changes in the cell volume of Xenopus oocytes expressing rubber aquaporin. A negative control (NC) oocyte injected with water, a positive control oocyte injected with HvPIP2;4, and tested rubber aquaporin-expressing oocytes were transferred into a hypotonic solution at time 0 and monitored every 20 s for up to 180 s or until they burst in the case of HbPIP2;1- or HvPIP2;4-expressing oocytes. For testing effects of the aquaporin inhibitor, oocytes were injected with the complementary RNA encoding the rubber HbPIP2;1 aquaporin and incubated for 10 min in the presence of 1 mm HgCl2 before analysis of swelling.
Table I.
Changes in Pf of oocytes injected with water or with HbPIP1;1, HbPIP2;1, and HbTIP1;1 complementary RNA (cRNA) and effect of HgCl2 on HbPIP2;1 activity
Pf was calculated from osmotic swelling data as described in “Materials and Methods.” For mercury inhibition, oocytes were preincubated in the buffer containing 1 mm HgCl2 for 10 min, before transfer to the hypotonic buffer. Data are means ± se (in parentheses, number of replicates, with one oocyte per well) calculated from a combination of at least two independent experiments. Different letters indicate values that are significantly different (Tukey's test, one-way ANOVA; P < 0.01).
| Oocytes | Pf |
|---|---|
| ×10−2 cm s−1 | |
| HbPIP2;1 cRNA injected | 2.16 ± 0.10 c (23) |
| HbPIP2;1 cRNA injected (1 mm mercury treated) | 1.99 ± 0.10 c (15) |
| HbTIP1;1 cRNA injected | 0.79 ± 0.06 b (29) |
| Water injected | 0.14 ± 0.01 a (28) |
| HvPIP2;4 cRNA injected | 2.40 ± 0.10 cd (5) |
These results clearly indicated that HbPIP2;1-encoded protein efficiently functions as a water channel, in an Hg-insensitive manner, and was far more efficient than the aquaporin encoded by the HbTIP1;1 gene, at least in Xenopus oocytes.
Kinetic Effects of Ethrel Bark Treatment on the Expression of the HbPIP1;1, HbPIP2;1, and HbTIP1;1 Genes in the Inner Liber and Laticifers
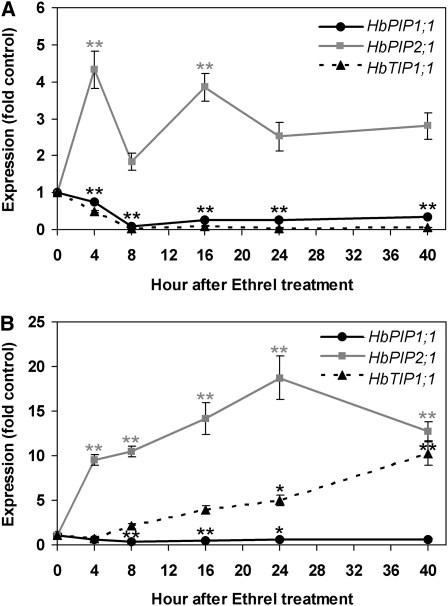
Since HbPIP2;1, HbTIP1;1, and HbPIP1;1 were previously shown to differentially respond to ethylene at 24 h after stimulation (Fig. 3), their tissue-dependent expression patterns were further examined in the same kinetic studies. As shown in Figure 6, the expression ratio of the HbPIP2;1 gene increased significantly as soon as 4 h and up to at least 40 h after treatment in both inner liber cells (Fig. 6A) and latex cells (Fig. 6B). This HbPIP2;1 gene up-regulation by ethylene was 2- to 4-fold and 10- to 17-fold higher than in the control, in the inner liber tissues and in the laticifers, respectively (i.e. about 5-fold more up-regulated in the laticifers than in the liber tissues).
Figure 6.
Kinetic changes in transcript levels of the HbPIP1;1, HbPIP2;1, and HbTIP1;1 genes in the inner liber (A) or latex (B) cells of mature virgin trees (PB217 rubber clone) in response to bark treatment with Ethrel. Batches of three mature virgin trees of the PB217 rubber clone were treated beneath the tapping cut with Ethrel at 4, 8, 16, 24, or 40 h before latex and bark sampling. Untreated trees were used as controls. 40S gene expression was used as an endogenous control. Expression levels are presented as expression ratio (treated to control), which was set at 1 for the control trees. Data correspond to means of three independent replicates and se (n = 9). Asterisks or double asterisks indicate significant differences in fold expression of transcripts between untreated control and Ethrel-treated trees (Tukey's test, one-way ANOVA; P < 0.05 or P < 0.01, respectively).
Conversely, the HbTIP1;1 gene exhibited tissue-specific differential expression in response to ethylene. It was early and markedly down-regulated in the inner liber tissues but progressively up-regulated in the laticifers, reaching about 10-fold higher expression in treated trees than in the controls at 40 h after stimulation.
The HbPIP1;1 gene was significantly down-regulated both in the inner bark and latex samples, reaching less than 20% of its expression level in the control, from 8 h to at least 40 h after the treatment.
Western-Blot Analysis of the HbPIP2;1 Protein in the Inner Bark Tissues
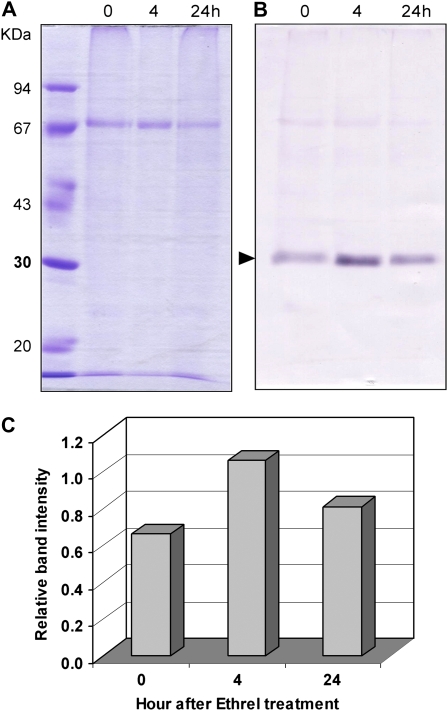
As the HbPIP2;1 gene was shown to be up-regulated by ethylene in both latex and bark tissues, it could be of major importance in controlling the water fluxes between these two tissues in response to ethylene. An antibody, raised against a synthetic peptide designed from its deduced amino acid sequence, was used for western-blot analysis of the microsomal fraction protein extracted from the inner bark of the Ethrel-stimulated (4 and 24 h) and control (Fig. 7B) rubber trees. The intensity of Coomassie Brilliant Blue-stained 67-kD protein (Fig. 7A) and the expected size of the approximately 31-kD immunolabeled band (Fig. 7B) enabled calculation of the 31-kD/67-kD intensity ratio (Fig. 7C) and confirmed that the HbPIP2;1 protein was around 2-fold enhanced as soon as 4 h after the treatment, then slightly decreased at 24 h. This protein accumulation pattern was related to the HbPIP2;1 transcript level pattern in bark tissues, as shown in Figure 6A.
Figure 7.
Western-blot verification and quantification of HbPIP2;1 aquaporin protein overexpression in Hevea inner liber after 4 and 24 h of bark treatment with Ethrel. The microsomal fraction from Hevea inner bark tissues, not treated (0) or treated with Ethrel for 4 and 24 h, was extracted, and 12 μL of each protein extract was separated by one-dimensional SDS-PAGE. One gel was stained for proteins with Coomassie Brilliant Blue (A). The proteins were transferred from another gel onto a polyvinylidene difluoride membrane and submitted to western blotting (B) using an antibody raised against a synthetic peptide fragment specific to the deduced protein sequence of HbPIP2;1. The 67-kD stained protein (A) and the HbPIP2;1 antibody-labeled band (B) at the expected size of about 31 kD (arrowhead) were quantified by scanning, and the relative band intensities (target 31-kD/67-kD protein) were calculated (C). [See online article for color version of this figure.]
Tissue Localization of HbPIP2;1 Gene Expression
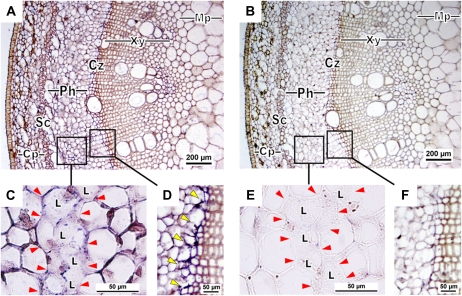
In situ hybridization was also performed to detect and localize the HbPIP2;1 transcripts within the tissues of untreated young rubber stems. As expected, the HbPIP2;1 transcripts were detected with the antisense probes only (Fig. 8, A, C, and D), giving the strongest signal in the phloem tissues (Fig. 8A), especially in the pericambial area (Fig. 8D). The young laticifers, which were identified as partially fused cells with granular contents, also exhibited significant staining (Fig. 8C). Controls performed with the sense probe (Fig. 8, B, E, and F) or without probe (data not shown) did not display significant signal in any tissue. These data suggested that, at least in the young stem, HbPIP2;1 expression was higher in the young phloem and in the maturating laticifers.
Figure 8.
In situ hybridization of HbPIP2;1 transcripts in young rubber stem. Transversal sections were hybridized with HbPIP2;1 antisense (A, C, and D) or sense (B, E, and F) digoxigenin probes. Transcripts of HbPIP2;1 are visualized by the intensity of blue to purple color developed by the reaction with anti-digoxigenin antibodies coupled to alkaline phosphatase using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate as substrates. The different images correspond to increasing magnifications in the same area. Sense probe control was used to indicate unspecific background signaling. Cp, Cortical parenchyma; Cz, cambium zone; L, laticiferous cell; Mp, medullar parenchyma; Ph, phloem; Sc, sclerenchyma; Xy, xylem vessel. The yellow arrowheads indicate the labeled precambial area in D. The red arrowheads indicate the shape of the fusing young latex cells (laticifers) in C and E. The probe concentration was 3 μg mL−1. Bars = 200 μm (A and B) and 50 μm (C–F).
Cloning and in Silico Characterization of the Promoter Regions of HbPIP2;1 and HbTIP1;1
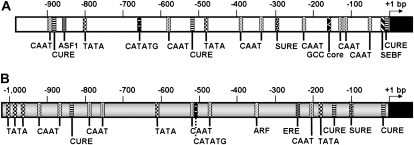
About 1-kb-long polynucleotide sequences upstream from the start codon of both the HbPIP2;1 and HbTIP1;1 genes were cloned (Fig. 9). TATA boxes as well as several CAAT box motifs were found in both promoter sequences, confirming that they contained all minimal elements reported to be necessary for accurate initiation of basal transcription in most plant species.
Figure 9.
Diagram of the promoter sequences of the HbPIP2;1 and HbTIP1;1 genes. The promoter sequences of HbPIP2;1 (A) and HbTIP1;1 (B) were cloned, sequenced, and analyzed for putative cis-acting sequences using the PLACE software. The CAAT and TATA boxes, as well as the ethylene-responsive (ERE), auxin-responsive (ARE), copper-responsive (CuRE), and sulfur-responsive (SuRE) elements, are indicated. The negative numbers represent the upstream ends of the promoter fragments.
Among the known cis-regulatory sequences, unexpectedly none of the most common ethylene-responsive elements (EREs) reported in many plant species was found in the sense orientation of the HbPIP2;1 promoter region. However, one GCC box (Ohta et al., 2000) on the other strand (GGCGGC in the antisense orientation of HbPIP2;1) was located at positions −172 to −167. Conversely, one classical ERE (5′-AWTTCAAA-3′; Rawat et al., 2005) was found at the right position, located −251 to −244 upstream from the HbTIP1;1 gene start codon.
Three auxin-responsive elements, one ASF-1-binding site (Niggeweg et al., 2000; Redman et al., 2002), one SEBF-binding site (Boyle and Brisson, 2001), and one CATATG box (Xu et al., 1997), as well as two others, one auxin-response factor-binding site and one CATATG box, could be identified in the HbPIP2;1 and HbTIP1;1 promoter sequences, respectively.
Several other cis-acting sequences were identified in both promoter sequences (Supplemental Figs. S2 and S3), among them those involved in response to copper (CuRE cores; Kropat et al., 2005) and sulfur (SuRE core; Maruyama-Nakashita et al., 2005).
The nucleotide sequences of the HbPIP2;1 and HbTIP1;1 promoter regions were submitted to the GenBank database under the accession numbers FJ851081 and FJ851082, respectively.
DISCUSSION
Ethrel Induced a Concomitant Increase in Latex Yield and Decrease in Latex TSC
To demonstrate the direct effects of ethylene, avoiding any interference with the tapping-induced latex regenerative metabolism in the laticifers, we studied the events induced by Ethrel upon the very first tapping of mature virgin trees only. The PB217 rubber clone was chosen because of its marked yield response to ethylene (Obouayeba et al., 1996). We confirmed through kinetic studies that Ethrel treatment induced a wide increase in latex yield concomitant with a marked decrease in its TSC (Fig. 1). Both effects were perceptible at 8 h and were significant from 16 to at least 40 h after the treatments.
As this ethylene-induced latex dilution could be due to water influx inside the resting laticifers, we addressed the possible role of aquaporins in this process.
Differential Expression of the HbPIP1;1, HbPIP2;1, and HbTIP1;1 Genes in Response to Ethylene
Among the various possible “housekeeping” genes we tested, the 40S gene was chosen as the reference gene, because its expression was not affected by Ethrel. However, it was about 100-fold higher expressed in latex than in the liber tissues (Supplemental Fig. S1).
Preliminary quantitative reverse transcription-PCR analyses showed that the HbPIP1;1, HbPIP2;1, and HbTIP1;1 genes were more than 100-fold higher expressed in the inner bark tissues than in the latex of the virgin control trees (Fig. 3). However, this relatively low expression in the laticifers could be explained by the above-mentioned higher expression of the 40S reference gene in the latex compared with in the liber and should not be considered as a strictly liber-specific expression. Thus, at least in the PB217 virgin control trees, these three aquaporin genes were considered to be expressed at the same order of magnitude in both tissues, being less than 10-fold lower expressed in the resting laticifers, compared with the active surrounding inner bark tissues.
Among the three aquaporin genes, HbTIP1;1 was expressed the highest, especially in the young inner liber cells of the control trees (Fig. 3). This is consistent with data reporting high TIP expression in the expanding cells in young tissues (Yamada et al., 1997), during seed germination (Fukuhara et al., 1999), or in growing tobacco (N. tabacum) cells in suspension culture (Reisen et al., 2003).
The three aquaporin genes were shown to be differentially regulated at 24 h after Ethrel bark treatment. HbPIP2;1 was up-regulated in both the laticifers and the inner liber tissues, and the up-regulation of this gene at the translational level in the phloem was confirmed by western-blot analysis. In contrast, HbTIP1;1 was up-regulated in the latex cells but very markedly down-regulated in the inner liber tissues. Conversely, HbPIP1;1 was down-regulated in both tissues.
There have been few reports on the effects of ethylene on aquaporin gene expression. Ethylene was reported to up-regulate aquaporin in grape (Vitis vinifera) berries (Chervin et al., 2008). Conversely, Ma et al. (2008) reported down-regulation of a PIP2;1 isoform by ethylene in petal epidermal cells. In accordance with the Ethrel-induced down-regulation of the HbTIP1;1 in Hevea inner liber, Xue et al. (2009) observed marked down-regulation by ethylene of the RhTIP1;1 gene in rose (Rosa hybrida ‘Samantha’) petals during flower opening.
The HbPIP2;1 and HbTIP1;1 Genes Encode Functional Water Channels and Harbor Several Hormones and Chemical-Responsive Elements
The full-length cDNAs of HbPIP2;1 and HbTIP1;1 were cloned and characterized. The deduced amino acid of HbPIP2;1 shared 91% sequence identity with PIP2 subfamily members from J. regia (JrPIP2;2 and JrPIP2;1), and HbTIP1;1 shared 91% protein sequence identity with γ-TIPs from R. communis (RcTIP1;1). It was confirmed (Fig. 4) that both harbored the consensus sequences that characterize the PIP2 and γ-TIP aquaporins: six conserved membrane-spanning domains, five connecting loops, NPA motifs (Chrispeels and Maurel, 1994; Maurel, 1997; Zhao et al., 2008), Ser phosphorylable residues, as well as His protonable residues, which are known to confer pH sensitivity to aquaporins (Fischer and Kaldenhoff, 2008). The HbPIP2;1 deduced protein sequence did not harbor the Cys residue adjacent to the second NPA loop that confers mercury sensitivity to most aquaporins (Preston et al., 1993; Zhang et al., 1993).
The functionality of both genes was confirmed in the X. laevis oocytes (Fig. 5). HbPIP2;1 exhibited the highest efficiency in promoting water exchange across the oocyte plasmalemma, at a similar level to that in the reference positive control HvPIP2;4 or HvPIP2;1 gene from barley (Katsuhara et al., 2002). As expected from its deduced amino acid sequence, the HbPIP2;1 aquaporin was confirmed to be insensitive to the HgCl2 classical aquaporin inhibitor at concentrations in the micromolar range (Macey, 1984; Chrispeels and Maurel, 1994). This result is consistent with a number of papers reporting that some aquaporins from different species are not sensitive to mercury (Hasegawa et al., 1994; Biela et al., 1999; Krajinski et al., 2000; Otto and Kaldenhoff, 2000).
The promoter region was cloned and characterized in silico for each of the two genes. A classical ERE was identified in the promoter region of the HbTIP1;1 gene (Fig. 9; Supplemental Fig. S3). Curiously, the HbPIP2;1 promoter sequence harbored the most common GCC-box ERE (Kitajima et al., 1998) but located on the second DNA strand (Fig. 9; Supplemental Fig. S2). Similar ERE misallocation has been reported for other gene promoter regions, such as the one for an osmotin isoform in tobacco, and have been suggested to bind the corresponding trans-acting factors, but giving eventually less efficient responses to the corresponding stimulus (Sato et al., 1996, Kitajima et al., 1998).
Interestingly, among the several cis-acting sequences found, typical sulfur- and copper-responsive elements were identified in both aquaporin gene promoters (Fig. 9; Supplemental Figs. S2 and S3). It is worth noting that before the discovery of ethylene as the best yield stimulant, sulfur was reported to favor latex production (Chapman, 1951; Baptist and de Jonge, 1955) and copper sulfate was used for many years to stimulate rubber yield (Tixier, 1951; d'Auzac, 1989c). Also, typical auxin-responsive elements were identified in both promoter sequences, while synthetic auxins have previously been reported and widely used to increase latex yield (Baptist and de Jonge, 1955; Tupy and Resing, 1969; d'Auzac, 1989c). Thus, it is not surprising that HbPIP2;1 and HbTIP1;1 may be regulated at the transcriptional level by ethylene, and it is possible that they may also be regulated by auxins, sulfur, and copper, leading to an increase in latex yield.
The HbPIP2;1 Gene Is Expressed in All Young Hevea Stem Inner Liber Cell Types, Including Laticifers
In situ hybridization performed on samples from rubber tree young stems suggested that the HbPIP2;1 gene was more expressed in the young inner liber, including in the maturating young laticifers (Fig. 8). Other studies of PIP2 tissue cell-specific expression, especially on roots of plant species such as maize (Zea mays; Hachez et al., 2006), rice (Oryza sativa; Sakurai et al., 2008), and grapevine (Vandeleur et al., 2009), showed strong signals in virtually all root cell types. Some authors reported high expression of PIP2 in other tissues such as leaves (Sakurai et al., 2008), stigmas (Bots et al., 2005), and flower petals (Ma et al., 2008). To our knowledge, this is the first report of in situ hybridization of PIP2 in the stem liber of trees.
Regulation of HbPIP2;1 and HbTIP1;1 Expression and Activity May Contribute to the Ethylene-Induced Decrease in Latex TSC and Increase in Yield
The kinetic studies confirmed up-regulation of the HbPIP2;1 gene by Ethrel, which reached maximum as soon as 4 h after treatment and continued for more than 40 h in both the inner bark and the latex cells (Fig. 6).
Conversely, the HbTIP1;1 gene, the promoter region of which harbored a classical ERE, exhibited tissue-specific high differential expression in response to ethylene, which was markedly, early, and durably down-regulated in the phloem tissues, whereas it was progressively up-regulated in the laticifers (Fig. 6).
These molecular events preceded the dilution of latex and increase in yield (Fig. 1). We propose that the differential expression of these aquaporins may contribute to the regulation of water transport and exchanges in the inner liber tissues and laticifers, hence to the control of latex flow and yield.
Differential Aquaporin Expression May Control Turgor Pressure in the Liber Tissues
At dawn and early in the morning, when the rubber trees are generally tapped, the turgor pressure (about 150 p.s.i.) in the trunk, especially in liber tissues, which contain the laticifer networks, has been reported to be directly responsible for the latex flow (Buttery and Boatman, 1964, 1966, 1967; Pakianathan, 1967; Yeang, 2005). Furthermore, upon bark tapping, the drop in pressure in the latex vessels (to about 15 p.s.i. near the tapping cut) has been known for many years to induce an influx of water in the laticifers. This so-called “latex dilution reaction” has been reported to occur at the expense of the water of the surrounding inner liber cells (Gooding, 1952; Buttery and Boatman, 1964, 1966, 1967; Pakianathan, 1967) and even of the cortical xylem tissues (Raghavendra et al., 1984), probably through the transversal vascular rays. These authors proposed that such an influx of water in the laticifers during the latex flow, leading to a further progressive decrease in the latex TSC, favored the latex flow itself and higher yield through both an increase in latex fluidity and by preventing the laticifers from collapsing under the pressure of the surrounding liber.
In the nonlaticifer inner liber tissues, the conducting phloem sieve tubes (via their companion cells), the parenchyma cells, and the transversal vascular ray cells are all interconnected via numerous plasmodesmata (Hébant and de Faÿ, 1980; de Faÿ et al., 1989), forming a wide symplastic network and favoring both cell-to-cell and between-tissue circulation of water and nutrients.
In the control virgin trees, the HbPIP2;1 gene was moderately expressed and the HbTIP1;1 gene was highly expressed in the inner liber cells; thus, their plasmalemma water conductance would be moderate and their tonoplast water conductance would be very high. The circulation of water via the symplastic route was probably dominant, thus favoring liber cell-to-cell water circulation. We suggest that, upon the first tapping of control virgin trees, the water coming from the xylem and inner liber tissues via this symplastic route may have hardly addressed the water demand from the laticifers expelling their cytoplasm, all the more so as water conductance of the liber cell and laticifer plasmalemma was relatively low. Furthermore, upon tapping, as the water conductance of the liber tissue tonoplast was very high, the inner liber cells may have been able to help fuel the water demand from the laticifers through the release of their vacuolar water. Such events should lead to a decrease in the turgor of the liber tissues surrounding the laticifers, thus discouraging latex flow and leading to low latex yield.
After Ethrel stimulation, the HbPIP2;1 gene was up-regulated in the inner liber tissues, and their plasmalemma water conductance would have been significantly increased. We propose that such a situation led to increased efficiency of water circulation through both the symplastic and their plasmalemma aquaporin pathways. This would greatly favor water exchanges within the liber cells and between the xylem and inner liber tissues, which could respond better to the water demand from the tapped laticifers. Furthermore, ethylene induced a very marked down-regulation of HbTIP1 in the liber tissues, undoubtedly resulting in a marked decrease in their tonoplast water conductance, thus allowing them to retain their vacuolar water. This could help maintain high enough turgor pressure in the liber cells surrounding the laticifers throughout latex flow, favoring the latex flow itself and resulting in a marked increase in latex yield.
It is worth noting here that, contrary to the liber cells, which harbor a large central vacuole, the vacuolar compartment of the laticifer, being under the form of polydispersed microvacuoles accounting for less than 20% of the total latex volume (d'Auzac et al., 1982), should not contribute much to laticifer turgor pressure.
Aquaporin Expression and Activity in the Laticifers Decrease Latex TSC
As mentioned earlier, the mature articulated laticifers are devoid of plasmodesmata (de Faÿ et al., 1989), and the only way for rapid water exchanges to occur with the surrounding inner liber cells would be via the PIP pathway. In the laticifers from virgin PB217 untreated trees, the expression of all three aquaporin genes was rather low, in particular a bit lower than in the phloem. Thus, the water conductance of both the laticifer plasmalemma and tonoplast would have been rather low in these resting latex cells. In these conditions, especially due to such relatively low HbPIP2;1 expression, the water exchanges between the laticifers and their surrounding inner liber tissues would be rather limited, maintaining high latex TSC and probably low latex dilution during the first tapping. This would result in low latex yield.
Ethrel induced up-regulation of HbPIP2;1 in the laticifers, up to about 20-fold. This should have significantly increased water conductance of the laticifer plasmalemma, favoring water influx and latex dilution in the laticifers. Increase in laticifer Suc loading a few hours after Ethrel bark treatment was first reported by Lacrotte et al. (1985). Furthermore, through same kinetic studies, Dusotoit-Coucaud et al. (2009) confirmed early up-regulation of sugar transporters (especially Suc transporters), specifically at the laticifer plasmalemma, in response to stimulation of rubber trees with Ethrel. Such ethylene-induced sugar loading should contribute to increasing the laticifer osmotic potential and in driving the PIP-dependent water influx in the laticifers, leading to decreased latex TSC, thus prolonging latex flow and increasing yield.
Apart from up-regulation of the aquaporins at the transcriptional level, aquaporin activity may be regulated at the posttranslational level in response to hormone treatments. In particular, pH has been reported to finely tune aquaporin activity through the protonation of some of their His residues by cytosolic H+, inducing their gating (Chaumont et al., 2005; Fischer and Kaldenhoff, 2008; Verdoucq et al., 2008). Bark treatments with synthetic auxins (Tupy, 1973) as well as with Ethrel (Coupé et al., 1977; Bzrozowska-Hanower et al., 1979; Amalou et al., 1992) have been shown to induce marked alkalinization of the latex cell cytosol, which should help increase laticifer PIP2 activity.
CONCLUSION
In this study, we propose, to our knowledge for the first time, that aquaporins are probably keys in the physiological mechanism of ethylene-induced increase in latex yield. From our experiments on the Ethrel stimulation of the PB217 virgin rubber trees, we propose that (1) HbPIP2;1 up-regulation and marked HbTIP1;1 down-regulation in the inner liber tissues both facilitate water circulation between xylem and phloem and within the inner liber tissues, thus probably contributing to maintain their high turgor pressure; and (2) HbPIP2;1 together with Suc transporter up-regulation in the laticifers, as well as their cytosolic alkalinization, facilitate water influx in these nonsymplastic cells. The combination of these events could account for the decrease in the latex TSC, resulting in higher latex fluidity (Supplemental Fig. S4). Even though more work remains to be performed on the cloning and characterization of other probable aquaporin isoforms from the Hevea liber and laticifers, we suggest that HbPIP2;1 and HbTIP1;1, as well as all the phenomena reported here, play an important role in facilitating the initial latex flow and its prolongation, accounting at least partly for the ethylene-induced increase in latex yield in virgin trees.
MATERIALS AND METHODS
Plant Materials and Field Experiments
The field experiment was performed with 8-year-old mature virgin rubber trees (Hevea brasiliensis) of the PB217 clone in the Bongo/Société Africaine de Plantations d'Hévéas plantation in southeastern Ivory Coast (West Africa). The trees, selected for their homogeneous girth (54 ± 1.5 cm), were treated and sampled on the same week in April, during the transition between the dry and wet seasons.
The study of the short-term kinetics of the ethylene effects on the various parameters was performed as described by Pujade-Renaud et al. (1997). Six batches of three trees were set up. Five of them were treated on a 1.5-cm large, lightly scraped suber strip beneath the next tapping cut with 1 g of 2.5% Ethrel in crude palm oil for 4, 8, 16, 24, and 40 h before the first tapping. The sixth batch was treated with 1 g of palm oil only as a control.
All trees were opened (tapped for the first time) on a half spiral, and the samples were collected early in the morning on the same day. Homogenous depth (approximately 1 mm from the cambium) of tapping was tested with a sharp calibrated punch.
Extraction of Total RNA from the Latex
Latex was collected from a pool of three trees per treatment. One volume (2 mL per tree) of latex was collected in 6 mL of 2× “fixation/extraction” buffer (50 mm Tris-HCl, 300 mm LiCl, 10 mm EDTA, and 10% SDS, pH 9.0). The latex samples were immediately deep frozen in liquid nitrogen and then stored at −80°C. Total RNA was extracted using the latex LiCl precipitation method described by Pujade-Renaud et al. (1994, 1997).
Extraction of Total RNA from Inner Soft Bark
Using a stainless steel cork borer (2.5 cm in diameter), pieces of bark were punched out up to the cambium, 5 to 15 cm below the treated area. The first 2 to 3 mm of the soft inner liber was quickly peeled off with a scalpel, and the thin shavings were immediately deep frozen in liquid nitrogen and then stored at −80°C. Total RNA was extracted from bark using the cesium chloride cushion method, adapted from Sambrook et al. (1989). Typically, 2 g of deep-frozen inner bark, ground into a fine powder under liquid nitrogen (Kika A10 high-speed grinder), was suspended in cold extraction buffer (4 m guanidine thiocyanate, 1% sarcosine, 1% polyvinylpyrrolidone, 20 mm ascorbic acid, and 3% [v/v] β-mercaptoethanol). The suspension was homogenized on ice for 30 s using an Ultraturax homogenizer and then centrifuged at 35,000g for 30 min at 4°C. The clear supernatant was transferred onto a 5.7 m cesium chloride cushion and centrifuged at 130,000g for 20 h at 20°C. The RNA pellet dissolved in diethyl pyrocarbonate-water was deproteinized by two phenol:chloroform:isoamyl alcohol (25:24:1, v/v) extractions. Total RNA was precipitated overnight at −80°C with sodium acetate/absolute ethanol and then pelleted by centrifugation. After rinsing in ethanol and air drying, the RNA pellet was resuspended in diethyl pyrocarbonate-water.
Construction of Inner Soft Bark-Directional Full-Length cDNA Libraries and Screening
Poly(A+) RNA isolation, cDNA synthesis [from 2.5 μg of poly(A+) RNA of inner bark mix samples from control and 4-, 8-, 16-, and 24-h ethylene-treated trees], construction of the directional cDNA library (Stratagene), as well as its screening (2.0 × 105 plaque-forming units) with the 32P-labeled EST cDNA probes were performed as described by Pujade-Renaud et al. (1997).
Cloning and Characterization of Promoter Regions
Genomic DNA was extracted from 0.1 g of young leaves of the PB217 clone using the cetyl-trimethyl-ammonium bromide method (Doyle and Doyle, 1990) and treated with RNase (30 μg mL−1 final concentration) at 37°C for 1 h. The proximal promoter region upstream from the 5′ untranslated region of the HbPIP2;1 and HbTIP1;1 genes was cloned by PCR amplification of the genomic DNA using the GenomeWalker Universal kit (Clontech) following the manufacturer's recommendations. The primary PCR was performed with the outer adaptor primer (AP1) and the outer gene-specific primer HbPIP2;1-R1 (5′-CACCGCCTTGTCCTCCAACTTCA-3′) or HbTIP1;1-R1 (5′-TGCCTCCTGGGGATGGCCTA-3′) using Advantage2 polymerase mix (Clontech). The secondary PCR was performed with the nested adaptor primer (AP2) and the nested gene-specific primer HbPIP2;1-R2 (5′-CCGTGACAGGATTTGGCAAGAGAAA-3′) or HbTIP1;1-R2 (5′-TCTGATCGGCATCTTACCTCGCGTA-3′). The PCR products cloned into the pGEM-T Easy vector (Promega) were analyzed for putative promoter cis-acting sequences using PLACE software (www.dna.affrc.go.jp/htdocs/PLACE/; Higo et al., 1999).
Analysis of Gene Expression by Real-Time PCR
After treatment with DNase I (Ambion), 2 μg of total RNA was used as template for the first-strand cDNA synthesis (SuperScript III; Invitrogen). The real-time PCR medium (40 μL) contained the following: 2 μL of a 1:40 dilution of the first-strand cDNA, 0.4 μm of each primer, 0.25 mm deoxyribonucleotide triphosphate mix, 2 mm MgCl2, 0.8 unit of Platinum Taq polymerase (Invitrogen), and a 1:1,000 dilution of SYBR Green I (Sigma) in 1× PCR buffer. The procedure included initial denaturation at 94°C for 3 min, followed by 35 cycles of two steps of PCR as denaturation at 94°C for 15 s and annealing and polymerization at 60°C for 1 min using an ABI-7500 real-time PCR machine. The relative quantity of rubber aquaporin transcripts, with the 40S gene as internal standard, was automatically calculated as 2−ΔΔCT by the ABI-7500 software. The PCR primer sets used for real-time PCR are shown in Supplemental Table S1. All first-strand cDNA syntheses and real-time PCR amplifications were performed in triplicate for each gene and time treatment. se calculation and ANOVA were used for statistical and significance analyses, respectively, taking into account all three-by-three replicates.
In Situ Hybridization
In situ hybridization was performed as described by Regan et al. (1999). Pieces (5 mm in diameter) of young rubber stem were fixed in 4% paraformaldehyde. After embedding in Paraplast (Oxford Labware), 8- to 10-μm sections were transversally cut and affixed to microscope slides. The sections were deparaffinized and dehydrated with successive ethanol/water steps. A 260-bp fragment probe corresponding to the conserved sequence of HbPIP2;1 was obtained from PCR amplification with the following primers: F, 5′-GATCGATGCCGAGGAGTTTA-3′; R, 5′-CCAAAGTCACAGCAGGGTTT-3′. The antisense- and sense-labeled probes were transcribed from linearized pGEM-T Easy-HbPIP2;1 plasmid with NcoI or SpeI using the digoxigenin DIG-RNA labeling kit (Roche) with either SP6 or T7 RNA polymerase. Sections were hybridized overnight at 50°C with the probe at a concentration of 0.4 to 3 μg mL−1 and then washed up to 0.1× SSC. Detection was performed by anti-digoxigenin alkaline phosphatase conjugate, followed by colorimetric detection of phosphatase activity (Bio-Rad). The images of sections were taken with bright-field optics using an Olympus BX51 microscope.
Functional Analysis of Hevea Aquaporins in Xenopus Oocytes
The coding regions of HbPIP2;1 or HbTIP1;1 cDNAs were inserted into the blunt-ended BglII site of the pXBG-ev1 expression vector (Preston et al., 1992). The constructs were linearized with BamHI, and complementary RNA with cap analog [m7G(5′)ppp(5′)G] was synthesized using the mMESSAGE mMACHINE T7 in vitro transcription kit (Ambion). Xenopus laevis oocytes were isolated and defolliculated as described by Katsuhara et al. (2002). Oocytes were injected with 50 nL of RNA solution (50 ng of RNA) or with 50 nL of RNase-free water, and oocytes were incubated at 20°C in modified Barth's solution (MBS). One day after cDNA injection, individual oocytes were transferred from full-strength (200 mOsm; Osmin) to 5-fold diluted (Osmout) MBS for measurement of the cell volume. Oocyte diameter changes were characterized with an inverted microscope (Cool SNAP) at 20-s intervals for 3 min as described by Mahdieh et al. (2008). The Pf was calculated using the equation Pf = V0 × [d(V/V0)/dt]/[S × Vw × (Osmin – Osmout)], where V0 is the initial oocyte volume (9 × 10−4 cm3), S is the initial surface area (0.045 cm2), and Vw is the molar volume of water (18 cm3 mol−1; Zhang and Verkman, 1991).
For Hg inhibition, oocytes were pretreated with MBS containing 0.01, 0.1, or 1 mm HgCl2 for 10 min before the water permeability assay (Maurel et al., 1993).
Western-Blot Analysis
The HbPIP2;1 purified antibody was obtained by immunization of rabbits with the specific peptide AKDVEVGGQGGEFQAKDYN, designed from the deduced amino acid sequence of HbPIP2;1 (Proteogenix).
Microsomal fractions, including plasma membranes, were extracted from the inner liber according to Bots et al. (2005). As protein concentrations were similar for all samples but at the limit of detection using the Bio-Rad kit (Supplemental Fig. S5), 12-μL extracts were separated by 10% SDS-PAGE. The proteins were either stained with Coomassie Brilliant Blue (Sambrook et al., 1989) or electroblotted onto a polyvinylidene difluoride membrane according to Towbin et al. (1979). Immunostaining was performed as described by Sookmark et al. (2002), except that the HbPIP2;1 antibody (1:500), the alkaline phosphatase-conjugated goat anti-rabbit antibody (1:5,000), and the 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium substrate (Roche) were used. The intensity of the protein bands was scanned using the Quantify One program version 4.2.3 (Bio-Rad).
Measurement of Latex Yield and TSC
A few hours after tapping, 2 mL of 5% formic acid was added to the preweighed cup and mixed with the latex. The next morning, the coagulated latex in the cup was weighed tree per tree using a portable laboratory electronic balance.
The latex TSC was also measured tree per tree. One milliliter of latex was collected in a preweighed screw-cap vial, which was weighed to determine the exact fresh matter and then left to dry at 75°C for at least 48 h in a ventilated oven until constant weight. The vial was weighed again to determine the dry matter. The TSC was calculated as dry matter to fresh matter and expressed as a percentage.
Statistical Analysis
Statistical analysis was tested for significance using a t test or one-way ANOVA (SPSS version 7.52 package). Differences were accepted as significant at P < 0.05.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers FJ851076, FJ851077, FJ851078, FJ851079, FJ851080, FJ851081, and FJ851082.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Fold basal expression of the 40S gene in the latex compared with the inner bark tissues from control and stimulated trees.
Supplemental Figure S2. The nucleotide sequence of the amplified 5′ upstream region of HbPIP2;1.
Supplemental Figure S3. The nucleotide sequence of the amplified 5′ upstream region of HbTIP1;1.
Supplemental Figure S4. Changes in the plasmalemma and tonoplast water conductance of the rubber tree liber cells and laticifers due to HbPIP2;1 and HbTIP1;1 aquaporin gene regulation in response to ethylene.
Supplemental Figure S5. Determination of the liber microsomal protein concentrations.
Supplemental Table S1. Gene-specific primer pairs used for real-time PCR experiments.
Supplementary Material
Acknowledgments
We are grateful to the staffs of the Société Internationale des Plantations d'Hévéa Headquarters and of the Bongo Rubber Plantation in Ivory Coast (West Africa) for their logistical and technical help in the preparation and monitoring of the field experiments as well as for allowing our team access to all their plant materials and laboratory facilities without any restriction. This article is part of K.T.'s doctoral degree study at Mahidol University.
This work was supported by the Franco-Thai Cooperation Program in Higher Education and Research 2005–2008, and a grant under an agreement between the Institut de Recherche pour le Développement and a consortium from the private sector (the Institut Français du Caoutchouc, the Société de Technologie Michelin, the Société Internationale des Plantations d'Hévéa, and the Société Financière des Caoutchoucs Consultant Services). K.T. was supported by the Royal Golden Jubilee Ph.D. program (grant no. PHD/0217/2547) of the Thailand Research Fund.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hervé Chrestin (chrestin@ird.fr).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G (2003) Over-expression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15: 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersson E, Fraysse L, Sjovall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59: 469–484 [DOI] [PubMed] [Google Scholar]
- Amalou Z, Bangratz J, Chrestin H (1992) Ethrel (ethylene releaser)-induced increases in the adenylate pool and transtonoplast ΔpH within Hevea latex cells. Plant Physiol 98: 1270–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiges I, Schaffner AR, Affenzeller MJ, Mas A (2002) Plant aquaporins. Physiol Plant 115: 175–182 [DOI] [PubMed] [Google Scholar]
- Baptist EDC, de Jonge P (1955) Stimulation of yield in Hevea brasiliensis. II. Effects of synthetic growth substances on yield and bark renewal. J Rubber Res Inst Malays 14: 362–382 [Google Scholar]
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18: 565–570 [DOI] [PubMed] [Google Scholar]
- Blackman LM, Overall RL (2001) Structure and function of plasmodesmata. Aust J Plant Physiol 28: 709–727 [Google Scholar]
- Boatman SG (1966) Preliminary physiological studies on the promotion of latex flow by plant growth regulators. J Rubber Res Inst Malays 19: 243–258 [Google Scholar]
- Bots M, Feron R, Uehlein N, Weterings K, Kaldenhoff R, Mariani T (2005) PIP1 and PIP2 aquaporins are differentially expressed during tobacco anther and stigma development. J Exp Bot 56: 113–121 [DOI] [PubMed] [Google Scholar]
- Boyle B, Brisson N (2001) Repression of the defense gene PR-10a by the single-stranded DNA binding protein SEBF. Plant Cell 13: 2525–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery BR, Boatman SG (1964) Turgor pressures in the phloem: measurement on Hevea latex. Science 145: 285–286 [DOI] [PubMed] [Google Scholar]
- Buttery BR, Boatman SG (1966) Manometric measurement of turgor pressures in laticiferous phloem tissues of Hevea brasiliensis. J Exp Bot 17: 283–296 [Google Scholar]
- Buttery BR, Boatman SG (1967) Effect of tapping, wounding and growth regulators on turgor pressure in Hevea brasiliensis. J Exp Bot 18: 644–659 [Google Scholar]
- Bzrozowska-Hanower J, Cretin H, Hanower P, Lioret C (1979) pH variations between the vacuolar and cytoplasmic compartments of the Hevea brasiliensis latex: seasonal influences and effect of bark treatment with Ethrel (an ethylene releaser). Physiol Veg 17: 889–905 [Google Scholar]
- Canny MJ (1995) Apoplastic water and solute movements: new rules for an old space. Annu Rev Plant Physiol Plant Mol Biol 46: 215–236 [Google Scholar]
- Chapman GW (1951) Plant hormones and yield in Hevea brasiliensis. J Rubber Res Inst Malays 13: 167–176 [Google Scholar]
- Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97: 749–764 [DOI] [PubMed] [Google Scholar]
- Chervin C, Tira-umphon A, Terrier N, Zouine M, Severac D, Roustan JP (2008) Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiol Plant 134: 534–546 [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Maurel C (1994) Aquaporins: the molecular basis of facilitated water movement through living plant cells. Plant Physiol 105: 9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Venisse JS, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S (2007) Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol 143: 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupé M, Chrestin H (1989) Physico-chemical and biochemical mechanisms of hormonal (ethylene) stimulation. In J d'Auzac, JL Jacob, H Chrestin, eds, Physiology of Rubber Tree Latex. CRC Press, Boca Raton, FL, pp 295–319
- Coupé M, Lambert C, Primot L, d'Auzac J (1977) Kinetic action of 2-chloroethyl-phosphonic acid (ethephon) on the latex polysomes of Hevea brasiliensis. Phytochemistry 16: 1133–1141 [Google Scholar]
- d'Auzac J (1989. a) Factors involved in the stopping of the latex flow after tapping. In J d'Auzac, JL Jacob, H Chrestin, eds, Physiology of Rubber Tree Latex. CRC Press, Boca Raton, FL, pp 257–285
- d'Auzac J (1989. b) Tapping systems and area of drained bark. In J d'Auzac, JL Jacob, H Chrestin, eds, Physiology of Rubber Tree Latex. CRC Press, Boca Raton, FL, pp 221–232
- d'Auzac J (1989. c) The hormonal stimulation of latex yield: historical account. In J d'Auzac, JL Jacob, H Chrestin, eds, Physiology of Rubber Tree Latex. CRC Press, Boca Raton, FL, pp 289–294
- d'Auzac J, Cretin H, Marin B, Lioret C (1982) A plant vacuolar system: the lutoids from the Hevea brasiliensis latex. Physiol Veg 20: 320–331 [Google Scholar]
- d'Auzac J, Ribaillier D (1969) Ethylene: a new stimulant of latex yield for Hevea brasiliensis. C R Acad Sci D Sci Nat 268: 3046–3049
- de Faÿ E, Sanier C, Hébant C (1989) Distribution of plasmodesmata in the phloem of Hevea brasiliensis in relation to laticifer loading. Protoplasma 149: 155–162 [Google Scholar]
- Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13–15 [Google Scholar]
- Dusotoit-Coucaud A, Brunel N, Kongsawadworakul P, Viboonjun U, Lacointe A, Julien JL, Chrestin H, Sakr S (2009) Sucrose importation into laticifers of Hevea brasiliensis, in relation to ethylene stimulation of latex production. Ann Bot (Lond) 104: 635–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kaldenhoff R (2008) On the pH regulation of plant aquaporins. J Biol Chem 283: 33889–33892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara T, Kirch HH, Bohnert HJ (1999) Expression of Vp1 and water channel proteins during seed germination. Plant Cell Environ 22: 417–424 [Google Scholar]
- Gohet E, Chantuma P, Lacote R, Obouayeba S, Dian K, Clément-Demange A, Kurnia D, Eschbach JM (2003) Latex clonal typology of Hevea brasiliensis: physiological modelling of yield potential and clonal response to ethephon stimulation. In Proceedings of the IRRDB Workshop on Exploitation Physiology, Kottayam, India. IRRDB, Kuala Lumpur, Malaysia, pp 199–217
- Gooding EGB (1952) Study in the physiology of latex. II. Latex flow on tapping Hevea brasiliensis associated changes in trunk diameter and latex concentration. New Phytol 51: 11–29 [Google Scholar]
- Guerrero FD, Jones JT, Mullet JE (1990) Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted: sequence and expression of three inducible genes. Plant Mol Biol 15: 11–26 [DOI] [PubMed] [Google Scholar]
- Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F (2006) Localization and quantification of plasma membrane aquaporin expression in maize primary root: a clue to understanding their role as cellular plumbers. Plant Mol Biol 62: 305–323 [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS (1994) Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem 269: 5497–5500 [PubMed] [Google Scholar]
- Hébant C (1981) Ontogeny of the primary laticiferous system of Hevea brasiliensis: an ultrastructural and cytochemical study. J Can Bot 59: 974–985 [Google Scholar]
- Hébant C, de Faÿ E (1980) Functional organization of the bark of Hevea brasiliensis (rubber tree): a structural and histo-enzymological study. Z Pflanzenphysiol 97: 391–398 [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Iorenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P (1998) Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10: 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuhara M, Akiyama Y, Koshio K, Shibasaka M, Kasamo K (2002) Functional analysis of water channels in barley roots. Plant Cell Physiol 43: 885–893 [DOI] [PubMed] [Google Scholar]
- Kitajima S, Koyama T, Yamada Y, Sato F (1998) Constitutive expression of the neutral PR-5 (OLP, PR-5d) gene in roots and cultured cells of tobacco is mediated by ethylene-responsive cis-element AGCCGCC sequences. Plant Cell Rep 18: 173–179 [DOI] [PubMed] [Google Scholar]
- Kongsawadworakul P, Chrestin H (2003) Laser diffraction: a new tool for identification and studies of physiological effectors involved in aggregation-coagulation of the rubber particles from Hevea latex. Plant Cell Physiol 44: 707–717 [DOI] [PubMed] [Google Scholar]
- Krajinski F, Biela A, Schubert D, Gianinazzi-Pearson V, Kaldenhoff R, Franken P (2000) Arbuscular mycorrhiza development regulates the mRNA abundance of Mtaqp1 encoding a mercury-insensitive aquaporin of Medicago truncatula. Planta 211: 85–90 [DOI] [PubMed] [Google Scholar]
- Kropat J, Tottey S, Birkenbihl RP, Depège N, Huijser P, Merchant S (2005) A regulator of nutritional copper signaling in Chlamydomonas is an SBP domain protein that recognizes the GTAC core of copper response element. Proc Natl Acad Sci USA 102: 18730–18735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacrotte R, Van De Sype H, Chrestin H (1985) Influence of ethylene on the uptake and use of exogenous sucrose, by the latex cells from Hevea brasiliensis: proposal for an action mechanism. Physiol Veg 23: 187–198 [Google Scholar]
- Lee CK, Tan H (1979) Daily variations in yield and dry rubber content in four Hevea clones. J Rubber Res Inst Malays 27: 117–126 [Google Scholar]
- Lustinec J, Langlois S, Resing W, Kim Chun C (1967) The yield stimulation of Hevea brasiliensis with chlorophenoxy-acetic acids and their effects on the drainage area. Rev Gen Caout Plast 43: 1645–1656 [Google Scholar]
- Lustinec J, Resing WL, Simmer J (1968) Distinction of two components of the drainage area at the trunk level of Hevea brasiliensis. Biol Plant 10: 284–293 [Google Scholar]
- Ma N, Xue JQ, Li YH, Liu XJ, Dai FW, Jia WS, Luo YB, Gao JP (2008) Rh-PIP2;1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiol 148: 894–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey RI (1984) Transport of water and urea in red blood cells. Am J Physiol 246: 195–203 [DOI] [PubMed] [Google Scholar]
- Mahdieh M, Mostajeran A, Horie T, Katsuhara M (2008) Drought stress alters water relations and expression of PIP-type aquaporin genes in Nicotiana tabacum plants. Plant Cell Physiol 49: 801–813 [DOI] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Nakamura Y, Watanabe-Takahashi A, Inoue E, Yamaya T, Takahashi H (2005) Identification of a novel cis-acting element conferring sulfur deficiency response in Arabidopsis roots. Plant J 42: 305–314 [DOI] [PubMed] [Google Scholar]
- Maurel C (1997) Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol 48: 399–429 [DOI] [PubMed] [Google Scholar]
- Maurel C, Chrispeels MJ (2001) Aquaporins: a molecular entry into plant water relations. Plant Physiol 125: 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Reizer J, Schroeder JI, Chrispeels MJ (1993) The vacuolar membrane protein g-TIP creates water specific channels in Xenopus oocytes. EMBO J 12: 2241–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59: 595–624 [DOI] [PubMed] [Google Scholar]
- Niggeweg R, Thurow C, Kegler C, Gatz C (2000) Tobacco transcription factor TGA2.2 is the main component of as-1-binding factor ASF-1 and is involved in salicylic acid- and auxin-inducible expression of as-1-containing target promoters. J Biol Chem 275: 19897–19905 [DOI] [PubMed] [Google Scholar]
- Obouayeba S, Boa D, Jacob JL (1996) Performance of the PB 217 Hevea clone in Cote d'Ivoire. Plantations Recherche Développement 3: 346–354 [Google Scholar]
- Ohta M, Ohme-Takagi M, Shinshi H (2000) Three ethylene-responsive transcription factors in tobacco with distinct transactivation functions. Plant J 22: 29–38 [DOI] [PubMed] [Google Scholar]
- Otto B, Kaldenhoff R (2000) Cell-specific expression of the mercury-insensitive plasma-membrane aquaporin NtAQP1 from Nicotiana tabacum. Planta 211: 167–172 [DOI] [PubMed] [Google Scholar]
- Pakianathan SW (1967) Determination of osmolarity of small latex samples by vapor pressure osmometer. J Rubber Res Inst Malays 20: 23–34 [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256: 385–387 [DOI] [PubMed] [Google Scholar]
- Preston GM, Jung JS, Guggino WB, Agre P (1993) The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem 268: 17–20 [PubMed] [Google Scholar]
- Pujade-Renaud V, Clement A, Perrot-Rechenmann C, Prévôt JC, Chrestin H, Jacob JL, Guern J (1994) Ethylene-induced increase in glutamine synthetase activity and mRNA levels in Hevea brasiliensis latex cells. Plant Physiol 105: 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade-Renaud V, Perrot-Rechenmann C, Chrestin H, Lacrotte R, Guern J (1997) Characterization of a full-length cDNA clone encoding glutamine synthetase from rubber tree latex. Plant Physiol Biochem 35: 85–93 [Google Scholar]
- Quist TM, Yokoi S, Bressan RA, Hasegawa PM, Joly RJ (2004) Differential regulation of plasma membrane aquaporin transcripts in Arabidopsis in response to environmental stress: proposed roles for aquaporins in regulating plant water balance. Recent Res Devel Biochem 5: 19–29 [Google Scholar]
- Raghavendra AS, Sulochanamma S, Rao GG, Mathew S, Satheesan KV, Sethuraj MR (1984) The pattern of latex flow in relation to clonal variation, plugging and drought tolerance. In Proceedings of the IRRDB Symposium “Exploitation, Physiology and Improvement of Hevea,” Montpellier, France. IRRDB, Kuala Lumpur, Malaysia, pp 205–226
- Rawat R, Xu ZF, Yao KM, Chye ML (2005) Identification of cis-elements for ethylene and circadian regulation of the Solanum melongena gene encoding cysteine proteinase. Plant Mol Biol 57: 629–643 [DOI] [PubMed] [Google Scholar]
- Redman J, Whitcraft J, Johnson C, Arias J (2002) Abiotic and biotic stress differentially stimulate as-1 element activity in Arabidopsis. Plant Cell Rep 21: 180–185 [Google Scholar]
- Regan S, Bourquin V, Touminen H, Sundberg B (1999) Accurate and high resolution in situ hybridization analysis of gene expression in secondary stem tissues. Plant J 19: 363–369 [DOI] [PubMed] [Google Scholar]
- Reisen D, Loborgne-Castel N, Ozalp C, Chaumont F, Marty F (2003) Expression of a cauliflower tonoplast aquaporin tagged with GFP in tobacco suspension cells correlates with an increase in cell size. Plant Mol Biol 52: 387–400 [DOI] [PubMed] [Google Scholar]
- Sakr S, Alves G, Morillon R, Maurel K, Decourteix M, Guilliot A, Fleurat-Lessard P, Julien JL, Chrispeels MJ (2003) Plasma membrane aquaporins are involved in winter embolism recovery in walnut tree. Plant Physiol 133: 630–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai J, Ahamed A, Murai M, Maeshima M, Uemura M (2008) Tissue and cell-specific localization of rice aquaporins and their water transport activities. Plant Cell Physiol 49: 30–39 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis TA (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sato F, Kitajima S, Koyama T, Yamada Y (1996) Ethylene-induced gene expression of osmotin-like protein, a neutral isoform of tobacco PR-5, is mediated by the AGCCGCC cis-sequence. Plant Cell Physiol 37: 249–255 [DOI] [PubMed] [Google Scholar]
- Sookmark U, Pujade-Renaud V, Chrestin H, Lacote R, Naiyanetr C, Seguin M, Romruensukharom P, Narangajavana J (2002) Characterization of polypeptides accumulated in the latex cytosol of rubber trees affected by the tapping panel dryness syndrome. Plant Cell Physiol 43: 1323–1333 [DOI] [PubMed] [Google Scholar]
- Tixier P (1951) The injection of trace elements, especially of copper in the form of copper sulfate into Hevea brasiliensis. J Rubber Res Inst Malays 13: 192–199 [Google Scholar]
- Tjasadihardja A, Kardjono W (1974) Clonal response to stimulation. Menara Perkebunan 42: 227–236 [Google Scholar]
- Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupy J (1973) The regulation of invertase activity in the latex of Hevea brasiliensis Muell. Arg.: the effects of growth regulators, bark wounding and latex tapping. J Exp Bot 24: 516–524 [Google Scholar]
- Tupy J, Resing WL (1969) Stimulation of latex yield in Hevea brasiliensis by bark treatments with plant growth substances at various distances from the tapping cut. Rev Gen Caout Plast 46: 479–482 [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149: 445–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gils GE (1951) Studies on the latex viscosity. I. Influence of the dry rubber content. Arch Rubbercult 28: 61–66 [Google Scholar]
- Varney GT, McCully ME, Canny MJ (1993) Sites of entry of water into the symplast of maize roots. New Phytol 125: 733–741 [DOI] [PubMed] [Google Scholar]
- Verdoucq L, Grondin A, Maurel C (2008) Structure-function analysis of plant aquaporin AtPIP2;1 gating by divalent cations and protons. Biochem J 415: 409–416 [DOI] [PubMed] [Google Scholar]
- Xu N, Hagen G, Guilfoyle T (1997) Multiple auxin response modules in the soybean SAUR 15A promoter. Plant Sci 126: 193–201 [Google Scholar]
- Xue J, Yang F, Gao J (2009) Isolation of RhTIP1;1, an aquaporin gene and its expression in rose flowers in response to ethylene and water deficit. Postharvest Biol Technol 51: 407–413 [Google Scholar]
- Yamada S, Komory T, Myers PM, Kuwata S, Imaseki H (1997) Expression of plasma membrane water channel genes under water stress in Nicotiana excelsior. Plant Cell Physiol 38: 1226–1231 [DOI] [PubMed] [Google Scholar]
- Yeang HY (2005) The kinetics of latex flow from the rubber tree in relation to latex vessels plugging and turgor pressure. J Rubber Res 8: 160–181 [Google Scholar]
- Yip E, Gomez JB (1980) Factors influencing the colloidal stability of fresh clonal Hevea latices as determined by the aerosol OT test. J Rubber Res Inst Malays 28: 86–106 [Google Scholar]
- Zhang R, van Hoek AN, Biwersi J, Verkman AS (1993) A point mutation at cysteine 189 blocks the water permeability of rat kidney water channel CHIP28k. Biochemistry 32: 2938–2941 [DOI] [PubMed] [Google Scholar]
- Zhang RB, Verkman AS (1991) Water and urea permeability properties of Xenopus oocytes: expression of mRNA from toad urinary bladder. Am J Cell Physiol 260: C26–C34 [DOI] [PubMed] [Google Scholar]
- Zhao CX, Shao HB, Chu LY (2008) Aquaporin structure-function relationships: water flow through plant living cells. Colloids Surf 62: 163–172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.