Abstract
Oligonucleotides containing Locked Nucleic Acids (LNA) to various extents and at various positions were evaluated for antisense activity, RNase H recruitment, nuclease stability and thermal affinity. In this work, two different diastereoisomers of LNA were studied: the beta-d-LNA and the alpha-l-LNA (abbreviated as β-d-LNA and α-l-LNA). Our findings show that the best antisense activity with 16mer gapmers containing β-d-LNA (oligonucleotides containing consecutive segments of LNA and DNA with a central DNA stretch flanked by two LNA segments, LNA–DNA–LNA) is found with gap sizes between 7 and 10 nt. The optimal gap size is motif-dependent, and requires the right balance between gap size and affinity. Compared to β-d-LNA, α-l-LNA shows superior stability against a 3′-exonuclease. The design possibilities of α-l-LNA were explored for different gapmers and other designs, collectively called chimeras. The placement of α-l-LNA in the junctions or in the flanks resulted in potent antisense oligonucleotides. Moreover, different chimeras with an alternate composition of DNA, α-l-LNA and β-d-LNA were evaluated in terms of antisense activity and RNase H recruitment. Chimeras with an interrupted DNA stretch with α-l-LNA still recruit RNase H and show good levels of antisense activity, while the same design with β-d-LNA results in a drop in antisense potency. Our findings indicate that α-l-LNA is a powerful and versatile nucleotide analogue for designing potent antisense oligonucleotides.
INTRODUCTION
The antisense strategy is based on the inhibition of gene expression as a result of the Watson–Crick complementary hybridization of oligonucleotides to single stranded RNA (1), thereby interfering with the expression of the encoded protein (2). Different mechanisms (3) have been described to account for the antisense activity, such as steric blockage of the translational machinery and RNase H based cleavage of the mRNA.
So far, phosphorothioate oligonucleotides have been the most widely used class of antisense compounds, and several phosphorothioate oligonucleotides are currently being tested in clinical trials. Despite their indisputable therapeutic potential, they have been associated with non-specific effects caused by interactions with intracellular and cell-surface proteins and non-specific cleavage of unintended targets (4).
Numerous sugar, base and backbone chemical modifications have been investigated to improve the binding affinity and increase the nuclease stability of antisense oligonucleotides. Modification of the 2′-O position of the ribose moiety has been the focus of much research (5). In general, 2′-O modified oligonucleotides cannot act as a substrate for RNase H, which so far seems to be the single important mechanism for control of gene expression. This potential drawback in the application of modified oligonucleotides for down-regulation has been remedied by the use of chimeric oligonucleotides (6), the so-called gapmers, which comprise a central stretch of RNase H recruiting nucleotides (typically phosphorothioates) flanked by modified nucleotides.
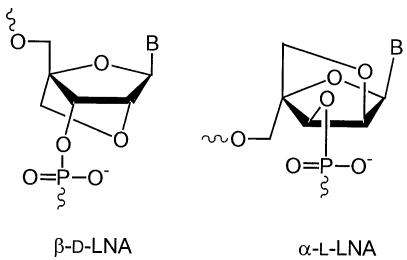
Much of the recent interest has been focused on the preparation of conformationally restricted derivatives. Beta-d-LNA (β-d-LNA, Locked Nucleic Acid), first described by Wengel and co-workers (7,8) and by Imanishi and co-workers (9), contains a methylene 2′-O, 4′-C linkage (Fig. 1). This bridge reduces the conformational flexibility and confers a RNA-like C3′-endo conformation to the sugar moiety of the nucleotide (10). This greatly enhances affinity towards DNA and RNA targets (Δ Tm values from 4.0 to 9.3°C per introduced LNA monomer compared to unmodified duplexes) (11).
Figure 1.
Chemical structures of β-d-LNA and α-l-LNA.
In 2002, Erdmann and co-workers (12) presented a systematic study on the nuclease stability and RNase H recruiting of β-d-LNA containing oligonucleotides using 18mers and concluded that a gap of 7 or 8 nt DNA is necessary for activation. The gapmers further exhibited increased stability against nucleases; as little as three β-d-LNAs at each end of the oligonucleotide increased the stability in human serum more than 10-fold.
The unprecedented thermal stability of duplexes involving β-d-LNA prompted researchers to study the properties of various LNA stereoisomers (13,14), especially alpha-l-LNA (α-l-LNA) (15–18) (Fig. 1). It is well established that β-d-LNA is locked in a N-type conformation and thus is yielding an A-form duplex with complementary DNA (17,19) and an almost canonical A-form (the natural form of double stranded RNA) with complementary RNA (10). Duplexes between α-l-LNA and DNA adopt a B-form (20) (the natural form of double stranded DNA), whereas duplexes α-l-LNA:RNA generate an intermediate structure between A and B form (16), which is structurally closer to the natural substrate of RNase H and thus is able to partially elicit RNase H activity (18). α-l-LNA has also been reported to confer increased stability towards nucleases (18).
Here we report the biochemical and biophysical properties of oligonucleotides containing α-l-LNA, β-d-LNA and chimeras with all three β-d-LNA, α-l-LNA and DNA. In particular, a systematic evaluation of different chimeric oligonucleotides of different size, design and load of LNA, in terms of RNase H activity, nuclease stability and down-regulation of target mRNA was carried out. We confirm the results of Erdmann and co-workers (12) and further complement them with more data regarding β-d-LNA.
MATERIALS AND METHODS
Monomer synthesis
The α-l-LNA monomers were prepared following published procedures (18) with the exception of the final phosphitylation step which was performed according to Pedersen et al. (21). The β-d-LNA monomers were obtained from Proligo (Boulder, CO).
Oligonucleotide synthesis
Oligonucleotides were synthesized using the phosphoramidite approach on an Expedite 8900/MOSS synthesizer (Multiple Oligonucleotide Synthesis System) at 1 µmol scale. At the end of the synthesis (DMT-on), the oligonucleotides were cleaved from the solid support using aqueous ammonia for 1–2 h at room temperature, and further deprotected for 4 h at 65°C. The oligonucleotides were purified by reverse phase HPLC (RP-HPLC). After the removal of the DMT-group, the oligonucleotides were characterized by AE-HPLC or RP-HPLC, and the molecular mass was further confirmed by ESI-MS.
UV-melting measurements
Melting curves were recorded with a Perkin Elmer UV/Vis spectrophotometer lambda 40 attached to a PTP-6 Peltier System. Oligonucleotides were dissolved in buffer (20 mM phosphate buffer, 100 mM NaCl, 0.1 nM EDTA, pH 7) using the two complementary strands at 1.5 µM and 1 cm path-length cells. Samples were denatured at 95°C for 3 min and slowly cooled to 20°C prior to measurements. Melting curves were recorded at 260 nm using a heating rate of 1°C/min, a slit of 2 nm and a response of 0.2 s. Tm values were obtained from the maxima of the first derivatives of the melting curves.
3′-Exonuclease stability study
Snake venom phosphodiesterase (SVPD, Amersham Biosciences) assays were performed using 26 µg/ml oligonucleotide, 0.3 µg/ml enzyme at 37°C in a buffer of 50 mM Tris–HCl, 10 mM MgCl2, pH 8. The enzyme was shown to maintain its activity under these conditions for at least 2 h. Aliquots of the enzymatic digestion were removed at the indicated times, quenched by heat denaturation for 3 min and stored at –20°C until analysis by RP-HPLC.
S1 endonuclease stability study
S1 endonuclease (Amersham Biosciences) assays were performed using 1.5 µM oligonucleotide and 16 U/ml enzyme at 37°C in a buffer of 30 mM NaOAc, 100 mM NaCl, 1 mM ZnSO4, pH 4.6. The enzyme was shown to maintain its activity under these conditions for at least 2 h. Aliquots of the enzymatic digestion were removed at the indicated times, quenched by lyophilization, and stored at –20°C until analysis by either RP-HPLC and ESI-MS or polyacrylamide electrophoresis.
Luciferase assay
The X1/5 HeLa cell line (ECACC Ref. No. 95051229) stably transfected with a Tet-Off luciferase system was used. In the absence of tetracycline, the luciferase gene is expressed constitutively. The expression was measured as light emission in a luminometer after addition of the luciferase substrate (luciferin). The X1/5 HeLa cell line was grown in Minimum Essential Medium Eagle (Sigma M2279) supplemented with 1× Non Essential Amino Acid (Sigma M7145), 1× Glutamax I (Invitrogen 35050–038), 10% FBS calf serum, 25 µg/ml Gentamicin (Sigma G1397), 500 µg/ml G418 (Invitrogen 10131–027) and 300 µg/ml Hygromycin B (Invitrogen 10687–010). The X1/5 HeLa cells were seeded at a density of 8000 cells/well in a white 96-well plate (Nunc 136101) the day before transfection. Before the transfection, the cells were washed once with OptiMEM (Invitrogen) followed by addition of 40 µl of OptiMEM with 2 µg/ml of Lipofectamine2000 (Invitrogen). The cells were incubated for 7 min before addition of the oligonucleotides. Then 10 µl of oligonucleotide solutions were added and the cells were incubated for 4 h at 37°C and 5% CO2. The cells were then washed once in OptiMEM and growth medium was added (100 µl). The luciferase expression was measured the day after. Luciferase expression was measured with the Steady-Glo luciferase assay system from Promega. 100 µl of the Steady-Glo reagent was added to each well and the plate was shaken for 30 s at 700 r.p.m. The plate was read in a Luminoscan Ascent instrument from ThermoLab systems after 8 min of incubation to complete total lysis of the cells. Luciferase expression was measured as Relative Light Units per second (RLU/s). The data were processed with Ascent software (v2.6) and graphs were drawn with SigmaPlot2001. All data were normalized to mock levels. No significant cell death was observed by microscopy for the duration of the assay.
RNase H assay
The RNA was radiolabeled (32P) at the 5′-end with T4 kinase (Invitrogen) following well-established procedures; 25 nM RNA was incubated in the presence of a 10-fold excess of various complementary oligonucleotides in 1× TMK-glutamate buffer [20 mM TrisOAc, 10 mM Mg(OAc)2 and 200 mM potassium glutamate, pH 7.25] supplied with 1 mM DTT in a reaction volume of 40 µl. The reactions were pre-incubated for 3 min at 65°C followed by 15 min at 37°C before addition of RNase H (Promega); 0.2 U of RNase H was added, and samples were withdrawn (6 µl) to formamide dye (3 µl) on ice at the time points 0, 10, 20 and 30 min after RNase H addition; 3 µl of the 0, 10, 20 and 30 min samples were loaded on a 15% polyacrylamide gel containing 6 M urea and 0.9× Tris borate/EDTA buffer. The gel was 0.4 mm thick and run at 35 W for 2 h. The gel was dried for 60 min at 80°C, followed by overnight exposure on a Kodak phosphorscreen. The Kodak phosphorscreen was read in a Bio-Rad FX instrument and the result was analysed with Bio-Rad Quantity One software.
RESULTS AND DISCUSSION
β-d-LNA gapmers: evaluation of the optimal gap size
Our first goal was to determine the optimal DNA stretch (gap size) for a 16mer β-d-LNA/DNA chimera for best antisense activity, and correlate it with the corresponding affinity against the complementary DNA strand.
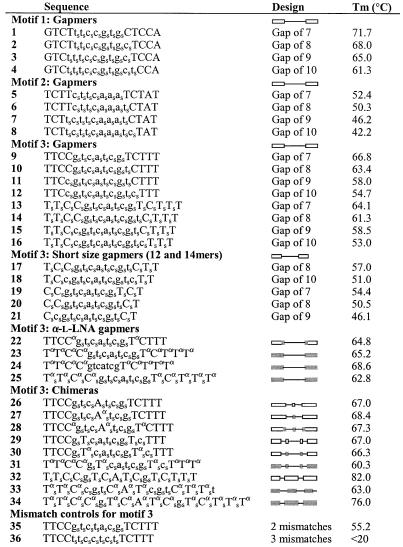
In a previous study, we tested different gapmers (16mers, 7 nt gap size) containing β-d-LNA against firefly luciferase mRNA by messenger walk screening, and observed moderate potencies for motifs 1, 2 and 3 in a dose–response manner.
The three different motifs of the mRNA of the firefly luciferase were targeted with 16mers with a PS gap and PO phosphate interlinkages in the β-d-LNA flanks. The DNA gap size varied from 7 to 10 nt with the β-d-LNA segment length adjusted accordingly to keep the overall length of the chimeric compound fixed (1–12 in Table 1).
Table 1. Sequences of the oligonucleotides used in this study.
The corresponding designs are also illustrated with drawings for the different gapmers and chimeras. Nomenclature for design column: line is DNA, the rectangle is β-d-LNA and the shaded rectangle is α-l-LNA residues. Upper case for LNA and lower case for DNA.
For all three studied motifs, it has been seen that the affinity drops with the reduction of LNA residues in the oligonucleotide and that the affinity is sequence dependent (Fig. 2). Gapmers (1–4) against motif 1 show a high effect in down-regulation with the increase in the gap size, with 10 nt being the best. This 10 nt gapmer (4) still presents a very high affinity (Tm 61.3°C). Gapmers (5 –8) against motif 2 exhibit a reduced effect in down-regulation as the gap size increases, 7 nt (5) being the most potent one (Tm 52.4°C). For this motif, the increase in gap size results in a critical loss of affinity resulting in less potent oligonucleotides. For motif 3, the 9 nt gap (11) (Tm 58.0°C) proves to be the most potent gapmer.
Figure 2.
Down-regulation of Luciferase expression by gapmers (1–12) with PS in the gap and PO in the flanks containing an increasing gap size from 7 to 10 nt directed against three different motifs (1, 2 and 3) at 2 nM. The melting temperatures (Tm) for the different gapmers are also included in the graph.
For motif 1, for which the affinity remains high enough, a larger gap (10 nt over 7, 8 and 9 nt) is an advantage in terms of RNase H recruitment and thus antisense activity, whereas motif 2 evidences that if the affinity lies within a low range, the gap size is not so determinant. Motif 3 affinities are apparently around the critical limit range; therefore both affinity and gap size play a role in determining the activity of these gapmers. These three motifs clearly demonstrate that there is a delicate balance between affinity and optimal gap size. In our assay, all gapmers showed extensive RNase H recruitment (vide infra).
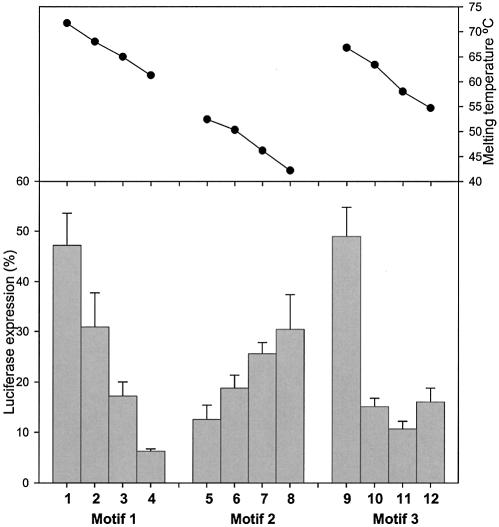
We also repeatedly observed for chimeric oligonucleotides containing β-d-LNA an overall trend of better down-regulation with an increase in the number of PS interlinkages for different gapmer designs. This is illustrated by motif 3 (gap PS) in Figure 2 compared to the 16mer (all PS) in Figure 3. Fully thiolated gapmers (13–16, all PS) perform better in the Luciferase assay than gapmers with a thiolated gap (9 –12, gap PS), and than the gapmers with all PO interlinkages (data not shown). The much lower activity of the all PO gapmers could be due to their rapid degradation by nucleases (vide infra).
Figure 3.
Down-regulation of Luciferase expression by fully thiolated oligonucleotides (all PS, 17–21) with reduced size and various gap sizes (12 and 14mers) at 2 nM. The 16mer (all PS, 13–16) is included as a reference. The melting temperatures (Tm) for the different gapmers are also included in the graph.
β-d-LNA gapmers: evaluation of the size and the gap size
The high affinity achieved by the introduction of β-d-LNA (11) should allow for the design of potent and short antisense oligonucleotides (<15 nt). Therefore, we evaluated different gap sizes for shorter oligonucleotides, 12 and 14mers, in terms of antisense activity, and correlated the data with the corresponding affinity against a complementary DNA strand. All oligonucleotides were also tested for the ability to direct target RNA cleavage by RNase H. Different 14mers (17, 18) and 12mers (19–21) against motif 3 (both ends-shortened oligonucleotides compared to the previously described 16mers) were prepared with all PS, and the DNA gap size varied with the β-d-LNA segment length adjusted accord ingly to keep the overall length of the chimeric compound fixed.
As it can be appreciated from Figure 3, the balance between affinity and gap size seems for these 14 and 12mers to prefer a gap of 8 nt (17 and 20). For the 14mer, an increased gap (10 nt, 18), and therefore a lower β-d-LNA substitution, corresponds to a decrease in affinity, which is reflected in a drop in antisense activity. Compared to the 16 and 14mers, the 12mers show both lower affinity and lower antisense activity, and present maximal activity for the 8 nt gap sized gapmer. As previously shown for the 16mers, a compromise between gap size and affinity determines the optimal gap size for a chimeric oligonucleotide containing β-d-LNA. Again in our assay, all gapmers showed extensive RNase H recruitment.
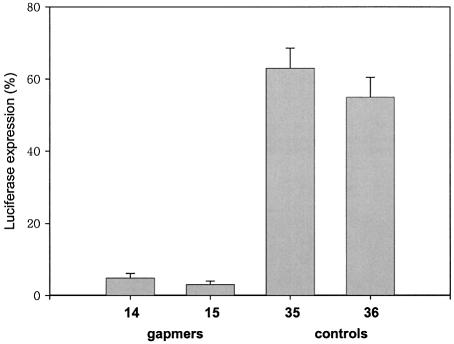
In order to prove that down-regulation is specific for a sequence-depending mechanism, we further tested two of our best performing antisense oligonucleotides (14 and 15) against motif 3 and compared them with two related mismatch-containing control oligonucleotides (35 and 36). It can be appreciated from Figure 4 that oligonucleotides 14 and 15 present more potent antisense activity than the corresponding mismatch-containing oligonucleotides 35 and 36. In particular, we see a 14–21-fold loss in down-regulation for the mismatch-containing controls, thus being supportive for a sequence-specific mode of action.
Figure 4.
Down-regulation of Luciferase expression by oligonucleotides 14 and 15, and the corresponding mismatch-containing control oligonucleotides 35 and 36 directed against motif 3 at 2 nM.
Nuclease stability of α-l-LNA and β-d-LNA
The protection of oligonucleotides with LNA against nucleases has been shown (12,18). However, no systematic study of end-protection with α-l-LNA had been presented, which is a relevant issue in the design of oligonucleotides containing α-l-LNA. Therefore, we prepared oligonucleotides with α-l-LNA at the 3′-end (t10Tαt and t9Tα2t) and evaluated their nucleolytic stability against a 3′-exonuclease (Snake Venom Phosphodiesterase, SVPD), and thus compared them with the corresponding β-d-LNA.
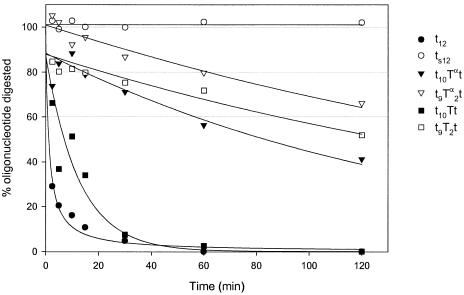
As previously noted (22), the introduction of just one β-d-LNA (t10Tt), gives no increase in stability compared to DNA (t12); however, the introduction of one α-l-LNA (t10Tαt) at the 3′-end of the oligonucleotide represents a significant gain in stability (40% full length oligonucleotide present after 2 h digestion) (Fig. 5). The introduction of two modifications (t9Tα2t) contributes even more to the stability of the oligonucleotide (65% full length after 2 h digestion) and is superior to the corresponding β-d-LNA (t9T2t).
Figure 5.
Kinetic study of oligonucleotides containing α-l-LNA at the 3′-end (t10Tαt and t9Tα2t) against a 3′-exonuclease (SVPD), in order to evaluate the nucleolytic stability. The graph also shows the corresponding controls (t12, ts12, t10Tt and t9T2t). The assays were performed using 26 µg/ml oligonucleotide, 0.3 µg/ml enzyme at 37°C in a buffer of 50 mM Tris–HCl, 10 mM MgCl2, pH 8. Aliquots of the enzymatic digestion were removed at the indicated times. In a separate assay, the enzyme was shown to maintain its activity under these conditions for at least 2 h (data not shown). The oligonucleotides were synthesized on deoxynucleoside-support, t. Upper case for LNA and lower case for DNA.
The gapmer is the most used design in antisense strategy with 2′-O modified nucleotides. This however has the disadvantage of turning the oligonucleotides more sensitive against endonucleases. Therefore, we wanted to evaluate the effect of an increasing deoxynucleotide gap on the stability against S1-endonuclease using 16mers with LNA containing flanks (Fig. 6).
Figure 6.
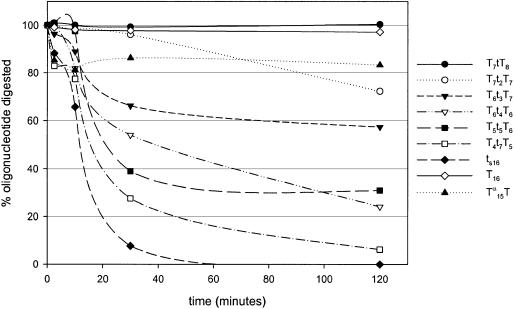
Kinetic study of different oligonucleotide gapmers (16mers) with an increasing deoxynucleotide gap against S1-endonuclease, in order to evaluate the nucleolytic stability. The graph also shows the stability of ts16, T16 and Tα15T against S1-endonuclease. The assay was performed using 1.5 µM oligonucleotide and 16 U/ml enzyme at 37°C in a buffer of 30 mM NaOAc, 100 mM NaCl, 1 mM ZnSO4, pH 4.6. Aliquots of the enzymatic digestion were removed at the indicated times. In a separate assay, the enzyme was shown to maintain its activity under these conditions for at least 2 h (data not shown). Upper case for LNA and lower case for DNA.
We saw that fully modified oligonucleotides with β-d-LNA or α-l-LNA are very stable against S1-endonuclease. After 2 h digestion, most of the β-d-LNA (T16) (85%) and α-l-LNA (Tα15T) (over 80%) remained, while the corresponding phosphorothioate could not be detected after 30 min digestion (Fig. 6).
Increasing the gap size results in a decrease in stability against S1-endonuclease (Fig. 6). As expected, no fragmentation of the LNA flanks was identified. It is important to note that with a gap of three DNAs, over 60% of the oligonucleotide is still full length after 120 min. This is significantly better than phosphorothioates, which in this assay are completely degraded in approximately 40 min.
This study was carried out with gapmers containing β-d-LNA in the flanks, but investigations with gapmers and chimeras (vide infra) containing α-l-LNA are currently under way.
Gapmers containing α-l-LNA
Bearing in mind that α-l-LNA affords more RNA:DNA like duplexes than β-d-LNA, we investigated the properties of oligonucleotides containing α-l-LNA both in the Luciferase assay and in the RNase H assay.
We started with a conservative approach, preparing different gapmers containing α-l-LNA, and evaluating them for their ability to down-regulate the target mRNA in the Luciferase assay. First, we substituted two β-d-LNA residues for two α-L-LNAs in a 16 gapmer against motif 3 with a thiolated 7 nt gap (PS gap), and placed the α-l-LNA in the junctions (the two residues in each flank next to the DNA gap, 22), in order to evaluate oligonucleotides that contain a low number of α-l-LNA. Then, we fully substituted both flanks with α-l-LNA and evaluated the effect (23–25).
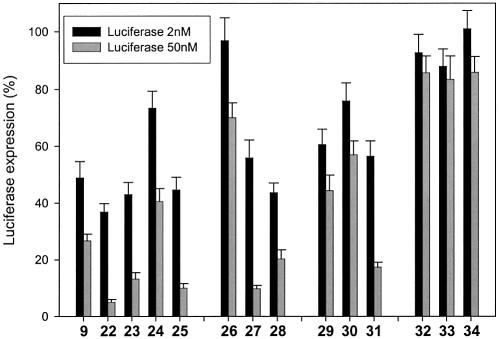
Oligonucleotide 22 shows potent antisense activity. It is actually 5-fold more potent than the corresponding all β-d-LNA gapmer (9, gap of 7 nt) (Fig. 7). Also, at 2 nM an increase in activity compared to the β-d-LNA reference is observed.
Figure 7.
Down-regulation of Luciferase expression by different gapmers (9, 22–25) and chimeras (26–34) containing α-l-LNA and β-d-LNA at 2 and 50 nM. Note: oligonucleotides with and without FAM were tested and compared, and no significant difference was appreciated between the free and FAM-labeled ones.
The second design (all α-l-LNA in both flanks, 23 and 25) presents slightly better down-regulation levels than observed for β-d-LNA gapmers, both at 2 and 50 nM. As observed for β-d-LNA gapmers, a higher load of PS interlinkages correlates with a better antisense activity (Fig. 7).
The introduction of α-l-LNA proves to be a potent tool for the construction of different gapmers, with good antisense activity. The placement of α-l-LNA in the junctions or in the flanks results in very potent oligonucleotides. Again all gapmers showed efficient RNase H recruitment (22 is shown in Fig. 8).
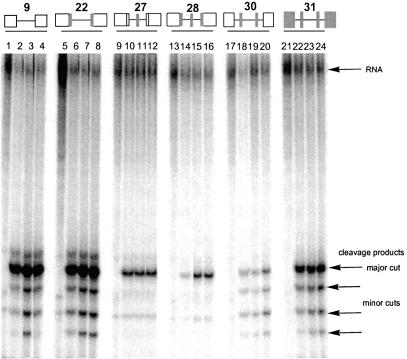
Figure 8.
Electrophoresis analysis of 32P-labeled target RNA degradation products. The chimeras (27, 28, 30, 31 and gapmer 22) containing α-l-LNA and the labeled RNA were incubated with E.coli RNase H1 and aliquots were taken at 0, 10, 20 and 30 min, electrophoresed and reaction products visualized by autoradiography. A gapmer (9) containing β-d-LNA was included in the gel for reference. In the drawing, the line is DNA, the rectangle LNA and the gray shadow corresponds to α-l-LNA residues.
Further design possibilities with α-l-LNA
We have shown that oligonucleotides containing α-l-LNA in a gapmer design present potent antisense activity, both with fully substitution of the flanks and with α-l-LNA only in the junctions of the flanks. Furthermore, since the ability of α-l-LNA for recruiting RNase H (18) was anticipated for different chimeras, we decided to further explore the possibilities. α-l-LNA in different chimeras (a chimera comprises an alternate composition of DNA, β-d-LNA and/or α-l-LNA) was tested for their antisense activity in the Luciferase assay and for RNase H recruitment, which will be described in the next section. The equivalent chimeras exclusively containing β-d-LNA were also prepared, in order to establish a comparison. Table 1 illustrates the chosen designs of chimeras against motif 3.
The first chimera consisted of a gapmer (with a 7 nt gap) containing β-d-LNA in the flanks and one β-d-LNA residue in the middle of the gap (26 in Table 1). This turns the 7 nt gap into two 3nt gaps and thus should provide the oligonucleotide with further stability against nucleases. The presence of just one β-d-LNA residue interrupting the stretch of DNAs in the gap results in a dramatic loss of down-regulation (26, Fig. 7). We evaluated the antisense activity of the corresponding chimera, presenting an α-l-LNA residue interrupting the DNA stretch (27). Using α-l-LNA, the design shows significant down-regulation at 50 nM concentration of oligonucleotide, thus achieving significant down-regulation with a DNA gapsize of only 3 nt residues (Fig. 7). Chimera 28, which contains two α-l-LNA residues at the edges of the flanks and one α-l-LNA residue interrupting the gap, also shows potent levels of down-regulation compared to 26.
Next, we evaluated the interruption of the DNA stretch with two LNA residues, comparing the effect of using β-d-LNA or α-l-LNA. In 29, we placed two β-d-LNA residues interrupting the gap. This resulted in poor antisense activity. No gain in antisense activity was recorded by placing two α-l-LNAs in the gap (30). However, a significant gain at 50 nM was obtained when both the two residues in the gap and all the flanks were substituted with α-l-LNA (31) (Fig. 7).
We also included highly substituted α-l-LNA oligonucleotides, in order to further explore the possibilities of α-l-LNA. Chimera 33 is based on a design containing 2′-deoxy-2′-fluoro-β-d-arabinonucleosides (2′-F-ANA) (23) for which Damha et al. claimed that the ability to elicit RNase H degradation of target RNA is significantly better than for the all DNA antisense oligonucleotides and for a 2′-F-ANA/DNA gapmer construct. Since α-l-LNA also shows partial RNase H recruitment, that design might also be a good design using α-l-LNA. Oligonucleotide 34 was also evaluated in terms of antisense activity. Interestingly, highly modified chimeras were inefficient in directing down-regulation (Fig. 7). Oligo nucleotide 33 was also evaluated in a RNase H assay and showed no cleavage under our experimental conditions (data not shown). This most probably accounts for its poor antisense activity.
The affinity (Tm) of all oligonucleotides containing α-l-LNA was measured against complementary DNA. Even though α-l-LNA is known to confer lower thermal stability to the oligonucleotide than β-d-LNA, it was still very high for all of them (Tm > 60°C).
RNase H recruitment of the chimeras containing α-l-LNA
Since RNase H recruitment is a significant property of an antisense oligonucleotide, most oligonucleotides of this study were further evaluated in a RNase H assay, as already mentioned. It was especially interesting to evaluate the chimeras containing α-l-LNA. In Figure 8, we can see a representative electrophoresis gel for different chimeras containing α-l-LNA.
Lanes 1–4 (Fig. 8) show the very rapid degradation of the targeted RNA with a β-d-LNA gapmer (9, reference). Lanes 5–24 show the activation of RNase H with different chimeras (27, 28, 30, 31 and gapmer 22) containing β-d-LNA and/or α-l-LNA in the flanks and α-l-LNA interrupting the gap. In all cases, RNase H activation is observed. However, preferences in cleavage sites varied depending on the design (minor cuts). Typically, they were at the residue complementary to the last DNA residue before the flank towards the 5′-end of the antisense oligonucleotide (major cut), and in the middle of the gap. Other minor cuts were found, always at the sites complementary to the DNA gap.
In our set-up, the RNase H assay has been shown to be not very discriminative, and has only been used as a first and non-restrictive filter in the selection of most potent antisense oligonucleotides. No prediction of antisense activity can be anticipated just from this assay.
CONCLUSIONS
From our study, we conclude that the optimal gap size for a gapmer (16mer) containing β-d-LNA lies between 7 and 10 nt. A further determination (discrimination between 7 and 10 nt) is motif-dependent and relates to a delicate balance between affinity and gap size. The high affinity caused by the introduction of β-d-LNA enables the use of potent antisense oligonucleotides as short as 12 and 14 nt in size. Compared to β-d-LNA, α-l-LNA shows superior stability against a 3′-exonuclease. α-l-LNA proved to be a potent tool enabling the construction of different gapmers and chimeras, which present potent antisense activity. The presence of α-l-LNA in the gap and in the junctions results in oligonucleotides with potent antisense activity, thus potentially conferring stability to the oligonucleotides against nucleases.
In conclusion, both β-d-LNA and α-l-LNA are shown to be excellent in the design of antisense oligonucleotides with potent antisense activity. Furthermore, α-l-LNA presents more versatile design possibilities.
Acknowledgments
ACKNOWLEDGEMENTS
The authors wish to thank Emine Bilgin for assistance in synthesizing oligonucleotides and Henrik Olsen for assistance in the Luciferase and RNase H assays.
REFERENCES
- 1.Zamecnik P.C. and Stephenson,M. (1978) Inhibition of Rous Sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl Acad. Sci. USA, 75, 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uhlmann E. and Peyman,A. (1990) Antisense oligonucleotides: A new therapeutic Principle. Chem. Rev., 90, 543–584. [Google Scholar]
- 3.Crooke S.T. (1999) Molecular mechanisms of action of antisense drugs. Biochim. Biophys. Acta, 1489, 31–44. [DOI] [PubMed] [Google Scholar]
- 4.Woolf T.M., Melton,D.A. and Jennings,C.G.B. (1992) Specificity of antisense oligonucleotides in vivo. Proc. Natl Acad. Sci. USA, 89, 7305–7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook P.D. (1999) Making drugs out of oligonucleotides: a review and perspective. Nucl. Nucl., 18, 1141–1162. [DOI] [PubMed] [Google Scholar]
- 6.Monia B.P., Lesnik,E.A., Gonzalez,C., Lima,W.F., McGee,D., Guinosso,C.J., Kawasaki,A.M., Cook,P.D. and Freier,S.M. (1993) Evaluation of 2′-modified oligonucleotides containing 2′-deoxy gaps as antisense inhibitors of gene expression. J. Biol. Chem., 268, 14514–14522. [PubMed] [Google Scholar]
- 7.Koshkin A.A., Singh,S.K., Nielsen,P., Rajwanshi,V.K., Kumar,R., Meldgaard,M., Olsen,C.E. and Wengel,J. (1998) LNA (Locked Nucleic Acids): Synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation and unprecedented nucleic acid recognition. Tetrahedron, 54, 3607–3630. [Google Scholar]
- 8.Singh S.K., Nielsen,P., Koshkin,A.A. and Wengel,J. (1998) LNA (locked nucleic acids): synthesis and high-affinity nucleic acid recognition. Chem. Commun., 455–456. [Google Scholar]
- 9.Obika S., Nanbu,D., Hari,Y., Andoh,J., Morio,K., Doi,T. and Imanishi,T. (1998) Stability and structural features of the duplexes containing nucleoside analogues with a fixed N-type conformation, 2′-O,4′-C -methyleneribonucleosides. Tetrahedron Lett., 39, 5401–5404. [Google Scholar]
- 10.Petersen M., Bondensgaard,K., Wengel,J. and Jacobsen,J.P. (2002) Locked Nucleic Acid (LNA) recognition of RNA: NMR solution structures of LNA:RNA hybrids. J. Am. Chem. Soc., 124, 5974–5982. [DOI] [PubMed] [Google Scholar]
- 11.Wengel J. (2001) LNA (Locked Nucleic Acid). In Crooke,S.T. (ed.), Antisense Drug Technology; Principles, Strategies and Applications. Marcel Dekker, Inc., New York, Basle, pp. 339–357. [Google Scholar]
- 12.Kurreck J., Wyszko,E., Gillen,C. and Erdmann,V.A. (2002) Design of antisense oligonucleotides stabilized by Locked Nucleic Acids. Nucleic Acids Res., 30, 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajwanshi V.K., Håkansson,A.E., Sørensen,M.D., Pitsch,S., Singh,S.K., Kumar,R., Nielsen,P. and Wengel,J. (2000) The eight stereoisomers of LNA (Locked Nucleic Acid): A remarkable family of strong RNA binding molecules. Angew. Chem. Int. Ed. Engl., 39, 1656–1659. [DOI] [PubMed] [Google Scholar]
- 14.Rajwanshi V.K., Kumar,R., Kofod-Hansen,M. and Wengel,J. (1999) Synthesis and restricted furanose conformations of three novel bicyclic thymine nucleosides: a xylo-LNA nucleoside, a 3′-O,5′-C-methylene linked nucleoside and a 2′-O,5′-C-methylene-linked nucleoside. J. Chem. Soc., Perkin Trans. 1, 1407–1414. [Google Scholar]
- 15.Rajwanshi V.K., Håkansson,A.E., Dahl,B.M. and Wengel,J. (1999) LNA stereoisomers: xylo-LNA (β-d-xylo configured locked nucleic acid) and α-l-LNA (α-l-ribo configured locked nucleic acid). Chem. Commun., 1395–1396. [Google Scholar]
- 16.Petersen M., Håkansson,A.E., Wengel,J. and Jacobsen,J.P. (2001) α-l-LNA (α-l-ribo configured Locked Nucleic Acid) recognition of RNA. A study by NMR spectroscopy and molecular dynamics simulations. J. Am. Chem. Soc., 123, 7431–7432. [DOI] [PubMed] [Google Scholar]
- 17.Wengel J., Petersen,M., Nielsen,K.E., Jensen,G.A., Håkansson,A.E., Kumar,R., Sorensen,M.D., Rajwanshi,V.K., Bryld,T. and Jacobsen,J.P. (2001) LNA (locked nucleic acid) and the diastereoisomeric α-l-LNA: conformational tuning and high-affinity recognition of DNA/RNA targets. Nucl. Nucl., 20, 389–396. [DOI] [PubMed] [Google Scholar]
- 18.Sørensen M.D., Kværnø,L., Bryld,T., Håkansson,A.E., Verbeure,B., Gaubert,G., Herdewijn,P. and Wengel,J. (2002) α-l-ribo configured Locked Nucleic Acid (α-l-LNA): Synthesis and properties. J. Am. Chem. Soc., 124, 2164–2176. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen C.B., Singh,S.K., Wengel,J. and Jacobsen,J.P. (1999) The solution structure of a locked nucleic acid (LNA) hybridized to DNA. J. Biomol. Struct. Dyn., 17, 175–191. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen K.E., Petersen,M., Håkansson,A.E., Wengel,J. and Jacobsen,J.P. (2002) α-l-LNA (α-l-ribo configured Locked Nucleic Acid) recognition of DNA: An NMR spectroscopic study. Chem. Eur. J., 8, 3001–3009. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen D.S., Rosenbohm,C. and Koch,T. (2002) Preparation of LNA phosphoramidites. Synthesis, 802–808. [Google Scholar]
- 22.Morita K., Hasegawa,C., Kaneko,M., Tsutsumi,S., Sone,J., Ishikawa,T., Imanishi,T. and Koizumi,M. (2002) 2′-O,4′-C-Ethylene-bridged Nucleic Acids (ENA): Highly nuclease-resistant and thermodynamically stable oligonucleotides for antisense drug. Bioorg. Med. Chem. Lett., 73–76. [DOI] [PubMed] [Google Scholar]
- 23.Min K., Viazovkina,E., Galarneau,A., Parniak,M.A. and Damha,M.J. (2002) Oligonucleotides comprised of alternating 2′-deoxy-2′-fluoro-β-d -arabinonucleosides and d-2′-deoxyribonucleosides (2′F-ANA/DNA ‘altimers’) induce efficient RNA cleavage mediated by RNase H. Bioorg. Med. Chem. Lett., 12, 2651–2654. [DOI] [PubMed] [Google Scholar]