Abstract
Plants produce reactive oxygen species (ROS) in response to environmental stresses sending signaling cues, which, if uncontrolled, result in cell death. Like other aerobic organisms, plants have ROS-scavenging enzymes, such as superoxide dismutase (SOD), which removes superoxide anion radical (O2−) and prevents the production and buildup of toxic free radicals. However, increasing the expression of cytosolic SODs is complex, and increasing their production in vivo has proven to be challenging. To avoid problems with endogenous regulation of gene expression, we expressed a gene from the archaeal hyperthermophile Pyrococcus furiosus that reduces O2−. P. furiosus uses superoxide reductase (SOR) rather than SOD to remove superoxide. SOR is a thermostable enzyme that reduces O2− in a one-electron reduction without producing oxygen. We show that P. furiosus SOR can be produced as a functional enzyme in planta and that plants producing SOR have enhanced tolerance to heat, light, and chemically induced ROS. Stress tolerance in the SOR-producing plants correlates positively with a delayed increase in ROS-sensitive transcripts and a decrease in ascorbate peroxidase activity. The SOR plants provide a good model system to study the impact of cytosolic ROS on downstream signaling in plant growth and development. Furthermore, this work demonstrates that this synthetic approach for reducing cytosolic ROS holds promise as a means for improving stress tolerance in crop plants.
Reactive oxygen species (ROS) such as singlet oxygen (1O2), superoxide anion radical (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH·) are produced as part of normal metabolism by organisms living in aerobic environments (Grene, 2002; Mittler, 2002; Apel and Hirt, 2004; Gapper and Dolan, 2006; Halliwell, 2006; Moller et al., 2007). While an increase in ROS can result in cell death, it is now well accepted that ROS also can function as signaling molecules (Foyer and Noctor, 2003, 2005a, 2005b; Mittler et al., 2004; Bailey-Serres and Mittler, 2006; Mittler, 2006; Kim et al., 2008). In this work, we have focused on the cytosolic O2− signal. Cytosolic O2− is normally metabolized by superoxide dismutase (SOD) to produce H2O2 and oxygen. H2O2 can elicit additional signals, and oxygen can serve as a substrate for further ROS production.
Several previous studies have indicated that increasing endogenous SODs enhances stress tolerance (McKersie et al., 1993, 1996, 1999; Samis et al., 2002). Overexpression of SOD targeted to chloroplast enhanced resistance to methyl viologen (Slooten et al., 1995) and increased oxidative stress tolerance (Van Camp et al., 1996; Van Breusegem et al., 1999; McKersie et al., 2000; Gupta et al., 1993a, 1993b). Additional evidence for the importance of organellar SOD for plant growth came from studies in which decreasing expression of mitochondrial manganese SOD resulted in reduction of root growth in young seedlings and a change in the redox balance (Morgan et al., 2008).
Altering cytosolic SOD also affects stress tolerance. Recently, new insights into the regulation of the copper/zinc (Cu/Zn) SOD were revealed through microRNA studies (Sunkar et al., 2006; Abdel-Ghany and Pilon, 2008; Dugas and Bartel, 2008). Both cytosolic and chloroplast Cu/Zn SOD are negatively regulated by miR398. Mutating or suppressing miR398 increased the production of both Cu/Zn SODs and was reported to enhance tolerance to high light, heavy metals, and other oxidative stresses (Sunkar et al., 2006); however, this phenotype appears to vary with the growth conditions of the seedlings (Dugas and Bartel, 2008). In summary, present data indicate that the functional temperature range and production of plant enzymes to remove O2− are limited by endogenous mechanisms regulating either enzyme function or gene expression, and compensatory mechanisms are needed to reduce secondary oxygen species (Grene, 2002; Foyer and Noctor, 2005b).
Our approach has been to use a heterologous system to constitutively dampen cytosolic O2− signaling and reduce ROS toxicity. To avoid endogenous regulatory mechanisms, we selected superoxide reductase (SOR), an enzyme found in anaerobic microorganisms that reduces superoxide in a one-electron reduction reaction. Pyrococcus furiosus normally lives in anaerobic hydrothermal vents (Fiala and Stetter, 1986). To avoid cellular damage arising from oxygen exposure when it is expelled into the cold, oxygenated seawater, P. furiosus uses the extremely efficient enzyme SOR to reduce O2− (Jenney et al., 1999; Grunden et al., 2005). There are three major types of SORs that are classified based on their N-terminal structures (Hazlett et al., 2002). For this work, we selected a class II SOR from the archaeal hyperthermophile P. furiosus. The class II SORs do not have an N-terminal iron-binding site. They contain only the C-terminal iron-binding center that reduces O2− (Jenney et al., 1999; Hazlett et al., 2002; Auchère et al., 2006). The sole product of the SOR reduction of O2− is H2O2.
Employing SOR to remove O2− has many advantages compared with SOD. First, in contrast to plant SODs, P. furiosus SOR reduces O2− without producing O2, thus lowering the potential for further ROS generation (Jenney et al., 1999; Jenney and Adams, 2001; Weinberg et al., 2004). Second, P. furiosus SOR is an extremely stable enzyme that has a functional temperature range of 4°C to 100°C (Jenney et al., 1999; Grunden et al., 2005). Third, SOR has a higher affinity for O2− and a higher Kcat than Escherichia coli iron SOD and bovine Cu/Zn SOD (Jenney et al., 1999; Emerson et al., 2003). Fourth, when the gene is expressed in heterologous systems, the active site ferrous ions of SOR will complex with ferrocyanide to reduce O2− to water without forming detectable H2O2 (Molina-Heredia et al., 2006; Kovacs and Brines, 2007). Fifth, because endogenous SODs (CSD1 and CSD2) are regulated by microRNA (Sunkar et al., 2006; Abdel-Ghany and Pilon, 2008; Dugas and Bartel, 2008) and because SOR is not a plant enzyme, it should not be regulated either transcriptionally or posttranscriptionally in the same manner as SOD.
Previously, we have shown that P. furiosus SOR can be expressed in tobacco (Nicotiana tabacum) cells (NT-1) and that it will produce a functional enzyme (Im et al., 2005). To test the efficacy of P. furiosus SOR in planta and to gain an understanding of the impact of dampening a cytosolic O2− signal on plant growth and development, we used another model plant system, Arabidopsis (Arabidopsis thaliana).
RESULTS
Generation of Transgenic Arabidopsis Expressing P. furiosus SOR
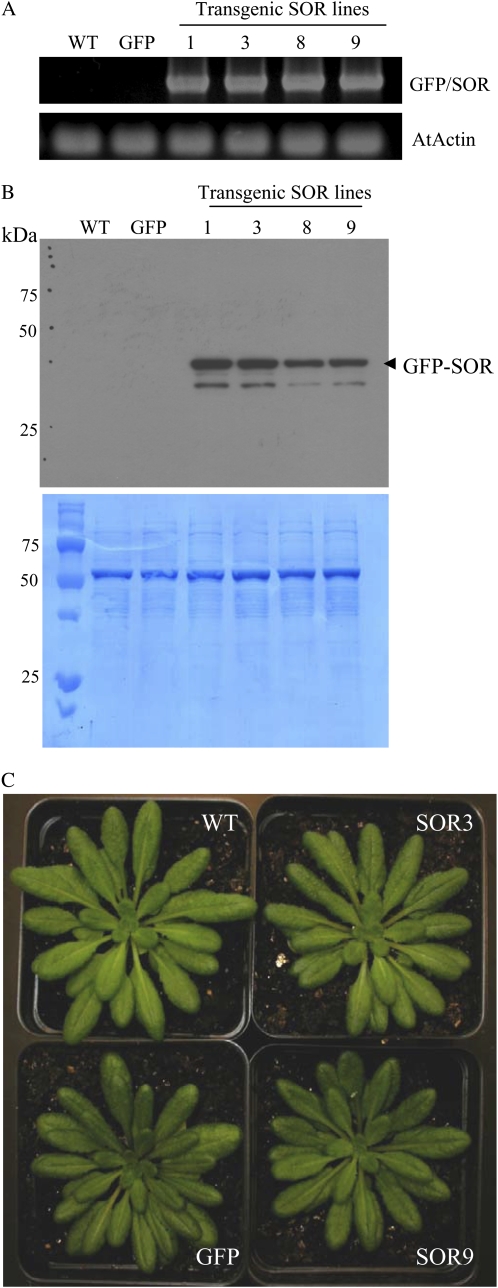
P. furiosus SOR was expressed in Arabidopsis plants as a GFP fusion under the control of the cauliflower mosaic virus 35S promoter using the Gateway vector construct pK7WGF2. We selected four independent homozygous lines (GFP-SOR1, -3, -8, and -9) and one line transformed with the 35S promoter containing only the GFP for further characterization. Expression of the GFP-SOR transgene was confirmed by reverse transcription (RT)-PCR using internal GFP forward and SOR reverse primers (Fig. 1A). With these primers, no transcript was detected in either the wild type or the GFP line. The full-length GFP-SOR (42-kD) protein was detectable in a soluble protein fraction using antibodies raised against P. furiosus SOR (Fig. 1B), and the GFP fluorescence is readily detectable in the cytosol of both root and leaf cells (Supplemental Fig. S1, A and B).
Figure 1.
Arabidopsis plants produce P. furiosus GFP-SOR and grow normally. A, Expression of GFP-SOR in 14-d-old transgenic Arabidopsis plants is shown by RT-PCR analysis using an internal GFP forward primer and a SOR-specific reverse primer to detect the fusion transcript (top panel). Primers specific for Arabidopsis actin were used for the loading control (bottom panel). B, Immunoblot analysis indicates that GFP-SOR is produced in transgenic Arabidopsis plants. Protein was detected with antibodies raised against P. furiosus SOR. The bottom panel is the amido black-stained polyvinylidene difluoride membrane showing the protein extracts from each line. An equal amount (25 μg of protein) of total soluble protein was used in each lane. The GFP-SOR protein (42-kD predicted molecular mass) and smaller proteolytic products are detected in all SOR transgenic lines. C, Plants grown under short-day conditions (8 h of light/16 h of dark) have no obvious phenotype. Leaf size (24.8 ± 1.7 mm for the wild type, 23.4 ± 0.8 mm for GFP, 24.8 ± 1.8 mm for SOR3, and 21.6 ± 1.5 mm for SOR9) and number (36.0 ± 1.7 for the wild type, 39.5 ± 1.4 for GFP, 41.0 ± 0.8 for SOR3, and 39.5 ± 2.5 for SOR9) of 56-d-old plants were not statistically different for any of the SOR lines. WT, Wild type. [See online article for color version of this figure.]
GFP-SOR plants (hereafter denoted as SOR plants) have no morphological differences compared with the wild type and grow similarly under normal growth conditions (8 h of light/16 h of dark; Fig. 1C). The number and size of rosettes are comparable in wild-type and SOR plants over their life cycle. Under short-day conditions, flowering in the SOR plants is usually delayed by 2 to 4 d compared with wild-type and GFP plants (data not shown). Delays in flowering are more pronounced (4–6 d) when plants are grown under continuous light (Supplemental Fig. S2). Continuous light leads to increased ROS and favors the transition from the vegetative to the reproductive phase in Arabidopsis (Gapper and Dolan, 2006). Delays in transition to reproductive growth indicate that flowering in SOR plants is less sensitive to continuous light.
SOR Is Functional in Arabidopsis Plants
SOR activity was quantified using a standard SOD assay that will measure both SOD and class II SOR activity (Im et al., 2005). The assay, denoted SOR/SOD, is based on the competition of these enzymes with cytochrome c for O2−. In the reaction, xanthine and xanthine oxidase are added to produce O2−, which will reduce ferricytochrome c. However, SOR will reduce the O2− generated by xanthine oxidase, thereby preventing the reduction of ferricytochrome c. We can use this assay because the class II SORs will not directly reduce ferricytochrome c (Jenney et al., 1999; Hazlett et al., 2002; Auchère et al., 2006).
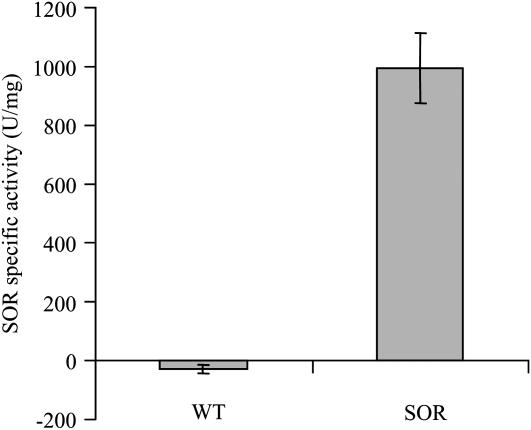
To obtain ample material for the assay, we used leaves from mature plants. Endogenous SOD present in the plant extract also decreases O2− levels, as seen in the basal activity in the wild-type leaves (Table I). Although we cannot distinguish between the SOD and SOR activities with this assay, because P. furiosus SOR is more heat stable than plant SODs, we could enrich for SOR activity by heat treating the plant extracts prior to assaying, as shown in Table I. Using this same protocol, we detected from 3.5- to 4-fold higher SOR/SOD activity in heat-treated samples of young seedlings from the two SOR lines compared with controls (Supplemental Table S1). Further evidence of P. furiosus SOR activity in the plant extracts is given in Supplemental Figure S3. The assay is based on the study from Jenney et al. (1999), which demonstrated that the SOR enzyme can be differentiated from SOD based upon SOR's ability to reoxidize ferrocytochrome c when it is present in reactions in excess amounts. Although the amount of SOR in the plant extracts is low compared with E. coli-expressed protein, the ability to reoxidize ferrocytochrome c is evident by the change in slope compared with the wild-type control.
Table I.
SOR/SOD activity in mature leaves from 56-d-old wild-type and transgenic Arabidopsis plants grown under continuous light for 14 d
One unit of SOR/SOD activity is defined as the amount of enzyme that inhibits the rate of reduction of cytochrome c by 50% (McCord and Fridovich, 1969). Specific activities for heat-treated (HT) samples were determined for cell extracts that were heat treated at 80°C for 15 min as described in “Materials and Methods.” Data are averages ± se of triplicate values from a single experiment.
| Sample | Specific Activity |
|---|---|
| units mg−1 | |
| Wild type | 13.32 ± 0.04 |
| SOR3 | 13.51 ± 1.21 |
| SOR9 | 14.26 ± 0.54 |
| NC906 ± P. furiosus SORa | 43.61 ± 3.76 |
| Wild type (HT) | 19.28 ± 1.66 |
| SOR3 (HT) | 48.08 ± 1.59 |
| SOR9 (HT) | 48.78 ± 1.06 |
Extract of E. coli strain NC906 (sod mutant) expressing P. furiosus SOR was used as a positive control.
Because chromophores in the extracts from green tissue interfered with the in vitro SOR/SOD assay, we assayed root tissue. Basal activity was higher in root samples not only because of a lack of chromophore but also because we did not dialyze the samples overnight prior to the assay. Importantly, roots expressing SOR had 26% to 56% more SOR/SOD activity (Table II). The reproducibly higher activities in the roots of the SOR lines in addition to the increased activity in leaves and seedlings after heat treatment (Table I; Supplemental Table S1) make a compelling argument that extracts from the SOR plants have greater ability to reduce O2− than control plant extracts.
Table II.
SOR/SOD activity in extracts of roots from 28- and 42-d-old wild-type and transgenic Arabidopsis plants
One unit of SOR/SOD activity is defined as the amount of enzyme that inhibits the rate of reduction of cytochrome c by 50% (McCord and Fridovich, 1969). The activity is reported as the average of at least three values from two experiments: one using 28-d-old roots and one using 42-d-old roots. The se is indicated.
| Sample | Specific Activity |
|---|---|
| units mg−1 | |
| Wild type | 273.4 ± 1.9 |
| GFP | 238.1 ± 27.7 |
| SOR3 | 344.8 ± 16.5 |
| SOR9 | 372.3 ± 2.7 |
Because of the endogenous SOD activity, however, it was important to try to purify the recombinant SOR from the plant extracts so that we could confirm its function. To this end, we immunoprecipitated the recombinant SOR from leaf extracts with antibodies raised against P. furiosus SOR (Supplemental Fig. S4). To demonstrate the function of the purified recombinant SOR protein, leaf extracts were heat treated, the recombinant SOR protein was immunoprecipitated, and activity was assayed using a more sensitive tetrazolium salt (WST-1 [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt]) to monitor O2−-mediated reduction (Dojindo Molecular Technologies; Fig. 2). The immunoprecipitant from the SOR transgenic plants had SOR activity, and the wild-type control had none. All of the above results support our thesis that P. furiosus SOR is produced in Arabidopsis as a functional enzyme.
Figure 2.
Immunoprecipitated SOR from the transgenic plants is functional. SOR was immunoprecipitated from heat-treated leaf extracts and assayed using the Dojindo WST-SOD assay kit according to the manufacturer's directions. Wild-type (WT) plants were used as a control. One unit of SOR activity is defined as the amount of enzyme that inhibits the rate of reduction of WST-1 by 50%.
SOR-Expressing Arabidopsis Seeds and Seedlings Have Increased Heat Stability
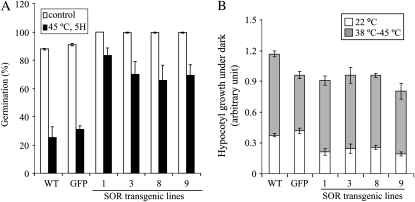
Heat stress can affect plant metabolism and major physiological processes by generating ROS (Larkindale and Knight, 2002; Mittler, 2002; Suzuki and Mittler, 2006; Miller et al., 2008). To determine whether expressing P. furiosus SOR in plants would enhance heat stress tolerance, seeds were used to test for basal thermotolerance and seedlings were used to test for acquired thermotolerance according to Hong and Vierling (2000) and Larkindale et al. (2005), respectively. For the seed germination assay, stratified seeds were treated at 45°C for 5 h, and germination was evaluated 2 d later. Overexpression of GFP itself was used, because GFP has been shown to generate H2O2 in some systems (Tsien, 1998) and, if this occurred in planta, it might thereby increase compensatory ROS-scavenging mechanisms. The SOR lines had a 2- to 3-fold increase in germination compared with both the wild type and GFP (Fig. 3A).
Figure 3.
A, Seeds from SOR transgenics have a higher percentage of germination after heat treatment. One hundred seeds per line were used for the seed germination assay. The data are means ± sd from two independent experiments. B, SOR seedlings also had increased acquired thermotolerance. Acquired thermotolerance was tested by the hypocotyl elongation assay. Briefly, 2.5-d-old etiolated dark-grown seedlings were either maintained at 22°C or treated at 38°C for 1.5 h followed by 45°C for 2 h. Seedlings were returned to 22°C for 2.5 d in the dark. Plates were photographed, and hypocotyl growth was measured using Adobe Photoshop and analyzed using Microsoft Excel. A total of 21 to 31 seedlings per line were used for the hypocotyl elongation assay. The data are means ± sd. WT, Wild type.
Previous work has shown that thermotolerance can vary with the growth stage (Hong and Vierling, 2000; Hong et al., 2003; Clarke et al., 2004; Larkindale et al., 2005); therefore, we investigated acquired thermotolerance with a hypocotyl elongation assay of young seedlings described by Hong and Vierling (2000). Hypocotyl elongation was measured and analyzed before and after treatment. The 2.5-d dark-grown SOR seedlings tended to have the same or slightly lower hypocotyl length for the first 2.5 d compared with wild-type and GFP seedlings (Fig. 3B). After heat treatment, hypocotyl length increased slightly more in the SOR lines such that the ratio of growth after treatment to total growth tended to be slightly (1.3- to 1.5-fold) greater compared with the wild type (Fig. 3B), but there was no dramatic change in acquired thermotolerance using the hypocotyl elongation assay.
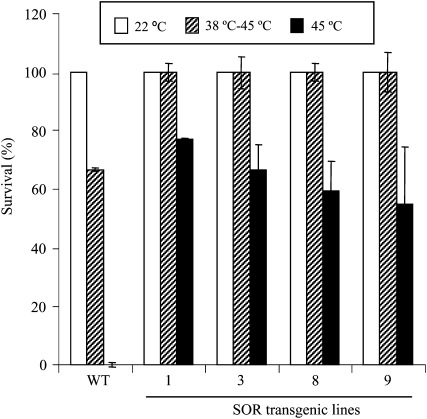
Vegetative-stage plants also were tested. In these experiments, 10-d-old seedlings growing under a short-day light regime were analyzed either for basal thermotolerance by heating directly to 45°C for 2 h or for acquired thermotolerance by acclimation at 38°C for 1.5 h and then incubating 2 h at 45°C. The percentage of seedlings that survived was determined 7 d after heat treatments. All the SOR lines showed a higher survival compared with wild-type plants. The survival for the SOR lines was approximately 50% to 75% for basal thermotolerance and 35% for acquired thermotolerance (Fig. 4). In contrast, none of the wild-type seedlings survived the basal thermotolerance assay. These results indicate that producing the P. furiosus-derived ROS-scavenging enzyme (SOR) in light-grown plants significantly enhanced their tolerance of heat stresses. Furthermore, the effect of SOR was more evident when assessing basal thermotolerance.
Figure 4.
SOR seedlings had a higher percentage of survival following heat treatment. Ten-day-old Arabidopsis seedlings were maintained at 22°C throughout the experiment, treated at 38°C for 1.5 h followed by 45°C for 2 h, or treated at 45°C for 2 h. Following heat stress, the experimental group was returned to the 22°C incubator and all plates were left at 22°C for 7 d. The number of seedlings that survived was counted after 7 d. Eighteen seedlings per line were used in each experiment. The data are means ± sd from three independent experiments. WT, Wild type.
One of the critical intracellular sites of oxidative damage in plants is the chloroplast, where heat-induced disruption of electron transport can take place. Heat stress results in photoinhibition and photobleaching of chlorophyll as well as an increase in ROS (H2O2) in the cytosol (Willekens et al., 1995). Even though SOR is localized in the cytosol, we reasoned that reducing ROS in the cytosol might enhance survival in response to chloroplast-generated ROS (Koussevitzky et al., 2008). To measure the impact of SOR on chlorophyll biosynthesis and stability, 2.5-d dark-grown seedlings were heat treated at 48°C for 30 min and were then exposed to light. Dramatic differences were evident in the SOR lines, as shown in the photograph in Supplemental Figure S5. When chlorophyll was extracted and the levels were measured, heat-treated wild-type seedlings had only 7% of the chlorophyll of non-heat-treated control seedlings, while heat-treated SOR seedlings had 48% to 100% of the control chlorophyll (Table III). The fact that dark-grown SOR lines had increased heat tolerance under high light indicates that the effects of SOR went beyond the cytosol.
Table III.
Etiolated SOR seedlings accumulate more chlorophyll after heat treatment
Etiolated seedlings were grown for 2.5 d in the dark at 22°C, exposed to 48°C for 30 min in the dark, and transferred to continuous light for 24 h. Total chlorophyll extracted from 25 seedlings is reported as a percentage of chlorophyll recovered from non-heat-treated controls. The data are means ± se from two independent experiments.
| Sample | Percentage Chlorophyll Content of the Respective Non-Heat-Treated Control Samples |
|---|---|
| Wild type | 7.0 ± 1.9 |
| GFP | 31.1 ± 18.5 |
| SOR1 | 48.5 ± 14.2 |
| SOR3 | 102.1 ± 1.0 |
| SOR8 | 108.6 ± 1.4 |
| SOR9 | 108.5 ± 13.3 |
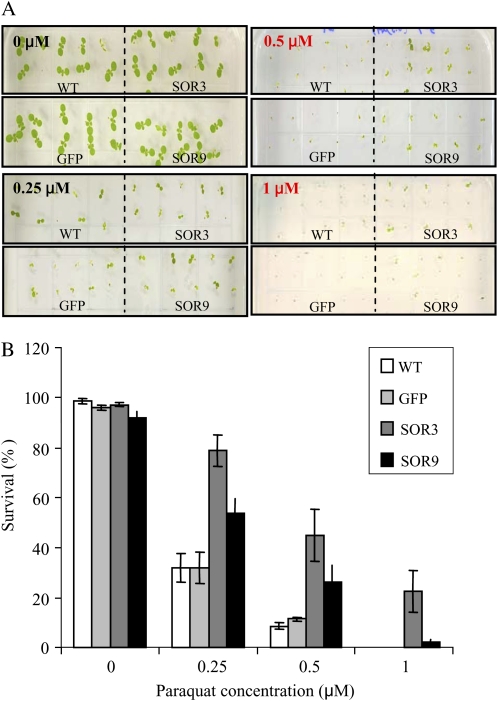
P. furiosus SOR Increases Plant Tolerance to Chemically Induced ROS in Vivo
Methyl viologen (paraquat), an effective electron acceptor that generates O2−, was used to generate ROS. Seeds were grown in medium with different concentrations of paraquat (0, 0.25, 0.5, and 1 μm) and maintained under continuous light. As indicated in Figure 5, SOR seedlings were more tolerant of chemically generated ROS. SOR3 and SOR9 germinated and survived 1.5- to 2.5-fold higher on 0.25 μm paraquat and 2- to 4-fold higher on 0.5 μm paraquat compared with wild-type and GFP lines after 14 d under continuous light. The SOR lines even germinated on 1 μm paraquat, unlike wild-type and GFP seeds. These data provide evidence that even though SOR is produced in the cytosol, it may increase the capacity of the plants to detoxify ROS generated from other organelles, such as the chloroplast or mitochondria. These data also help to explain the resilience of the SOR plants after heat and light stress shown in Supplemental Figure S5 and Table III.
Figure 5.
P. furiosus SOR increases plant tolerance to chemically induced ROS in vivo. A, Arabidopsis seeds (25 seeds of each line) were sterilized and plated on the same plate of MS medium containing different concentrations of paraquat (0, 0.25, 0.5, and 1 μm) as indicated. B, The survival (number of green seedlings) was calculated for each line after 14 d under continuous light. Results are reported as percentage of each control (100%) and show means ± sd from three independent experiments. WT, Wild type. [See online article for color version of this figure.]
Response to Heat Stress Is Delayed in SOR Seedlings
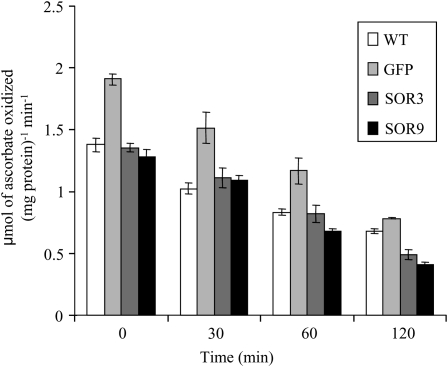
To further investigate how the SOR plants were coping with heat-induced ROS, we analyzed in more detail the response of light-grown seedlings (8 h of light/16 h of dark) to heat stress. For these studies, we performed biochemical analyses of cytosolic ascorbate peroxidase (APX) activity (Mittler and Zilinskas, 1991). We also monitored the presence of ROS-sensitive proteins and analyzed transcripts that are known to be induced in response to increased ROS and heat using RT-PCR and quantitative PCR. Time-course studies were conducted during an acute, 45°C heat stress in the light. Ten-day-old seedlings were incubated at 45°C for 0, 30, 60, or 120 min.
The fact that APX activity does not increase in SOR plants indicates that APX activity is not limiting in this system and suggests that basal H2O2 is not elevated (Fig. 6, zero time point). In contrast, the GFP line has higher APX activity. This is consistent with reports that GFP can generate H2O2 (Haseloff and Amos, 1995; Tsien, 1998) and may contribute to the slightly more robust phenotype of GFP transgenic seedlings.
Figure 6.
APX activity does not increase in SOR seedlings prior to or after stress. Seedlings were grown in 8 h of light and 16 h of dark for 10 d (zero time point) and then incubated at 45°C for 30, 60, or 120 min. Seedlings were harvested from before treatment (zero time point) and after heat treatment. APX activity was measured. Numbers are means of three values from two experiments. WT, Wild type.
Panchuk et al. (2002) reported that APX activity decreased in response to acute heat stress. We found that APX activity decreased in all lines (wild-type, GFP, and SOR plants) in a similar manner in response to heat stress. Importantly, SOR plants were always more resilient than GFP or wild-type plants and yet had lower APX activity, indicating that APX activity was not a major factor contributing to heat stress tolerance in SOR plants.
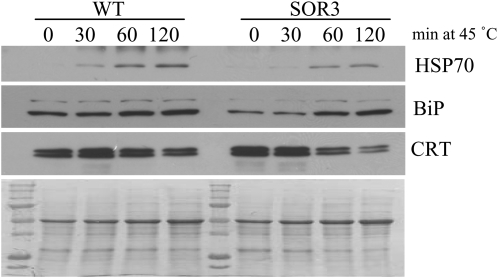
To determine how other ROS- and heat stress-sensitive proteins were affected in the SOR seedlings, we monitored the relative abundance of HSP70 (a heat shock protein), BiP (an endoplasmic reticulum [ER] chaperone), and calreticulin (CRT; an ER chaperone and calcium-binding protein). As shown in Figure 7, similar trends are seen in protein patterns for both wild-type and SOR seedlings; however, for BiP and HSP70, changes in protein abundance appear to be less in SOR seedlings. That is, induction of HSP70 and BiP are delayed in response to heat stress in SOR seedlings. CRT decreased rather than increased with heat stress, and loss of CRT protein was faster in SOR seedlings compared with wild-type seedlings.
Figure 7.
HSP70, BiP, and CRT are less abundant in response to heat stress in SOR plants. Total protein was isolated, and an equal amount of protein (20 μg) was separated by SDS-PAGE, immunoblotted, and visualized with antibodies specific for the proteins indicated (top panels). The bottom panel is an amido black-stained polyvinylidene difluoride membrane showing the protein extracts from each line. The data are representative of two biological replicates that gave similar results. WT, Wild type.
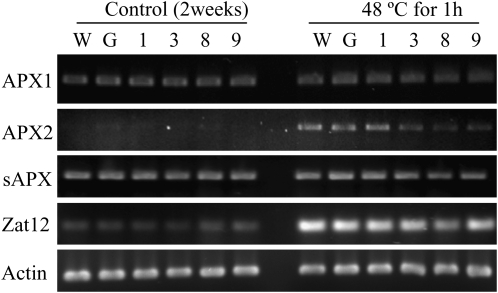
A very sensitive measure of changes in oxidative status is the induction of ROS-sensitive transcripts such as Zat12. Zat12 is a H2O2-sensitive transcription factor (Rizhsky et al., 2004; Davletova et al., 2005b). As predicted, heat stress (48°C for 1 h) increased the Zat12 transcript in wild-type and all of the SOR seedlings (Fig. 8). The transcript levels of H2O2-scavenging cytosolic enzymes, ascorbate peroxidases (APX1 and APX2), were monitored. We did not detect altered expression of APX1 basal levels between wild-type and SOR seedlings or between control and heat-treated samples. APX2, a cytosolic APX, is normally hard to detect but increases readily with heat and high-light stress (Rossel et al., 2002; Davletova et al., 2005b; Bechtold et al., 2008). As anticipated, APX2 increased in response to stress in wild-type and GFP lines; however, in the most highly expressing SOR lines, SOR3, -8, and -9, there was a decrease in APX2 transcript (Fig. 8). Furthermore, the chloroplast stromal APX transcript was less abundant in SOR8 and -9 after 1 h of heat stress (Fig. 8). These data suggested that either the levels of these transcripts and/or endogenous ROS scavenging enzymes were adequate and other compensatory pathways were induced or SOR seedlings did not produce as much H2O2 under these conditions.
Figure 8.
The SOR lines had lower heat-induced transcripts. RNA was collected from 14-d-old Arabidopsis seedlings. cDNA was generated and then amplified using PCR with gene-specific primers for APXs and Zat12 (H2O2-sensitive transcription factor). Actin primers were used as loading controls. PCR samples were analyzed by gel electrophoresis. W, Wild-type; G, GFP; 1, 3, 8, and 9, SOR lines 1, 3, 8, and 9.
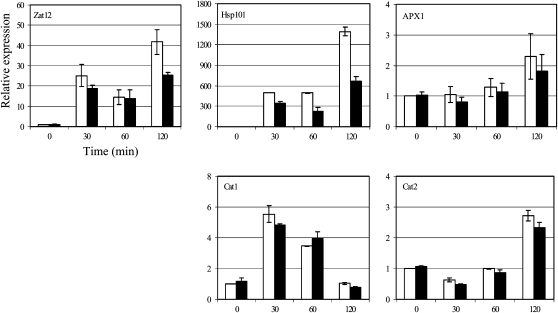
More quantitative measurements of transcript levels of known heat-induced (Hsp101) and oxidation-inducible genes, including Zat12, APX1, Catalase1 (Cat1), and Cat2, were conducted during time-course experiments using wild-type and SOR seedlings. Transcript levels of each gene monitored are expressed as the fold change compared with the level of expression in the wild-type zero time point. Quantitative RT-PCR analysis of wild-type and SOR seedlings confirmed that Hsp101, Zat12, APX1, Cat1, and Cat2 are all induced in response to heat stress (Fig. 9). Hsp101 is a well-characterized, heat stress response transcript (Queitsch et al., 2000). In the SOR lines, there was a delay in the increase of this transcript. The response of Hsp101 at the 2-h time point showed 30% to 50% less induction in the SOR lines. These data are consistent with delayed production of heat stress response proteins (HSP70 and BiP) and oxidation-induced transcripts (Zat12) in the heat-tolerant SOR lines. All H2O2-scavenging enzymes, including catalase and APX, show similar profiles in wild-type and SOR lines, indicating a normal, although in some instances delayed, ability for these enzymes to sense and respond to oxidative stress at the transcriptional level.
Figure 9.
The SOR lines had lower heat-induced transcript (Hsp101) and delayed induction of Zat12. Expression levels of genes encoding ROS-sensitive proteins (Hsp101 and Zat12) and ROS-scavenging enzymes (APX1, Cat1, and Cat2) were detected by quantitative RT-PCR. The raw data (cycle threshold values) were normalized using Actin2/8 as an internal control. White bars indicate the wild type and black bars indicate the SOR3 line. The experiment was reproduced at least twice.
Importantly, basal levels of all of these ROS- and stress-induced transcripts were not elevated in SOR plants under nonstressed conditions. Purified, recombinant P. furiosus SOR produces H2O2 when it reduces superoxide in vitro (Grunden et al., 2005). If this occurred when P. furiosus SOR was produced in the cytosol of Arabidopsis plants, one would anticipate that the endogenous catalase or perdoxidase activity would increase to compensate for an increase in H2O2 production. However, there was no significant difference in the basal APX activity of the seedlings under nonstressed conditions, as shown in Figure 6. Nor were there differences in catalase activity or H2O2 using the in vitro assay (Table IV). There also were no significant increases in the ratio NAD/NADH (7.9 ± 1.1 for the wild type, 4.8 ± 1.2 for GFP, 6.4 ± 0.5 for SOR3, and 5.0 ± 0.1 for SOR9) or NADP/NADPH (1.0 ± 0.2 for the wild type, 0.6 ± 0.4 for GFP, 0.6 ± 0.3 for SOR3, and 0.8 ± 0.1 for SOR9), which implies that, as was found with E. coli expressing SOR (Molina-Heredia et al., 2006), there was no increased demand on cellular reductant in SOR plants. Taken together, these data suggest that in planta either SOR is not very active under nonstressed conditions, and therefore excess H2O2 is not being produced, or SOR is functional all the time and endogenous enzymes are capable of reducing the H2O2 produced. A third explanation also is quite possible. When produced in planta, SOR might form a complex that reduces superoxide completely to water without producing H2O2, as was reported when P. furiosus SOR was produced in E. coli (Molina-Heredia et al., 2006).
Table IV.
H2O2 levels in wild-type and SOR transgenic Arabidopsis plants
The data are reported as the average of two separate experiments with 10 replicates for each experiment. se for each line is indicated.
| Sample | Average |
|---|---|
| nmol H2O2 g−1 fresh weight | |
| Wild type | 12.9 ± 0.5 |
| GFP | 14.4 ± 0.7 |
| SOR3 | 14.5 ± 0.5 |
| SOR9 | 16.9 ± 0.9 |
DISCUSSION
All aerobic organisms have multiple, interacting pathways for reducing ROS. Plants, as sessile organisms, predictably have developed plasticity in their ability to remove ROS and, as a result, present a challenge to biologists attempting to identify key regulatory factors in ROS-mediated signaling and responses. We constitutively expressed the SOR gene from P. furiosus in plants to enhance ROS scavenging and potentially reduce basal ROS. Such a synthetic system, in which O2− is rapidly reduced, should in theory decrease severe responses to stress and enhance survival.
Heat stress leads to the production of ROS and oxidative damage in cells, and many ROS-mediated heat stress responses have been characterized (Mittler, 2006; Suzuki and Mittler, 2006; Volkov et al., 2006). There is a large family of heat shock factors in plants. Some function as corepressors or coactivators, and some appear to function as direct sensors of ROS (Miller and Mittler, 2006; Kant et al., 2007). Many downstream effectors, such as downstream transcription factors, heat shock proteins, and ROS-scavenging enzymes such as APX, are known to be a part of the heat stress response and are ROS sensitive (Panchuk et al., 2002; Davletova et al., 2005a).
Paradoxically, cytosolic APX genes, specifically APX2, which is heat and high-light inducible (Rossel et al., 2002; Bechtold et al., 2008), were expressed at lower levels in the SOR transgenic plants compared with controls in response to heat stress. These data and activity assays indicate that an increase in APX activity did not contribute to heat tolerance. In addition, the increase in the Zat12 transcript was delayed in response to heat stress in SOR plants compared with wild-type plants. Zat12 is a H2O2 sensor that is required for induction of cytosolic APX1 expression in response to oxidative (Rizhsky et al., 2004), osmotic, high-light, and heat (Davletova et al., 2005b) stresses. It is unlikely that compensatory H2O2-scavenging mechanisms similar to those reported in the apx double mutants (Miller et al., 2007) were produced in the SOR plants, because SOR plants showed no evidence of increased anthocyanin biosynthesis (Supplemental Fig. S2). Furthermore, SOR plants were more tolerant of chemical, light, and heat stress, and their growth is not stunted as in the apx double mutants (Miller et al., 2007).
The data also make a compelling argument that stress tolerance of the SOR seedlings does not result from an increase in HSP70 or BiP. The promoter region of HSP70A contains independent cis-elements that can be activated by heat and ROS (H2O2 and 1O2; Shao et al., 2007). It is likely that increased scavenging of cytosolic ROS in the SOR plants contributed to delayed induction of HSP70 and the ER stress sensor, BiP.
While it is well accepted that ROS affect plant growth and development, underlying mechanisms controlling ROS are not well understood (Gapper and Dolan, 2006). Pharmacological approaches such as the addition of exogenous O2− to study ROS signaling are nonselective and generate secondary ROS species before they penetrate plant cell membranes (Gapper and Dolan, 2006; Halliwell, 2006). The challenges presented by the short life and low membrane permeability of ROS (especially O2−) make a compelling argument for model systems in which one can selectively produce and/or dampen specific ROS signals in order to dissect interacting sensing and response pathways (Laloi et al., 2007). The SOR plants should prove to be a good model for studying O2−-mediated events.
In summary, we have shown that P. furiosus SOR can be produced as a functional protein in Arabidopsis. We also demonstrate that expressing P. furiosus SOR delays the response to heat stress and enhances survival under conditions known to produce increased ROS. Future genomic and metabolomic analyses will be required to understand the impact of SOR on basal plant metabolism and to fully characterize the effects on downstream events mediated by cytosolic O2− in planta.
MATERIALS AND METHODS
Generation and Selection of SOR Transgenic Plants
The gene encoding Pyrococcus furiosus SOR (accession no. AE010234) was cloned into pK7WGF2 (Functional Genomics Division, Department of Plant Systems Biology, Ghent University, Ghent, Belgium; Im et al., 2005). Recombinant plasmids were transformed into Agrobacterium tumefaciens EHA105 using the freeze-thaw method (Chen et al., 1994) and then transformed into Arabidopsis (Arabidopsis thaliana ecotype Columbia) by the floral dip method (Clough and Bent, 1998). Four independent transformed lines were further selected. Stable expression of the transgene was monitored by RT-PCR and immunoblotting as described below.
RT-PCR and Quantitative RT-PCR
RNA was isolated using the RNeasy kit (Qiagen), with an additional DNase I treatment to remove contaminating genomic DNA. RT was carried out to generate cDNA using Omniscript reverse transcriptase enzyme (Qiagen). GFP-fused SOR transcripts were detected by PCR as described by Im et al. (2005) using internal GFP forward and gene-specific primers (SOR reverse and actin-specific primers). APX-specific primers described by Panchuk et al. (2002) and Zat12-specific primers (forward, 5′-AACACAAACCACAAGAGGATCA-3′; reverse, 5′-CGTCAACGTTTTCTTGTCCA-3′) were used to determine the levels of APXs and Zat12 transcript. Quantitative RT-PCR was carried out using Full Velocity SYBR-Green QPCR Master Mix (Stratagene) on an MX3000P thermocycler (Stratagene). Gene-specific primers for select genes were designed with the help of AtRTPrimer, a database for generating specific RT-PCR primer pairs (Han and Kim, 2006). PCR was optimized, and reactions were performed in duplicate. The primers for different genes were as follows: Hsp101 (At1g74310), 5′-GGCTTGTGCGAATGTGAGAGTCC-3′ (forward) and 5′-GAGGCTGAAGCTTGTCTCTCAGGTC-3′ (reverse); Zat12 (At5g59820), 5′-AACACAAACCACAAGAGGATCA-3′ (forward) and 5′-CGTCAACGTTTTCTTGTCCA-3′ (reverse); APX1 (At1g07890), 5′-TGCACCCATCATGGTCCGACTC-3′ (forward) and 5′-CCCTGATGGGGTCCAACAACCTAAG-3′ (reverse); Cat1 (At1g20630), 5′-CGCCGATTTGCGAGATACACACAG-3′ (forward) and 5′-GACCTCGAGTTCCGACAGTCAAAGA-3′ (reverse); Cat2 (At4g35090), 5′-TCCCGTCGAGGTATGACCAGGTT-3′ (forward) and 5′-CTTGCCAGCTTCTGTCCCAAAGACT-3′ (reverse). Transcript levels were standardized based on cDNA amplification of the reference gene ACTIN2/8. Relative gene expression data were generated using the 2−ΔΔCt method (Livak and Schmittgen, 2001) using the wild-type zero time point as the reference. PCR conditions were one cycle of 95°C for 10 min, 40 cycles of 95°C for 1 min for DNA denaturation, 55°C for 30 s for DNA annealing and extension, and 95°C for 15 s, and 60°C for 30 s to see the dissociation curve.
Protein Isolation and Immunoblotting
Total protein extract was obtained from plants frozen in liquid N2 or seedlings grown as described by Weigel and Glazebrook (2002). Protein concentrations were quantified as described by Bradford (1976). Protein was separated by 10% (w/v) SDS-PAGE and detected with antibodies raised in rabbits against P. furiosus SOR (at 1:2,000 dilution) or antibodies raised against HSP70, BiP, and CRT (at 1:1,000 dilution). Immunoreactivity was visualized with either horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibody (Pierce).
SOR/SOD Activity Assay
Samples were ground with liquid nitrogen and lysed as described previously (Im et al., 2005). Samples were centrifuged at 27,000g at 4°C for 30 min, and the resulting supernatants were passed through a 0.45-μm filter unit to remove cellular debris. Extracts were dialyzed overnight in 50 mm phosphate buffer. To reduce the plant SOD background activity of dialyzed samples, samples were heat treated as indicated in Table I and Supplemental Table S1 and centrifuged at 21,000g for 15 min. Roots were harvested from seedlings grown for 28 or 42 d on Murashige and Skoog (MS) medium containing 1% Suc in a growth chamber (8 h of light/16 h of dark) and analyzed without dialysis or heat treatment. The heat treatments used were sufficient to inactivate some endogenous plant SOD activity, allowing for greater discrimination between SOD and SOR activity in the transgenic plants.
Unless otherwise indicated, the standard SOR/SOD assay was performed as described by Im et al. (2005). One unit of SOR/SOD activity is defined as the amount of enzyme that inhibits the rate of reduction of cytochrome c by 50% (McCord and Fridovich, 1969).
To confirm activity of the recombinant protein, SOR was immunoprecipitated from the heat-treated (80°C for 15 min) leaf extracts with P. furiosus SOR antibodies using protein A-Sepharose beads as described previously (Shank et al., 2001). Because of the low amount of protein recovered with immunoprecipitation, SOR activity was assayed by monitoring the reduction of the more sensitive tetrazolium salt, WST-1, rather than cytochrome c (Dojindo Molecular Technologies). For this assay, one unit of SOR activity is defined as the amount of enzyme that inhibits the rate of reduction of WST-1 by 50%.
H2O2 Measurements (Ferrous Ammonium Sulfate/Xylenol Orange Assay)
A ferrous ammonium sulfate/xylenol orange (FOX) method was used to quantify H2O2 in plant extracts (Wolff, 1994). The original FOX method was modified by addition of an acidification step, where 1 mL of 25 mm H2SO4 was added to each sample to allow for precipitation of interfering substances (sugars, starches, polysaccharides) for 15 min on ice and centrifuged at 9,700g for 15 min at 4°C. The cell-free extract was collected and passed through a 0.45-μm filter unit. A total of 100 μL was added to 1 mL of the FOX reagent, mixed, and incubated at room temperature for 20 min. The concentration of H2O2 in the reagent was calibrated using A240 and an extinction coefficient of 43.6 m−1 cm−1. The results presented are from two independent experiments with a total of 20 replicates for each sample. The concentration of H2O2 is measured in nmol H2O2 g−1 fresh weight cells.
APX Activity Assay
APX activity was determined as described previously (Nakano and Asada, 1981). Fifty micrograms of the extract was used in a 3-mL APX assay, and the reaction proceeded for 2 min. APX activity was expressed as μmol ascorbate oxidized mg−1 protein min−1. Additional confirmation of APX activity was indicated by an in-gel assay as described by Panchuk et al. (2002; Supplemental Fig. S6).
Seed Germination and Plant Growth
Arabidopsis seeds were surface sterilized as described by Weigel and Glazebrook (2002). Seeds and seedlings, unless otherwise noted, were incubated in a growth chamber under short-day conditions (8 h of light/16 h of dark) at 21°C with light intensity of approximately 150 μmol m−2 s−1. For the hypocotyl elongation assay, seeds were sown on medium (MS medium containing 1% Suc and 0.8% agar) and plates were placed in a vertical position in the dark under conditions as described above. For experiments with mature plants, plants were grown under similar conditions in the North Carolina State University Phytotron.
Thermotolerance Assays
To test seed basal thermotolerance, stratified seeds were treated at 45°C for 5 h and germination was evaluated 2 d later following the protocol of Larkindale et al. (2005). The hypocotyl elongation assay was carried out as described by Hong and Vierling (2000). Growth after the heat treatment was measured and compared with that of seedlings receiving no heat treatment. For tests of vegetative-stage plants, 10-d-old seedlings were used as described by Hong and Vierling (2000). Heat-treated plates were returned to the 22°C incubator, and all plates were left at 22°C for 7 d. The number of seedlings that survived was counted after 7 d.
Chlorophyll Quantification
Seedlings were ground with liquid nitrogen and extracted with 80% (v/v) acetone by shaking until the leaves became breach. The chlorophyll content was quantified spectrophotometrically based on A663 as described (Burke et al., 2000). Data are reported as total chlorophyll per 25 seedlings.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Images of plants and cells expressing P. furiosus GFP-SOR and GFP alone.
Supplemental Figure S2. Images of 56-d-old, short-day plants grown under continuous light for 4 d.
Supplemental Figure S3. SOR activity is measured by showing the reoxidation of ferrocytochrome c.
Supplemental Figure S4. Immunoprecipitation of GFP-SOR using antibodies raised against P. furiosus SOR.
Supplemental Figure S5. Etiolated SOR seedlings accumulate more chlorophyll after heat treatment.
Supplemental Figure S6. In-gel assay showing APX activity.
Supplemental Table S1. SOR/SOD activity in 14-d-old SOR and GFP transgenic Arabidopsis seedlings.
Supplementary Material
Acknowledgments
We thank Dr. Becky Boston (North Carolina State University) for kindly providing the HSP70, BiP, and CRT antibodies. We also thank former undergraduate students, Caroline Smith, Leslie Hewes, Carla Pistole, and Casey Lowder, for technical help.
This work was supported by a grant from the National Aeronautics and Space Administration Institute for Advanced Concepts to A.M.G. and W.F.B., by the U.S. Department of Agriculture (Cooperative State Research, Education, and Extension Service grant no. 35318–05024 to A.M.G., W.F.B., and Mary M. Peet), and by the North Carolina Agricultural Research Service.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Wendy F. Boss (wendy_boss@ncsu.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abdel-Ghany SE, Pilon M (2008) MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem 283: 15932–15945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Auchère F, Pauleta SR, Tavares P, Moura I, Moura JJ (2006) Kinetics studies of the superoxide-mediated electron transfer reactions between rubredoxin-type proteins and superoxide reductases. J Biol Inorg Chem 11: 433–444 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Mittler R (2006) The roles of reactive oxygen species in plant cells. Plant Physiol 141: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold U, Richard O, Zamboni A, Gapper C, Geisler M, Pogson B, Karpinski S, Mullineaux PM (2008) Impact of chloroplastic- and extracellular-sourced ROS on high light-responsive gene expression in Arabidopsis. J Exp Bot 59: 121–133 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Burke JJ, O'Mahony PJ, Oliver MJ (2000) Isolation of Arabidopsis mutants lacking components of acquired thermotolerance. Plant Physiol 123: 575–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Nelson RS, Sherwood JL (1994) Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. Biotechniques 16: 664–668, 670 [PubMed] [Google Scholar]
- Clarke SM, Mur LAJ, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38: 432–447 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005. a) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Schlauch K, Coutu J, Mittler R (2005. b) The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol 139: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas DV, Bartel B (2008) Sucrose induction of Arabidopsis miR398 represses two Cu/Zn superoxide dismutases. Plant Mol Biol 67: 403–417 [DOI] [PubMed] [Google Scholar]
- Emerson JP, Coulter ED, Phillips RS, Kurtz DM Jr (2003) Kinetics of the superoxide reductase catalytic cycle. J Biol Chem 278: 39662–39668 [DOI] [PubMed] [Google Scholar]
- Fiala G, Stetter KO (1986) Pyrococcus-furiosus Sp-Nov represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch Microbiol 145: 56–61 [Google Scholar]
- Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119: 355–364 [Google Scholar]
- Foyer CH, Noctor G (2005. a) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28: 1056–1071 [Google Scholar]
- Foyer CH, Noctor G (2005. b) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141: 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grene R (2002) Oxidative Stress and Acclimation Mechanisms in Plants. American Society of Plant Biologists, Rockville, MD [DOI] [PMC free article] [PubMed]
- Grunden AM, Jenney FE Jr, Ma K, Ji M, Weinberg MV, Adams MW (2005) In vitro reconstitution of an NADPH-dependent superoxide reduction pathway from Pyrococcus furiosus. Appl Environ Microbiol 71: 1522–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, Heinen JL, Holaday AS, Burke JJ, Allen RD (1993. a) Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA 90: 1629–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, Webb RP, Holaday AS, Allen RD (1993. b) Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol 103: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B (2006) Reactive species and antioxidants: redox biology is a fundamental theme of aerobic life. Plant Physiol 141: 312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Kim D (2006) AtRTPrimer: database for Arabidopsis genome-wide homogeneous and specific RT-PCR primer-pairs. BMC Bioinformatics 7: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J, Amos B (1995) GFP in plants. Trends Genet 11: 328–329 [DOI] [PubMed] [Google Scholar]
- Hazlett KR, Cox DL, Sikkink RA, Auch'ere F, Rusnak F, Radolf JD (2002) Contribution of neelaredoxin to oxygen tolerance by Treponema pallidum. Methods Enzymol 353: 140–156 [DOI] [PubMed] [Google Scholar]
- Hong SW, Lee U, Vierling E (2003) Arabidopsis hot mutants define multiple functions required for acclimation to high temperatures. Plant Physiol 132: 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Vierling E (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA 97: 4392–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im YJ, Ji M, Lee AM, Boss WF, Grunden AM (2005) Production of a thermostable archaeal superoxide reductase in plant cells. FEBS Lett 579: 5521–5526 [DOI] [PubMed] [Google Scholar]
- Jenney FE Jr, Adams MW (2001) Rubredoxin from Pyrococcus furiosus. Methods Enzymol 334: 45–55 [DOI] [PubMed] [Google Scholar]
- Jenney FE Jr, Verhagen MF, Cui X, Adams MW (1999) Anaerobic microbes: oxygen detoxification without superoxide dismutase. Science 286: 306–309 [DOI] [PubMed] [Google Scholar]
- Kant P, Kant S, Gordon M, Shaked R, Barak S (2007) Stress response suppressor1 and stress response suppressor2, two DEAD-box RNA helicases that attenuate Arabidopsis responses to multiple abiotic stresses. Plant Physiol 145: 814–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Meskauskiene R, Apel K, Laloi C (2008) No single way to understand singlet oxygen signalling in plants. EMBO Rep 9: 435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R (2008) Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem 283: 34197–34203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J, Brines L (2007) Understanding how the thiolate sulfur contributes to the function of the non-heme iron enzyme superoxide reductase. Acc Chem Res 40: 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K (2007) Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc Natl Acad Sci USA 104: 672–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138: 882–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049–6055 [PubMed] [Google Scholar]
- McKersie BD, Bowley SR, Harjanto E, Leprince O (1996) Water-deficit tolerance and field performance of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol 111: 1177–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie BD, Bowley SR, Jones KS (1999) Winter survival of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol 119: 839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie BD, Chen Y, de Beus M, Bowley SR, Bowler C, Inze D, D'Halluin K, Botterman J (1993) Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.). Plant Physiol 103: 1155–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie BD, Murnaghan J, Jones KS, Bowley SR (2000) Iron-superoxide dismutase expression in transgenic alfalfa increases winter survival without a detectable increase in photosynthetic oxidative stress tolerance. Plant Physiol 122: 1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Mittler R (2006) Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann Bot (Lond) 98: 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133: 481–489 [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R (2007) Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol 144: 1777–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11: 15–19 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Mittler R, Zilinskas BA (1991) Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiol 97: 962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Heredia FP, Houee-Levin C, Berthomieu C, Touati D, Tremey E, Favaudon V, Adam V, Niviere V (2006) Detoxification of superoxide without production of H2O2: antioxidant activity of superoxide reductase complexed with ferrocyanide. Proc Natl Acad Sci USA 103: 14750–14755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58: 459–481 [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Lehmann M, Schwarzlander M, Baxter CJ, Sienkiewicz-Porzucek A, Williams TCR, Schauer N, Fernie AR, Fricker MD, Ratcliffe RG, et al (2008) Decrease in manganese superoxide dismutase leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis. Plant Physiol 147: 101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22: 867–880 [Google Scholar]
- Panchuk II, Volkov RA, Schoffl F (2002) Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol 129: 838–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queitsch C, Hong SW, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12: 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Davletova S, Liang H, Mittler R (2004) The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J Biol Chem 279: 11736–11743 [DOI] [PubMed] [Google Scholar]
- Rossel JB, Wilson IW, Pogson BJ (2002) Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol 130: 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samis K, Bowley S, McKersie B (2002) Pyramiding Mn-superoxide dismutase transgenes to improve persistence and biomass production in alfalfa. J Exp Bot 53: 1343–1350 [PubMed] [Google Scholar]
- Shank KJ, Su P, Brglez I, Boss WF, Dewey RE, Boston RS (2001) Induction of lipid metabolic enzymes during the endoplasmic reticulum stress response in plants. Plant Physiol 126: 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao N, Krieger-Liszkay A, Schroda M, Beck CF (2007) A reporter system for the individual detection of hydrogen peroxide and singlet oxygen: its use for the assay of reactive oxygen species produced in vivo. Plant J 50: 475–487 [DOI] [PubMed] [Google Scholar]
- Slooten L, Capiau K, Van Camp W, Van Montagu M, Sybesma C, Inze D (1995) Factors affecting the enhancement of oxidative stress tolerance in transgenic tobacco overexpressing manganese superoxide dismutase in the chloroplasts. Plant Physiol 107: 737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R, Kapoor A, Zhu JK (2006) Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18: 2051–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126: 45–51 [Google Scholar]
- Tsien RY (1998) The green fluorescent protein. Annu Rev Biochem 67: 509–544 [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Slooten L, Stassart JM, Moens T, Botterman J, Van Montagu M, Inze D (1999) Overproduction of Arabidopsis thaliana FeSOD confers oxidative stress tolerance to transgenic maize. Plant Cell Physiol 40: 515–523 [DOI] [PubMed] [Google Scholar]
- Van Camp W, Capiau K, Van Montagu M, Inze D, Slooten L (1996) Enhancement of oxidative stress tolerance in transgenic tobacco plants overproducing Fe-superoxide dismutase in chloroplasts. Plant Physiol 112: 1703–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov RA, Panchuk II, Mullineaux PM, Schoffl F (2006) Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Biol 61: 733–746 [DOI] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J (2002) Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Weinberg MV, Jenney FE Jr, Cui X, Adams MW (2004) Rubrerythrin from the hyperthermophilic archaeon Pyrococcus furiosus is a rubredoxin-dependent, iron-containing peroxidase. J Bacteriol 186: 7888–7895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willekens H, Inzé D, Van Montagu M, Van Camp W (1995) Catalases in plants. Mol Breed 1: 207–228 [Google Scholar]
- Wolff SP (1994) Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol 233: 182–189 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.