Abstract
A great number of plants synchronize flowering with day length. In rice (Oryza sativa), photoperiod is the primary environmental cue that triggers flowering. Here, we show that the s73 mutant, identified in a γ-irradiated Bahia collection, displays early flowering and photoperiodic insensitivity due to a null mutation in the PHOTOPERIOD SENSITIVITY5 (SE5) gene, which encodes an enzyme implicated in phytochrome chromophore biosynthesis. s73 mutant plants show a number of alterations in the characteristic diurnal expression patterns of master genes involved in photoperiodic control of flowering, resulting in up-regulation of the floral integrator Heading date3a (Hd3a). Early heading date1 (Ehd1), an additional rice floral activator, was also highly expressed in the s73 mutant, suggesting that SE5 represses Ehd1 in wild-type plants. Silencing of Ehd1 in both Bahia and s73 backgrounds indicated that SE5 regulates Ehd1 expression. The data also indicate that SE5 confers photoperiodic sensitivity through regulation of Hd1. These results provide direct evidence that phytochromes inhibit flowering by affecting both Hd1 and Ehd1 flowering pathways.
Flowering in many plants is dependent upon day length. Long-day (LD) and short-day (SD) photoperiod-sensitive plants promote flowering based on a critical threshold related to the proportion of diurnal hours that are experienced. In Arabidopsis (Arabidopsis thaliana) and rice (Oryza sativa) LD and SD plants, respectively, several genes implicated in flowering have already been identified. Arabidopsis FLOWERING LOCUS T (FT) and its rice ortholog Heading date3a (Hd3a) are master genes that act as strong floral promoters, and encode a transmissible signal that moves from leaves to the apex, where it causes the transition from vegetative to floral meristem (Corbesier et al., 2007; Tamaki et al., 2007). FT expression is regulated by both circadian clock and light through mediation of CONSTANS (CO), which encodes a zinc-finger transcriptional activator (Putterill et al., 1995; Kardailsky et al., 1999). CO mRNA abundance is regulated by the circadian clock and accumulates late in the day when plants growing under LD are exposed to light (Suárez-López et al., 2001). On the other hand, there is evidence of posttranscriptional regulation of CO by photoreceptors: Phytochrome B (phyB) promotes the degradation of CO protein early in the day, whereas cryptochromes and phyA promote the stabilization in the evening, allowing the activation of FT (Valverde et al., 2004). In rice, two independent photoperiod pathways, involving the floral regulators Hd1, the rice ortholog of CO, and Early heading date1 (Ehd1), have been defined as controlling heading date through the regulation of Hd3a.
It has been shown that, under SD conditions, Hd1 activates Hd3a thereby promoting flowering, whereas it acts as a repressor under noninductive LD conditions (Izawa et al., 2002). This evidence comes from the early and late heading dates displayed by the hd1 mutant under LD and SD conditions, respectively (Yano et al., 2000). According to the external coincidence model, the integration of signals mediated by both photoreceptors and phases determined by the circadian cycle leads to induction or suppression of flowering (Hayama et al., 2003; Simpson, 2003; Izawa, 2007a). Hd1 expression is predominantly regulated by the circadian clock through OsGI, a key gene that positively controls Hd1 expression (Hayama et al., 2003). The highest expression levels of Hd1 occur around 14 h after dawn, and may coincide either with daylight or with dark, depending on day length. Flowering inhibition exerted by Hd1 under LD conditions can be explained by a model similar to that proposed for Arabidopsis (Turck et al., 2008): During LD, highest Hd1 expression occurs during daylight hours, and therefore coincides with the presence of light-active phytochromes that could modify HD1 to act as an inhibitor of Hd3a transcription, thus inhibiting flowering. On the other hand, the maximal levels of Hd1 mRNA, under SD, would be reached in darkness and, under such a scenario, HD1 would activate transcription of Hd3a, promoting flowering (Izawa et al., 2002; Hayama and Coupland, 2004; Izawa, 2007a). Taken together, these observations lead to the conclusion that flowering inhibition by Hd1 appears to be mediated by phytochromes.
PHOTOPERIOD SENSITIVITY5 (SE5) encodes a heme oxygenase with high similarity to HY1 from Arabidopsis, which is implicated in phytochrome chromophore biosynthesis (Izawa et al., 2000). The se5 mutant is deficient in active phytochromes and exhibits very early heading under both SD and LD conditions. It also shows insensitivity to photoperiod (Izawa et al., 2000). In the se5 mutant, Hd1 expression levels are only slightly different to those of the wild type, whereas Hd3a is highly expressed. Early flowering under LD may be explained by an absence of functional phytochromes, which could lead to continuous activation of Hd3a expression by Hd1 (Izawa et al., 2002). In contrast to the se5 mutant, the hd1 mutant does not show high expression of Hd3a under LD conditions, although it exhibits an earlier heading date than wild type, but flowers later than se5. Furthermore, the se5 hd1 double mutant flowers at the same time as the se5 single mutant under conditions of continuous light, whereas hd1 plants flower much later (Izawa et al., 2002). These data hint at the existence of an additional mechanism of flowering signaling mediated by phytochromes.
Ehd1 codes for a B-type response regulator that, in an Hd1-independent manner, induces flowering under LD and strongly under SD (Doi et al., 2004). Interestingly, Ehd1 is unique to the flowering pathway in rice. Expression analysis using transgenic plants overexpressing Ehd1 indicate that Ehd1 activates transcription of Hd3a in rice, and activates additional rice orthologs of Arabidopsis FT genes (Doi et al., 2004; Izawa, 2007b). The expression pattern of Ehd1 was clearly distinct from that of Hd1. Hd1, which functions as a circadian clock mediator, shows similar patterns of expression under both SD and LD conditions, whereas Ehd1 is only induced under SD conditions (Doi et al., 2004). This suggests that the daily pattern of expression of Ehd1 is probably regulated by a circadian mechanism. Several genes have been identified that regulate Ehd1 expression. For example, OsLFL1 (O. sativa LEC2 and FUSCA3-Like1) represses Ehd1 transcription by direct binding to its promoter, and therefore its ectopic expression delays flowering (Peng et al., 2007, 2008). Furthermore, OsGI regulates, in an indirect manner, Ehd1 expression under SD conditions, via OsMADS51 (Kim et al., 2007). These observations imply that, in rice, Hd1 promotes flowering under SD and inhibits flowering under LD, whereas Ehd1 promotes flowering under both conditions, although to a greater extent under SD conditions (Doi et al., 2004; Izawa, 2007a, 2007b). In other words, Hd1 and Ehd1 are antagonistic under LD, but are synergistic under SD. It has been suggested that the integration of these pathways results in the modulation of FT-like genes, such as Hd3a, conferring the SD phenotype in rice (Izawa, 2007a, 2007b). Recently, it has been demonstrated that Ghd7, encoding a CCT domain protein having no homolog in Arabidopsis, acts upstream of Ehd1 and Hd3a in the photoperiod flowering pathway (Xue et al., 2008).
In this study we characterize s73, a null mutation of SE5, to provide new insight into the regulation of the photoperiodic control of flowering in rice. By expression analysis of the major regulators of the flowering pathway in the mutant, we demonstrate that phytochromes inhibit flowering through both Hd1 and Ehd1 flowering pathways.
RESULTS
The s73 Mutant Displays Early Flowering and Insensitivity to Photoperiod
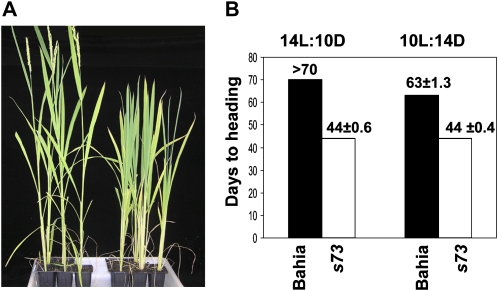
The s73 mutant was identified as a recessive, early flowering mutant in field screenings employing an irradiated Bahia mutant population (Domingo et al., 2007). Bahia is a japonica type cultivar, widely grown across the Mediterranean region. The mutant flowers at 44 d after germination (DAG), 28 d earlier than the wild-type Bahia plants (Fig. 1A). Exposure to different photoperiods showed that s73 mutant plants are insensitive to photoperiod. Mutants flowered at the same time under both LD (14 h light/10 h dark) and SD (10 h light/14 h dark) conditions, whereas Bahia flowered later under LD than under SD conditions (Fig. 1B).
Figure 1.
Comparison of the flowering phenotypes of the s73 mutant and the Bahia wild type. A, Phenotypes of s73 (left) and Bahia wild type (right). Plants were grown for 50 d under natural LD conditions (14 h light/10 h dark). s73 plants showed spikelets, whereas Bahia plants were still in the vegetative stage, and showed no signs of flowering. B, Flowering time under different photoperiod conditions. Days to flowering time were scored from germination to emergence of panicle (heading day) from the main culms under LD (14 h light/10 h dark [14L:10D]) and SD (10 h light/14 h dark [10L:14D]) conditions.
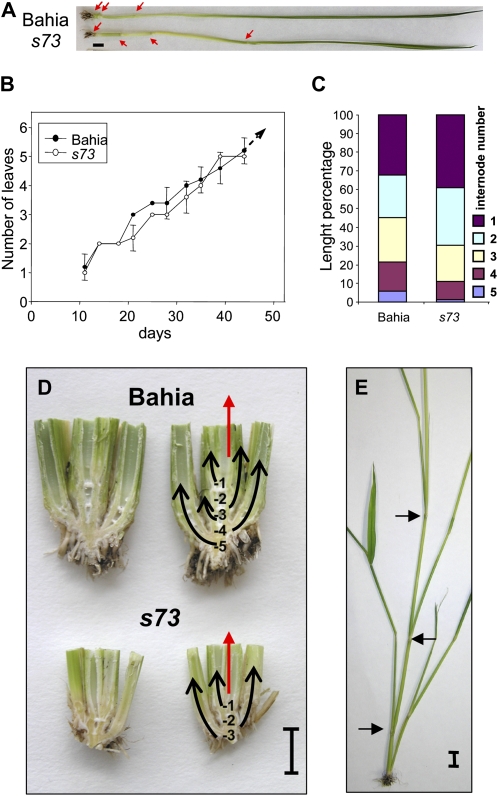
The s73 mutant shows weak growth, with few stems and a yellowish color, when grown under LD conditions. In controlled growth-chamber environments, s73 plants displayed faster internode elongation during the first weeks following germination, although leaf emergence time was not affected until flowering (Fig. 2, A and B). This resulted in a different pattern of internode elongation at the mature stage (Fig. 2C). The youngest internodes in s73 plants were clearly longer than in Bahia, whereas the oldest ones were shorter (Fig. 2A). Differences were also seen for the basal internodes with little elongation (Fig. 2D), since in Bahia there were five visible internodes, but in s73 only three. s73 plants produced fewer tillers than Bahia, and some of them were generated from the aerial nodes of the main stem (Fig. 2E), a phenomenon rare in Bahia.
Figure 2.
Comparison of developmental phenotypes of the s73 mutant with the Bahia wild type. A, Internode elongation of main culm of s73 and Bahia plants grown for 35 d. Leaves were removed to show the pattern of node elongation. Arrows indicate node positions (bar = 1 cm). B, Comparison of leaf emergence rates between Bahia and s73. The leaf number of individual plants was scored on the days indicated until panicle emergence in s73. The average leaf number under LD is plotted. Similar results were obtained under SD conditions (black circles, Bahia; white circles, s73). C, Internode lengths from adult plants expressed as a percentage of the final plant height. D, Longitudinal sections from basal portions of the main and secondary culms (red and black arrows, respectively). Numbers indicate node positions. E, s73 adult plant showing secondary stems in aerial nodes. Leaves were removed to reveal nodes (indicated by arrows). Bars in D and E = 1 cm.
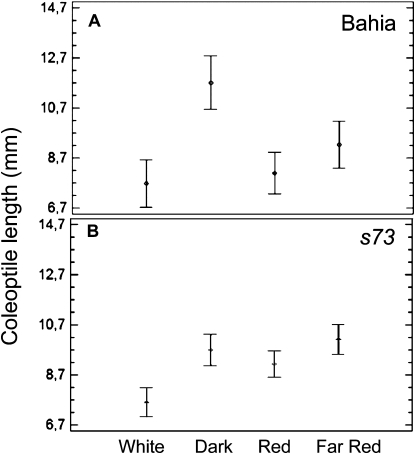
The deficiency of both phyA- and phyB-type phytochromes in se5 produces a lack of light responses in the mutant (Izawa et al., 2000). To examine whether s73 mutant shows deficiencies in light perception or signaling mediated by phytochromes, we analyzed its response to light signals by measuring coleoptile elongation. Bahia seedlings, exposed to red (660 nm), far-red (730 nm), or white light, showed shorter coleoptiles compared to those of dark-grown seedlings (Fig. 3). Although significant differences were not observed across all treatments, it seems that inhibition of elongation was less marked under far-red treatment. The coleoptiles of s73 seedlings elongated to similar extents under both red and far-red light conditions, as well as in darkness. These results are consistent with se5 behavior under the different light treatments (Izawa et al., 2000), and with the concept that s73 is a phytochrome-deficient mutant.
Figure 3.
Photomorphogenic responses of Bahia (A) and s73 (B) plants, grown in darkness, under white light or under red (15 min) or far-red (25 min) light treatments. Coleoptile lengths were measured after each treatment, and lsd intervals are provided (P = 0.05; n = 10).
s73 Mutant Shows a Point Mutation in SE5
To identify the gene mutated in s73, a DNA microarray experiment was performed. Bahia and s73 leaves were collected from plants, grown under LD conditions, 1 h after dawn. Out of the 166 genes that showed significant differential expression, 49 (29.5%) had no Gene Ontology (GO) assignation and, of the remaining 117 genes, 72 were up-regulated and 44 down-regulated. Biological process classification according to GO annotation of the differentially expressed gene set revealed no obviously enriched groups, except for 17 up-regulated genes encoding ribosomal proteins. Only five genes coding for transcription factors were detected (Table I). This group included PIL5 (log fold change = −0.7), which has been implicated in physiological processes mediated by phytochromes (Oh et al., 2006). Within the set of down-regulated genes, it is worthy to mention MAX2 (M = −0.9), which encodes a protein containing an F-box/LRR repeat involved in the formation of secondary meristems (Stirnberg et al., 2002), an observation that might be related to ectopic branching of the mutant.
Table I.
List of genes coding for transcription factor groups showing differential expression between s73 and Bahia plants
LogFC, Logarithm of fold change.
| Locus | Putative Function | Domain Family | LogFC |
|---|---|---|---|
| LOC_Os03g21800 | Transcription factor RF2b, putative, expressed | bZIP | 0.8 |
| LOC_Os02g54050 | Transcriptional factor TINY, putative, expressed | ERF/AP2 | 0.5 |
| LOC_Os03g43810 | PIL5, putative, expressed | bHLH | –0.7 |
| LOC_Os12g40890 | OsIAA30: auxin-responsive Aux/IAA gene family member, expressed | Aux/IAA | –1.0 |
| LOC_Os11g04720 | OsRR9: rice type-A response regulator, expressed | RR | –1.0 |
| LOC_Os01g72370 | ORG3, putative, expressed | bHLH | –1.6 |
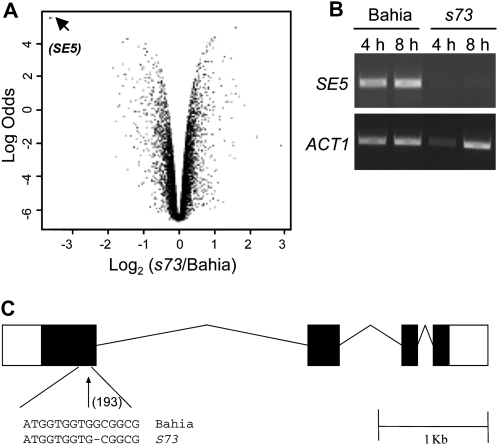
Examination of the differentially expressed genes using a volcano plot revealed that SE5 was the most down-regulated gene, with statistical evidence higher than 99% (Fig. 4A). Further reverse transcription (RT)-PCR analysis of SE5 confirmed that this gene was not expressed in the s73 mutant (Fig. 4B). Analysis of the SE5 sequence showed a 1-bp deletion in position 193 that caused a frame shift and resulted in a premature stop codon in s73 (Fig. 4C).
Figure 4.
Volcano plot analysis, mRNA expression, and genomic structure of SE5. A, Volcano plot analysis of the differentially expressed genes detected in the microarray experiment. Statistical significance (ordinate) is plotted against fold change (abscissa) for each probe. Fold changes are provided as a log2 scale [log2(s73/Bahia)]. The log odds score (ordinate) represents the probability that the gene is differentially expressed. Arrowhead corresponds to SE5 score. B, SE5 expression in Bahia and s73 plants. Adult plants were grown for 1 week under 12 h light/12 h darkness conditions. RNA was isolated from leaf samples taken at 4 and 8 h after light was turned on and SE5 expression was analyzed by semiquantitative RT-PCR. Act1 was used as the control. C, Genomic structure of SE5. s73 shows a 1-bp deletion at position 193, located inside the first exon. Black boxes represent exons; white boxes represent the 5′-untranslated region (left) and the 3′-untranslated region (right), respectively.
Overexpression of SE5 in s73 Mutant Restores Wild-Type Phenotype
To verify that the s73 mutant phenotype was due to the disruption of the SE5 sequence, we overexpressed SE5 in s73, driven by the constitutive Actin1 (Act1) promoter. Overexpression of SE5 restored the wild-type phenotype and s73 transgenic plants produced more stems, were taller, and flowered at the same time as the Bahia control, under both SD and LD conditions (Fig. 5). s73 flowered at 54 DAG in both SD and LD conditions, whereas Bahia and s73 plants overexpressing SE5 flowered at 75 and 108 DAG under SD and LD conditions, respectively. In addition, overexpression of SE5 in Bahia plants did not produce a longer vegetative cycle since transgenic plants flowered at approximately the same time as Bahia plants (Fig. 5).
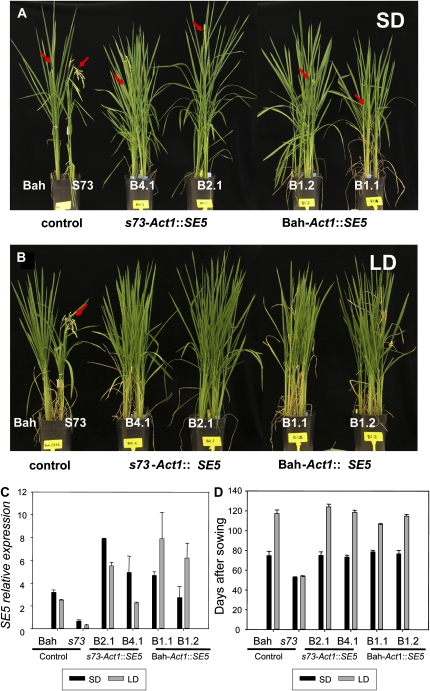
Figure 5.
Phenotypic comparisons (left to right) of Bahia (Bah), s73, s73-Act1∷SE5 (lines B4.1 and B2.1), and Bahia-Act1∷SE5 (lines B1.2 and B1.1) plants overexpressing SE5. A and B, Plants grown under SD (A) and LD (B) conditions for 11 weeks. Bahia plants are at the heading stage. s73 plants showed mature panicles, whereas Bahia, s73-Act1∷SE5, and Bahia-Act1∷SE5 showed emergent panicles, indicated by red arrows. C, SE5 relative mRNA levels in Bahia, s73, and transgenic plants overexpressing SE5 quantified by quantitative RT-PCR. Plants were grown under neutral day (12 h light/12 h dark) conditions for 2 weeks, and for an additional week under SD (10 h light/14 h dark; dark bars) or LD (14 h light/10 h dark; open bars) conditions. Relative mRNA levels were normalized to total RNA amounts. D, Days to heading of the Bahia, s73, and transgenic plants overexpressing SE5 grown under SD or LD conditions (n = 8).
It is worth noting that although the Act1 promoter drives constitutive expression (Zhang et al., 1991), the SE5 mRNA level in s73 plants overexpressing SE5 was higher when plants were grown under SD, as seen in Bahia plants. However, expression levels of SE5 transcripts in Bahia overexpressing SE5 plants showed the opposite pattern, i.e. higher mRNA levels were seen under LD conditions. Morphologically, s73 and Bahia transgenic plants overexpressing SE5 were similar, and slightly more vigorous than Bahia plants under SD and LD conditions (Fig. 5).
Diurnal Rhythmic Expression of OsGI, Hd1, Ehd1, and Hd3a Is Altered in s73 Mutant
Differences in flowering time between s73 and Bahia plants could be reflected in the expression patterns of genes known to be involved in the photoperiod regulatory pathway. We compared the behavior of genes associated with the circadian clock and those involved in photoperiod flowering, by analyzing the daily rhythms of their mRNA levels in 4-week-old plants under LD and SD conditions. It has been reported that rice OsGI, Hd1, and Hd3a mRNA levels exhibit diurnal oscillation (Hayama et al., 2003). We observed that the expression pattern of OsGI was not affected by the absence of SE5 function, although its mRNA levels were lower in the s73 mutant under LD (Fig. 6). Hd1 mRNA changes followed the same pattern, and a decrease in its level was also detected in s73 plants, especially under LD conditions. Both OsGI and Hd1 expression levels were similar in s73 and Bahia under SD conditions, suggesting the differences between these two genes in the s73 mutant is probably not a consequence of variation in general circadian clock function, but of the absence of SE5.
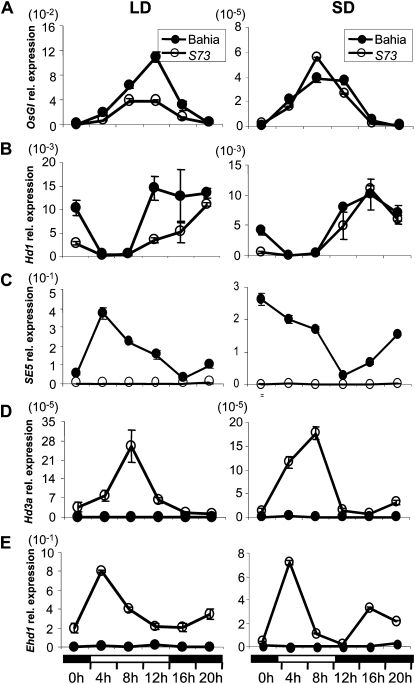
Figure 6.
Diurnal expression patterns of OsGI (A), Hd1 (B), SE5 (C), Hd3a (D), and Ehd1 (E) in Bahia (black circles) and s73 (white circles) plants as indicated by quantitative RT-PCR results. Plants were grown under neutral day (12 h light/12 h dark) conditions for 3 weeks, and for an additional week under SD (10 h light/14 h dark) or LD (14 h light/10 h dark) conditions. In all sections, the mean of each point is based on the average of three biological replicates. Black bars indicate dark periods, and white bars indicate light periods. Relative mRNA levels normalized to total RNA amounts are shown. The values presented are the mean of two biological replicates. Error bars indicate sd from the mean.
On the other hand, Hd3a mRNA levels were increased in s73 plants and displayed maximal levels 8 h after dawn. Ehd1 transcripts also increased during the day cycle in the mutant. Under SD conditions, Hd3a and Ehd1 showed much higher levels of expression in s73 than in Bahia, and were higher than those observed under LD conditions. Under both photoperiodic conditions, Ehd1 expression level reached maximal levels a few hours after dawn, although under SD conditions a second peak of expression, almost undetectable under LD, was detected at midnight. The fact that Ehd1 and Hd1 are regulated independently (Doi et al., 2004), and the observation that SE5 produces elevated levels of Ehd1, led us to conclude that SE5 negatively controls Ehd1 expression in Bahia plants.
Sensitivity to Photoperiod Is Conferred by SE5 through Hd1
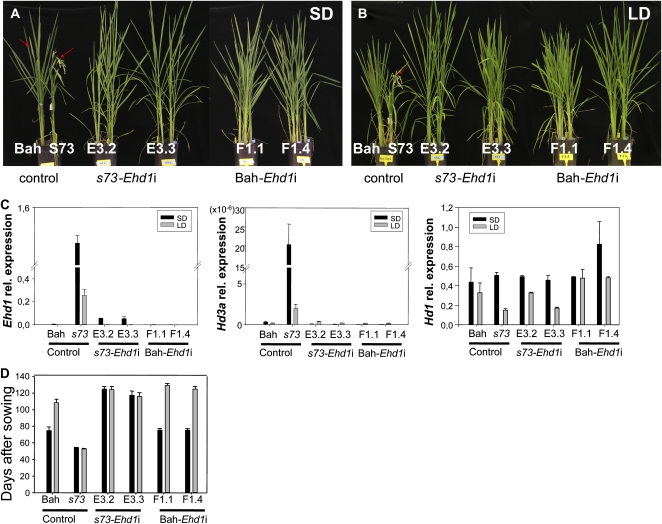
To evaluate the interaction between Ehd1 and SE5, Ehd1-silenced s73 and Bahia transgenic plants were generated and employed in flowering-time experiments. Under both SD and LD conditions, s73-Ehd1i plants exhibited delayed flowering (Fig. 7), indicating that the high level of expression of Ehd1 in the s73 mutant is required for the early flowering phenotype. Interestingly, s73-Ehd1i plants flowered at the same time under both SD and LD conditions, indicating that loss of Ehd1 function does not restore photoperiod sensitivity to s73. Under LD conditions, silencing of Ehd1 in s73-Ehd1i plants restored the wild-type phenotype in the s73 mutant. These plants showed the same flowering time as Bahia or s73 plants overexpressing SE5 (Figs. 5 and 7). However, under SD conditions, s73-Ehd1i plants flowered later than the wild type. The data therefore imply that the up-regulation of Ehd1 in the s73 mutant is the main factor responsible for the early flowering phenotype, but that it does not control photoperiod sensitivity in the mutant. This leads to the conclusion that the lack of photoperiod sensitivity might be attributed to the absence of functional SE5 in both s73 and s73-Ehd1i plants. This is consistent with the restoration of response to day length observed in the s73 transgenic lines overexpressing SE5 (Fig. 5). Because flowering is promoted through the Hd1 pathway in the absence of Ehd1, differences observed in LD and SD flowering time between s73-Ehd1i and Bahia-Ehd1i plants leads to the conclusion that sensitivity to photoperiod is conferred by SE5 through the Hd1 pathway. On the other hand, under LD conditions, Bahia-Ehd1i plants flower later than both Bahia and s73-Ehd1i plants. Under SD conditions, Bah-Ehd1i plants flowered at the same time as the Bahia control, but earlier than s73-Ehd1i plants.
Figure 7.
Phenotypic comparisons (from left to right) of Bahia (Bah), s73, s73-Ehd1i (lines E3.2 and E3.3), and Bahia-Ehd1i (lines F1.1 and F1.4) plants. A, Plants growing under SD conditions. Pictures were taken when Bahia plants initiated the heading (11 weeks). s73 plants show mature panicles, whereas Bahia, s73-Ehd1i, and Bahia-Ehd1i show emergent panicles, indicated by red arrows. B, Plants growing under LD conditions. Pictures were taken 11 weeks after sowing. C, Ehd1, Hd3a, and Hd1 mRNA levels in Bahia, s73, and transgenic plants quantified by quantitative RT-PCR. Plants were grown under neutral day (12 h light/12 h dark) conditions for 2 weeks, and for an additional week under SD (10 h light/14 h dark) or LD (14 h light/10 h dark) conditions. RNA was isolated from leaf samples 4 h after the lights were turned on for Ehd1 and Hd3a analysis or 16 h for Hd1 analysis. Relative mRNA levels normalized to total RNA amounts are shown. Error bars indicate sd. D, Days to flowering of the Bahia, s73, s73-Ehd1i, and Bahia-Ehd1i p1ants grown under SD or LD conditions.
Levels of Ehd1 were reduced drastically in s73 transgenic lines, although silencing was not complete and minor mRNA amounts were detected (Fig. 7). As might be expected, the level of Hd3a mRNA was also reduced in s73-Ehd1i plants, confirming that Ehd1 regulates expression of Hd3a (Doi et al., 2004). This observation identifies Ehd1 as a primary factor responsible for the induction of Hd3a in the s73 mutant. The differences in flowering time between s73-Ehd1i and Bahia-Ehd1i under SD conditions could be explained by variations in their Hd1 mRNA levels. Expression levels of Hd1 in plants grown under LD conditions were significantly higher in Bahia-Ehd1i than in Bahia or s73-Ehd1i plants, which could be responsible for the enhanced inhibition on flowering promotion observed under LD conditions. No changes could be observed in levels of Hd1 in Bahia-Ehd1i plants grown under SD conditions. These facts indicate that SE5 could have influence on regulation of Hd1 expression, under LD condition but not under SD condition, and interesting observation that needs further investigation elsewhere.
DISCUSSION
In this work, we report that Ehd1 is negatively regulated by SE5 at the level of transcription. This results in up-regulation of Hd3a and early flowering, under all conditions tested. This fact indicates that light-activated phytochromes affect the two main photoperiod flowering pathways, respectively through the repression of Ehd1 transcription and through the modification of the HD1 protein (Doi et al., 2004; Turck et al., 2008).
It has been proposed that flowering in rice is controlled by two different regulatory pathways governed by the Ehd1 and Hd1 genes in an independent manner. These pathways converge through modulation of the expression of Hd3a, the master gene that switches on the flowering process. Hd1 regulates Hd3a expression by perceiving signals both from the circadian clock and also from light through the action of phytochrome. Mutations in either PHYB or PHYC accelerate flowering under LD conditions, indicating that the presence of phyB or phyC inhibits flowering under LD (Takano et al., 2005). It has been proposed that the presence of phytochromes under LD conditions could increase the modification of HD1 that leads to inhibition of flowering (Hayama and Coupland, 2004). Ehd1 expression is regulated by a distinct mechanism. In part, the circadian clock exerts its influence through OsMADS51 that transmits a SD promotion signal from OsGI to Ehd1 (Kim et al., 2007). However, Ehd1 expression also responds to other signals: Under LD conditions, Ghd7 delays flowering through its enhanced expression most likely repressing Ehd1 (Xue et al., 2008), and, via a different pathway, Ehd2/OsId1 up-regulates Ehd1 expression under both SD and LD conditions (Matsubara et al., 2008; Park et al., 2008). Ehd2/OsId1 is required for the expression of Ehd1, even though it seems unlikely that it is directly involved in the photoperiodic response of Ehd1 for flowering (Park et al., 2008). It has also been suggested that phytochromes might control Ehd1 through SE5 (Izawa, 2007a). Our work provides evidence supporting this contention, in that an increase in the mRNA levels of Ehd1 under both SD and LD is associated with SE5 disruption. This provides evidence for a novel regulatory step in flowering, one mediated by phytochromes through Ehd1.
According to this proposal, SE5 controls Ehd1 transcription negatively and thereby the influence of phytochromes in this signaling pathway. SE5 encodes a heme oxygenase that functions in phytochrome chromophore biosynthesis. Mutations in this gene lead to a decrease or an absence of photochemical activity of functional phytochromes (Izawa et al., 2000; Emborg et al., 2006). Phytochromes are involved in a number of processes that affect plant development. Mutations in heme oxygenase genes have been previously described in rice (se5), Arabidopsis (hy1), pea (Pisum sativum; pcd1), tomato (Solanum lycopersicum; yg-2), and tobacco (Nicotiana tabacum; pew1; Emborg et al., 2006; Linley et al., 2006). Most of these mutants share phenotypic characteristics, such as abnormal stem or hypocotyl elongation, a decrease in red and far-red light responses during deetiolation, and a yellowish color. Mutants deficient in phyC flower earlier under LD conditions, and those deficient in phyB under both SD and LD conditions (Takano et al., 2005). Phytochromes, as for other photoreceptors, are proteins having significant roles in the photoperiod control of flowering (Valverde et al., 2004; Liu et al., 2008). In rice, the mutation in SE5 produces not only early flowering, but also a definite insensitivity to photoperiod (Izawa et al., 2000). It has been proposed that phytochromes may explain the antagonistic role displayed by Hd1 depending on the length of the day (Hayama and Coupland, 2004; Turck et al., 2008). In this sense, under LD conditions, the maximal daily expression levels of Hd1 overlap with light and, thus, with the presence of light-activated phytochromes leading to the modification of HD1 and to the inhibition of flowering by repression of Hd3a. In contrast, under SD conditions, Hd1 is expressed during darkness when no active phytochrome is available and, under such conditions, HD1 would activate Hd3a, promoting flowering. As seen in our work, the s73 line has a mutated null allele of SE5 that encodes a truncated SE5 protein, causing increased Ehd1 and Hd3a expression. The early flowering of s73 could be due to the unbalanced expression of Hd1 and Ehd1 in the mutant, resulting in higher levels of Hd3a under both SD and LD conditions. It has been proposed that competition could exist between Ehd1 and Hd1 in the binding site within the Hd3a promoter (Doi et al., 2004; Izawa, 2007a). Thus, in s73, the increased expression of Ehd1 and the lack of functional phytochromes to modify HD1 apparently promote flowering under LD.
The fact that s73 plants flower earlier than do Bahia plants under SD conditions probably is due to an additive effect of both increased Ehd1 expression level and normal induction of Hd1. Under LD conditions, the early flowering phenotype of s73 reverts when Ehd1 is silenced, indicating both that Ehd1 acts downstream SE5 and that the increased levels of Ehd1 in the mutant are responsible for the flowering phenotype. Under SD conditions, suppression of Ehd1 expression in s73 also delayed flowering time but, in Bahia, did not result in major changes in flowering time. Bahia and s73 delay flowering under LD conditions when Ehd1 is silenced, indicating that the absence of Ehd1 expression might promote a delay in flowering by enhancing the Hd1 pathway. However, under SD, Bahia-Ehd1i flowers earlier than S73-Ehd1i, indicating that Hd1 may be also requiring active phytochromes to induce flowering. The absence of Ehd1 results in a slight increase in Hd1 transcript levels under LD conditions but not under SD conditions, suggesting that a second factor dependent on light is needed to regulate Hd1 transcription. Further investigation will be needed to fully elucidate the mechanism of regulation of Hd1 expression by light.
Another piece of valuable information provided by this work relates to the observation that sensitivity to photoperiod is lost in s73. This is not recovered by silencing Ehd1, producing plants that do not distinguish between SD and LD. Ehd1 silencing in Bahia still preserves photoperiodicity, and produces plants that flower later under LD conditions. These still contain a functional allele of SE5 and an active Hd1 pathway. This leads us to conclude that the response to day length is controlled by SE5 through Hd1 pathway. This is in accordance with previous studies, showing that plants carrying a deficient Ehd1 functional gene but active SE5 products still respond to photoperiod (Doi et al., 2004). It is worth pointing out that all plants studied in this work in which SE5 was mutated were insensitive to photoperiod, independently of Ehd1 expression, supporting the observation that SE5 is responsible for photoperiodicity.
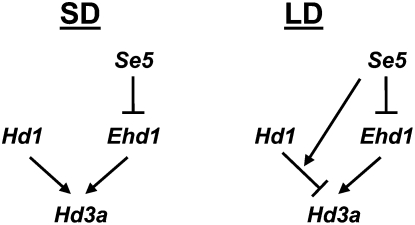
Our results indicate that in the absence of functional phytochromes, as in the se5 and s73 mutants, Ehd1 is the major genetic factor responsible for the elevated level of Hd3a expression and consequently for early flowering. This defines a new phytochrome-mediated mechanism of flowering repression through transcriptional regulation of Ehd1. In addition, Ehd1 is regulated by other factors than phytochromes. Thus, Ehd1 has been shown to have a rhythmical pattern of expression, characteristic of genes controlled by the circadian clock. Furthermore, it has been demonstrated that OsGI, one of the master genes in the circadian clock pathway, is able to down-regulate Ehd1 expression through OsMADS51, a MADS-box transcriptional factor (Kim et al., 2007). Additionally, Ehd2/OsId1 and Ghd7, respectively, up- and down-regulate Ehd1, supposedly through two different pathways, but it is not clear yet what signals govern these pathways (Matsubara et al., 2008; Xue et al., 2008). Our results indicate that Ehd1 is also regulated by phytochromes, and this finding clarifies the photoperiodic control of flowering in rice (Fig. 8): Ehd1 is transcriptionally regulated by phytochromes, decreasing its expression under LD conditions due to a long exposure to light. On the contrary, under SD conditions, Ehd1 expression is increased because of a shorter exposure to phytochrome that, thus, promotes flowering. At the same time, under LD conditions, Hd1 acts as a flowering repressor, while under SD conditions acts as an inducer. Through this mechanism, as suggested previously (Izawa, 2007a), the balance between these different states of expression modulates the photoperiodic response.
Figure 8.
Schematic representation of the flowering-signal genetic pathways in rice in which SE5 acts as a repressor of Ehd1 expression. Under LD condition SE5 inhibits transcription of Hd3a, in an indirect manner through HD1.
MATERIALS AND METHODS
Plant Materials and Growing Conditions
The s73.04 mutant line was generated by γ-ray irradiation (250 Gy) of plants of rice (Oryza sativa var. japonica ‘Bahia’). To monitor the effect of flowering time, plants were cultured in controlled growth rooms under SD (10 h light/14 h dark) or LD (14 h light/10 h dark) conditions at 25°C.
Photomorphogenetic assays were performed as described previously (Biswas et al., 2003), with few modifications. White, red (R), and far-red (FR) lights were used to treat seedlings. The white light sources were white fluorescent tubes (Philips Sylvania, 150 μmol−2·s−1). R and FR light was obtained by passing white light from an incandescent source (Olympus Highlight 3100) through appropriate colored filters (Cheshire Optical), producing R light (660 nm, 12–14 μmol−2·s−1) or FR light (730 nm, 6–8 μmol−2·s−1).
RNA Isolation, Semiquantitative RT-PCR, and Quantitative Real-Time PCR
Total RNA was isolated using the RNeasy plant mini kit (QIAgen; ref. 74904), following the manufacturer's instructions. First-strand cDNA was synthesized from 3 μg of total RNA, using the ThermoScript RT-PCR system (Invitrogen; ref. 11146–016), according to the manufacturer's instructions. Synthesized cDNAs were used for real-time, quantitative RT-PCR. One-step real-time PCR assays were performed as previously described (Domingo et al., 2009). The real-time PCR procedure involved an incubation at 48°C for 30 min, followed by 45 cycles at 95°C for 2 s, 60°C for 8 s, and 72°C for 8 s. The identities of the amplicons and the specificity of the reaction were verified by melting curve analysis and by sequencing the reaction product. The sequences of the primers, extension times, and number of cycles are provided in Supplemental Table S2.
RNA Amplification, Microarray Hybridization, and Data Analysis
Four independent dye-swap experiments were performed using RNA isolated from mutant and wild-type plants, which was amplified and labeled using the AminoAllyl MessageAmp II aRNA amplification kit (Ambion) according to the manufacturer's instructions. One microgram of total RNA of each sample was used for amplification. The final concentration of aRNA was determined using a NanoDrop ND1000 spectrophotometer. For the dye-coupling reaction, 10 μg of aRNA of each sample were employed following the dye coupling and labeled aRNA cleanup protocol provided by Ambion. A total of four independent biological samples of each genotype (Bahia and s73.04) were labeled with Cy3 and Cy5, alternatively, and used for the microarray hybridization.
Labeled samples were hybridized to 45,000-element whole-genome oligonucleotide microarrays provided by the University of Arizona (http://www.ag.arizona.edu/). The hybridized microarray slides were washed and scanned using a Gene Pix Autoloader 4200AL (Axon/Molecular Devices). Spot finding and data extraction was done using GenePix Pro 6 software (Axon/Molecular Devices). Quality control, normalization, and the determination of differentially expressed genes were performed in R using the Limma package (Smyth, 2005) of Bioconductor (http://www.bioconductor.org), with local background subtraction and Lowess normalization performed for each microarray slide. Linear models and empirical Bayes methods from the Limma package of Bioconductor were applied to derive a P value, false discovery rate (FDR; P adjusted), and mean of log2-based ratio across four slides. Functional category classifications were grouped using the Blast2GO software (Conesa et al., 2005). The microarray data were deposited in the Gene Expression Omnibus at the National Center for Biotechnology Information (GSE16796).
In total, 166 genes represented on the rice DNA microarray showed differential expression of >1.5-fold (−0.5 > log fold change > 0.5 with an adjusted P value [FDR] < 0.05); 96 and 70 genes were up- and down-regulated, respectively (Supplemental Table S1). Microarray data was validated using quantitative RT-PCR analysis (Supplemental Fig. S1). Functional classification of genes was performed using the Plant GOSlim Assignment of Rice Proteins from Rice Genome Annotation Project (http://rice.plantbiology.msu.edu).
Vector Construction and Transformation
SE5 was amplified by PCR from Bahia cultivar cDNA using the following primers: (forward) 5′-TCATCGATGGCGCCCGCGGCAGC-3′ and (reverse) 5′-CAGGTACCATGACCTCTGCCCAGTGTCC-3′. The PCR product was digested with ClaI and KpnI and cloned under the control of the Act1 promoter (Zhang et al., 1991) in pCAMBIA1305.1 (Cambia) to generate pACT1∷SE5. To construct the Ehd1 RNAi molecule, a 410-pb fragment of the Ehd1 gene from Bahia cDNA was first amplified, using the following primers: (sense-F) 5′-GGCTCGAGATTTACTTCTGAAGTGCAG-3′ and (sense-R) 5′-CGGAATTCGGTGGCCGTTGATCTCG-3′, for the sense fragment, and (antisense-F) 5′-GCTCTAGATTTACTTCTGAAGTGCAG-3′ and (antisense-R) 5′-CGAAGCTTCGGTGGCCGTT-3′, for the antisense fragment. The resultant amplicons were digested with XhoI/EcoRI and HindIII/XhoI, for the sense and antisense fragments, respectively, and cloned in pHANNIBAL (Cambia) under the control of the cauliflower mosaic virus 35S promoter. Next, the expression cassette was excised using SacI and PstI and subcloned in pCAMBIA1305.1 to generated pEhd1-RNAi. The resultant plasmids were transformed into Agrobacterium tumefaciens EHA105 by electroporation, and used for the transformation of rice Bahia cultivar (sp. japonica). Rice transformations were performed according to Hiei et al. (1994).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AP008212.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Comparison between gene expression analyzed by quantitative RT-PCR and microarrays, expressed as log2 (s73/Bahia).
Supplemental Table S1. List of differentially expressed genes obtained in the microarray analysis of s73 plants.
Supplemental Table S2. Sequences of primers and temperature used in quantitative RT-PCR analysis.
Supplementary Material
Acknowledgments
We thank Matilde Sancho and Angel Boix for technical assistance.
This work was supported by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (to C.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Concha Domingo (domingo_concar@gva.es).
The online version of this article contains Web-only data.
References
- Biswas KK, Neumann R, Haga K, Yatoh O, Iino M (2003) Photomorphogenesis of rice seedlings: a mutant impaired in phytochrome-mediated inhibition of coleoptile growth. Plant Cell Physiol 44: 242–254 [DOI] [PubMed] [Google Scholar]
- Conesa A, Gotees S, García-Gómez JM, Terol J, Talon M, Robles M (2005) Blast2GO: a universal annotation and visualization tool in functional genomics research. Application note. Bioinformatics 21: 3674–3676 [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Gend Dev 18: 926–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo C, Andrés F, Iglesias D, Tharreau D, Talón M (2009) Depletion of auxin content mediated by OsGH3.1 overexpression activates defense response and enhances resistance to fungal pathogen in rice. Mol Plant Microbe Interact 22: 201–210 [DOI] [PubMed] [Google Scholar]
- Domingo C, Andrés F, Talón M (2007) Rice cv. Bahia mutagenized population: a new resource for rice breeding in the Mediterranean basin. Span J Agric Res 5: 341–347 [Google Scholar]
- Emborg TJ, Walker JM, Noh B, Viestra D (2006) Multiple heme oxygenase family members contribute to the biosynthesis of the phytochrome chromophore in Arabidopsis. Plant Physiol 140: 856–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama R, Coupland G (2004) The molecular basis of diversity in the photoperiodic flowering responses of Arabidopsis and rice. Plant Physiol 135: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K (2003) Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422: 719–722 [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence boundaries of the T-DNA. Plant J 6: 271–282 [DOI] [PubMed] [Google Scholar]
- Izawa T (2007. a) Daylength measurements by rice plants in photoperiodic short-day flowering. Int Rev Cytol 256: 191–222 [DOI] [PubMed] [Google Scholar]
- Izawa T (2007. b) Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J Exp Bot 8: 3091–3097 [DOI] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Tokutomi S, Okuno K, Shimamoto K (2000) Phytochromes confer the photoperiodic control of flowering in rice (a short-day plant). Plant J 22: 391–399 [DOI] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K (2002) Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev 16: 2006–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kim SL, Lee S, Kim HJ, Nam HG, An G (2007) OsMADS51 is a short-day flowering promoter that function upstream of Ehd1, OsMAD14, and Hd3a. Plant Physiol 145: 1484–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley PJ, Landsberger M, Kohchi T, Cooper JB, Terry MJ (2006) The molecular basis of heme oxygenase deficiency in the pcd1 mutant of pea. FEBS J 273: 2594–2606 [DOI] [PubMed] [Google Scholar]
- Liu LJ, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, Wang L, Yanga HQ (2008) COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 20: 292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K, Yamanouchi U, Wang ZX, Minobe Y, Izawa T, Yano M (2008) Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1. Plant Physiol 148: 1425–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Kamiya Y, Bae G, Chung WI, Choi G (2006) Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J 47: 124–139 [DOI] [PubMed] [Google Scholar]
- Park SJ, Kim SL, Lee S, Je BI, Piao HL, Park SH, Kim CM, Ryu CH, Park SH, Xuan YH, et al (2008) Rice Indeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J 56: 1018–1029 [DOI] [PubMed] [Google Scholar]
- Peng LT, Shi ZY, Li L, Shen GZ, Zhang JL (2007) Ectopic expression of OsLFL1 in rice represses Ehd1 by binding on its promoter. Biochem Biophys Res Commun 360: 251–256 [DOI] [PubMed] [Google Scholar]
- Peng LT, Shi ZY, Li L, Shen GZ, Zhang JL (2008) Overexpression of transcription factor OsLFL1 delays flowering time in Oryza sativa. J Plant Physiol 165: 876–885 [DOI] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80: 847–857 [DOI] [PubMed] [Google Scholar]
- Simpson G (2003) Evolution of flowering in response to day length: flipping the CONSTANS switch. Bioessays 25: 829–832 [DOI] [PubMed] [Google Scholar]
- Smyth GK (2005) Limma: linear models for microarray data. In R Gentleman, V Carey, S Duboit, R Irizarry, W Huber, eds, Bioinformatics and Computational Biology Solutions using R and Bioconductor, Springer, New York, pp 397–420
- Stirnberg P, van de Sande K, Leyser O (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141 [DOI] [PubMed] [Google Scholar]
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410: 1116–1120 [DOI] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T, Yano M, Nishimura M, Miyao A, Hirochika H, et al (2005) Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 17: 3311–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316: 1033–1036 [DOI] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59: 573–594 [DOI] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303: 1003–1006 [DOI] [PubMed] [Google Scholar]
- Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, et al (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40: 761–767 [DOI] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, et al (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12: 2473–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, McElroy D, Wu R (1991) Analysis of rice Act1 5′-region activity in transgenic rice plants. Plant Cell 3: 1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.