Abstract
While the possible importance of the tricarboxylic acid (TCA) cycle reactions for leaf photosynthesis operation has been recognized, many uncertainties remain on whether TCA cycle biochemistry is similar in the light compared with the dark. It is widely accepted that leaf day respiration and the metabolic commitment to TCA decarboxylation are down-regulated in illuminated leaves. However, the metabolic basis (i.e. the limiting steps involved in such a down-regulation) is not well known. Here, we investigated the in vivo metabolic fluxes of individual reactions of the TCA cycle by developing two isotopic methods, 13C tracing and fluxomics and the use of H/D isotope effects, with Xanthium strumarium leaves. We provide evidence that the TCA “cycle” does not work in the forward direction like a proper cycle but, rather, operates in both the reverse and forward directions to produce fumarate and glutamate, respectively. Such a functional division of the cycle plausibly reflects the compromise between two contrasted forces: (1) the feedback inhibition by NADH and ATP on TCA enzymes in the light, and (2) the need to provide pH-buffering organic acids and carbon skeletons for nitrate absorption and assimilation.
Illuminated leaves simultaneously assimilate (gross photosynthetic assimilation) and produce (photorespiration and day respiration) CO2. While the general metabolic scheme of the respiratory pathway is known, the regulation of day respiration is one conundrum of plant photosynthetic biology. In fact, day respiration is the cornerstone for nitrogen assimilation by leaves simply because carbon assimilation produces organic materials (carbohydrates) that are in turn converted to nitrogen acceptors by respiration. Unsurprisingly then, intense efforts are currently devoted to elucidate the metabolic basis of the regulation of day respiration, with the optimization of nitrogen assimilation for a better yield of crop plants as an ultimate goal (Lawlor, 2002).
It is well accepted that leaf respiration is often inhibited by light; that is, day respiration is lower than dark respiration (Atkin et al., 2000; Tcherkez et al., 2005, and refs. therein). While there is very little evidence of regulation at the genetic transcription level (Rasmusson and Escobar, 2007), the fundamental reasons invoked to explain such an inhibition by light are enzymatic (posttransductional or biochemical). It has indeed been shown in the unicellular alga Selenastrum minutum that pyruvate kinase is inhibited in the light by the high level of cytoplasmic dihydroxyacetone phosphate (Lin et al., 1989). In addition, the mitochondrial pyruvate dehydrogenase complex is partly inactivated by (reversible) phosphorylation in extracts from illuminated leaves (Budde and Randall, 1990; Tovar-Mendez et al., 2003). Photorespiration is probably also involved in the inhibition of pyruvate dehydrogenase, as it has been shown that this enzyme is down-regulated by NH3, which is a by-product of the photorespiratory Gly decarboxylation (Krömer, 1995). Enzymes of the tricarboxylic acid (TCA) cycle are also assumed to be inhibited in the light because of the high mitochondrial NADH/NAD+ ratio due to photorespiratory Gly decarboxylation (Gardeström and Wigge, 1988). Additionally, it has been shown that the mitochondrial isocitrate dehydrogenase is inhibited by the high NADPH/NADP+ ratios that may occur in the light (Igamberdiev and Gardeström, 2003; Kasimova et al., 2006). Accordingly, Gly decarboxylase antisense lines of potato (Solanum tuberosum) have larger day respiratory decarboxylation rates in the light (as revealed by 14C-labeling experiments), and the ATP/ADP as well as the NADH/NAD+ ratios are both smaller than in the wild type (Bykova et al., 2005). In addition, the predominance of NADH production by Gly oxidation over that by the TCA cycle has been shown using isolated mitochondria under ADP-limiting conditions (Day et al., 1985); this might reduce the availability of NAD+ for the mitochondrial dehydrogenation steps of the TCA cycle. Such a scenario is consistent with the larger in vivo decarboxylation rates of 13C-enriched TCA substrates under very low, nonphysiological oxygen conditions (400 μmol mol−1 CO2, 2% O2) as compared with high oxygen O2 conditions (1,000 μmol mol−1 CO2, 21% O2; Tcherkez et al., 2008).
Physiological experiments have further shown that the inhibition of day respiration in the light is associated with lower TCA cycle activity. When detached illuminated leaves of Phaseolus vulgaris were supplied with [13C]1-pyruvate, 13CO2 was produced in the light, showing the in vivo activity of pyruvate dehydrogenase; however, when supplied with [13C]3-pyruvate, the 13C labeling in both day-respired CO2 and citrate was very modest, showing the weak activity of the malic enzyme and enzymes of the TCA cycle (Tcherkez et al., 2005). Oxygen consumption measurements with isolated mitochondria extracted from illuminated spinach (Spinacia oleracea) leaves and supplied with exogenous malate, succinate, or citrate showed that citrate gives the lowest respiration rate; in addition, when malate was supplied, it was mainly converted to citrate and pyruvate, with less than 1% isocitrate or fumarate (Hanning and Heldt, 1993). Although the latter results do suggest that TCA cycle reactions were very slow, they also indicate that the phosphoenolpyruvate carboxylase (PEPC), malic enzyme, citrate synthase, and malate dehydrogenase were still active in the light. It remains plausible that such data obtained in vitro may have been influenced by the experimental extraction-purification of mitochondria as well as the artificial addition of exogenous substrates.
Consequently, then, there is at present no clear picture of day-respiratory fluxomics in illuminated leaves. As an aid to clarifying metabolic fluxes through respiratory reactions and enzymatic regulations in folio, we conducted 13C-labeling experiments in detached leaves of Xanthium strumarium (cocklebur) with 13CO2, [13C]Glc, or [13C]pyruvate under either D2O or H2O conditions. We took advantage of known H/D enzymatic isotope effects to study the metabolic control and the effectiveness of metabolic reactions (Rose, 1961; Rose et al., 1962; Breker et al., 1999; Hochuli et al., 2000). Samples were fixed with the freeze-clamp technique, and the 13C enrichment in metabolites was analyzed with 13C-NMR. Both (1) the effect of D2O on the 13C distribution and (2) the 13C fluxomic analysis in H2O indicate that the TCA pathway is not cyclic in the light and is accompanied by rate-limiting steps such as 2-oxoglutarate dehydrogenase and citrate synthase.
RESULTS
Photosynthesis and Transpiration
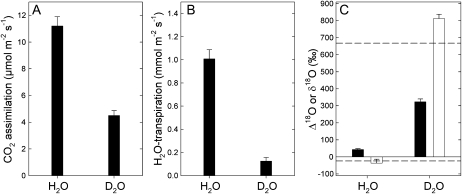
Figure 1A shows the photosynthetic net CO2 assimilation value in either H2O or D2O, as measured with the open gas-exchange system. Clearly, there was a H2O/D2O solvent isotope effect on assimilation of around 2 that corresponds to the H/D isotope effect associated with the carboxylase activity of Rubisco, as already observed by Tcherkez and Farquhar (2008). Both H2O transpiration measured by the infrared gas analyzer of the gas-exchange system (Fig. 1B) and the estimated δ18O of water at the evaporative sites (Fig. 1C) demonstrate that leaf water was completely replaced by heavy water in D2O-fed leaves. (1) In D2O-fed leaves, transpiration represented less than 10% of that in H2O-fed leaves; the small value of 0.1 mmol m−2 s−1 came from the residual infrared absorption of D2O measured by the infrared analyzer (D2O absorbance is about 20% of H2O absorbance at the wavelengths of interest, 2.6–2.8 μm). (2) The estimated oxygen isotope composition of water at the evaporative sites was very close to that of source water (Fig. 1C, dashed lines). As a consequence, all of the recent photosynthetic products formed in the light in D2O were deuterium labeled. As such, their processing through leaf metabolism was influenced by deuteriation (H/D isotope effects), as will become apparent below.
Figure 1.
Net photosynthetic assimilation (A), H2O transpiration (B), photosynthetic 18O/16O isotope fractionation (C; Δ18O, black bars), and 18O/16O isotope composition of transpired water (C; δ18O, white bars) of detached X. strumarium leaves, as photosynthesis operates in H2O or D2O under 400 μmol mol−1 CO2, 21% O2, 400 μmol m−2 s−1 photosynthetically active radiation, and 21°C. Dashed lines stand for the δ18O value of source water (H2O, bottom dashed line; D2O, top dashed line).
Leaf CO2 Evolution
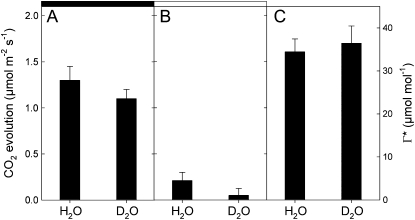
When measured with the method of Laisk (1977; i.e. with CO2 response curves of net assimilation at different light levels), the CO2 compensation point in the absence of day respiration (Γ*) was similar in natural and heavy water (Fig. 2A). In other words, under a fixed internal CO2 mole fraction, both H2O- and D2O-incubated leaves had the same partitioning factor of Rubisco (vc/vo, the carboxylase-to-oxygenase activity ratio); that is, when normalized to CO2 gross assimilation, photorespiration was similar in both H2O and D2O. While there is a small effect of D2O on the night respiration rate (Fig. 2A), the day respiration rate was reduced by heavy water (Fig. 2B), suggesting that respiratory substrates and/or the metabolic pathway involved in CO2 evolution (different solvent isotope effects) were not similar in the light and in the dark. In other words, day respiration involved rate-limiting reactions that were isotopically sensitive to deuterium.
Figure 2.
CO2 evolution of detached X. strumarium leaves in either H2O or D2O in the dark (A) or in the light (B) at 21°C and 21% O2. The CO2 compensation point in the absence of day respiration (ci-based Γ*) is shown in C. The rate of day respiration (CO2 evolution in the light) and Γ* were measured with the Laisk method (Laisk, 1977).
Day Decarboxylation of Pyruvate
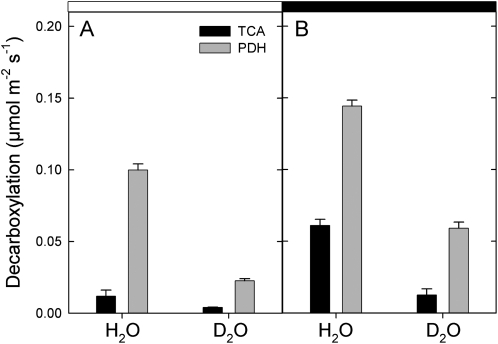
Detached leaves were fed with 13C-enriched pyruvate in the light under either H2O or D2O conditions. The decarboxylation rate of [13C]3-pyruvate and [13C]1-pyruvate via the TCA cycle and the pyruvate dehydrogenase (PDH) was measured using the deviation of the 12C/13C on-line photosynthetic isotope fractionation Δobs due to the production of 13CO2 in the light (Fig. 3). In H2O, the PDH-catalyzed decarboxylation was reduced in the light to a small extent (by nearly 30%) compared with darkness. The decarboxylation associated with the TCA cycle was mostly inhibited (by nearly 75%) by light. In D2O, both decarboxylations were smaller compared with H2O either in the dark or in the light.
Figure 3.
Rate of decarboxylation of 13C-enriched pyruvate by the TCA cycle (black bars) or pyruvate dehydrogenase (gray bars) of detached X. strumarium leaves either in the light (A) under 400 μmol mol−1 CO2, 21% O2, 400 μmol m−2 s−1 photosynthetically active radiation, and 21°C or in the subsequent dark period (B). The experiments were done in either H2O or D2O. The decarboxylation rates were measured using the [13C]pyruvate enrichment technique and subsequent 13C analysis of evolved CO2 (for details, see “Materials and Methods”).
In the light, there was a solvent isotope effect on decarboxylation rates of about 4 (PDH) or 3 (TCA cycle). The deuterium isotope effect associated with citrate synthase is 3 (Fig. 4; Supplemental Table S1), suggesting that the entry into the TCA cycle was controlled by this very enzyme (similar isotope effect of 3). The deuterium isotope effect of the PDH is smaller (1.7; Fig. 4; Supplemental Table S1) than that observed here (4), so additional deuterium isotope effects occurred, thereby decreasing the 13CO2 production from [13C]1-pyruvate. The latter effect may have come from the refixation of 13CO2 by the PEPC that impeded the apparent CO2 evolution by the PDH (see below).
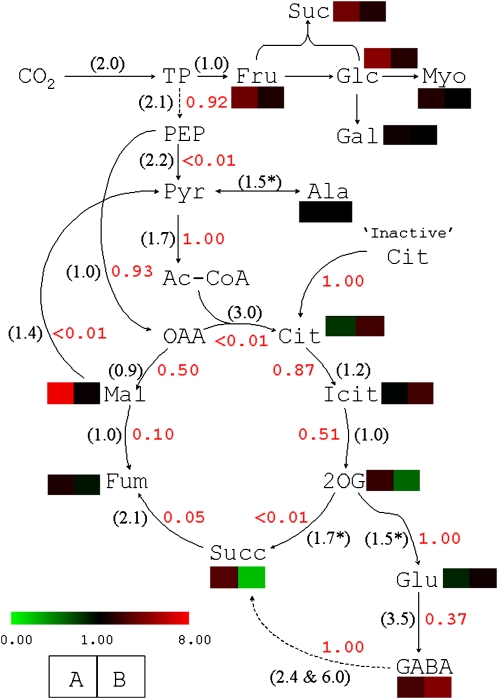
Figure 4.
Respiratory fluxome analysis after 13CO2 labeling and measurement of 13C enrichments in C-atom positions in metabolites with 13C-NMR on detached X. strumarium leaves. CO2 labeling was done under 400 μmol mol−1 CO2, 21% O2, 400 μmol m−2 s−1 photosynthetically active radiation, and 21°C. Transmission coefficients of metabolic reactions (Fj values) are indicated in red. The in vitro H/D isotope effects associated with the enzymes of interest are indicated in parentheses. Colored cells indicate the observed H2O/D2O effect on the average molecular 13C enrichment (cell A) and the metabolite concentration measured with gas chromatography coupled to time-of-flight mass spectrometry (cell B). Asterisks indicate that the isotope effect has not been measured, so assumed values obtained with comparable enzymes are shown. Corresponding references for H/D isotope effects are given in Supplemental Table S1.
In darkness, the PDH-catalyzed decarboxylation was significantly less affected by D2O than in the light, with a solvent isotope effect of around 2, which is close to the in vitro deuterium isotope effect (1.7). The TCA cycle is associated with a large isotope effect (nearly 4) in the dark. This value is larger than that of citrate synthase (3), indicating that other effects occurred in D2O, such as the concatenation of H/D isotope effects along the TCA cycle (i.e., there were cumulative H/D isotope effects on citrate synthase, succinate dehydrogenase, etc.). Therefore, at this stage, the results suggest that under D2O conditions, the TCA cycle was mainly restricted by citrate production in the light and by several steps in the dark.
Day Respiratory Fluxome
In order to get insight into actual metabolic fluxes associated with day respiration, leaves were labeled with 13CO2 in the light and sampling was done with the freeze-clamp technique. The 13C distribution in metabolites was analyzed by NMR. The probability coefficients were back-calculated from the 13C distribution after 13CO2 photosynthetic labeling in H2O. They are shown in Figure 4 (in red). Such calculations included a citrate store at natural abundance (1.1% 13C). Without such a pool, the sum of squares could not be minimized satisfactorily (S > 0.01; see “Materials and Methods”). Under the citrate-store assumption, the probability that such a citrate reserve was used to feed the TCA cycle was 1 and the sum of squares was less than 10−4.
Clearly, two rate-limiting reactions appeared within the glycolytic/respiratory pathway, namely, pyruvate kinase and citrate synthase; their probability coefficient was lower than 0.01 (Fig. 4). In other words, the very modest 13C labeling in citrate and metabolites downstream (e.g. succinate) was such that the calculated probability coefficient of citrate synthase was very small. Both phosphoenolpyruvate and 2-oxoglutarate were associated with two competitive reactions, for which the PEPC-catalyzed carboxylation and the GOGAT-catalyzed Glu production were much more likely (probability coefficient of 0.93 and 1.00, respectively) than pyruvate kinase and 2-oxoglutarate decarboxylation (probability coefficient < 0.01), respectively. Unsurprisingly, then, the 13C-labeling pattern observed in succinate was consistent with the involvement of the γ-aminobutyrate (GABA) shunt; that is, we found probability coefficients of 1 for 2-oxoglutarate amination and GABA conversion to succinate and of 0.37 for Glu decarboxylase. Noteworthy, oxaloacetate molecules were not fully committed to the citrate synthase reaction but, rather, to malate production (probability coefficient of 0.5) through the reverse operation of the malate dehydrogenase, which was further followed by the fumarase (probability coefficient of 0.1). This result was further confirmed by the use of two transfer coefficients (forward and backward) for both the fumarase and the malate dehydrogenase. Under the assumption that reversibility occurred, the forward transfer coefficients obtained were zero for both reactions with no change of backward values, thereby pointing out that these reactions did operate in a reverse manner (toward fumarate and malate, respectively). As both malate and succinate gave fumarate, fumarate tended to accumulate, as already observed in this species (Tcherkez et al., 2008).
Such a pattern was consistent with the effect of D2O on the distribution of the 13C label and on metabolite concentrations (Fig. 4, colored cells). First, the most visible effects of D2O were on sugars and malate. In the latter metabolite, this was due to the lower 13C enrichment in phosphoenolpyruvate (PEPC substrate) because of the deuterium isotope effect on photosynthetic CO2 assimilation. Citrate appeared slightly more 13C enriched though slightly less abundant in D2O. This suggests the involvement of the malic enzyme, which produced 13C-enriched pyruvate from PEPC-derived malate, while the deuterium isotope effect of citrate synthase restricted even more the citrate production rate, thereby reducing its content. Succinate tended to accumulate (larger content; Fig. 4, green color) simply because of the deuterium isotope effect on succinate dehydrogenase. The effect of D2O on fumarate was very slight but similar to that in malate (slightly less 13C enriched; Fig. 4).
13C Distribution after Pyruvate or Glc Labeling
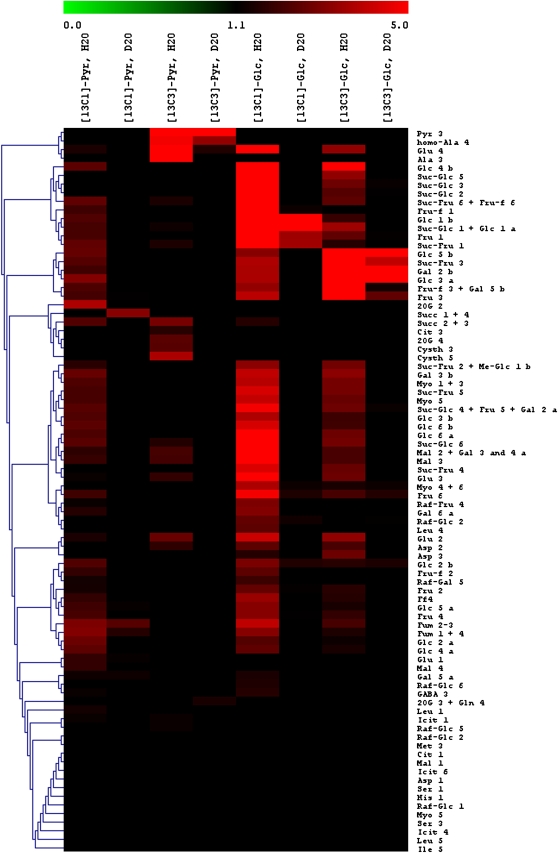
The 13C percentage in C-atom positions detected with NMR after [13C]pyruvate or [13C]Glc labeling in either H2O or D2O is shown in Figure 5, in which values are represented with colors in an isotopomic array. As expected, hexoses, Suc, raffinose, and myoinositol were labeled after [13C]Glc labeling in H2O. After [13C]1-pyruvate labeling in H2O, several atom positions were labeled, including sugars, indicating the refixation of 13CO2 evolved from [13C]1-pyruvate. Fumarate was 13C labeled after [13C]Glc feeding in H2O and not in D2O. Such labeling may have originated from either the photosynthetic input through the PEPC activity or the TCA cycle, as CO2 assimilation, glycolysis, and the citrate synthase are affected by a deuterium isotope effect. Nevertheless, with [13C]1-pyruvate in H2O, fumarate was labeled through refixation, while this was not the case with [13C]3-pyruvate, demonstrating that the TCA cycle activity was very small and that the PEPC activity was responsible for fumarate production. Consistently, fumarate was labeled by [13C]1-pyruvate in D2O, and this was most likely due to the PEPC (re)fixation, which was not affected by deuterium (no H/D isotope effect; Fig. 4), in clear contrast with the TCA cycle. In D2O, the 13C enrichment in malate showed a quite similar pattern, with (1) labeling in the C-2 and C-3 atom positions by [13C]Glc (through the PEPC activity) and, accordingly, (2) labeling in the C-4 atom position via the refixation of 13CO2 evolved from [13C]1-pyruvate.
Figure 5.
Isotopomic array representation of 13C enrichment in C-atom positions detected with 13C-NMR after [13C]Glc or [13C]pyruvate labeling of X. strumarium leaves in either H2O or D2O in the light under 400 μmol mol−1 CO2, 21% O2, 400 μmol m−2 s−1 photosynthetically active radiation, and 21°C. Cit, Citrate; Cysth, cystathionine; Fum, fumarate; Icit, isocitrate; Mal, malate; Me-Glc, O-methyl-Glc; Myo, myoinositol; Pyr, pyruvate; Raf, raffinose; Succ, succinate; 2OG, 2-oxoglutarate. Fru is designated as Fru (pyranic form) or Fru-f (furanic form). Carbon atom positions are indicated with numbers. Suc-Glc indicates the Glc moiety of Suc, Raf-Glc indicates the Glc moiety of raffinose, and so on. Lowercase a and b indicate α- and β-configurations of hexoses, respectively.
By contrast, succinate was not labeled with [13C]3-Glc, whereas it appeared labeled by either [13C]1-pyruvate (PEPC refixation) or [13C]3-pyruvate (TCA cycle activity). Citrate showed the same pattern, as did 2-oxoglutarate and Glu. The C-2 and C-3 atom positions of Glu appeared in the same cluster as the C-2 and C-3 atom positions in malate and many C atom positions in carbohydrates, while the C-1 atom of Glu was very close the C-4 atom position in malate. This indicated the important PEPC anapleurotic contribution to providing carbon atoms for Glu synthesis. On the other hand, the labeling of the C-4 atom position in Glu, mainly by [13C]3-pyruvate, was inherited from the labeling in 2-oxoglutarate formed by the TCA cycle. In addition, the C-4 atom position in Glu formed a cluster with Ala and homo-Ala, which are immediate sink metabolites after [13C]pyruvate feeding. In other words, Glu was one major carbon sink to which organic acids (citrate, isocitrate, 2-oxoglutarate) of the TCA cycle were channeled.
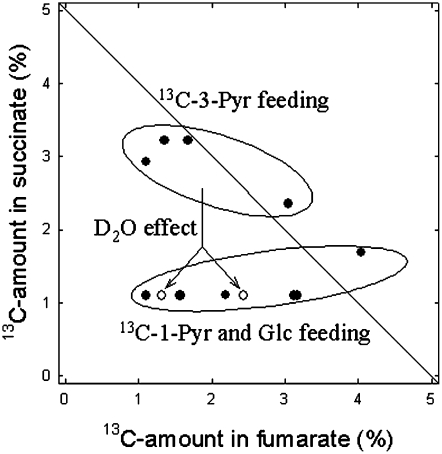
The 13C Relationship between Succinate and Fumarate
As an aid in clarifying the metabolic relationship between TCA metabolites, a graph showing the 13C enrichment in fumarate versus that in succinate (data redrawn from Fig. 5) is shown in Figure 6. These two metabolites were chosen here because fumarate follows succinate within the TCA cycle, while they may have different C-atom origins: the PEPC activity and the backward catalysis by fumarase, or the forward operation of the TCA cycle. It is clear that fumarate and succinate did not have a similar 13C enrichment pattern, some data being far from the 1:1 relationship. Rather, two different cases were observed: (1) a weak labeling in succinate combined with an increasing labeling in fumarate (Fig. 6, bottom group), and (2) a nearly constant 13C labeling in succinate combined with a similar or lower labeling in fumarate (Fig. 6, top group). Such groups matched two different labeling conditions: [13C]3-pyruvate (top group) and [13C]Glc or [13C]1-pyruvate (bottom group). This coincidence indicates that the 13C label in products of CO2 (re)fixation and carbohydrates could reach fumarate molecules but not succinate. By contrast, the production of excess [13C]acetyl-CoA after [13C]3-pyruvate labeling fed TCA intermediates downstream, such as succinate. In other words, there was a disequilibrium of the 13C label distribution between the PEPC-derived fumarate and the forward steps of the TCA cycle that produce succinate. After [13C]3-pyruvate labeling in D2O, the 13C labeling in succinate virtually disappeared (Fig. 6, white circles), likely because of the citrate synthase restriction (H/D isotope effect). This is not the case for fumarate for one datum (approximately 2.5% 13C), indicating again that the 13C enrichment in fumarate was weakly citrate synthase dependent.
Figure 6.
Relationship between the 13C enrichment (percentage) in C-atom positions in fumarate and the symmetrical C-atom positions in succinate (i.e. C-2 + C-3 and C-1 + C-4). Only data in which one of these two metabolites was labeled (13C > 1.1%) are shown. Black circles, H2O conditions; white circles, D2O conditions. Pyr, Pyruvate.
DISCUSSION
Although it has become widely accepted that leaf day respiration is inhibited in the light, the importance of respiratory TCA cycle reactions for leaf photosynthesis has been recognized (Nunes-Nesi et al., 2007); furthermore, there are many uncertainties on whether the TCA cycle biochemistry is similar in the light compared with the dark. Here, we investigated the metabolic fluxes of individual reactions of the TCA cycle in intact detached leaves of X. strumarium with two isotopic methods (13C fluxomics and H/D isotope effects).
The TCA Cycle Comprises Rate-Limiting Steps
The respiratory fluxome was calculated from the 13C enrichment in metabolites after 13CO2 feeding. While we recognize that such 13C enrichment values are associated with whole-cell metabolite pools and not specific mitochondrial, chloroplastic, or cytoplasmic pools, several limiting steps clearly accompanied CO2 evolution in the light at the leaf level: pyruvate kinase, citrate synthase, and 2-oxoglutarate dehydrogenase (probability coefficient < 0.01; Fig. 4). Accordingly, phosphoenolpyruvate was consumed by the PEPC, 2-oxoglutarate was consumed by the GOGAT to produce Glu, and the citrate pool was supplemented by the remobilization of stored citrate (Fig. 4). Similar conclusions were reached with the use of heavy water. First, day respiration was strongly affected by D2O, indicating that rate-limiting respiratory reactions are sensitive to deuterium (H/D isotope effect; Fig. 2). Second, [13C]pyruvate decarboxylation by the TCA cycle (13CO2 production from [13C]3-pyruvate) was associated with a H2O/D2O isotope effect of nearly 3 in the light, a value similar to that of citrate synthase (Fig. 3). Third, [13C]pyruvate decarboxylation by the PDH (13CO2 production from [13C]1-pyruvate) was associated with a H2O/D2O isotope effect of nearly 4 in the light, much larger than that of the PDH alone (1.7). This indicates that in D2O, 13CO2 evolved from [13C]1-pyruvate was either consumed by PEPC-catalyzed refixation or diluted by 12CO2 evolved from malate by malic enzyme. Accordingly, there is a low deuterium effect on both enzymes, thereby increasing their relative contribution to carbon fluxes as compared with the PDH. Fourth, the 13C distribution after 13CO2 labeling in D2O showed (1) a lower enrichment in malate, consistent with the involvement of the PEPC that used less 13C-enriched phosphoenolpyruvate (due to the deuterium effect on photosynthetic assimilation), and (2) a depletion of the citrate pool, revealing the rate-limiting nature of citrate synthase for the entry of neosynthesized acetyl-CoA into the TCA cycle.
Such conclusions are consistent with published biochemical data already discussed in the introduction. In fact, pyruvate kinase has been found inhibited by dihydroxyacetone phosphate in the light (Lin et al., 1989). Furthermore, the PEPC is known to be activated by phosphorylation in the light (Duff and Chollet, 1995), and protein phosphorylation is not affected by deuterium (Stein et al., 1978; Zhou and Adams, 1997). In addition, 2-oxoglutarate dehydrogenase is believed (1) to compete with the PDH for acetyl-CoA molecules and the E3 subunit of the enzymatic complex (Dry and Wiskich, 1987; Budde et al., 1991; Millar et al., 1999) and (2) to be inhibited by the NADH redox poise (Igamberdiev and Gardeström, 2003) and pyruvate (Dry and Wiskich, 1985). In other words, the oxidation of 2-oxoglutarate by this enzyme is not favorable in the light, as its substrates are more efficiently consumed by competing enzymes.
There is currently no convincing evidence that citrate synthase is controlled by allosteric phosphorylated effectors or NAD(P)H (Wiegand and Remington, 1986) except for a weak inhibition by ATP at pH less than 8 (large Ki of 5 mmol L−1; Iredale, 1979). Neither is citrate synthase activity (1) influenced by posttranslational modifications (Wiegand and Remington, 1986) or (2) limited by the amount of substrate acetyl-CoA. Acetyl-CoA synthesis by the PDH is believed to exceed consumption by the TCA (Tcherkez et al., 2005), and respiring mitochondria are thought to have very large acetyl-CoA/free CoA ratios (Budde et al., 1991). Nevertheless, it should be recognized that in vitro citrate synthase activities measured from leaf extracts have been found to be limited, with values as low as approximately 0.3 μmol m−2 s−1 in tobacco (Nicotiana tabacum), which is less than that of the PEPC (near 5 μmol m−2 s−1) or the isocitrate dehydrogenase (1–2 μmol m−2 s−1; Scheible et al., 2000). Quite importantly, citrate synthase is inhibited by citrate, with a Ki value of 3 to 5 mmol L−1 (Matsuoka and Srere, 1973). Plausibly, therefore, the involvement of citrate synthase as a limiting step (see above) stemmed from both the inhibition by and the contribution of night-stored [12C]citrate (Fig. 4) that impeded neosynthesized citrate from entering the TCA cycle. Other studies have indeed suggested that citrate is mainly produced during the night and used in the subsequent light period (Scheible et al., 2000; for a specific discussion, see Tcherkez and Hodges, 2008).
The TCA Cycle Is Bidirectional
The low rates of pyruvate kinase, 2-oxoglutarate dehydrogenase, and citrate synthase activities in vivo were associated with the redirection of oxaloacetate molecules to malate and then fumarate (Fig. 4). As such, the TCA cycle appeared not to operate like a proper cycle but rather as two different and weakly connected branches fed by the PEPC and stored citrate. Such a scenario agrees with the distribution of the 13C label after pyruvate or Glc labeling. In fact, fumarate could be labeled with [13C]1-pyruvate but not [13C]3-pyruvate, demonstrating that the entry of [13C]acetyl-CoA into the TCA cycle was small and that the PEPC activity was responsible for fumarate accumulation (Fig. 5). Accordingly, such a pattern was weakly sensitive to D2O due to the absence of a deuterium isotope effect on the PEPC. Conversely, succinate, 2-oxoglutarate, and citrate were labeled by [13C]3-pyruvate while fumarate was not (Fig. 5). Unsurprisingly therefore, when plotted against each other, fumarate and succinate had contrasting labeling patterns, with two main groups that coincided with 13C labeling in succinate under [13C]3-pyruvate feeding on the one hand and the disappearance of [13C]succinate under other labeling conditions on the other hand (Fig. 6).
The TCA “cycle” thus appeared as noncyclic, due to the lack of metabolic connection between 2-oxoglutarate (or succinate) and fumarate. In such a framework, the role of the PEPC as an anapleurotic source for supplying oxaloacetate molecules to feed both fumarate accumulation and the small citrate synthase flux is critical. Other studies provided further evidence of this using either biochemical measurements or stable isotope abundances (for review, see Tcherkez and Hodges, 2008).
The cyclic nature of the TCA pathway is restored during the night. In fact, the night activity of the TCA cycle was associated with a larger D2O effect on [13C]3-pyruvate decarboxylation (Fig. 3). Accordingly, 14C labeling and 14CO2 evolution measurements also showed that, contrary to the light, leaf dark respiration is associated with the consumption of pyruvate by the TCA cycle (Gibbs and Beevers, 1955). That is, oxaloacetate is regenerated from citrate through the breakdown of pyruvate into CO2. In addition, there was little effect of D2O on the rate of night respiration (Fig. 2), indicating that enzymes with a low deuterium dependency were involved in CO2 evolution after illumination, such as the malic enzyme. The latter has indeed been found to be involved in CO2 production in Ricinus communis leaves after the light-to-dark transition; accordingly, the malate pool decreases and the PEPC activity is down-regulated in the dark (Gessler et al., 2009).
Rationale and Perspectives
The carbon flow restriction through the TCA cycle and the fragmentation of the latter into backward and forward segments reflect imperatives caused by the metabolic control upon illumination: (1) the inhibition of TCA enzymes such as isocitrate dehydrogenase by large redox ratios [NAD(P)H/NAD(P)] within the mitochondrial matrix (Gardeström and Wigge, 1988; Igamberdiev and Gardeström, 2003); (2) the decrease of the glycolytic input of acetyl-CoA molecules because of elevated ATP and triose phosphates and the inhibition of both the pyruvate kinase and the PDH; and (3) the apparent reduction of TCA cycle activity by high dihydroxyacetone phosphate-to-Glc phosphate ratios (Tcherkez et al., 2008). Quite similarly, it has been shown in baker's yeast (Saccharomyces cerevisiae) with 13C fluxomics techniques under fermentation conditions (in which large NADH/NAD levels occur) that the TCA cycle is reorchestrated into two opposite branches, producing fumarate and 2-oxoglutarate (Camarasa et al., 2003). In leaves, the large NADH/NAD ratio is believed to be caused by photorespiratory Gly decarboxylation (Igamberdiev and Gardeström, 2003) and the export of excess reductive power from the chloroplast (Noctor et al., 2007).
As a consequence, organic acids such as malate and fumarate accumulate, and both the small influx of neosynthesized citrate and the citrate reserve are consumed. A major fate of such citrate molecules is certainly Glu, as evidenced by the quite large production of [13C]Glu after [13C]3-pyruvate feeding, as well as [13C]GABA (through the GABA shunt; Figs. 4 and 5). That is, Glu is one major carbon sink to which TCA-related organic acids are channeled and, as such, nitrogen metabolism appears cornerstone for day respiratory carbon metabolism. Accordingly, malate and fumarate may act as counteranions for pH regulation during nitrate assimilation. This view is consistent with that of Scheible et al. (2000), who argued that diurnal changes in both transcript levels and TCA enzyme activities correlated with nitrogen (NH3) availability. In addition, it has been shown in short-term manipulations of CO2/O2 ratios that the TCA cycle activity was higher when photorespiration increased, thereby responding to a larger need of Glu molecules for the NH2 transfer cycle during the recovery of photorespiratory cycle intermediates (Tcherkez et al., 2008).
Our conclusions may have pervading consequences such that likely nitrogen assimilation (Glu production) weakly correlates to instantaneous carbon assimilation because of the buffering effect of stored citrate and the use of PEPC-derived oxaloacetate molecules for fumarate production. Further experimental assessment that uses simultaneous 15N and 13C labeling is thus needed and will be addressed in a subsequent study.
MATERIALS AND METHODS
Plant Material
Xanthium strumarium (Asteraceae) plants were grown in the greenhouse from seed in 100-mL pots of potting mix and transferred to 3-L pots after 2 weeks. Minimum photosynthetic photon flux density during a 16-h photoperiod was kept at approximately 400 μmol m−2 s−1 by supplementary lighting. Temperature and vapor pressure deficit were maintained at approximately 25.5°C/18.5°C and 1.4/1.2 kPa day/night, respectively. The carbon isotope composition (δ13C) of CO2 in the greenhouse air was −9.5‰ ± 0.3‰. The third or fourth leaves (from the apical bud) were used for all measurements.
Gas-Exchange Measurements
Open System (Photosynthesis and On-Line Carbon Isotope Discrimination)
The photosynthesis system is similar to that described by Tcherkez et al. (2008). Briefly, a purpose-built assimilation chamber was connected in parallel to the sample air hose of the LI-6400 (LI-COR). Leaf temperature was controlled at 21°C with a water bath and was measured with a copper-constantan thermocouple plugged to the thermocouple sensor connector of the LI-6400 chamber/infrared gas analyzer. Inlet air was adjusted to approximately 10 mmol mol−1 H2O and passed through the chamber at a rate of 30 L h−1, monitored by the LI-6400. Light (400 μmol m−2 s−1) was supplied by light-emitting diodes (Spot-LED PE5730-904; Pearl Diffusion). Inlet CO2 was obtained from a gas cylinder (Alphagaz N48; Air Liquide) with a δ13C of −45.3‰ ± 0.2‰. The outlet air of the chamber was regularly shunted and was sent to a sampling loop to measure the 13C/12C and 18O/16O isotope composition and thus the on-line isotopic discrimination (Δobs). The gas inside the loop was introduced into an NA-1500 elemental analyzer (EA; Carlo-Erba), as described by Tcherkez et al. (2005). When the isotopic analysis was triggered, the loop was shunted and the gas inside was introduced into the EA with helium for gas chromatography. The connection valve between the EA and the isotope ratio mass spectrometer (Optima; GV Instruments) was opened when the CO2 peak emerged from the EA. Δobs during photosynthesis was measured following the method described by Evans et al. (1986). The isotope composition of dark-respired CO2 was similarly measured with the open system.
Sampling
Leaves were cut (petiole under water) at the end of the light period in the greenhouse and incubated in the dark (15 h) in either H2O or D2O at 21°C. Such an incubation period of 15 h allowed the full replacement of leaf water by D2O and was enough for accumulated dark respiratory intermediates (such as citrate) to be fully deuterated (the proton/deuteron exchange time is about 0.4–0.7 × 10−3 s at ambient temperature). The light was then turned on. Two types of 13C-labeling experiments were carried out: 99% 13C enrichments followed by NMR analyses, and near-natural 13C abundance enrichments coupled to isotope-ratio mass spectrometry. The photosynthetic conditions were exactly similar in both (light level, temperature, O2 and CO2 mole fraction). In all experiments, photosynthesis was allowed to stabilize for 100 min before labeling, and then leaves were labeled for 2 h ([13C]Glc and [13C]pyruvate labeling) or 50 min (13CO2 labeling). For NMR analyses, the leaf was immediately frozen at the end of the labeling period with a freeze-clamp system and then kept at −80°C. Such a modest duration of 13CO2 labeling (50 min) avoided the general buildup of 13C signals and so allowed the accurate identification and quantification of 13C peaks by NMR. Separate identical experiments were carried out for isotopic isotope-ratio mass spectrometry measurements, because in the latter case, the 13C enrichment in labeling substrates (Glc and pyruvate) was lower (near-natural abundance) and the carbon isotope composition of dark-respired CO2 was measured after illumination on dark-adapted leaves (30 min after the light was switched off).
Day Respiration Rate and Γ*
The day respiration rate and Γ* were measured with the Laisk method (Laisk, 1977). Γ* values obtained in D2O were corrected for the solvent isotope effect associated with CO2 and O2 dissolution in water (αH/D of 0.994 and 1.097, respectively; Wilhelm et al., 1977; Scharlin and Battino, 1992).
NMR Analyses
NMR measurements were carried out as described by Tcherkez et al. (2005) from perchloric acid extracts prepared from 2.5 g of frozen leaf material. Spectra were obtained using a Bruker spectrometer (AMX 400) equipped with a 10-mm multinuclear probe tuned at 100.6 MHz (13C-NMR). The assignment of 13C resonance peaks was carried out according to Gout et al. (1993). Identified compounds were quantified from the area of their resonance peaks using fully relaxed conditions for spectra acquisition (pulses at 20-s intervals). Peak intensities were normalized to a known amount of the internal reference compound (maleate for 13C) that was added to the sample (internal standard). When 13C-13C or 13C-D spin-spin interactions occurred, the total 13C signal of the C-atom position of interest was the sum of the peak intensities of the multiplet. Two types of control were done for NMR analyses: experiments with water (no labeling substrates) and those with 12C substrate. The latter control was required to normalize the 13C signal after 13C labeling so as to calculate the 13C percentage.
Chemicals
Heavy water (D2O 99.9%; D-175 CDN-isotopes) was from Cluzeau Info Labo. D2O was also enriched in 18O at a near-natural abundance level (δ18O = +668‰ ± 5‰ versus SMOW). The positional 13C-labeled molecules (99% 13C in the considered position) were purchased from Eurisotop. Pyruvate was dissolved in distilled water, and the pH was adjusted to 6.7 with NaOH. To obtain nonfully labeled solutions (near-natural abundances for Δobs experiments), the labeled compounds were mixed with industrial pyruvate (δ13C = −21‰) from Sigma. The resulting overall composition of pyruvate solutions was checked to be 1,400‰. In all case, the final concentration was 0.015 mol L−1. The solutions were fed to leaves through the transpiration stream.
Calculations
The procedure used to calculate the decarboxylation rates of 13C-enriched substrates in the light from apparent Δobs values has already been explained in detail by Tcherkez et al. (2005, 2008). Briefly, the difference between apparent Δobs values obtained with and without substrate addition is considered to reflect the additional decarboxylation flux in the light. Using mass balance equations, it can be shown that the decarboxylation rate rday has the following form:
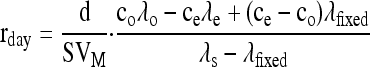 |
where d is airflow, S is leaf surface area, VM is the molar volume at air temperature, and ce and co are the CO2 mole fractions in inlet and outlet air, respectively. λ values are 13C percentages (using δ values is not possible because of large 13C enrichments) in inlet CO2 (subscript e), outlet CO2 (subscript o), net fixed CO2 (subscript fixed), and 13C-enriched added substrate (subscript s). This equation holds for homogeneously labeled substrates; it is somewhat changed for positional enrichments to take into account the different origin of decarboxylated CO2. This occurs when pyruvate is added: the C-1 atom of pyruvate is decarboxylated by PDH, while the C-2 and C-3 positions are decarboxylated by the TCA cycle.
A similar procedure applies to dark-respired CO2 measurements. In other words, CO2 that is produced in darkness (13C percentage; λglobal) after a light period with 13C-enriched substrate feeding comes from respiratory oxidation of new photosynthates (the 13C percentage in the net fixed carbon; λfixed), photosynthates from the previous light period in the greenhouse (13C percentage; λprevious), and additional C coming from the 13C-enriched substrate fed to the leaf (13C percentage; λs). The night decarboxylation rate has the following form:
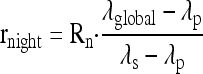 |
where λp is a linear combination of λprevious and λfixed. Rn is the dark respiration rate. It is equal to 0.6 λprevious+ 0.4 λfixed after 2 to 3 h in the light under ordinary CO2/O2 conditions (during which 200–400 mmol C m−2 have been fixed; Nogués et al., 2004). It should be noted that possible variations in these coefficients only introduce negligible errors in the estimate of the 13C-enriched substrate decarboxylation (rnight) because of the strong 13C enrichment of the substrate (i.e. the λp value is always very small compared to λglobal or λs and may be neglected). Again, that relationship is somewhat modified with positional enrichments to take into account the different origin of decarboxylated CO2 (Tcherkez et al., 2005).
Oxygen Isotope Calculations
The oxygen isotope fractionation (18Δobs) was measured on-line as described above. The oxygen isotope composition (δ18O, versus SMOW) of natural (H2O) and heavy (D2O) water used here was −24‰ ± 0.4‰ and +668‰ ± 5‰. The δ18O value of water at the evaporative sites (δe) was back-estimated from 18Δobs with the following equations (Flanagan et al., 1991):
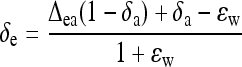 |
where ɛw is the 16O/18O isotope fractionation factor associated with H2O-CO2 oxygen exchange (41.9‰ at 21°C). δa is the δ18O value of water vapor in air. Δea is the 18O enrichment of leaf water compared with water vapor in air. If we assume a full 18O isotopic equilibrium between H2O and CO2, we have:
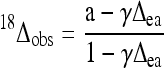 |
that rearranges to:
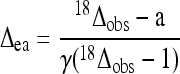 |
where γ is the ratio cc/(ca − cc), with ca and cc being the CO2 mole fraction outside the leaf and in mesophyll cells. a is the 16O/18O isotope fractionation associated with CO2 diffusion. As inlet air was completely dry in the gas exchange system used here, the water vapor in outlet air only came from transpired water. We thus made the further assumption (mass balance): δa ≈ δe − ɛ+, where ɛ+ is the 16O/18O isotope fractionation factor associated with the liquid-vapor water equilibrium (−9.5‰ at 21°C). This gives:
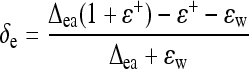 |
The value of Δea was obtained from 18Δobs (equation above) and the cc value estimated with the internal mesophyll conductance measured in both natural and heavy water by Tcherkez and Farquhar (2008).
Metabolomic Measurements
Gas chromatography coupled to time-of-flight mass spectrometry was performed on a LECO Pegasus III with an Agilent 6890 N gas chromatography system and an Agilent 7683 automatic liquid sampler. The column was an RTX-5 w/integra-Guard (30 m × 0.25 mm i.d. + 10-m integrated guard column; Restek). Leaf samples (20 mg of powder from freeze-dried material) were ground in a mortar in liquid N2 and then in 2 mL of 80% methanol in which ribitol (100 μmol L−1) was added as an internal standard. Extracts were transferred to 2-mL Eppendorf tubes and centrifuged at 10,000g and 4°C for 15 min. Supernatants were transferred to fresh tubes and centrifuged again. Several aliquots of each extract (0.1 mL, 3 × 0.2 mL, and 0.4 mL) were spin-dried under vacuum and stored at −80°C until analysis. Methoxyamine was dissolved in pyridine at 20 mg mL−1, and 50 μL of this mixture was used to dissolve the dry sample (from the 0.2-mL aliquot; see above). Following vigorous mixing, samples were incubated for 90 min at 30°C with shaking. Eighty microliters of N-methyl-N-(trimethyl-silyl)trifluoroacetamide was then added, and the mixture was vortexed and incubated for 30 min at 37°C with shaking. The derivatization mix was then incubated for 2 h at room temperature. Before loading into the gas chromatography autosampler, a mix of a series of eight alkanes (chain lengths of C10–C36) was included.
Analyses were performed by injecting 1 μL in splitless mode at 230°C injector temperature. The chromatographic separation was performed in helium as a carrier gas at 1 mL min−1 in the constant flow mode and using a temperature ramp ranging from 80°C to 330°C between 2 and 18 min, followed by 6 min at 330°C. The total run time per injection was 30 min. Ionization was made by electron impact at 70 eV, and the mass spectra acquisition rate was 20 spectra s−1 over the m/z range 80 to 500, as described previously (Weckwerth et al., 2004). Peak identity was established by comparison of the fragmentation pattern with mass spectra available databases (National Institute of Standards and Technology), using a match cutoff criterion of 700/1,000, and by retention index using the alkane series as retention standards. The integration of peaks was performed using the LECO Pegasus software. Because automated peak integration was occasionally erroneous, integration was verified manually for each compound in all analyses.
Clustering Analysis
The 13C-NMR data were represented as an isotopomic array as described by Tcherkez et al. (2007). The positional isotopic abundances (in 13C percentage) relative to the natural 13C abundance (1.1%) are indicated by colors, so that black cells indicate near-natural abundance and green and red cells indicate lower and larger than natural 13C abundance. The clustering analysis was carried out with MeV 4.1 software (Saeed et al., 2003) and based on the cosine correlation method.
Fluxomics Calculations from NMR Data
In the following, we describe how flux rates (here expressed as probability coefficients) associated with the reactions of respiratory metabolism have been calculated from 13C signals (measured with NMR) in C-atom positions of metabolites. In other words, the 13C signals were used as input variables and a set of equations (as described below) was used to calculate probability coefficients. The principle of the computation was to minimize residuals (sum of squares).
As this study used 13C pulse experiments and not steady-state isotopic labeling, fluxomics calculations were based on 13C amounts brought out by 13C labeling (denoted as ui for any C-atom position i) and not 13C percentages. That is, the 13C signal obtained with the control 12CO2 experiment (denoted as 12ui) was subtracted from that obtained with the 13CO2 experiment (denoted as 13ui): ui = 13ui − 12ui. 12ui is ordinarily not equal to zero simply because of the natural 13C abundance (1.1%). The 13C amount brought by labeling was then normalized with the total assimilated 13C (denoted as U). The corresponding value is denoted as Pi:
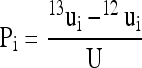 |
where i is the C-atom position of interest. Similarly, U is the total 13C amount brought by the labeling; that is, U = 13U − 12U. The 13C distribution (set of pi values for all detected C-atom positions in NMR spectra) was then analyzed using a set of equations similar to those of Dieuaide-Noubhani et al. (1995) and Rontein et al. (2002) and exemplified by the equation below. We did not use flux rates here but instead probability coefficients from source C to the C-atom position i: any metabolic reaction j was associated with a probability coefficient (transfer coefficient) Fj. The transfer probability through two subsequent reactions, j and k, is assumed to be equal to the product FjFk. The probability that the 13C signal of a C-atom position within a given metabolite was detected not only depends on the production rate but is also the result of its commitment into the subsequent reaction m; as such, we used (1 − Fm) as a multiplying factor. For example, the C-1 atom position in pyruvate (Pyr) is formed through either glycolysis from triose phosphates (TP) or the malic enzyme from malate (Mal). We thus obtained the calculated 13C amount brought by labeling (pi) as follows:
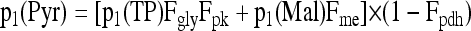 |
where Fgly , Fpk, Fme, and Fpdh are the transfer coefficients associated with glycolytic PEP production, pyruvate kinase, malic enzyme, and pyruvate dehydrogenase, respectively. Similar equations were written for the other metabolites, so that the general equation for the C-atom position i of metabolite x is:
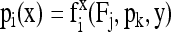 |
where Fj, pk, and y are transfer coefficients, 13C amounts, and other C-atom positions, respectively. fix is the particular mathematical function associated with the position i in x. The Fj values were computed with the method derived from Römisch-Margl et al. (2007); that is, by minimizing the sum of squares:
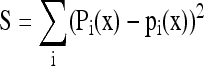 |
where Pi(x) is the observed value of the 13C amount in C-atom position i in metabolite x.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. H/D isotope effects of the enzymes of interest.
Supplementary Material
This work was supported by the Institut Fédératif de Recherche and the Agence Nationale de la Recherche (Young Researcher Project contract no. JC08–330055 to G.T.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Guillaume Tcherkez (guillaume.tcherkez@u-psud.fr).
The online version of this article contains Web-only data.
References
- Atkin OK, Millar AH, Gärdestrom P, Day DA (2000) Photosynthesis, carbohydrate metabolism and respiration in leaves of higher plants. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis, Physiology and Metabolism. Kluwer Academic Publishers, London, pp 203–220
- Breker L, Weber H, Griengl H, Ribbons DW (1999) In situ proton-NMR analyses of E. coli HB101 fermentations in 1H2O and D2O. Microbiology 145: 3389–3397 [DOI] [PubMed] [Google Scholar]
- Budde RJA, Fang TK, Randall DD, Miernyk JA (1991) Acetyl-coenzyme A can regulate activity of the mitochondrial pyruvate dehydrogenase complex in situ. Plant Physiol 95: 131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde RJA, Randall DD (1990) Pea leaf mitochondrial PDH complex is inactivated in vivo in a light-dependent manner. Proc Natl Acad Sci USA 87: 673–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykova NV, Keerberg O, Pärnik T, Bauwe H, Gardeström P (2005) Interaction between photorespiration and respiration in transgenic potato plants with antisense reduction in glycine decarboxylase. Planta 222: 130–140 [DOI] [PubMed] [Google Scholar]
- Camarasa C, Grivet JP, Dequin S (2003) Investigation by 13C-NMR and tricarboxylic acid (TCA) deletion mutant analysis of pathways for succinate formation in Saccharomyces cerevisiae during anaerobic fermentation. Microbiology 149: 2669–2678 [DOI] [PubMed] [Google Scholar]
- Day DD, Neuburger M, Douce R (1985) Biochemical characterization of chlorophyll-free mitochondria from pea leaves. Aust J Plant Physiol 12: 119–130 [Google Scholar]
- Dieuaide-Noubhani M, Raffard G, Canioni P, Pradet A, Raymond P (1995) Quantification of compartmented metabolic fluxes in maize root tips using isotope distribution from C-13 or C-14 labeled glucose. J Biol Chem 270: 13147–13159 [DOI] [PubMed] [Google Scholar]
- Dry IB, Wiskich JT (1985) Inhibition of 2-oxoglutarate oxidation in plant mitochondria by pyruvate. Biochem Biophys Res Commun 133: 397–403 [DOI] [PubMed] [Google Scholar]
- Dry IB, Wiskich JT (1987) 2-Oxoglutarate dehydrogenase and pyruvate dehydrogenase activities in plant mitochondria: interaction via a common coenzyme A pool. Arch Biochem Biophys 257: 92–99 [DOI] [PubMed] [Google Scholar]
- Duff SMG, Chollet R (1995) In vivo regulation of wheat-leaf phosphoenolpyruvate carboxylase by reversible phosphorylation. Plant Physiol 107: 775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13: 281–292 [Google Scholar]
- Flanagan LB, Bain JF, Ehleringer JR (1991) Stable oxygen and hydrogen isotope composition of leaf water in C3 and C4 plant species under field conditions. Oecologia 88: 394–400 [DOI] [PubMed] [Google Scholar]
- Gardeström P, Wigge B (1988) Influence of photorespiration on ATP/ADP ratios in the chloroplasts, mitochondria, and cytosol, studied by rapid fractionation of barley (Hordeum vulgare) protoplasts. Plant Physiol 88: 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler A, Tcherkez G, Karyanto O, Keitel C, Ferrio JP, Ghashghaie J, Farquhar GD (2009) On the metabolic origin of the carbon isotope composition of CO2 evolved from darkened light-acclimated leaves in Ricinus communis. New Phytol 181: 385–398 [DOI] [PubMed] [Google Scholar]
- Gibbs M, Beevers H (1955) Glucose dissimilation in the higher plant: effect of age of tissue. Plant Physiol 30: 343–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E, Bligny R, Pascal N, Douce R (1993) The C-13 nuclear magnetic resonance studies of malate and citrate synthesis and compartmentation in higher plant cells. J Biol Chem 286: 3986–3992 [PubMed] [Google Scholar]
- Hanning I, Heldt HW (1993) On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea L.) leaves. Plant Physiol 103: 1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochuli M, Szyperski T, Wüthrich K (2000) Deuterium isotope effect on the central carbon metabolism of E. coli cells grown on a D2O-containing medium. J Biomol NMR 17: 33–42 [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Gardeström P (2003) Regulation of NAD and NADP dependent isocitrate dehydrogenases by reduction levels of pyridine nucleosides in mitochondria and cytosol of pea leaves. Biochim Biophys Acta 1606: 117–125 [DOI] [PubMed] [Google Scholar]
- Iredale SE (1979) Properties of citrate synthase from Pisum sativum mitochondria. Phytochemistry 18: 1057–1059 [Google Scholar]
- Kasimova MR, Grigiene J, Krab K, Hagedorn PH, Flyvbjerg H, Andersen PE, Möller IM (2006) The free NADH concentration is kept constant in plant mitochondria under different metabolic conditions. Plant Cell 18: 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krömer S (1995) Respiration during photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 46: 45–70 [Google Scholar]
- Laisk AK (1977) Kinetics of Photosynthesis and Photorespiration in C3 Plants. Nauka, Moscow
- Lawlor DW (2002) Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J Exp Bot 53: 773–787 [PubMed] [Google Scholar]
- Lin M, Turpin DH, Plaxton WC (1989) Pyruvate kinase isozymes from the green alga Selenastrum minutum: kinetic and regulatory properties. Arch Biochem Biophys 269: 228–238 [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Srere PA (1973) Kinetic studies of citrate synthase from rat kidney and rat brain. J Biol Chem 248: 8022–8030 [PubMed] [Google Scholar]
- Millar AH, Hill SA, Leaver CJ (1999) Plant mitochondrial 2-oxoglutarate dehydrogenase complex: purification and characterisation in potato. Biochem J 343: 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, De Paepe R, Foyer CH (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12: 125–134 [DOI] [PubMed] [Google Scholar]
- Nogués S, Tcherkez G, Cornic G, Ghashghaie J (2004) Respiratory carbon metabolism following illumination in intact French bean leaves using 13C/12C isotope labeling. Plant Physiol 136: 3245–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Nesi A, Sweetlove LJ, Fernie AR (2007) Operation and function of the tricarboxylic acid cycle in the illuminated leaf. Physiol Plant 129: 45–56 [Google Scholar]
- Rasmusson AG, Escobar MA (2007) Light and diurnal regulation of plant respiratory gene expression. Physiol Plant 129: 57–67 [Google Scholar]
- Römisch-Margl W, Schramek N, Radykewitz T, Hettenhuber C, Eylert E, Huber C, Römisch-Margl L, Schwarz C, Dobner M, Demmel N, et al (2007) 13CO2 as a universal metabolic tracer in isotopologue perturbation experiments. Phytochemistry 68: 2273–2289 [DOI] [PubMed] [Google Scholar]
- Rontein D, Dieuaide-Noubhani M, Dufourc EJ, Raymond P, Rolin D (2002) The metabolic architecture of plant cells: stability of central metabolism and flexibility of anabolic pathways during the growth cycle of tomato cells. J Biol Chem 277: 43948–43960 [DOI] [PubMed] [Google Scholar]
- Rose IA (1961) The use of kinetic isotope effects in the study of metabolic control. J Biol Chem 236: 603–609 [PubMed] [Google Scholar]
- Rose IA, Kellermeyer R, Stjernholm R, Wood HG (1962) The distribution of 14C in glycogen from deuterated glycerol-14C as a measure of the effectiveness of triosephosphate isomerase in vivo. J Biol Chem 237: 3325–3331 [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378 [DOI] [PubMed] [Google Scholar]
- Scharlin P, Battino R (1992) Solubility of 13 non-polar gases in deuterium oxide at 15-45°C and 101.325 kPa: thermodynamics of transfer of non-polar gases from H2O to D2O. J Solution Chem 21: 67–91 [Google Scholar]
- Scheible WR, Krapp A, Stitt M (2000) Reciprocal diurnal changes of PEPc expression, cytosolic pyruvate kinase, citrate synthase and NADP-isocitrate dehydrogenase expression regulate organic acid metabolism during nitrate assimilation in tobacco leaves. Plant Cell Environ 23: 1155–1167 [Google Scholar]
- Stein RL, Romero R, Bull HG, Cordes EH (1978) A kinetic α-deuterium isotope effect for the binding of purine nucleosides to calf spleen purine nucleoside phosphorylase: evidence for catalysis by distortion. J Am Chem Soc 100: 6249–6251 [Google Scholar]
- Tcherkez G, Cornic G, Bligny R, Gout E, Ghashghaie J (2005) In vivo respiratory metabolism of illuminated leaves. Plant Physiol 138: 1596–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Cornic G, Bligny R, Gout E, Mahé A, Hodges M (2008) Respiratory metabolism of illuminated leaves depends on CO2 and O2 conditions. Proc Natl Acad Sci USA 105: 797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Farquhar GD (2008) On the effect of heavy water (D2O) on carbon isotope fractionation in photosynthesis. Funct Plant Biol 35: 201–212 [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Ghashghaie J, Griffiths H (2007) Methods for improving the visualization and deconvolution of isotopic signals. Plant Cell Environ 30: 887–891 [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Hodges M (2008) How isotopes may help to elucidate primary nitrogen metabolism and its interactions with (photo)respiration in C3 leaves. J Exp Bot 59: 1685–1693 [DOI] [PubMed] [Google Scholar]
- Tovar-Mendez A, Miernyk JA, Randall DD (2003) Regulation of pyruvate dehydrogenase complex activity in plant cells. Eur J Biochem 270: 1043–1049 [DOI] [PubMed] [Google Scholar]
- Weckwerth W, Wenzel K, Fiehn O (2004) Process for the integrated extraction, identification and quantification of metabolites, proteins and RNA to reveal their co-regulation in biochemical networks. Proteomics 4: 78–83 [DOI] [PubMed] [Google Scholar]
- Wiegand G, Remington SJ (1986) Citrate synthase: structure, control and mechanism. Annu Rev Biophys Biophys Chem 15: 97–117 [DOI] [PubMed] [Google Scholar]
- Wilhelm E, Battino R, Wilcock RJ (1977) Low-pressure solubility of gases in liquid water. Chem Rev 77: 219–262 [Google Scholar]
- Zhou J, Adams JA (1997) Is there a catalytic base in the active site of cAMP dependent protein kinase? Biochemistry 36: 2977–2984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.