Abstract
The isotopic composition of mercury (Hg) was determined in cinnabar ore, mine-waste calcine (retorted ore), and leachates obtained from water leaching experiments of calcine from two large Hg mining districts in the U.S. This study is the first to report significant mass-dependent Hg isotopic fractionation between cinnabar ore and resultant calcine. Data indicate that δ202Hg values relative to NIST 3133 of calcine (up to 1.52‰) in the Terlingua district, Texas, are as much as 3.24‰ heavier than cinnabar (−1.72‰) prior to retorting. In addition, δ202Hg values obtained from leachates of Terlingua district calcines are isotopically similar to, or as much as 1.17‰ heavier than associated calcines, most likely due to leaching of soluble, byproduct Hg compounds formed during ore retorting that are a minor component in the calcines. As a result of the large fractionation found between cinnabar and calcine, and because calcine is the dominant source of Hg contamination from the mines studied, δ202Hg values of calcine may be more environmentally important in these mined areas than the primary cinnabar ore. Measurement of the Hg isotopic composition of calcine is necessary when using Hg isotopes for tracing Hg sources from areas mined for Hg, especially mine water runoff.
Short abstract
Mercury isotopic composition varies from unprocessed ore, retorted ore, and leachates of mine waste at two mercury mines in the United States.
Introduction
Mercury is a common contaminant in the environment due to both natural and anthropogenic sources. It is of environmental concern because elevated concentrations can be toxic to living organisms (1). Mercury has no known metabolic function in animals and is not easily eliminated by organisms, including humans (2). Thus, exposure to Hg is considered undesirable and potentially hazardous (1).
Areas around mined and unmined deposits of Hg contain some of the highest concentrations of Hg worldwide (3−5). Abandoned and inactive Hg mines are of environmental concern because of the high concentration of Hg present in discarded wastes at these sites. There are presently no Hg mines operating in the United States, primarily because of low demand for Hg and environmental concerns (6).
Cinnabar (hexagonal, HgS) is the dominant Hg-bearing ore mineral in Hg mines worldwide, although minor ore minerals such as elemental Hg (Hg0), metacinnabar, (isometric HgS, metastable relative to cinnabar), calomel (Hg2Cl2), kleinite (Hg2N(Cl,SO4)·n(H2O)), montroydite (HgO), and terlinguaite (Hg+Hg2+ClO)) are found in some deposits (7−10). Extraction of Hg during mining is generally carried out in a retort or a rotary furnace where Hg ore is heated to 600−700 °C, which converts cinnabar to elemental Hg0, the final Hg product that is sold commercially (6,9,11−13). Retorting of Hg-bearing ore is known to be an incomplete process, and as a result, calcine found at most Hg mines contains unconverted cinnabar, elemental Hg0, metacinnabar, and other ionic Hg byproduct compounds formed during ore retorting (5,7). One consequence of Hg mining is that considerable calcine is generated during ore processing and this calcine is typically discarded on the surface at mined sites. Even after many years of inactivity, Hg mining areas continue to be characterized by highly elevated Hg concentrations as a result of the incomplete Hg extraction process (3,14). Microscopic fine-grained cinnabar and several byproduct Hg compounds found in calcine can adversely affect surrounding environments due to sediment and water runoff containing high concentrations of Hg. Minor byproduct Hg compounds such as chlorides, oxychlorides, and sulfates, which are water-soluble are generally released downstream in mine runoff, potentially contaminating nearby ecosystems (15−17). Unprocessed ore and tailings containing cinnabar also remain at some mines, and can be a significant source of Hg to surrounding environments.
New analytical methods for isotopic analysis have been developed to help characterize environmental cycling of Hg. Measurements of the isotopic composition of Hg hold promise as a basis for tracing sources of Hg contamination in the environment and evaluation of isotopic fractionation during geochemical processes (18−22). Before Hg isotopes can be evaluated as a tracer of Hg in mined areas, the variability in Hg isotopic composition of the Hg sources involved must be measured. Therefore, the objective of this study was to measure the isotopic compositions of Hg in (1) cinnabar from two areas of past Hg mining to evaluate the variability of Hg isotopic composition within Hg deposits; (2) the minor Hg ore minerals metacinnabar, calomel, kleinite, montroydite, and terlinguaite to evaluate potential isotopic fractionation during formation of these Hg minerals; (3) calcine to evaluate potential fractionation of Hg resulting from retorting of ore during mining; and, (4) water leachates of calcine to evaluate the variability in isotopic composition of the Hg in simulated runoff from Hg mines. Two mining districts of significant past Hg production, McDermitt, Nevada, and Terlingua, Texas were selected for study.
Study Areas
Terlingua, Texas
Mines of the Terlingua district are found in southwest Texas, in and around the town of Terlingua (29° 19.170′, 103° 37.000′). Mercury was mined in this area from 1888 until 1973 (23,24). Total production from this region was >5 000 000 kg of Hg (∼150 000 flasks of 34.5 kg each) (23). Consequently, of the Hg mines in the U.S., the Terlingua district produced the third largest amount of Hg and only mines in the California Coast Ranges (120 000 000 kg of Hg) and McDermitt, Nevada (10 000 000 kg of Hg) produced more (4,25). The Terlingua district includes over 30 separate mines (10), but our work focused on the Mariscal, Study Butte, Mariposa, and Terlingua (also known as the Chisos) mines. As is true for most Hg mines worldwide, the Hg ore in the Terlingua district is dominantly cinnabar, but minor Hg minerals, including metacinnabar, elemental Hg0, calomel, montroydite, terlinguaite and kleinite were identified at some mines (10,23). The district has been estimated to contain over 2 000 000 m3 of calcine (15). This calcine contains Hg concentrations as high as 19 000 μg/g (15), some of which are water-soluble (5,7). Leaching, runoff, and downstream transport of Hg from calcine potentially deliver significant Hg to surroundings streams, which eventually flow into the Rio Grande (15).
McDermitt, Nevada
Mercury was mined at McDermitt (also known as Cordero, 41° 54.970′, 117° 48.600′) from 1935 to 1992, when the mine closed (6). McDermitt was the largest Hg mine in Nevada, and production of Hg was about 10 000 000 kg (25). Ore was dominantly cinnabar, but minor elemental Hg0 and oxychlorides (e.g., Hg2ClO and Hg4Cl2O) were found locally (9). Numerous piles of calcine are scattered throughout the McDermitt mine site, but have been estimated as >1 000 000 m3(8). Similar to the Terlingua mines, high Hg concentrations (up to 1400 μg/g) in calcine at McDermitt potentially lead to Hg leaching and transport of Hg downstream from the mined areas (8).
Experimental Section
Study Materials
All of the calcine and most of the cinnabar samples from the McDermitt mine and Terlingua district analyzed in this study were collected during previous studies (8,15,26). All calcine was collected as grab samples about 25−50 cm below the surface to avoid the highly oxidized near-surface environment, and generally at this depth, the calcines were dark gray in color as opposed to more oxidized red-brown at the surface. All McDermitt mine cinnabar samples were physically separated from bulk ore by crushing and hand picking. Samples of metacinnabar, calomel, cinnabar, terlinguaite, kleinite, and montroydite from the Terlingua district were obtained from the Colorado School of Mines (CSM, Golden, Colorado) Geology Museum. These minerals were physically separated from the host rock and other minerals. Previously separated and powdered calomel, montroydite, and a mixed terlinguaite-kleinite sample from the Terlingua district were obtained from a U.S. Geological Survey (USGS, Denver, Colorado) mineral collection. The terlinguaite−kleinite sample from the USGS was fine-grained and these minerals could not be separated, thus, this sample was analyzed as a mixed mineral.
Analytical Methods
Total Hg
The concentration of total Hg (THg) was determined in pulverized ore and calcine samples using a 3:1 HCl:HNO3 (aqua-regia) leach (1.0 g sample leached in 10 mL aqua-regia) and cold-vapor atomic fluorescence spectrometry following EPA method 1631 (27). Quality control for THg was established using method blanks, blank spikes, matrix spikes, standard reference materials (SRMs), and sample duplicates. Recoveries for THg on blank and matrix spikes were 93−107%. The relative percent difference in calcine sample duplicates was ≤18% for THg. For the SRM’s, NIST 2704 and PACS-2 analyzed in this study, recoveries ranged from 96 to 107% of certified values for THg. Method blanks were below the THg limit of determination, which was 0.005 μg/g.
Isotopic Analysis of Hg
Pulverized calcine and Hg mineral samples were leached in aqua-regia (3:1 HCl:HNO3) overnight in borosilicate glass test tubes (1.0 g sample leached in 10 mL aqua-regia) at room temperature and diluted immediately prior to analysis with 3% (v/v) HCl to a final Hg concentration of 1 μg/L (all minerals and calcines) or 2 μg/L (calcine leachates). A blank digestion was performed, diluted to 50 mL with 3% (v/v) HCl and confirmed to contain no Hg. Hg was introduced into a multiple collector inductively coupled plasma mass spectrometer (MC-ICP-MS) (Nu Plasma HR, Nu Instruments, Wrexham, North Wales, UK) as a cold vapor, which was generated from Hg2+ in solution using stannous chloride in a commercially available cold vapor (CV) generation system (HGX-200, Cetac, Omaha, NE). Argon carrier gas (sample gas) was introduced into the CV generator at a flow rate of 50−60 mL/min and stannous chloride reductant and sample solution, each at a rate of 1.5 mL/min were mixed with the Ar gas. A makeup Ar gas flow of 0.9−1.0 L/min was added prior to introduction into the plasma. Background signal (3% (v/v) HCl blank) was measured on each isotope peak for 60 s and subtracted from the peak integrated data. Signal data were measured via time-resolved analysis software for approximately eight minutes and data were integrated for approximately five minutes. The sensitivity of the MC-ICP-MS for 202Hg was 700−1000 mV (μg/L) and data were collected using solution THg concentrations of 1−2 μg/L. Each sample was bracketed by NIST 3133 Hg standard at the same THg as the sample and sample data were corrected for mass bias using standard sample bracketing correction. The standard sample bracket method was optimized to ensure that the precision was comparable to previously published studies that used either standard-sample bracketing or Tl correction for mass bias (18,28,29). Sensitivity for 202Hg was comparable to or greater than that of other published methods (28−30). Studies were performed to determine how closely standard and sample concentrations and matrix should to be matched to obtain optimal precision. Common matrix constituents did not cause mass bias in the measurements when no matrix matching between standards and samples was performed. No mass bias was introduced when sample and standard concentrations were varied over a 25-fold range, but method precision decreases as sample concentration is decreased. Also, when concentrations greater than 2 μg/L were used washout times increased. Isotopic ratios202Hg/198Hg, 201Hg/198Hg, 200Hg/198Hg, and 199Hg/198Hg were measured and Hg isotopic compositions are reported in standard delta notation as δxHg in per mil (‰), referenced against NIST 3133 (18) where x = 199, 200, 201, or 202. Values of δxHg are calculated as follows:
All δxHg values and 2 standard deviation (SD) reproducibility of replicate isotopic measurements are reported in Supporting Information (SI) Table S-1, but only δ202Hg data are discussed below. All data were assessed for mass independent fractionation (MIF) by calculating Δ199Hg, Δ200Hg, and Δ201Hg using a method previously described (18). The long-term reproducibility of the method was determined to be ± 0.09‰ (2SD) for δ202Hg and ± 0.05‰ (2SD) for Δ199Hg by measurement of the isotopic composition of 2 μg/L THg in secondary standard UM-Almadén (18), but was ± 0.24‰ (2SD) for δ202Hg and ± 0.10‰ (2SD) for Δ199Hg at a THg concentration of 1 μg/L (31) (SI Table S-1 contains all δ and Δ values for this standard).
Water Leaching Experiments of Mine-Waste Calcine
Laboratory water leaching studies were conducted on samples of calcine using an operationally defined procedure modified from EPA Method 1312 (32). Eight mine-waste calcine samples (seven from Terlingua and one from McDermitt) of 30−50 g were leached with 150 mL of deionized water acidified with ultrapure H2SO4/HNO3 to a pH of 5.0. Leachate fluid of pH 5 is recommended to simulate rainwater pH in the western U.S. (32). The samples were leached on a shaker table using 250 rpm for 18 h. The leachate was isolated and filtered using 0.45 μm nitro-cellulose disposable filters. Concentrated ultrapure HCl was added to the filtrate to yield 3% (v/v) HCl content. Immediately prior to analysis, leachates were diluted with 3% (v/v) HCl to yield a Hg concentration in the range of 1−2 μg/L.
Results and Discussion
Isotopic Composition of Cinnabar from the McDermitt Mine
To establish the variability of Hg isotopic composition of cinnabar within a single Hg deposit, Hg isotopic compositions were determined in (1) several cinnabar crystals separated from a single sample of ore from the McDermitt mine and (2) several additional cinnabar samples collected throughout the McDermitt mine site. The total range in the isotopic composition of Hg measured for a single sample of McDermitt cinnabar was −0.42 to −0.69‰, which is indistinguishable within analytical precision (±0.24‰, SI Tables S-1 and S-2). A smaller range in isotopic Hg compositions was found for the multiple samples of cinnabar, −0.57 to −0.70‰, also indistinguishable within analytical precision. No statistically significant MIF was observed in these samples.
Previous studies report δ202Hg compositions for various Hg-bearing mineralized rocks and ore deposits in California and Nevada as −3.88 to 2.10‰, a variation of 5.98‰ (21,33). Other studies reported a smaller variation in δ202Hg values for cinnabar samples collected from numerous Hg mines and deposits worldwide, −1.73 to 1.33‰, a variation of 3.06‰ (20,31). It is unclear if the δ202Hg compositions measured in Hg-bearing mineralized rocks (21,33) show a wide variation due to isotopic Hg sources from varying rock types or isotopic fractionation during ore formation. Although the above studies reported a wide variation in δ202Hg compositions for various Hg deposits worldwide, our Hg isotopic analysis of numerous samples of cinnabar collected throughout the McDermitt mine indicates that cinnabar at the McDermitt mine has an isotopically narrow range of δ202Hg composition of −0.60 ± 0.20‰.
Hg Isotopic Composition of Cinnabar and Other Hg Ore Minerals from the Terlingua District
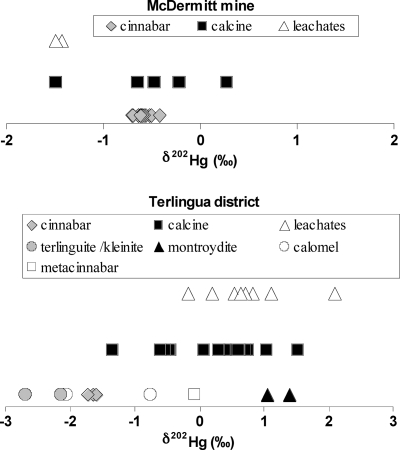
The range in δ202Hg was −1.60 to −1.72‰, indicating a statistically insignificant variation of 0.12‰, for various cinnabar samples from the Terlingua district (Figure 1). The δ202Hg of other Hg-bearing ore minerals from the Terlingua district (montroydrite, kleinite, terlinguaite, metacinnabar, and calomel) ranged from −2.70 to 1.39‰, a variation of 4.09‰ (Figure 1), a much wider variation than found for the Terlingua district cinnabar samples. In addition, mass-independent fractionations in Δ199Hg ranging from −0.09 to 0.36‰ were observed in these Hg minerals (SI Table S-1, Figure 2). The differences in Hg isotopic composition among the various Hg minerals from the Terlingua district are statistically significant (Figure 1).
Figure 1.
Isotopic compositions of Hg for various sources of Hg from the McDermitt mine and the Terlingua district. Data shown for cinnabar, metacinnabar, terlinguaite, and kleinite are isotopic measurements made in duplicate or triplicate on one sample of each mineral and symbols represent the average. For calomel and montroydite, duplicate or triplicate isotopic measurements were made on two separate samples of each mineral from different sources (Colorado School of Mines Museum and U.S. Geological Survey) and are plotted as separate averages.
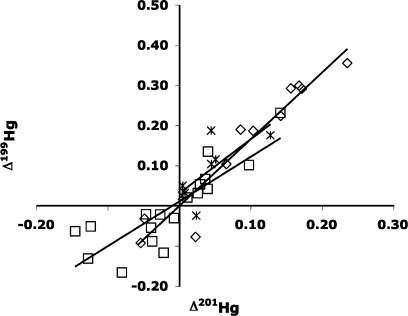
Figure 2.
Δ199 Hg vs Δ201Hg of minerals, mine waste calcine, and leachates of mine waste calcine. The slope of the line is 1.66 for the minerals and 1.11 for the calcines. The slope of the line for the leachates is 1.35 although the MIF is not statistically significant for any of the leachates based on the method reproducibility and 2σ of the mean.
The Hg-bearing ore minerals of the Terlingua district are primary, hypogene in origin, formed by hydrothermal mineralizing fluids (34). Deposition of ore minerals from a hydrothermal fluid is complex, potentially resulting from several geochemical processes including changes in pH, temperature, fluid boiling and mixing, redox reactions, and reactions with surrounding wallrocks (35). Formation conditions are unknown for the individual Hg ore minerals in the Terlingua deposit, and thus, it is not possible to explain their wide varying isotopic compositions. However, possible factors controlling mass-dependent isotopic variability in these minerals include varying formation temperatures, paragenesis, or variable redox states or bond strengths among the different minerals (36,37).
Mass independent isotopic variability is generally caused by the nuclear field shift effect or the magnetic isotope effect. Previous studies have shown MIF due to photochemical reduction of Hg2+ and methyl-Hg to Hg038,39. When Δ199 Hg vs Δ201Hg is plotted for each of these photochemical reduction processes a slope of 1.36 is obtained for methyl-Hg and 1.00 for Hg2+ reduction (39). The same plot of the Terlingua Hg minerals has a slope of 1.66 indicating that the source of MIF for these minerals is not photochemical reduction (Figure 2). The specific cause of mass independent isotopic variability in these minerals is unknown(37).
The δ202Hg values of two samples of calomel vary significantly between the two samples analyzed (−0.75‰ and −2.05‰). Calomel is a minor, but widespread, ore mineral in the Terlingua district. It is formed under a variety of temperatures, and is also known to contain microscopic amounts of elemental Hg0(40). The δ202Hg values of calomel suggest isotopic fractionation as a result of variable formational processes, formation temperatures, or redox conditions. The difference in δ202Hg values also could indicate the presence of elemental Hg0 in different proportions to calomel in the samples. This Hg would be expected to have a different isotopic composition than calomel, causing variations in the measured bulk Hg isotopic composition of the calomel samples.
Hg Isotopic Composition of Mine-Waste Calcines
Terlingua, Texas
The δ202Hg data obtained from the analysis of calcine in the Terlingua district show isotopic fractionation of the cinnabar due to the retorting process. The δ202Hg values for Terlingua calcine vary from −1.34‰ (Study Butte mine) to 1.52‰ (Mariscal mine), a variation of 2.86‰ (Figure 1). Terlingua cinnabar has an average δ202Hg of −1.66 ± 0.12‰ (Figure 1). Thus, the δ202Hg values for samples of Terlingua calcine are isotopically heavier than the original cinnabar, recording Hg isotopic fractionation. MIF in Δ199Hg range from −0.17 to 0.23‰ in the Terlingua calcines (Figure 2), which is small or statistically insignificant. During the retorting process, Hg2+ in cinnabar is reduced to Hg0(gas) and volatilized from the ore. This Hg0(gas) is then cooled, condensed, and captured as Hg0(liquid)(9). The retorting process is often incomplete, and not all of the cinnabar is converted to Hg0. Thus, some of the original cinnabar survives the retorting process and is present as microscopic grains in calcine, often as fine-grain cinnabar encapsulated in quartz and calcite gangue, but there is also metacinnabar present in calcine (5,7). Concentrations of THg up to 19 000 μg/g in samples of calcine from the Terlingua district probably are due to the presence of residual cinnabar (15) (see SI Table S-3 for Hg concentrations in calcines and calcine leachates). Therefore, calcines with δ202Hg values (i.e., Study Butte mine calcine sample 03SB3, −1.34‰) that are isotopically similar to Terlingua cinnabar (−1.66 ± 0.12‰), may contain significant residual cinnabar that has not been altered by the retort process. Conversely, Terlingua calcines that are enriched in the heavy isotopes of Hg (relative to cinnabar) require that the volatilized Hg0 was enriched in the light isotopes. However, no correlation between THg in calcines and isotopic composition was found (SI Figure S-1). The lack of correlation between THg in calcines and δ202Hg values is partly due to a cinnabar “nugget effect”. For example, a calcine containing cinnabar may have the isotopic composition of cinnabar, but samples with more residual cinnabar will have a higher THg, but also have the isotopic composition of cinnabar, thus, δ202Hg will not generally correlate with higher THg in many calcine samples.
Calcine contains byproduct Hg compounds formed when cinnabar is converted to elemental Hg0 during the retorting process (12,15) and these newly formed Hg compounds are enriched in the heavier isotopes of Hg. Consequently, previous research has shown that there are several natural and anthropogenic processes that will fractionate Hg isotopes and result in the formation of an isotopically light Hg0(gas) including (1) biotic, photochemical, and chemical reduction of Hg2+ to Hg038,39,41,42, (2) volatilization of Hg0(gas) from aqueous solution and Hg0(liquid)43−45, and (3) retorting of Hg ore (31). The results for calcines in this study are consistent with previous studies that have shown that the Hg2+ remaining after incomplete reduction and volatilization will be enriched in the heavier isotopes of Hg. In addition, theoretical calculations indicate that heavier isotopes of Hg will be preferentially partitioned into the oxidized species (Hg2+) relative to the reduced species (Hg0) (46).
If a kinetic process during retorting causes Rayleigh-type fractionation, then a range in δ202Hg of 0.28−9.25‰ represents fractionation between the ore and calcine for retort efficiencies between 50 and 99% (47), using published fractionation factors for reduction and volatilization of Hg over a temperature range of 22−37 °C (38,39,41−43). A much smaller range of fractionation is observed in this study for retorting, which is expected since the retorting process is carried out at much higher temperatures. While the observed fractionation of Hg in the calcine relative to cinnabar is consistent with reduction and volatilization as the relevant processes, there could be other processes that fractionate Hg in the calcine. For instance, formation of other Hg minerals such as metacinnabar during retorting also could cause fractionation.
McDermitt, Nevada
Similar to the results for the Terlingua calcine samples, the δ202Hg data obtained for calcine samples from the McDermitt mine also reflect fractionation processes due to ore retorting. The δ202Hg values vary from −0.64 to −0.22‰ for three of the five McDermitt calcine samples (Figure 1); these samples are isotopically similar to the McDermitt cinnabar (−0.60 ± 0.20‰). MIF in Δ199Hg range from −0.05 to 0.17‰ and are not statistically significant (Figure 2). These three δ202Hg compositions indicate the presence of significant residual cinnabar that has not been altered isotopically by the retort process in these McDermitt calcine samples. Another McDermitt calcine sample was isotopically heavier (δ202Hg = 0.28‰) than McDermitt cinnabar, and similar to the results for the Terlingua calcine samples, this is likely a result of Hg isotopic fractionation during retorting (as discussed above). Conversely, the fifth McDermitt calcine sample has a significantly lighter δ202Hg value of −1.49‰. Previous studies have reported the presence of elemental Hg0 in calcine from the McDermitt mine due to inefficient removal of elemental Hg0 during retorting (48). We interpret the lighter δ202Hg in this calcine sample to be due to the presence of elemental Hg0.
Leachates of Calcine Samples
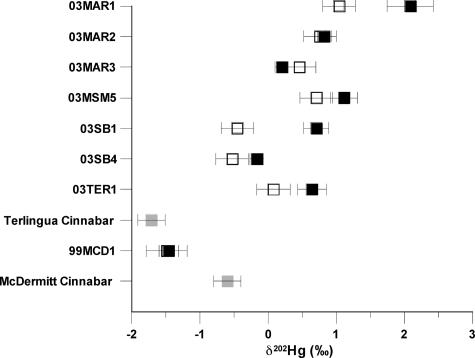
Leaching experiments were designed to examine the isotopic composition of Hg minerals that are soluble in weakly acidic water (pH 5, which is similar to rainwater pH in this region of the US) and likely to be mobilized during weathering of the calcine piles. The δ202Hg values for leachates of Terlingua calcine vary from −0.17‰ to 2.09‰, and in general, the leachates have δ202Hg values that are isotopically heavier than the associated bulk calcine (Figure 3). In only one sample (03MAR3, Mariposa mine) was the leachate δ202Hg (0.20‰) isotopically lighter than the bulk calcine sample (0.46‰). MIF in Δ199Hg ranged from −0.02 to 0.19‰ and were small or statistically insignificant. The results of the calcine leachate studies suggest the possibility that minor, soluble, byproduct Hg compounds, formed during retorting and present in the calcine, were leached during these experiments. This study was designed to determine the isotopic composition of rainwater leachate of the calcine, not that of any particular soluble compounds present in the calcine, thus, our data only indicate that these leachates are isotopically heavy in comparison to the δ202Hg of the associated calcine and cinnabar from an individual mine. For example, for sample 03SB1 (Study Butte mine) the leachate δ202Hg was 0.71‰, whereas the calcine δ202Hg was −0.46‰. Thus, the δ202Hg measured for the leachate was 1.17‰ heavier than that of the calcine sample that was leached.
Figure 3.
Variability in isotopic composition of total Hg in mine-waste calcine (open box) and pH 5 water-leachable Hg (filled box) of those calcines from mines of the Terlingua district and McDermitt mine. MAR = Mariposa mine, MSM = Mariscal mine, SB = Study Butte mine, and MCD = McDermitt mine. Error bars represent 2σ of the mean. Cinnabar from the Terlingua district and McDermitt mine (gray box) are shown for reference.
Because byproduct Hg compounds are a minor component of the calcines, these compounds are often difficult to identify. However, previous studies using edge extended X-ray absorption fine structure analysis have identified several Hg oxides, chlorides, oxychlorides, and sulfates (12) in various calcine samples, and many of these Hg compounds are water-soluble. In this study, calcine samples were leached with weak acidic water and compounds such as sulfates, chlorides, and oxychlorides were dissolved, whereas cinnabar has limited solubility at this pH. In summary, the results of our leachate studies indicate that runoff from the Hg mines studied is most likely to carry a δ202Hg isotopic composition that is similar to or heavier than that of calcine present at the site due to the presence of the minor, byproduct Hg compounds formed during or after retorting of the calcine. Because of the high solubility of these compounds at the pH of the leachate used, they dominate the δ202Hg composition in the water runoff.
Recent studies reported that the Hg isotopic compositions of river sediment collected downstream from the Idrija Hg mine, Slovenia, were similar to that of cinnabar ore at the mine upstream (49). In the Idrija study, downstream river sediments were found to contain large amounts of cinnabar, and thus, isotopic Hg tracing was used successfully to link downstream Hg contamination to cinnabar from the Idrija mine (49). We have not measured the isotopic Hg compositions of stream sediments proximal to the mines studied, however, we focused on examining anthropogenic Hg isotopic fractionation in calcine at these sites. Calcines are voluminous and primary cinnabar ore minor at the mines studied (e.g., >2 000 000 m3 in the Terlingua district), and sediment transport away from such mines, especially in dry, desert environments also is generally minor (15). Therefore, due to the large volume of calcine at these Hg mines, the Hg isotopic composition of water runoff is most likely to be dominated by the Hg isotopic composition of calcine, not the primary cinnabar ore. Studies using Hg isotopic tracing in Hg mined areas should include the measurement of the Hg isotopic composition of calcine in areas where calcine predominates volumetrically over primary cinnabar ore. The measurement of the Hg isotopic compositions of cinnabar and leachates of the calcine are also necessary to adequately trace Hg sources from areas mined for Hg. In addition, tracing of mining-related Hg may be complicated by microbial, chemical, and photochemical transformations in water and sediment (16,17,50) that potentially fractionate Hg isotopes. Such fractionation processes have not been well characterized in ecosystems downstream from areas mined for Hg.
Acknowledgments
We are grateful to the U.S. Geological Survey Mineral Resources and Energy Resources Programs for their support of this project. We also thank Bruce Geller and Ed Raines of the Colorado School of Mines Geology Museum and Heather Lowers of the U.S. Geological Survey for supplying Hg mineral samples for isotopic analysis. The review comments of Ian Ridley and Jim Crock (USGS, Denver) were helpful in the preparation of this paper as were suggestions from three reviewers for ES&T. The research described in this paper has been funded in part by the United States Environmental Protection Agency (EPA) under the Greater Research Opportunities (GRO) Graduate Program. EPA has not officially endorsed this publication and the views expressed herein may not reflect the views of the EPA. The use of brand or trade names in this report is for descriptive purposes only and does not constitute endorsement by the U.S. Geological Survey.
Supporting Information Available
Tables of Hg isotopic data for all study materials, Hg concentrations in calcine and leachates, and pH of leachates, as well as a graph of various isotopic compositions vs Hg concentrations. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- An Assessment of Mercury in the Environment. National Academy of Sciences, National Research Council: Washington, D.C., 1978; p 185. [Google Scholar]
- Eisler R.. In Mercury Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review, Biological Report 85(1.10); U.S. Fish and Wildlife Service: Washington, DC, 1987; p 90. [Google Scholar]
- Gray J. E.; Theodorakos P. M.; Bailey E. A.; Turner R. R. Distribution, speciation, and transport of mercury in stream-sediment, stream-water, and fish collected near abandoned mercury mines in southwestern Alaska, USA. Sci. Total Environ. 2000, 260, 21–33. [DOI] [PubMed] [Google Scholar]
- Rytuba J. J. Mercury mine drainage and processes that control its environmental impact. Sci. Total Environ. 2000, 260, 57–71. [DOI] [PubMed] [Google Scholar]
- Kim C. S.; Bloom N. S.; Rytuba J. J.; Brown G. E. Jr. Mercury speciation by X-ray absorption fine structure spectroscopy and sequential chemical extractions: a comparison of speciation methods. Environ. Sci. Technol. 2003, 37, 5102–5108. [DOI] [PubMed] [Google Scholar]
- Brooks W. E.; Matos G. R.. Mercury Recycling in the United States in 2000, Circular 1196-U; U.S. Geological Survey: Washington, DC, 2005; p 21. [Google Scholar]
- Gray J. E.; Hines M. E.; Higueras P. L.; Adatto I.; Lasorsa B. K. Mercury speciation and microbial transformations in mine wastes, stream sediments, and surface waters at the Almaden mining district, Spain. Environ. Sci. Technol. 2004, 38, 4285–4292. [DOI] [PubMed] [Google Scholar]
- Gray J. E.Leaching, Transport, And Methylation of Mercury in and around Abandoned Mercury Mines in the Humboldt River Basin and Surrounding Areas, Nevada, Bulletin B 2210-C; U.S. Geological Survey: Washington, DC, 2003; p 15. [Google Scholar]
- Bailey E. H.; Phoenix D. A.. Quicksilver Deposits in Nevada, Geology and Mining Series No. 41; University of Nevada: Reno, NV, 1944; p 206. [Google Scholar]
- Ross C. P. The quicksilver deposits of the Terlingua region, Texas. Econ. Geol. 1941, 35, 115–142. [Google Scholar]
- Mercury Study Report to Congress, I-VIII, EPA-452/R-97-003; U.S. Environmental Protection Agency: Washington, DC, 1997. [Google Scholar]
- Kim C. S.; Brown G. E. Jr.; Rytuba J. J. Characterization and speciation of mercury-bearing mine wastes using X-ray absorption spectroscopy. Sci. Total Environ. 2000, 261, 157–168. [DOI] [PubMed] [Google Scholar]
- Rytuba J. J. Mercury from mineral deposits and potential environmental impact. Sci. Total Environ. 2003, 43, 326–338. [Google Scholar]
- Gosar M.; Pirc S.; Bidovec M. Mercury in the Idrijca River sediments as a reflection of mining and smelting activities of the Idrija mercury mine. J. Geochem. Explor. 1997, 58, 125–131. [Google Scholar]
- Gray J. E.; Hines M. E.; Biester H. Mercury methylation influenced by areas of past mercury mining in the Terlingua district, Southwest Texas, USA. Appl. Geochem. 2006, 21, 1940–1954. [Google Scholar]
- Slowey A. J.; Brown G. E. Jr. Transformations of mercury, iron, and sulfur during the reductive dissolution of iron oxyhydroxide by sulfur. Geochim. Cosmochim. Acta 2007, 71, 877–894. [Google Scholar]
- Slowey A. J.; Rytuba J. J.; Brown G. E. Jr. Speciation of mercury and mode of transport from placer gold mine tailings. Environ. Sci. Technol. 2005, 39, 1547–1554. [DOI] [PubMed] [Google Scholar]
- Blum J. D.; Bergquist B. A. Reporting of variations in the natural isotopic composition of mercury. Anal. Bioanal. Chem. 2007, 388, 353–359. [DOI] [PubMed] [Google Scholar]
- Ridley W. I.; Stetson S. J. A review of isotopic composition as an indicator of the natural and anthropogenic behavior of mercury. Appl. Geochem. 2006, 21, 1889–1899. [Google Scholar]
- Hintelmann H.; Lu S. Y. High precision isotopic ratio measurements of mercury isotopes in cinnabar ores using multi-collector inductively coupled plasma mass spectrometry. The Analyst 2003, 128, 635–639. [DOI] [PubMed] [Google Scholar]
- Smith C. N.; Kesler S. E.; Bjorn K.; Blum J. D. Mercury isotope fractionation in fossil hydrothermal systems. Geology 2005, 33, 825–828. [Google Scholar]
- Lamborg C. A new twist for Mercury. Science 2007, 318, 402–403. [DOI] [PubMed] [Google Scholar]
- Sharp R. D.Development of the mercury mining industry Trans-Pecos Texas, Mineral Resource Circular 64; Texas Bureau of Economic Geology: Austin, 1980. [Google Scholar]
- Avery D. W.; Moyle P. R.; Reisenburg R. M.; Fay J. M.. Preliminary Assessment and Site Inspection, The Mariscal Mine and Mill Site, Big Bend National Park, Brewster County, Texas, U.S. Bureau of Mines, Site Characterization Section; U.S. Bureau of Mines: Washington, DC, 1996. [Google Scholar]
- Noble D. C.; McCormack J. K.; McKee E. H.; Silberman M. L.; Wallace A. B. Time of mineralization in the evolution of the McDermitt caldera complex, Nevada-Oregon, and the relation of Middle Miocene mineralization in the northern Great Basin to coeval regional basaltic magmatic activity. Econ. Geol. 1988, 83, 859–863. [Google Scholar]
- Gray J. E.; Crock J. G.; Fey D. L. Environmental geochemistry of abandoned mercury mines in west-central Nevada, USA. Appl. Geochem. 2002, 17, 1069–1079. [Google Scholar]
- Bloom N. S.; Fitzgerald W. F. Determination of volatile mercury species at the picogram level by low-temperature gas chromatography with cold-vapour atomic fluorescence detection. Anal. Chim. Acta 1988, 208, 151–161. [Google Scholar]
- Ghosh S.; Xu Y.; Humayun M.; Odom L.. Mass-independent fractionation of mercury isotopes in the environment. Geochem. Geophys. Geosyst. 2008, 9(3), Q03004, DOI: 10.1029/2007GC001827. [Google Scholar]
- Foucher D.; Hintelmann H. High-precision measurement of mercury isotope ratios in sediments using cold-vapor generation multi-collector inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2006, 384, 1470–1478. [DOI] [PubMed] [Google Scholar]
- Jackson T. A.; Whittle D. M.; Evans M. S.; Muir D. C. G. Evidence for mass-independent and mass-dependent fractionation of the stable isotopes of mercury by natural processes in aquatic ecosystems. Appl. Geochem. 2008, 23, 547–571. [Google Scholar]
- Stetson S. J. Analytical Methods for the Evaluation of Environmental Contamination by Perchlorate and Mercury. Ph.D. Dissertation, Colorado School of Mines, Golden, Colorado, 2009.
- Test Methods for Evaluating Solid Waste, 3rd ed.; U.S. Environmental Protection Agency: Washington, DC, 1986; Vol HI and II (SW-846).
- Smith C. N.; Kesler S. E.; Blum J. D.; Rytuba J. J. Isotope geochemistry of mercury in source rocks, mineral deposits and spring deposits of the California Coast Ranges, USA. Earth. Planet. Sci. Lett. 2008, 269, 399–407. [Google Scholar]
- Yates R. G.; Thompson G. A.. Geology and Quicksilver Deposits of the Terlingua District, Texas, Professional Paper 312; U.S. Geological Survey: Washington, DC, 1959; p 114. [Google Scholar]
- Barnes H. L., Geochemistry of Hydrothermal Ore Deposits. 3rd ed.; John Wiley and Sons: New York, 1997; p 972. [Google Scholar]
- Schauble E. A. Applying stable isotope fractionation theory to new systems. Rev. Mineral. Geochem. 2004, 55, 65–111. [Google Scholar]
- Sherman L. S.; Blum J. D.; Nordstrom D. K.; McCleskey R. B.; Barkay T.; Vetriani C. Mercury isotopic composition of hydrothermal systems in the Yellowstone Plateau volcanic field and Guaymas Basin sea-floor rift. Earth. Planet. Sci. Lett. 2009, 279, 86–96. [Google Scholar]
- Yang l.; Sturgeon R. E. Isotopic fractionation of mercury induced by reduction and ethylation. Anal. Bioanal. Chem. 2009, 393, 377–385. [DOI] [PubMed] [Google Scholar]
- Bergquist B. A.; Blum J. D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 2007, 318, 417–420. [DOI] [PubMed] [Google Scholar]
- Hillebrand W. F.; Schaller W. T.. The Mercury Minerals from Terlingua, Texas, Bulletin 405; U.S. Geological Survey: Washington, DC, 1909. [Google Scholar]
- Kritee K.; Blum J. D.; Johnson M. W.; Bergquist B. A.; Barkay T. Mercury stable isotope fractionation during reduction of Hg(II) and Hg(0) by mercury resistant microorganisms. Environ. Sci. Technol. 2007, 41, 1889–1895. [DOI] [PubMed] [Google Scholar]
- Kritee K.; Blum J. D.; Barkay T. Mercury stable isotope fractionation during reduction of Hg(II) by different microbial pathways. Environ. Sci. Technol. 2008, 42, 9171–9177. [DOI] [PubMed] [Google Scholar]
- Zheng W.; Foucher D.; Hintelmann H. Mercury isotope fractionation during volatilization of Hg(0) from solution into the gas phase. J. Anal. At. Spectrom. 2007, 22, 1097–1104. [Google Scholar]
- Sonke J. E.; Zambardi T.; Toutain J. P. Indirect gold trap-MC-ICP-MS coupling for Hg stable isotope analysis using a syringe injection interface. J. Anal. At. Spectrom. 2008, 23, 569–573. [Google Scholar]
- Estrade N.; Carignan J.; Sonke J. E.; Donard O. F. X. Mercury isotope fractionation during liquid-vapor evaporation experiments. Geochim. Cosmochim. Acta 2009, 73, 2693–2711. [Google Scholar]
- Schauble E. A. Role of nuclear volume in driving equilibrium stable isotope fractionation of mercury, thallium, and other very heavy elements. Geochim. Cosmochim. Acta 2007, 71, 2170–2189. [Google Scholar]
- Biester H.; Gosar M.; Muller G. Mercury speciation in tailings of the Idrija mercury mine. J. Geochem. Explor. 1999, 65, 195–204. [Google Scholar]
- Gray J. E.; Hines M. E.; Biester H.; Larsorsa B. K. Mercury methylation in mine wastes collected from abandoned mercury mines in the USA. J. Phys. IV 2003, 107, 573–576. [Google Scholar]
- Foucher D.; Ogrinc N.; Hintelmann H. Tracing mercury contamination from the Idrija mining region (Slovenia) to the Gulf of Trieste using Hg isotope ratio measurements. Environ. Sci. Technol. 2009, 43, 33–39. [DOI] [PubMed] [Google Scholar]
- Slowey A. J.; Johnson S. B.; Rytuba J. J.; Brown G. E. Jr. Role of organic acids in promoting colloidal transport of mercury from mine tailings. Environ. Sci. Technol. 2005, 39, 7869–7874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.