Abstract
Endometriosis is characterized by endometrial tissue growth outside the uterus, due primarily to survival, proliferation, and neoangiogenesis of eutopic endometrial cells and fragments refluxed into the peritoneal cavity during menses. Although various signaling molecules, including cAMP, regulate endometrial proliferation, survival, and embryonic receptivity in endometrium of women without endometriosis, the exact molecular signaling pathways in endometrium of women with disease remain unclear. Given the persistence of a proliferative profile and differential expression of genes associated with the MAPK signaling cascade in early secretory endometrium of women with endometriosis, we hypothesized that ERK1/2 activity influences cAMP regulation of the cell cycle. Here, we demonstrate that 8-Br-cAMP inhibits bromodeoxyuridine incorporation and cyclin D1 (CCND1) expression in cultured human endometrial stromal fibroblasts (hESF) from women without but not with endometriosis. Incubation with serum-containing or serum-free medium resulted in higher phospho-ERK1/2 levels in hESF of women with vs. without disease, independent of 8-Br-cAMP treatment. The MAPK kinase-1/2 inhibitor, U0126, fully restored cAMP down-regulation of CCND1, but not cAMP up-regulation of IGFBP1, in hESF of women with vs. without endometriosis. Immunohistochemistry demonstrated the highest phospho-ERK1/2 in the late-secretory epithelial and stromal cells in women without disease, in contrast to intense immunostaining in early-secretory epithelial and stromal cells in those with disease. These findings suggest that increased activation of ERK1/2 in endometrial cells from women with endometriosis may be responsible for persistent proliferative changes in secretory-phase endometrium.
Increased activation of MEK/ERK inhibits cAMP-dependent cell cycle regulation and promotes sustained cell proliferation in human endometrial stromal fibroblasts of women with endometriosis.
Endometriosis is a benign gynecological disorder that affects 6–10% of reproductive-age women and up to 50% of women with infertility and pelvic pain (1). The pathogenesis of endometriosis is believed to derive from retrograde transplantation of fragments and cells of eutopic uterine endometrium shed into the peritoneal cavity during menses (2). Attachment, growth, neoangiogenesis, and survival of these cells on the peritoneum, ovaries, bowel, and other organs are attributed to intrinsic abnormalities of the shed eutopic endometrium, proinflammatory environment of the ectopic sites, and compromised immune clearance of the lesions (2). The fact that eutopic endometrium differs at the molecular level in women with vs. without endometriosis is now well established, as studies from our group and others have demonstrated incomplete transitioning of the endometrium from proliferative (estrogen-dominant) to secretory [progesterone and protein kinase A (PKA)-dominant] phases, a phenotype of enhanced cellular survival, and attenuation of progesterone-induced down-regulation of DNA synthesis and cellular mitosis, compared with women without disease (3,4,5,6,7,8). Because successful embryo implantation requires proper timing and synchrony between the developing embryo and endometrium and involves sequential endometrial cell proliferation and differentiation (9,10), persistence of the proliferative phenotype in secretory endometrium of women with endometriosis may contribute, in part, to the observed infertility associated with this disorder.
Cell proliferation and differentiation (decidualization) of human endometrial stromal fibroblasts are regulated by various factors, including cAMP and progesterone (11,12,13). cAMP predominantly signals through PKA, but it may also bind to cyclic-nucleotide-gated ion channels and guanine-nucleotide exchange factors (GEFs), e.g. repressor/activator protein GEF 3 [RapGEF3, also referred as exchange protein activated by cAMP (EPAC1)], to regulate downstream targets (14). cAMP inhibits proliferation in several cell lines by repressing cyclin D1 (CCND1) expression (15,16). Although CCND1 is critical for early checkpoint regulation at the G1 phase of the cell cycle (17), and its down-regulation is essential for cAMP inhibition of cell-cycle progression (18), the mechanism involved in cAMP regulation of CCND1 is still largely unknown.
In addition to cAMP, MAPK has also been shown to play a crucial role in regulating cell proliferation (19). Indeed, persistent activation of the MAPK ERK1/2 (also referred as p42/p44) is required to pass G1 phase restriction of the cell cycle by transcriptionally up-regulating CCND1 expression (20,21,22,23). Interestingly, our previous results demonstrated that eutopic endometrium of women with endometriosis has decreased expression of genes associated with inactivation of MAPK signaling cascades relative to women without disease (3). Some of these genes include ERBB receptor feedback inhibitor 1 (ERRFI1) (also referred as MIG-6/RALT/Gene33), which is a negative regulator of MAPK signaling, and regulators of G protein signaling 1 (RGS1), which is an activator of GTPases that rapidly switches off G protein-coupled receptor signaling pathways.
Given that expression of cell cycle genes persists in secretory endometrium of women with endometriosis and expression of MAPK-associated genes is dysregulated in eutopic endometrial tissue of women with vs. without disease, we hypothesized that ERK1/2/MAPK activity may influence cAMP regulation of the cell cycle gene CCND1 in human endometrial stromal fibroblasts (hESF) of women with endometriosis. Herein, we show increased ERK1/2 activity in vivo and in vitro and inhibitory actions of ERK1/2 activity on cAMP down-regulation of CCND1 expression in endometrial stromal cells from women with vs. without endometriosis.
Materials and Methods
Human endometrial samples
Human eutopic endometrial tissue samples were obtained from 24- to 50-yr-old women undergoing endometrial biopsy or hysterectomy for diagnosis or treatment of pelvic pain, fibroids, prolapse, and/or endometriosis (Table 1). Presence or absence of endometriosis was confirmed by laparoscopic visualization and histological analysis of peritoneal lesions. Staging of endometriosis was defined according to the revised American Fertility Society classification system (24). All patients did not use hormonal medication within 3 months before surgery. Samples were obtained through the University of California, San Francisco, National Institutes of Health Human Endometrial Tissue and DNA Bank with appropriate institutional review, approvals, and written informed consent from all participating subjects, as approved by the University of California San Francisco Committee on Human Research and the Stanford University Committee on the Use of Human Subjects in Research. Endometrial samples were processed for immunohistochemistry or cell culture experiments.
Table 1.
Subject characteristics
| Cell culture
|
Immunohistochemistry
|
|||
|---|---|---|---|---|
| Sample ID | Diagnosis | Sample ID | Cycle phase | Diagnosis |
| 298 | No endo (P) | 441 | PE | No endo (Fb, M) |
| 316 | No endo (P, A, Fb) | 426 | PE | No endo (Dm, Dp, M) |
| 229 | No endo (PP) | 539 | PE | No endo (Fb) |
| 236 | No endo (POP) | 588 | PE | Endo, stage II |
| 275 | No endo (Fb) | 634 | PE | Endo, stage II |
| 277 | No endo (Fb) | 697 | PE | Endo, stage IV |
| 285 | No endo (Fb) | 114 | PE | Endo, stage IV |
| 293 | No endo (Fb) | 123 | PE | Endo, stage IV |
| 326 | No endo (Fb) | |||
| 310 | No endo (Fb, Ec) | 650 | ES | No endo (Fb, M) |
| 456 | ES | No endo (Fb, Dm, Mm) | ||
| 233 | Endo, stage II | 458 | ES | No endo (M) |
| 272 | Endo, stage II–IV (A) | 680 | ES | No endo (UVP) |
| 242 | Endo, stage I–II | 24 | ES | No endo (M) |
| 243 | Endo, stage II–III (A, Fb) | 607 | ES | Endo, stage IV |
| 279 | Endo, stage II–IV | 599 | ES | Endo, stage IV |
| 288 | Endo, stage IV | 591 | ES | Endo, stage I |
| 307 | Endo, stage IV | 625 | ES | Endo, unstaged |
| 314 | Endo, stage IV | 471 | ES | Endo, stage III–IV |
| 375 | Endo, stage IV | 127 | ES | Endo, stage IV |
| 381 | Endo, stage IV | |||
| 271 | Endo, stage IV (A) | 626 | MS | No endo (Cc, Rc) |
| 305 | Endo, stage IV (Fb) | 465 | MS | No endo (Fb) |
| 665 | MS | No endo (Fb, M, PP, A) | ||
| 635 | MS | Endo, stage II | ||
| 546 | MS | Endo, stage II (A) | ||
| 37 | MS | Endo, stage III | ||
| 526 | MS | Endo, stage III–IV (A) | ||
| 462 | LS | No endo (SUI) | ||
| 614 | LS | No endo (UVP) | ||
| 648 | LS | No endo (Fb, SUI) | ||
| 576 | LS | No endo (POC) | ||
A, Adenomyosis; Cc, cystocele; Dm, dysmenorrhea; Dp, dysparenuria; Ec, enterocele; Endo, endometriosis; Fb, uterine fibroids; M, menorrhagia; Mm, menometrorrhagia; No endo, without endometriosis; P, polyp; POC, paratubal ovarian cyst; POP, pelvic organ prolapse; PP, pelvic pain; Rc, rectocele; SUI, stress urinary incontinence; UVP, uterovaginal prolapse.
hESF isolation and culture
hESF from control (n = 10) and endometriosis (n = 12) subjects were isolated by digesting endometrial tissue samples with collagenase, followed by filtration, as previously described (25,26). Isolated hESF were then cultured in growth medium [phenol red-free medium of 3:1 high-glucose DMEM/MCDB-105, 0.676 mm sodium pyruvate, 10% charcoal-stripped (CS) fetal bovine serum (FBS), 1% antibiotic-antimycotic mix, 50 μg/ml gentamycin, and 5 μg/ml insulin], up to three to seven passages. Medium was replaced every 2–3 d. This method produces 99% pure stromal fibroblasts (26,27). For RT-PCR and Western blot experiments, confluent cells were serum starved for 24 h in serum-free medium (3:1 high-glucose DMEM/MCDB-105, 0.75 mm sodium pyruvate, 50 μg/ml gentamycin, 50 μg/ml ascorbic acid, 10 μg/ml apo-transferrin) before treatment or collection. For cAMP experiments, serum-starved cells were treated with vehicle [dimethylsulfoxide (DMSO); Sigma-Aldrich, St. Louis, MO], 1–10 μm of the PKA inhibitor H89, or 10 μm of the ERK1/2 inhibitor U0126 (Calbiochem, San Diego, CA) for 30 min in 2% CS-FBS-containing medium, according to the doses previously described (28). Treatment with 10 μm H89 resulted in a high percentage of cell death (detachment of cells from plate), whereas treatment of H89 at 1 or 5 μm did not result in visible morphological changes in hESF. Hence, experiments herein used 1 or 5 μm H89. After addition of inhibitors, cells were treated with or without 0.5 mm 8-bromoadenosine cAMP (Sigma-Aldrich) for 96 h, optimal conditions for decidualization, previously determined (26). Samples without any treatment were collected at d 0 to serve as time-0 controls. Culture media with inhibitors and cAMP were refreshed every 48 h. All sample treatments were done in independent experiments to ensure reproducibility of results.
Quantitative RT-PCR
Total RNA was isolated from cultured hESF using the RNeasy Plus Mini Kit following the manufacturer’s protocol (QIAGEN, Valencia, CA). Total RNA was quantified by UV spectrophotometry and analyzed for integrity with the Agilent 2100 Bioanalyzer (Agilent Biotechnologies, Palo Alto, CA). RNA samples were reverse transcribed using random primers and the iScript RT reagents following the manufacturer’s protocol (Bio-Rad Laboratories, Hercules, CA). mRNA levels were determined by real-time quantitative RT-PCR using the Mx 3005 Pro (Stratagene, La Jolla, CA). The primer sets (0.3 μm) were as follows: 1) CCND1, 5′-GTGGGTGTGCAAGCCAGGT-3′ and 5′-TTCCTGTCCT ACTACCGCCT-3′; 2) IGF-binding protein 1 (IGFBP1), 5′-CTATGATGGCTC GAAGGCTC-3′ and 5′-TTCTTGTTGCAGTTTGGCAG-3′; and 3) ribosomal protein L19 (RPL19), 5′-GCAGATAATGGGAGGAGCC-3′ and 5′-GCCCATCTTTGATGAGCTTC-3′. cDNA samples (1 μg RNA in 160 μl RT solution) were amplified by iQ SYBR Supermix (Bio-Rad): 1) DNA polymerase activation at 95 C for 15 min and 2) 40 cycles of denaturation at 95 C for 15 sec, annealing at 60 C (IGFBP1, RPL19) or 59 C (cyclin D1) for 45 sec, and extension at 72 C for 60 sec. Absence of primer dimers was routinely determined by performing a cycle of denaturation at 95 C for 1 min and 35 cycles of increasing temperature (1 C) from 65 C to 97 C for 33 sec at the end of each run. Relative expression was computed using (1+EFF)−Ct, where the efficiency (EFF) of the cDNA standards is from 90–110%. Samples were assayed in duplicate. Relative gene expression was normalized with RPL19 (as the internal reference) and time-zero controls within each group. Fold changes were calculated relative to the average expression of untreated hESF (no endometriosis, control) for respective time periods.
Western blot
Cell lysates were isolated using RIPA lysis buffer as previously described (25). Protein quantity in the cell lysates was evaluated by the Bradford protein assay following the manufacturer’s instructions (Bio-Rad). Twenty micrograms of total protein lysates were loaded onto 4–20% SDS-polyacrylamide gels, transferred to nitrocellulose membranes (Whatman), and blocked with 5% nonfat dry milk in Tris-buffered saline/0.1%Tween solution as previously described (25). After blocking, membranes were incubated with 1:1000 mouse monoclonal antihuman phospho-ERK1/2 (pERK1/2) antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA), rabbit antihuman ERK1/2 (Santa Cruz), or goat antihuman actin (Santa Cruz) at room temperature for 2 h or incubated with 1:500 rabbit antihuman cyclin D1 (Santa Cruz) at 4 C overnight. After washes of Tris-buffered saline/0.1%Tween solution, membranes were incubated with 1:1000 horseradish peroxidase (HRP)-conjugated sheep antimouse (GE Health Care, Buckinghamshire, UK), HRP-conjugated donkey antirabbit (Amersham Pharmacia Biotech, Inc., Piscataway, NJ), or HRP-conjugated rabbit antigoat (Santa Cruz) antibodies at room temperature for 1 h. Bound antibodies were detected by ECL plus Western blotting detection system (Amersham Pharmacia Biotech) and exposed to x-ray films (GE Health Care). Western blots were scanned by HP Scanjet 5590 and quantified by Image J.
Bromodeoxyuridine (BrdU) incorporation assay
Cultured hESF were plated at 5 × 104 cells per well in 96-well plates and serum starved for 24 h before incubation with or without cAMP for 96 h. Cells were incubated with BrdU for the last 18 h. Cell proliferation was measured by the BrdU incorporation assay, according to the manufacturer’s instructions (Roche Diagnostics GmbH, Mannheim, Germany), using a 96-well plate reader at 450 nm wavelength (Bio-Rad).
ELISA
Conditioned media were collected after incubation with cultured hESF and treated as described above. Samples were then subjected to ELISA to quantify IGFBP1 secretion following the manufacturer’s instructions (Diagnostic Systems Laboratories, Webster, TX). Individual samples were assayed in duplicate, and a standard curve was generated in each experiment. IGFBP1 production by hESF was normalized to total RNA simultaneously collected from the same hESF sample. Inter- and intraassay coefficients of variation for the IGFBP1 ELISA were 5.0–7.4 and 2.4–3.4%, respectively.
Immunohistochemistry
Full-thickness human endometrial tissues from control (n = 14) and endometriosis (n = 16) subjects during proliferative (PE), early secretory (ES), midsecretory (MS), and late secretory (LS) phases of the menstrual cycle (Table 1) were fixed in 10% buffered formalin and embedded in paraffin. Samples were then cut into 4-μm sections and mounted onto glass slides. Sections were deparaffinized in xylene (Sigma-Aldrich), rehydrated in decreasing concentrations of ethanol, quenched with enzyme block (Dako, Carpinteria, CA), and blocked with 3% BSA/0.1% Tween solution. For pERK1/2 staining, slides were sequentially incubated with 1) 1 μg/ml mouse antihuman pERK1/2-Tyr204 (Santa Cruz) or preimmunized IgG2A (negative control; Sigma-Aldrich) at room temperature for 1 h, 2) 10 μg/ml biotinylated horse antimouse IgG (Vector Laboratories, Inc., Burlingame, CA) at room temperature for 30 min, and 3) 5 μg/ml streptavidin-HRP conjugate at room temperature for 10 min, with PBS washes between steps. All slides were then stained with, 3,3′-diaminobenzidine (Vector Laboratories), counterstained with hematoxylin, dehydrated with increasing concentrations of ethanol, and mounted with Permount solution (Sigma-Aldrich) for examination under the DM5000B microscope (Leica Microsystems, Ltd., Wetzlar, Germany). Immunopositive cells (intense brown stain) and a total of approximately 500 stromal and 300 epithelial cells were counted on average from four randomly selected fields (×200 magnification) per slide and expressed as percent positive staining (number of immunopositive cells/total number of cells × 100).
Data analysis
Data are presented as least square means ± sem and were subjected to statistical analysis using Student’s t test, Mann-Whitney U test, Kruskal-Wallis ANOVA, or two-way ANOVA with (nonparametric) or without (parametric) ranks as indicated in each figure legend. Differences between means in Kruskal-Wallis ANOVA and two-way ANOVA were further analyzed by Dunn’s multiple comparison test and Bonferroni post hoc test, respectively. P < 0.05 were considered statistically significant.
Results
cAMP regulation of cell proliferation
hESF from women without endometriosis
Previously, our microarray analysis of cAMP-treated hESF (n = 2) (13) demonstrated down-regulation of several cell cycle-associated genes, including cyclin B and E2. To confirm and explore the inhibitory action of cAMP on proliferation of nonendometriotic hESF, we isolated and cultured hESF from women without endometriosis. Because induction of CCND1 is the rate-limiting step in cell proliferation that is essential for G1 progression (29), we evaluated CCND1 expression in hESF of women without endometriosis before and after cAMP treatment. As a result, cAMP significantly reduced CCND1 protein levels by about 63% in hESF (Fig. 1A) and inhibited CCND1 mRNA expression by about 74% (Fig. 1B). This phenomenon was observed even up to seven passages and does not seem to be dependent on the menstrual cycle stage when the cells were isolated (data not shown), consistent with responses in earlier observations (26).
Figure 1.
cAMP regulation of CCND1 expression in endometrial stromal fibroblasts from women without vs. with endometriosis. A, Endometrial stromal fibroblasts from patients without (no endo, gray bars, n = 4) endometriosis were treated with vehicle (Ctrl) or 0.5 mm 8-bromo-cAMP (cAMP) for 4 d in 2% serum-containing medium, and CCND1 protein expression was analyzed by Western blot. B, Endometrial stromal fibroblasts from patients without (n = 6) or with (endo, white bars, n = 6) endometriosis were treated as above, and CCND1 mRNA expression was analyzed by quantitative RT-PCR. All values were normalized by RPL19 for total actin and mRNA expression for protein levels. C, Endometrial stromal fibroblasts from patients without (n = 5) or with (n = 4) endometriosis were treated as above, and cell proliferation was analyzed by BrdU incorporation. Bar graphs (least square means ± sem) represent fold changes relative to control (Ctrl, no endo, without cAMP). Means with asterisks and same letters indicate significant differences at P < 0.05 by Student’s t test and two-way ANOVA followed by Bonferroni post hoc test, respectively.
hESF from women with endometriosis
To determine whether persistent in vivo expression of cell cycle-related genes in eutopic endometrium of women with vs. without endometriosis during ES phase (3) occurs in vitro, we compared cAMP-dependent regulation of CCND1 in hESF of women with relative to without endometriosis. Although cAMP significantly inhibited CCND1 mRNA levels in hESF of women without endometriosis, cAMP did not completely down-regulate CCND1 mRNA levels in hESF of women with disease (Fig. 1B), suggesting an attenuated response to cAMP in hESF of women with endometriosis.
BrdU incorporation
To confirm whether dysregulation of CCND1 mRNA expression is associated with abnormal cell proliferation in hESF of women with endometriosis, cell proliferation was measured by BrdU incorporation. Although cAMP significantly inhibits cell proliferation in hESF of women without disease, this significant difference was lost in hESF of women with endometriosis (Fig. 1C), consistent with the attenuated regulation of CCND1 expression by cAMP in hESF of women with disease. The data further support the abnormal phenotype observed in the proliferative-to-secretory transition in women with vs. without endometriosis.
Inhibition of PKA by H89
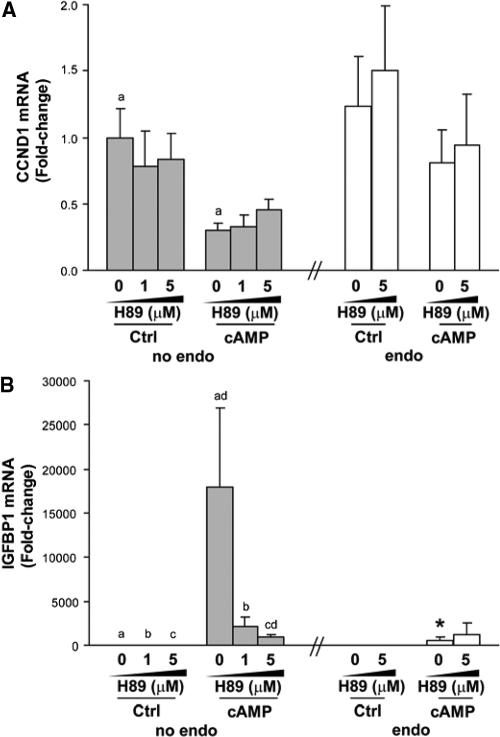
To determine whether cAMP regulation of CCND1 gene expression occurs through PKA, hESF were treated with varying doses of the PKA inhibitor H89. Inhibition of PKA by H89 at 1 and 5 μm (higher levels were toxic to cells) did not affect basal mRNA expression of CCND1 in hESF of women without endometriosis (Fig. 2A). Treatment with cAMP significantly inhibited CCND1 mRNA expression in hESF of women without disease, but H89 addition at 1 and 5 μm (higher levels were toxic to cells) did not significantly reverse cAMP action on CCND1 mRNA expression (Fig. 2A). Likewise, H89 did not influence CCND1 transcript levels in hESF of women with endometriosis, in the presence or absence of cAMP treatment (Fig. 2A, right). Treatment with cAMP increased mRNA expression of the decidualization marker, IGFBP1, in hESF of women without endometriosis to a greater extent than those of women with disease (Fig. 2B), consistent with previous reports (26,30). Interestingly, H89 at 5 μm but not at 1 μm significantly reversed cAMP induction of IGFBP1, which is a known cAMP/PKA target gene, in hESF of women without endometriosis (Fig. 2B), demonstrating the efficacy of H89 to block PKA in these cells. H89 failed to further inhibit the comparably lower cAMP induction of IGFBP1 mRNA expression in hESF of women with vs. without endometriosis (Fig. 2B), further confirming reduced cAMP/PKA action in these cells.
Figure 2.
Inhibition of PKA by H89 in endometrial stromal fibroblasts from women without vs. with endometriosis. A and B, Endometrial stromal fibroblasts from patients without (no endo, gray bars, n = 6) or with (endo, white bars, n = 6) endometriosis were treated with DMSO vehicle (no inh), 1 μm H89, or 5 μm H89 for 30 min in 2% serum-containing medium, followed by addition of vehicle (Ctrl) or 0.5 mm cAMP for 4 d. Treatments and medium were refreshed every 2 d. CCND1 (A) and IGFBP1 (B) mRNA expression were analyzed by quantitative RT-PCR. All values were normalized by RPL19. Bar graphs (least square means ± sem) represent fold changes relative to control (no inh/no endo). Means with same letters indicate significant differences within treatments at P < 0.05 using two-way ANOVA by ranks, followed by Bonferroni post hoc test. Means with asterisks indicate significant differences in endo vs. corresponding no endo treatment at P < 0.05 using Mann-Whitney U test.
pERK1/2 levels in hESF from women with or without endometriosis
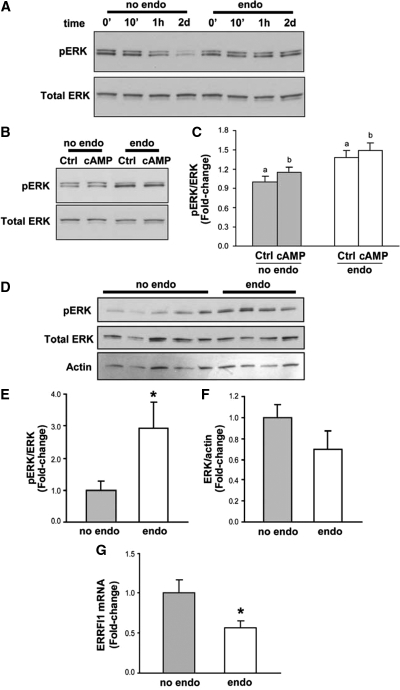
Cyclin D1 induction has frequently been associated with ERK1/2 activation (31). To explore the ERK1/2 signaling in hESF from women with endometriosis, we first evaluated the levels of pERK1/2 in hESF of women without and with endometriosis. Incubation of hESF with 2% serum-containing medium for up to 2 d resulted in decreased levels of pERK1/2/total ERK1/2 ratio in hESF of women without but not with endometriosis (Fig. 3A). Treatment with cAMP for 2 d did not affect pERK1/2/total ERK1/2 ratio in hESF of women without or with endometriosis, and pERK levels remained higher in hESF of women with relative to without endometriosis (Fig. 3, B and C). Because serum contains several growth factors that may affect pERK1/2 levels in cell lysates, confluent hESF were serum starved for 24 h and then cultured in serum-free medium. This resulted in an even greater difference in pERK1/2 levels (approximately 3-fold greater) between hESF of women without endometriosis vs. with endometriosis (Fig. 3, D and E). Expression of total ERK1/2 and actin in hESF remained the same, independent of disease or no disease (Fig. 3, D and F).
Figure 3.
ERK phosphorylation in endometrial stromal fibroblasts incubated with 2% serum-containing or serum-free medium. A, Endometrial stromal fibroblasts from patients without (no endo, gray bars, n = 6) or with (endo, white bars, n = 6) endometriosis were incubated in 2% serum-containing medium for 0 min, 10 min, 1 h, or 2 d without refreshing the medium (A), treated with vehicle (Ctrl) or 0.5 mm 8-bromo-cAMP (cAMP) in 2% serum-containing medium for 2 d without refreshing the medium (B and C), or incubated in serum-free containing medium for 24 h (D). Cell lysates were analyzed for Tyr204-phosphorylated ERK (pERK) and total ERK by Western blot. Values for pERK (C and E) and total ERK (F) were normalized by total ERK and total actin protein, respectively. Representative blots (A, B, and D) and graphical representations (C, E, and F) are shown as least square means ± sem and presented as fold change relative to control (Ctrl, no endo). Means with asterisks and same letters indicate significant differences at P < 0.05 using Student’s t test and two-way ANOVA followed by Bonferroni post hoc test, respectively. G, Endometrial stromal fibroblasts from patients without (no endo, n = 6) or with (endo, n = 6) endometriosis were incubated in serum-free medium for 24 h. ERRFI1 mRNA expression was analyzed by quantitative RT-PCR. Values were normalized by RPL19 gene. Bar graphs (least square means ± sem) represent fold changes relative to control (no endo). Means with asterisks indicate significant differences at P < 0.05 by Student’s t test.
Previously, our microarray analysis indicated lower in vivo expression of ERRFI1, a negative regulator of MAPK signaling, in eutopic endometrium of women with moderate/severe endometriosis, compared with those without disease during the ES phase of the menstrual cycle (3). Interestingly, increased pERK1/2 in cultured hESF was also accompanied by decreased mRNA expression of ERRFI1 (Fig. 3G), suggesting a correlation between increased pERK1/2 levels and diminished ERRFI1 expression in the pathophysiology of endometriosis.
Contribution of pERK1/2 to hESF response to cAMP
ERK1/2 is phosphorylated through a three-component kinase module in which Raf activates MAPK and ERK kinase 1/2 (MEK), and MEK activates ERK (32). To determine the consequence of persistently high pERK1/2 levels in cAMP regulation of CCND1, we treated hESF with the MEK1/2 inhibitor U0126. Treatment with U0126 resulted in comparably diminished pERK1/2/ERK1/2 ratio levels in hESF, and this was independent of disease state (data not shown). cAMP, but not U0126, decreased CCND1 expression in hESF of women without endometriosis, and individual treatments of cAMP and U0126 did not affect CCND1 expression in hESF of women with endometriosis (Fig. 4A). Remarkably, whereas addition of U0126 did not further affect cAMP-dependent down-regulation of CCND1 in hESF of women without endometriosis, U0126 successfully rescued the insensitivity of cAMP down-regulation of CCND1 expression in hESF of women with endometriosis (Fig. 4A), demonstrating an inhibitory effect of MEK1/2/ERK1/2 on cAMP-mediated inhibition of CCND1 in the setting of endometriosis.
Figure 4.
Contribution of MEK/ERK to cAMP regulation of CCND1 and IGFBP1 expression in cultured endometrial stromal fibroblasts. A–C, Endometrial stromal fibroblasts from patients without (no endo, gray bars, n = 9) or with (endo, white bars, n = 7) endometriosis were treated with DMSO (vehicle, no inh) or 10 μm U0126 for 30 min in 2% serum-containing medium, followed by addition of vehicle (Ctrl) or 0.5 mm 8-bromo-cAMP for 4 d. Treatments and medium were refreshed every 2 d. CCND1 (A) and IGFBP1 (B) mRNA expression was analyzed by quantitative RT-PCR, and values were normalized to the RPL19 gene. C, IGFBP1 protein secretion was analyzed by ELISA, and values were normalized to total RNA. All bar graphs (least square means ± sem) represent fold changes relative to control (Ctrl, no endo and no treatment). Means with same letters indicate significant differences within treatments at P < 0.05 using two-way ANOVA by ranks, followed by Bonferroni post hoc test. Means with asterisks indicate significant differences in endo vs. corresponding no endo treatment at P < 0.05 using Mann-Whitney U test.
Because inhibition of proliferation in hESF by cAMP is accompanied by stromal differentiation (decidualization) (13), we evaluated the contribution of ERK1/2 signaling to cAMP-mediated stromal differentiation, using IGFBP1 as a decidualization marker. There is significant interaction between cAMP treatments and U0126 addition with regard to IGFBP1 expression in hESF of women without endometriosis (P = 0.04), but this interaction is lost in hESF of women with endometriosis (P = 0.10). Treatment of hESF with cAMP, but not with vehicle or U0126, increased IGFBP1 mRNA expression in hESF of women without endometriosis to a greater extent than those of women with disease (Fig. 4B). Addition of U0126 further increased cAMP-induced IGFBP1 mRNA expression in hESF of women without endometriosis, but it failed to rescue the blunted IGFBP1 mRNA expression in hESF of women with endometriosis (Fig. 4B). This suggests that inhibition of cAMP action by MEK1/2/ERK1/2 activity may be gene specific, because MEK1/2/ERK1/2 inhibits cAMP regulation of CCND1 but not IGFBP1 mRNA expression in hESF of women with endometriosis. The gene-specific inhibitory action of MEK1/2/ERK1/2 on cAMP transcriptional activity is consistent with the seemingly distinct mechanism involved in the regulation of CCND1 and IGFBP1 expression by cAMP (Fig. 2, A and B). Moreover, similar trends, albeit less significant, in IGFBP1 mRNA regulation by cAMP and U0126 were observed on the protein level, when secreted IGFBP1 was measured in conditioned medium from hESF of women with or without endometriosis (Fig. 4C).
pERK1/2 in human eutopic endometrium
To determine whether increased pERK1/2 in endometriosis occurs in vivo, as observed in vitro, we analyzed pERK1/2 immunostaining in eutopic endometrium of women with vs. without endometriosis. We selected random fields to include pERK staining in both the functionalis and basalis layer to have sufficient number of cells for quantification. In human uterine tissues, endometrial stromal cells of women without endometriosis had the highest pERK1/2 expression at LS and lowest expressions at PE, ES, and MS phases (Fig. 5, A and B). In contrast, stromal fibroblasts of women with endometriosis had the highest pERK1/2 expression at ES and lowest expression at PE phase. Stromal fibroblasts of women with endometriosis had relatively higher pERK1/2 expression in ES phase and have a numerically higher trend, although not significant, of pERK1/2 at MS phase than those of women without endometriosis (Fig. 5, A and B). Endometrial epithelial cells of women without endometriosis had comparable pERK1/2 expression throughout the menstrual cycle, whereas epithelial cells of women with endometriosis had significantly increased pERK1/2 expression at ES after the low expression at PE phase (Fig. 5, A and C). Epithelial cells of women with endometriosis demonstrated significantly higher pERK1/2 expression in the ES phase than those of women without disease (Fig. 5, A and C). Because hysterectomies are rarely performed in the LS phase of the menstrual cycle, we were unable to obtain a sufficient number of samples to analyze pERK1/2 expression in endometrium of women with endometriosis in the LS phase.
Figure 5.
Immunohistochemistry for ERK phosphorylation in eutopic endometrium of women with or without endometriosis. A, Representative photomicrographs of immunostaining for Tyr204-phosphorylated ERK (pERK) (brown) in paraffin sections of human endometrium from women without (no endo, white bars) vs. with (endo, gray bars) endometriosis during different phases of the menstrual cycle. Panels are shown at ×200 magnification. B and C, Graphical representation of pERK staining in stromal cells (B) and epithelial cells (C) is shown as least square means ± sem and presented as percent pERK-positive cells. Means with same letters indicate significant differences across the menstrual cycle at P < 0.05 using Kruskal-Wallis ANOVA, followed by Dunn’s multiple comparison test. Means with asterisks indicate significant differences in endo vs. corresponding no endo treatment at P < 0.05 using Mann-Whitney U test. For PE phase, no endo n = 3 and endo n = 5; for ES, no endo n = 5 and endo n = 6; for MS, no endo n = 3 and endo n = 4); for LS, no endo n = 4. EP, Epithelial cells; ST, stromal cells.
Discussion
The present study describes the potential role of MEK1/2/ERK1/2 signaling in the pathophysiology of endometriosis. We show for the first time that 1) hESF from women with endometriosis have high in vivo and in vitro levels of pERK1/2 relative to women without disease, resulting in impaired cAMP regulation of CCND1 mRNA in endometriosis, 2) increased pERK1/2 in hESF of women with endometriosis is accompanied by diminished expression of the endogenous MAPK inhibitor ERRFI1, and 3) regulation of cAMP-mediated transcriptional activity by MEK1/2/ERK1/2 signaling differs depending on the target gene (CCND1 or IGFBP1). Overall, we demonstrate the consequential effects of elevated pERK1/2 levels on cAMP action in eutopic endometrium of women with endometriosis, which lays the foundation to understand the interplay between MEK1/2/ERK1/2 and cAMP signaling in the pathophysiology of this disorder.
Association of MAPK activity with the pathogenesis of endometriosis was first described in ectopic lesions, where higher levels of phospho-p38 MAPK were observed, relative to eutopic endometrium of the same patients (33). A more recent study also demonstrated up-regulation of pERK1/2 MAPK and Src homology 2 domain-containing transforming protein 1 (SHC1) in ectopic endometrium of women with ovarian endometriosis relative to eutopic endometrium of women without disease (34). Up-regulation of MAPK subfamilies are thought to play important roles in regulating growth and maintenance of ectopic endometrial tissues by influencing expression and function of various cytokines. Activation of MEK1/2/ERK1/2 is necessary for TNFα-induced IL-6 expression and IL-1β-induced COX-2 expression, and activation of p38, c-jun N-terminal kinase (JNK), and ERK1/2 are important for IL-1β-induced IL-8 secretion in ectopic endometriotic stromal fibroblasts (33,35,36). Moreover, inhibition of p38 by FR167653 in an endometriosis mouse model successfully reduced the size of endometriotic lesions, without affecting the number of lesions, uterine weight, or body weight (37). Although several studies on MAPK and endometriosis have focused on ectopic sites, the present study demonstrated that pERK1/2 is also up-regulated in eutopic endometrium of women with endometriosis. Interestingly, a more recent paper has identified similar increased pERK1/2 levels in eutopic endometrium of women with endometriosis (38). Although there are some variations in their findings and ours, i.e. increased pERK1/2 in eutopic endometrial glandular epithelial cells of women with endometriosis at early-mid PE phase but not at ES-MS phase, they also observed increased pERK1/2 levels in eutopic endometrial stromal cells of women with endometriosis in the ES phase. Consistency in these observations underscores the potential involvement of increased stromal pERK in the pathophysiology of endometriosis. Indeed, our data demonstrate that increased MEK1/2/ERK1/2 activity influences cAMP signaling within the eutopic endometrial stromal cells of women with endometriosis. To our knowledge, this paper provides the first indication of the inhibitory role of MEK1/2/ERK1/2 on cAMP-mediated transcriptional activity in human endometrial stromal fibroblasts.
Murk et al. (38) further demonstrated that estrogen induces pERK1/2 levels within 10 min in hESF of women with but not without endometriosis. Here, we demonstrated persistently high pERK1/2 levels in hESF of women with vs. without endometriosis even in the absence of estrogen after 2 d incubation with 2% CS-serum-containing medium or after 24 h incubation with serum-free medium after 10% CS-serum-containing medium (phenol red-free). The reason for the increased pERK level in hESF of women with vs. without endometriosis in these estrogen-free culture conditions is not clear. It is tempting to speculate that preincubation or incubation with serum may contribute to persistence of pERK in hESF of women with endometriosis. Further investigation is required to understand the role of estrogen and other serum factors in regulating pERK levels.
The ability of H89 to significantly attenuate cAMP activation of IGFBP1 but not inhibition of CCND1 expression suggests that cAMP transcriptional activity may involve distinct molecular mechanisms depending on gene targets: IGFBP1 regulation predominantly through PKA signaling and CCND1 regulation involving an essential alternative pathway. Confirmation of the exact signaling pathways of IGFBP1 and CCND1 regulation by cAMP is currently under investigation in our laboratory. The complexity of cAMP signaling has also been described in cell cycle regulation. cAMP can positively or negatively regulate G1 phase progression depending on cell type (39,40). Here, we demonstrate that cAMP can inhibit CCND1 expression in hESF that allows repression of cell cycle progression. Inhibition of cell cycle genes by cAMP requires repression of MEK1/2/ERK1/2 in several cell types, e.g. H-Ras-mutated C643 cells (41,42,43). However, the present study demonstrates an inhibitory action of cAMP on the cell cycle gene CCND1 in hESF without affecting pERK1/2 levels, similar to other cell types, e.g. B-Raf-mutated cell lines (B-CPAP and 8505C cells) (41,44,45,46). Although cAMP inhibition of cell cycle genes in hESF does not appear to be through pERK down-regulation, our present data show that higher expression of pERK in hESF of women with vs. without endometriosis can negatively influence cAMP regulation of CCND1.
Activation of K-Ras, which is an upstream regulator of the Raf/MEK1/2/ERK1/2 module, in the ovarian surface epithelium of a transgenic mouse results in benign epithelial lesions, reminiscent of the epithelial component of endometriosis (47). However, the lack of association between the common variants of K-Ras and risk of endometriosis (48) suggests that persistent activation or reduced inhibition of the Ras/MEK/ERK pathway in endometriosis may involve an unknown mechanism other than mutation of the K-ras gene. Here, we showed that persistently elevated pERK1/2 levels in hESF of women with vs. without endometriosis were accompanied by reduction in expression of the MAPK inhibitor ERRFI1. Overexpression of ERRFI1 has previously been shown to inactivate ERK1/2 in the NIH3T3 fibroblast cell line without affecting initial activation of ERK1/2 (49). Moreover, the ability of ERRFI1 to inhibit ERK1/2 activity is consistent with the endometrial hyperplasia phenotype observed in the uterus of ERRFI1-null mice (50). It is tempting to speculate that persistently high levels of pERK1/2 in hESF of women with endometriosis may be in part a consequence of decreased ERRFI1 expression. Future studies on ERRFI1 and pERK in endometriosis may provide important new approaches in alleviating the negative contribution of pERK on cAMP signaling in endometriosis.
Mouse models of endometriosis demonstrate that transplantation of normal mouse endometrium in an ectopic location leads to gene expression changes (decreased HOXA10 and IGFBP1) in eutopic endometrium, suggestive of a weakly receptive endometrium (51). This suggests that systemic factors produced by ectopic endometrium in endometriosis lead to abnormalities in eutopic endometrium, characterized by aberrant hormonal response and reduced embryo receptivity. Our present and earlier data demonstrate that in vitro cultured hESF from women with vs. without endometriosis still retain an abnormal response to cAMP and persistent elevation of pERK1/2 levels, even up to several passages (26). Ability of eutopic hESF from women with endometriosis to remember its impaired endometrial response has also been observed by others (30,52), suggesting that the possible retention of defect in these cells may be a consequence of aberrant gene imprinting. Indeed, hypermethylation of implantation-associated genes, e.g. Hoxa10 and PR-B, has been observed in eutopic endometrium of a mouse model for endometriosis (51,53), whereas hypomethylation of steroidogenic factor 1 (SF-1) and ERβ genes were reported in ectopic vs. eutopic endometrium from women with endometriosis (54,55). Whether the impaired cAMP response and elevated levels of pERK in eutopic endometrium of women with endometriosis are associated with hypermethylation of MAPK-associated genes warrants further investigation.
We previously demonstrated that simultaneous activation of MEK1/2/ERK1/2 and PI3K/AKT pathways by high doses of insulin (1 ng/ml) inhibits estrogen/progesterone-induced IGFBP1 expression in normal hESF and that simultaneous inhibition of both pathways is necessary to fully restore IGFBP1 expression in these cells (56). Our current data demonstrated that exclusive inhibition of MEK1/2/ERK1/2 activity by the U0126 compound is sufficient to elevate cAMP-induced IGFBP1 mRNA expression in insulin-free cultures of normal (nonendometriosis) hESF but not endometriosis hESF. Dominance of MEK1/2/ERK1/2 action on regulating cAMP-induced IGFBP1 expression in insulin-free normal hESF may be due to absence of AKT/phosphatidylinositol 3-kinase (PI3K) activity in these cells. Indeed, cAMP inhibits AKT/PI3K activity in normal hESF (57) without influencing the low basal level of pERK1/2 (current data). Interestingly, although higher expression of pERK1/2 is consistent with lower IGFBP1 expression in hESF of women with vs. without endometriosis, inhibition of pERK1/2 by itself is not sufficient to restore cAMP-induced IGFBP1 mRNA expression in endometriosis hESF. It is tempting to speculate that cAMP-treated hESF of women with endometriosis may also have persistently high levels of phospho-AKT and that simultaneous inhibition of MAPK and AKT is necessary to restore cAMP-induced IGFBP1 mRNA expression in these cells. Future studies on the contribution of AKT/PI3K and MEK1/2/ERK1/2 activity on regulation of IGFBP1 mRNA level by cAMP are currently underway in our laboratory.
In conclusion, our data demonstrate the inhibitory role of MEK1/2/ERK1/2 on cAMP-dependent cell cycle regulation in the pathophysiology of endometriosis. Whether elevated pERK1/2 levels in eutopic endometrial stromal cells are a consequence or a precursor of endometriosis is uncertain and requires future studies. Identification of pathways involved in the pathophysiology of endometriosis underscores potential targets for therapeutics to inhibit progression and potentially minimize pain and infertility associated with this disorder.
Acknowledgments
We thank Ms. Kim Chi Vo for assistance in tissue procurement, the University of California, San Francisco National Institutes of Health (NIH) Human Endometrial Tissue and DNA Bank for tissue samples, and Drs. Ramsey McIntire and Akiko Kobayashi and Ms. Angela Rojas for helpful comments on the manuscript.
Footnotes
This work was supported by National Institute of Child Health and Human Development/NIH through cooperative agreement 1U54HD055764-02 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (L.C.G.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 9, 2009
Abbreviations: BrdU, Bromodeoxyuridine; CCND1, cyclin D1; CS, charcoal-stripped; DMSO, dimethylsulfoxide; ERRFI1, ERBB receptor feedback inhibitor 1; ES, early secretory; FBS, fetal bovine serum; GEF, guanine-nucleotide exchange factor; hESF, human endometrial stromal fibroblast; HRP, horseradish peroxidase; IGFBP1, IGF-binding protein 1; LS, late secretory; MEK, MAPK and ERK kinase 1/2; MS, midsecretory; PE, proliferative; pERK1/2, phospho-ERK1/2; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A; RPL19, ribosomal protein L19.
References
- Eskenazi B, Warner ML 1997 Epidemiology of endometriosis. Obstet Gynecol Clin North Am 24:235–258 [DOI] [PubMed] [Google Scholar]
- Burney RO, Giudice LC 2008 The pathogenesis of endometriosis. In: Nezhat C, Nezhat C, Nezhat FR, eds. Nezhat’s operative gynecologic laparoscopy and hysteroscopy. 3rd ed. Cambridge, New York: Cambridge University Press; 251–257 [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC 2007 Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148:3814–3826 [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC 2003 Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 144:2870–2881 [DOI] [PubMed] [Google Scholar]
- Kamat AA, Younes PS, Sayeeduddin M, Wheeler TM, Simpson JL, Agoulnik AI 2004 Protein expression profiling of endometriosis: validation of 2-mm tissue microarrays. Fertil Steril 82:1681–1683 [DOI] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL 1994 Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab 79:643–649 [DOI] [PubMed] [Google Scholar]
- Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA 1992 Integrin adhesion molecules in the human endometrium: correlation with the normal and abnormal menstrual cycle. J Clin Invest 90:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HS, Bagot C, Kardana A, Olive D, Arici A 1999 HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 14:1328–1331 [DOI] [PubMed] [Google Scholar]
- Tranguch S, Daikoku T, Guo Y, Wang H, Dey SK 2005 Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci 62:1964–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich K, Fauser BC, Devroey P, Griesinger G 2007 The role of the endometrium and embryo in human implantation. Hum Reprod Update 13:365–377 [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens J 2003 Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 178:357–372 [DOI] [PubMed] [Google Scholar]
- Tierney EP, Giudice LC 2004 Role of activin A as a mediator of in vitro endometrial stromal cell decidualization via the cyclic adenosine monophosphate pathway. Fertil Steril 81(Suppl 1):899–903 [DOI] [PubMed] [Google Scholar]
- Tierney EP, Tulac S, Huang ST, Giudice LC 2003 Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics 16:47–66 [DOI] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL 2002 Cyclic nucleotide research: still expanding after half a century. Nat Rev Mol Cell Biol 3:710–718 [DOI] [PubMed] [Google Scholar]
- Sewing A, Burger C, Brusselbach S, Schalk C, Lucibello FC, Muller R 1993 Human cyclin D1 encodes a labile nuclear protein whose synthesis is directly induced by growth factors and suppressed by cyclic AMP. J Cell Sci 104(Pt 2):545–555 [DOI] [PubMed] [Google Scholar]
- Ward AC, Csar XF, Hoffmann BW, Hamilton JA 1996 Cyclic AMP inhibits expression of D-type cyclins and cdk4 and induces p27Kip1 in G-CSF-treated NFS-60 cells. Biochem Biophys Res Commun 224:10–16 [DOI] [PubMed] [Google Scholar]
- Pagano M, Theodoras AM, Tam SW, Draetta GF 1994 Cyclin D1-mediated inhibition of repair and replicative DNA synthesis in human fibroblasts. Genes Dev 8:1627–1639 [DOI] [PubMed] [Google Scholar]
- L'Allemain G, Lavoie JN, Rivard N, Baldin V, Pouyssegur J 1997 Cyclin D1 expression is a major target of the cAMP-induced inhibition of cell cycle entry in fibroblasts. Oncogene 14:1981–1990 [DOI] [PubMed] [Google Scholar]
- Terada Y, Nakashima O, Inoshita S, Kuwahara M, Sasaki S, Marumo F 1999 Mitogen-activated protein kinase cascade and transcription factors: the opposite role of MKK3/6–p38K and MKK1-MAPK. Nephrol Dial Transplant 14(Suppl 1):45–47 [DOI] [PubMed] [Google Scholar]
- Winston JT, Coats SR, Wang YZ, Pledger WJ 1996 Regulation of the cell cycle machinery by oncogenic ras. Oncogene 12:127–134 [PubMed] [Google Scholar]
- Lavoie JN, L'Allemain G, Brunet A, Müller R, Pouysségur J 1996 Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem 271:20608–20616 [DOI] [PubMed] [Google Scholar]
- Pagès G, Lenormand P, L'Allemain G, Chambard JC, Meloche S, Pouysségur J 1993 Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA 90:8319–8323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, Rivard N, L'Allemain G, Pouysségur J 1996 A temporal and biochemical link between growth factor-activated MAP kinases, cyclin D1 induction and cell cycle entry. Prog Cell Cycle Res 2:49–58 [DOI] [PubMed] [Google Scholar]
- The American Fertility Society 1985 Revised American Fertility Society classification of endometriosis. Fertil Steril 43:351–352 [DOI] [PubMed] [Google Scholar]
- Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC 2006 Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab 91:1453–1461 [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC 2009 Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod 80:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayisli UA, Aksu CA, Berkkanoglu M, Arici A 2002 Estrogenicity of isoflavones on human endometrial stromal and glandular cells. J Clin Endocrinol Metab 87:5539–5544 [DOI] [PubMed] [Google Scholar]
- Tang M, Mazella J, Zhu HH, Tseng L 2005 Ligand activated relaxin receptor increases the transcription of IGFBP-1 and prolactin in human decidual and endometrial stromal cells. Mol Hum Reprod 11:237–243 [DOI] [PubMed] [Google Scholar]
- Coqueret O 2002 Linking cyclins to transcriptional control. Gene 299:35–55 [DOI] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ 2006 Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril 85:564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Assoian RK 2001 Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci 114:2553–2560 [DOI] [PubMed] [Google Scholar]
- Kolch W 2005 Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6:827–837 [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y 2004 Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am J Reprod Immunol 52:306–311 [DOI] [PubMed] [Google Scholar]
- Honda H, Barrueto FF, Gogusev J, Im DD, Morin PJ 2008 Serial analysis of gene expression reveals differential expression between endometriosis and normal endometrium: possible roles for AXL and SHC1 in the pathogenesis of endometriosis. Reprod Biol Endocrinol 6:59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi N, Harada T, Taniguchi F, Yoshida S, Iwabe T, Terakawa N 2004 Tumor necrosis factor-α induced the release of interleukin-6 from endometriotic stromal cells by the nuclear factor-κB and mitogen-activated protein kinase pathways. Fertil Steril 82(Suppl 3):1023–1028 [DOI] [PubMed] [Google Scholar]
- Wu MH, Wang CA, Lin CC, Chen LC, Chang WC, Tsai SJ 2005 Distinct regulation of cyclooxygenase-2 by interleukin-1β in normal and endometriotic stromal cells. J Clin Endocrinol Metab 90:286–295 [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Koga K, Hirota Y, Hirata T, Ruimeng X, Na L, Yano T, Tsutsumi O, Taketani Y 2006 FR 167653, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis in a murine model. J Reprod Immunol 72:85–93 [DOI] [PubMed] [Google Scholar]
- Murk W, Atabekoglu CS, Cakmak H, Heper A, Ensari A, Kayisli UA, Arici A 2008 Extracellularly signal-regulated kinase activity in the human endometrium: possible roles in the pathogenesis of endometriosis. J Clin Endocrinol Metab 93:3532–3540 [DOI] [PubMed] [Google Scholar]
- Stork PJ, Schmitt JM 2002 Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258–266 [DOI] [PubMed] [Google Scholar]
- Dumaz N, Marais R 2005 Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft fur Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J 272:3491–3504 [DOI] [PubMed] [Google Scholar]
- Rocha AS, Paternot S, Coulonval K, Dumont JE, Soares P, Roger PP 2008 Cyclic AMP inhibits the proliferation of thyroid carcinoma cell lines through regulation of CDK4 phosphorylation. Mol Biol Cell 19:4814–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SJ, McCormick F 1993 Inhibition by cAMP of Ras-dependent activation of Raf. Science 262:1069–1072 [DOI] [PubMed] [Google Scholar]
- Dumaz N, Marais R 2003 Protein kinase A blocks Raf-1 activity by stimulating 14–3–3 binding and blocking Raf-1 interaction with Ras. J Biol Chem 278:29819–29823 [DOI] [PubMed] [Google Scholar]
- Graves LM, Bornfeldt KE, Raines EW, Potts BC, Macdonald SG, Ross R, Krebs EG 1993 Protein kinase A antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc Natl Acad Sci USA 90:10300–10304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaz N, Light Y, Marais R 2002 Cyclic AMP blocks cell growth through Raf-1-dependent and Raf-1-independent mechanisms. Mol Cell Biol 22:3717–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmanno K, Millar T, McMahon M, Cook SJ 2003 ΔRaf-1:ER* bypasses the cyclic AMP block of extracellular signal-regulated kinase 1 and 2 activation but not CDK2 activation or cell cycle reentry. Mol Cell Biol 23:9303–9317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T 2005 Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med 11:63–70 [DOI] [PubMed] [Google Scholar]
- Zhao ZZ, Nyholt DR, Le L, Martin NG, James MR, Treloar SA, Montgomery GW 2006 KRAS variation and risk of endometriosis. Mol Hum Reprod 12:671–676 [DOI] [PubMed] [Google Scholar]
- Fiorentino L, Pertica C, Fiorini M, Talora C, Crescenzi M, Castellani L, Alemà S, Benedetti P, Segatto O 2000 Inhibition of ErbB-2 mitogenic and transforming activity by RALT, a mitogen-induced signal transducer which binds to the ErbB-2 kinase domain. Mol Cell Biol 20:7735–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N, Gilbert JL, Broaddus RR, Demayo FJ, Jeong JW 2007 Generation of a Mig-6 conditional null allele. Genesis 45:716–721 [DOI] [PubMed] [Google Scholar]
- Lee B, Du H, Taylor HS 2009 Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod 80:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minici F, Tiberi F, Tropea A, Orlando M, Gangale MF, Romani F, Campo S, Bompiani A, Lanzone A, Apa R 2008 Endometriosis and human infertility: a new investigation into the role of eutopic endometrium. Hum Reprod 23:530–537 [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Wang Y, Halverson G, Jailwala P, Guo SW 2006 Genomic alterations in ectopic and eutopic endometria of women with endometriosis. Gynecol Obstet Invest 62:148–159 [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE 2007 Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod 77:681–687 [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE 2007 Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab 92:3261–3267 [DOI] [PubMed] [Google Scholar]
- Lathi RB, Hess AP, Tulac S, Nayak NR, Conti M, Giudice LC 2005 Dose-dependent insulin regulation of insulin-like growth factor binding protein-1 in human endometrial stromal cells is mediated by distinct signaling pathways. J Clin Endocrinol Metab 90:1599–1606 [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Hirota Y, Koga K, Yano T, Tsutsumi O, Taketani Y 2003 Akt as a possible intracellular mediator for decidualization in human endometrial stromal cells. Mol Hum Reprod 9:265–269 [DOI] [PubMed] [Google Scholar]