Abstract
Knowledge of altered maternal nutrition effects on growth-regulating systems is critical to understanding normal and abnormal fetal development. There are many reports of hepatic fetal IGF system responses to maternal nutrient restriction (MNR) during pregnancy in rodents and sheep but none in nonhuman primates. We determined effects of MNR on the fetal baboon hepatic IGF system. Social groups of female baboons were fed ad libitum, controls, or 70% controls (MNR) from 0.16 to 0.5 gestation and fetuses delivered by cesarean section. Fetal liver tissue was analyzed for IGF-I, IGF-II, and IGF binding protein (IGFBP)-3 mRNA by in situ hybridization and quantitative RT-PCR and protein by immunohistochemistry (IHC); IGF-I receptor, IGF-II receptor by quantitative RT-PCR and IHC and IGFBP-1 by in situ hybridization and IHC. MNR did not alter fetal body or liver weight. Fetal hepatic glycogen staining increased with MNR. MNR reduced fetal hepatic IGF-I and IGF-II and increased IGFBP-1 mRNA and decreased IGF-I, IGF-II, IGF-I receptor, and IGF-II receptor protein and increased protein for IGFBP-1 and IGFBP-3. MNR increased caspase-3, indicating apoptosis and decreased Akt staining, indicating decreased nutrient sensing. In conclusion, whereas fetal body and liver weights did not change in response to moderate MNR during the first half of baboon pregnancy, the major indices of function of the hepatic IGF system measured were all reduced.
Moderate global reduction in maternal nutrient intake decreases activity of the IGF system in the fetal baboon liver, providing a mechanism to explain altered growth and differentiation in the placenta and key fetal organs.
The IGF system has important metabolic, mitogenic, and differentiative actions and is a major regulator of growth and development of the placenta and fetal organs (1). The various components of this system are produced widely by the fetus and placenta and act as autocrine, paracrine, and endocrine factors to promote cell cycle progression, increase DNA and protein synthesis, and stimulate cell differentiation in cultured embryos and fetal cell lines in vitro. Fetal IGF plasma concentrations correlate positively with birth weight in many species including humans, primates, sheep, pigs, rabbits, and rodents (2).
Mice in which IGF-I or IGF-II genes are deleted have birth weights approximately 60% of normal littermates (3). The IGFs manifest their actions after IGF-I and/or IGF-II binding to IGF-I receptor (IGF-IR) and the insulin receptor. In contrast, it is generally considered that IGF-II receptor (IGF-IIR) binds only IGF-II and that this binding does not result in altered cell signaling and serves to clear IGF-II from the plasma, thereby decreasing its bioavailability, although recent studies suggest that the IGF-IIR may link to functional postmembrane signaling (4). IGF-IR gene deletion in mice eliminates activities of both IGF-I and -II, producing offspring only 45% of the birth weight of normal mice (5).
Although IGF-I and -II are produced in most tissues in postnatal life, the liver is the major site of production of both IGFs and their binding proteins (IGFBPs) (1). However, specific liver IGF-I gene deletion in mice has little effect on birth weight or postnatal development, even though circulating IGF-I is reduced, indicating that at least in rodents, extrahepatic IGF-I production can maintain fetal and early postnatal growth (6). Rodents are altricial species and show very different pre- and postnatal growth patterns from precocial species such as primates and studies on the IGF system during fetal primate development are few (7).
The goal of the present study was to determine the effects of carefully controlled and monitored global nutrient restriction on the fetal baboon hepatic IGF system. We previously demonstrated that in baboons of similar phenotype, maternal consumption of 70% of the global ad libitum diet fed from 0.16 to 0.5 d of pregnancy decreases maternal body weights by 11% but does not significantly alter overall fetal growth or total placental, fetal liver, or kidney weight. However, this moderate level of maternal nutrient restriction does alter the detailed fetal renal histology and expression of key genes (8) as well as producing major changes in the placental IGF system (9). We hypothesized that this same level of maternal nutrient restriction (MNR) would decrease activity of the fetal IGF axis within the liver, a major site of both nutrient regulation and IGF production (1).
Materials and Methods
Animal care
Fourteen baboons (Papio species) from the Southwest National Primate Research Center were studied. Animals were housed in outdoor gang cages providing full social and physical activity. Housing and environmental enrichment have been published (10). All procedures were approved by the University of Texas Health Science Center and Southwest National Primate Research Center Institutional Animal Care and Use Committees.
System for controlling and recording individual feeding
Nonpregnant morphometric details of the mothers have been published. Before pregnancy the two groups did not differ in morphometric characteristics (8). Animals were fed between 0700 and 0900 h or 1100 and 1300 h as described (10). At feeding time, all baboons exited their group cage and passed along a chute and into individual feeding cages. Each baboon’s weight was obtained while crossing an electronic scale (GSE 665; GSE Scale Systems, Milwaukee, MI). Water was continuously available in the feeding cages via individual waterers (Lixit, Napa, CA). Animals ate Purina Monkey Diet 5038 (Purina, St. Louis, MO). At the start of the feeding period, ad libitum-fed control (CTR) baboons were given 60 biscuits in their individual cage. Biscuits remaining were counted after baboons returned to their group cage. Nutrient-restricted animals (MNR) were fed 70% of food eaten by contemporaneous controls on a per-kilogram basis.
Study design
Healthy female baboons of similar body weights (10–15 kg) were placed into two group cages in social groups of 10–16 randomly assigned nonpregnant females and a vasectomized male. After acclimation to the feeding cages (30 d), a fertile male was substituted. Pregnancy was dated initially by timing of ovulation and changes in sex skin color and confirmed by ultrasound at 30 d of gestation (dG). Over a period of 6 months, eight CTR females and six MNR females became pregnant. MNR began on the day of pregnancy confirmation.
Cesarean sections
Standard cesarean sections were performed at 90 dG (11). Baboons were premedicated with ketamine hydrochloride (10 mg/kg, im). After intubation, isoflurane (2%) was used to maintain anesthesia throughout surgery and umbilical cord blood sampling. After hysterotomy, the umbilical cord was identified and elevated to the incision to enable fetal exsanguination, an American Veterinary Medical Association-approved method. We have demonstrated that tissue microstructure is more normal than that produced after euthanasia solution administration, which produces marked histological changes, especially in the liver (12). The placenta and fetus were removed from the uterus and immediately submitted for morphometric measurements, complete pathologic evaluation, and tissue sampling. Postoperative maternal analgesia was provided (buprenorphine hydrochloride, 0.015 mg/kg · d) for 3 d (Buprenex injectable; Reckitt Benckiser Health Care Ltd., Hull, UK) as described in detail (11).
RNA isolation from tissue
RNA was isolated from the fetal liver right lobe, using TrIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The RNA precipitate was washed with 1 ml of 75% ethanol and centrifuged at 4 C at 7500 × g for 5 min. The RNA was resuspended in 100 μl diethylpyrocarbonate-treated water and stored at −80 C. RNA quantity and purity was determined spectrophometrically.
Quantitative real-time PCR (QRT-PCR) quantification of target gene abundance
To measure IGF-I, IGF-II, IGF-IR, IGF-IIR, and IGFBP-3 mRNA levels, we used Assays-on-Demand products (Applied Biosystems, Foster City, CA). Although no baboon-specific primers or probes are available, we successfully used the Assays-on-Demand system for more than 40 different baboon genes using human probe sets. We quantified mRNA according to the manufacturer’s instructions. In brief, total RNA (50 ng) was reverse transcribed in a 100-μl reaction using a high-capacity cDNA archive kit (Applied Biosystems). cDNA synthesis was followed by QRT-PCR using gene-specific primers provided by the manufacturer, TaqMan universal PCR master mix (Applied Biosystems), and the target cDNA. The 18s rRNA was quantified as an endogenous control using the human Assays-on-Demand probe set. All samples were assayed in triplicate. The Assay-on-Demand kits used were Hs00153126_m1 (IGF-I), Hs00181385_m1 (IGF-IR), Hs00171254_m1 (IGF-II), Hs00181419_m1 (IGF-IIR), and Hs00181211_m1 (IGFBP-3).
For relative quantification of gene expression, the comparative threshold cycle (Ct) method was used (see user bulletin 2 for ABI PRISM 7700 sequence detection systems; Applied Biosystems). The value obtained for Ct represents the PCR cycle at which an increase in reporter fluorescence above a background signal can first be detected (10 times the sd of the baseline). Using this approach, the endogenous control Ct values were subtracted from the gene of interest Ct values to derive a ΔCt value.
In situ hybridization (ISH)
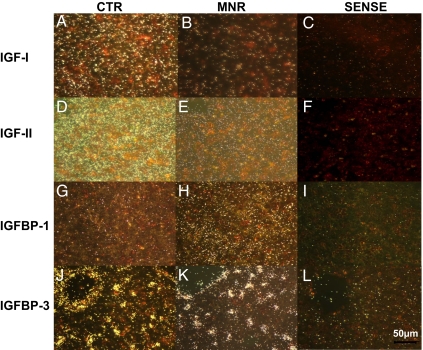
Central liver lobe paraffin sections (5 μm) from CTR and MNR fetuses were analyzed for expression of IGF-I, IGF-II, IGFBP-1, and IGFBP-3 mRNAs by in situ hybridization using 35S-labeled antisense riboprobes (13,14). The supplemental materials table, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals. org, lists probes used. Briefly, tissue sections were deparaffinized, rehydrated, and treated with proteinase-K. After prehybridization, tissue sections were hybridized with 35S-labeled antisense riboprobes overnight at 42–45 C specific for each probe. Excess probe was washed and the tissue sections were treated with ribonuclease to cleave unhybridized probe. Tissue sections then underwent washes in decreasing salt buffers (1.0–0.1× saline sodium citrate) at specific temperatures for each probe. After dehydration, slides were exposed to x-ray film overnight at −80 C to determine the signal intensity that provided guidance for the length of exposure of the nuclear track emulsion. Slides were then coated with nuclear track emulsion and exposed at 4 C under desiccating conditions. Slides were developed, fixed, counterstained with hematoxylin and eosin, and coverslipped (Permount; Fisher Scientific, Rochester, NY). Tissue sections were viewed under both dark and bright fields, and appropriate photomicrographs were obtained a ×20 and ×40 magnifications using ImagePro Plus 4.5 software (Media Cybernetics, Bethesda, MD). Figure 1 shows the sense and antisense reactions for IGF-I (Fig. 1, A–C), IGF-II (Fig. 1, D–F), IGFBP-1 (Fig. 1, G–I), and IGFBP-3 (Fig. 1, J–L) probes to demonstrate that the appropriate controls were performed.
Figure 1.
ISH photomicrographs of liver at 90 d gestation from fetuses of mothers who were fed ad libitum (CTR): A, D, G, and J; MNR (fed 70% CTR diet from 30 to 90 d of gestation): B, E, H, and K; sense negative control: C, F, I, and L; IGF-I: A–C; IGF-II: D–F; IGFBP-1: G–I; and IGFBP-3: J–L in dark field. Bar (lower right) applies to all panels.
Immunohistochemistry (IHC)
Fixed sections also from the central lobe of fetal liver were deparaffinized in xylene, rehydrated in descending grades of alcohol (100, 70, and 45%) to water, immersed in citrate buffer [0.01 m citrate buffer (pH 6.0)], and heated to boiling for 10–15 min for antibody retrieval. After cooling for 15 min, the sections were rinsed in potassium PBS [KPBS; 0.04 m K2HPO4, 0.01 m KH2PO4, 0.154 m NaCl (pH 7.4); seven times, 6 min each)] and washed for 10 min in a solution of 1.5% H2O2/methanol and then for 5 min in KPBS. Sections were placed in diluted (10%) normal serum for 20 min and covered with primary antibody overnight at 4 C. The table in the supplemental material lists the antibodies used. After overnight incubation, sections were rinsed in KPBS and incubated for 1 h at room temperature with secondary antibody. We used all biotinylated secondary antibodies from Vector Laboratories (Burlingame, CA; see supplemental material): goat antirabbit, catalog no. BA-1000; horse antimouse, catalog no. BA-2000; and rabbit antigoat, catalog no. BA-5000. All were used at a 1:1000 dilution). The appropriate preabsorbed negative controls were also run in which the antigen peptide was available (IGF-II, IGF-IIR). With the other proteins and peptides the primary antibody was omitted and normal serum was used for the negative control as shown in the individual figures. After serial dehydration in 50, 70, 95, and 100% ethanol and Histoclear (three times, 2–5 min each), sections were mounted and coverslipped (Histomount; National Diagnostics, Atlanta, GA).
Quantification of IHC
Images were acquired with a SPOT cooled camera (Diagnostic Instruments, Inc., Sterling Heights, MI) at a magnification of ×40. For caspase-3, images were taken at all of the 12 o’clock hour positions. For all other quantification, six pictures per animal were obtained at 2, 4, 6, 8, 10, and 12 o’clock and analyzed with Simple PCI imaging software (C Imaging, Compix Inc., Cranberry Township, PA). Photomicrographs were analyzed for fraction = area immunostained/region of interest × 100%, with maximum thresholds set at: red, 200, green, 200, blue, 255 for all images.
Histological staining for liver glycogen
Liver glycogen was stained with periodic acid-Schiff. Sections were hydrated in decreasing ethanol concentration, oxidized in 0.5% periodic acid solution for 5 min, rinsed in distilled water, placed in Schiff reagent (U973-01; JT Baker, Phillipsburg, NJ) for 15 min, washed in lukewarm water for 5 min, counterstained in Harris’ hematoxylin (SH30-4D; Fisher Scientific, Fair Lawn, NJ), dehydrated in increasing alcohols to xylene and coverslipped. PAS staining was quantified by measuring staining density and fraction as performed above with an Image J system (National Institutes of Health, Bethesda, MD).
Statistical analysis
Analyte values were log transformed before analysis. All data are expressed as mean ± sem. Parameters measured in CTR and MNR animals were compared with Student’s nonpaired two-tail t test. The relationship between histological findings and plasma IGF levels was assessed using Pearson correlation on fraction stained and ln-transformed plasma IGF values. Significance was set at P < 0.05 unless stated. Where P value approached 0.05, the actual value is presented. Unless otherwise specified, n = 8 CTR and n = 6 MNR.
Results
Morphometric measurements
Fetal body and liver weights
At 90 dG, body weights of CTR and MNR group fetuses were not different (100.9 ± 3.4 vs. 95.4 ± 3.3 g, respectively). Likewise, fetal liver weights were not different between CTR and MNR group fetuses (3.3 ± 0.2 vs. 3.2 ± 0.2 g), and fetal liver weight as a proportion of fetal weight did not differ between the groups.
Placental weight
Placental weights (CTR 70.4 ± 5.1 vs. MNR 62.9 ± 1.5 g) were not different between groups.
mRNA expression measured by QRT-PCR in CTR fetal liver compared with CTR placenta, brain, and kidney
The right liver lobe of CTR fetuses showed greater expression of IGF-I mRNA, as measured by QRT-PCR, than CTR placenta and CTR fetal brain and kidney (P < 0.05), whereas IGF-II mRNA was similar in CTR placenta, fetal liver, and kidney but 30-fold lower in CTR fetal brain than in fetal liver (Table 1; P < 0.05).
Table 1.
ΔCt QRT-PCR values by Assay-On-Demand at 90 dG in fetal liver, kidney, brain, and placenta
| CTR (n = 8)
|
MNR (n = 6)
|
P value | |||
|---|---|---|---|---|---|
| Mean | sem | Mean | sem | ||
| IGF-I | |||||
| Liver | 16.3 | 0.29 | 17.4 | 0.46 | 0.06 |
| Placenta | 18.0a | 0.34 | |||
| Kidney | 17.4a | 0.25 | |||
| Brain | 20.9a | 0.32 | |||
| IGF-II | |||||
| Liver | 7.3 | 0.13 | 7.8 | 0.50 | 0.24 |
| Placenta | 6.6 | 0.48 | |||
| Kidney | 6.8 | 0.03 | |||
| Brain | 16.4a | 0.71 | |||
| IGF-1R | |||||
| Liver | 15.6 | 0.16 | 16.2 | 0.61 | 0.27 |
| IGF-2R | |||||
| Liver | 17.1 | 0.15 | 17.7 | 0.49 | 0.21 |
| IGFBP-3 | |||||
| Liver | 11.2 | 0.17 | 11.8 | 0.58 | 0.27 |
CTR, Livers of fetuses of ad libitum-fed mothers; MNR, livers of fetuses of nutrient-restricted mothers (fed 70% CTR diet from 30 to 90 dG).
P < 0.05 vs. liver.
Effects of MNR on fetal liver gene and protein expression of IGF axis components
IGF-I
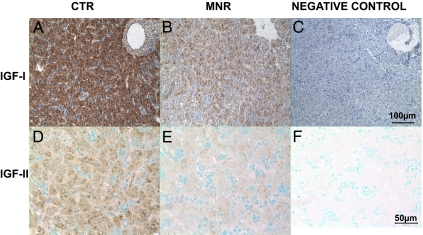
IGF-I mRNA (Fig. 1) and protein (Fig. 2) were distributed evenly throughout the fetal liver parenchyma with no appearance of a lobular regional distribution. IGF-I mRNA was similarly decreased when measured by QRT-PCR (P = 0.06, Table 2) and ISH (P = 0.05, Table 2 and Fig. 1) in liver of fetuses of MNR compared with CTR mothers. IGF-I protein was reduced in the liver of MNR fetuses compared with CTR (P < 0.01; Fig. 2 and Table 2).
Figure 2.
Immunohistochemistry for IGF-I (A–C) and IGF-II (D–F). Photomicrographs of liver at 90 d gestation from fetuses whose mothers were fed ad libitum (CTR; A and D), MNR (fed 70% CTR diet from 30 to 90 d of gestation; B and E); or negative control (C and F). Bar (right panel) applies to panels in that row.
Table 2.
In situ hybridization and immunochemistry at 90 dG in fetal liver, kidney, brain, and placenta
| ISH
|
IHC
|
|||||
|---|---|---|---|---|---|---|
| CTR (n = 8) | MNR (n = 6) | P value | CTR (n = 8) | MNR (n = 6) | P value | |
| IGF-I | 4.5 ± 0.71 | 2.6 ± 0.47 | 0.05 | 82.5 ± 4.26 | 35.2 ± 11.78 | <0.01 |
| IGF-II | 16.3 ± 2.06 | 5.2 ± 1.60 | <0.01 | 15.0 ± 4.77 | 2.6 ± 1.02 | <0.04 |
| IGF-1R | N/A | N/A | N/A | 1.9 ± 0.29 | 0.2 ± 0.08 | <0.01 |
| IGF-2R | N/A | N/A | N/A | 45.7 ± 8.53 | 14.9 ± 6.70 | <0.01 |
| IGFBP-1 | 2.5 ± 0.4 | 5.5 ± 1.48 | <0.02 | 9.8 ± 2.5 | 62.6 ± 10.7 | <0.01 |
| IGFBP-3 | 8.6 ± 1.76 | 6.9 ± 1.30 | 0.45 | 2.8 ± 0.73 | 27.9 ± 4.87 | <0.001 |
CTR, Livers of fetuses of ad libitum-fed mothers; MNR, livers of fetuses of nutrient restricted mothers (fed 70% CTR diet from 30 to 90 dG). Quantification of area positive for mRNA by ISH or protein by IHC in 90 dG livers of baboon fetuses of CTR or MNR mothers; P = CTR vs. MNR.
IGF-II
IGF-II mRNA and protein were also evenly distributed throughout the liver parenchyma with no appearance of a lobular regional distribution (Figs. 1 and 2). IGF-II mRNA abundance was not reduced when measured by QRT-PCR (Table 1), but the extent of its distribution was reduced when measured by ISH (P < 0.01; Table 2) in liver tissue of MNR fetuses compared with CTR. IGF-II protein was lower in liver tissue of fetuses of MNR mothers compared with CTR fetuses (P < 0.05; Fig. 2 and Table 2).
IGF-IR
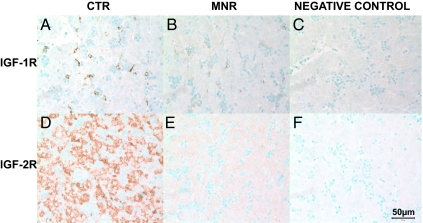
The distribution of IGF-IR immunoreactivity was sparse and restricted to the cytoplasm of nucleated red cells and cells lining the sinusoids, which by their location appeared to be hepatic stellate cells (Fig. 3). IGF-IR mRNA measured by QRT-PCR was not different between the livers of the two groups of fetuses (Table 1). Immunostaining for IGF-IR protein was reduced in liver of MNR fetuses compared with CTR (Fig. 3 and Table 2).
Figure 3.
Immunohistochemistry for IGF-1R (A–C) and IGF-IIR (D–F). Photomicrographs of liver at 90 d gestation from fetuses whose mothers were fed ad libitum (CTR; A and D) MNR (fed 70% CTR diet from 30 to 90 d gestation; B and E); or negative control (C and F). Bar (lower right) applies to all panels.
IGF-IIR
IGF-IIR protein was distributed evenly in hepatocytes throughout the liver (Fig. 3). Liver IGF-IIR measured by QRT-PCR was not different between groups (Table 1), whereas IGF-IIR protein was reduced in liver of MNR fetuses compared with CTR (Fig. 3 and Table 2).
IGFBP-1
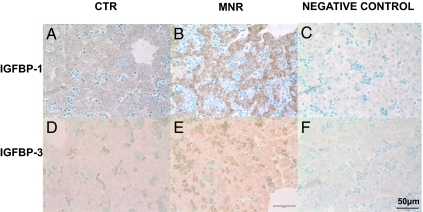
IGFBP-1 mRNA and protein were distributed evenly within hepatocytes throughout the hepatic parenchyma (Figs. 1 and 4). IGFBP-1 mRNA and protein abundance were increased in liver tissue of MNR group fetuses compared with CTR fetuses (Figs. 1 and 4 and Table 2).
Figure 4.
Immunohistochemistry for IGFBP-1 (A–C) and IGFBP-3 (D–F). Photomicrographs of liver at 90 d gestation from fetuses whose mothers were fed ad libitum (CTR; A and D); MNR (fed 70% CTR diet from 30 to 90 d gestation; B and E); or negative control (C and F). Bar (lower right) applies to all panels.
IGFBP-3
IGFBP3 mRNA was present within hepatocytes with an appearance of increased synthesis around the central vein (Fig. 1), but the majority of IGFBP-3 immunoreactivity was distributed in the cytoplasm of circulating nucleated erythrocytes, although some protein staining was present over hepatocytes (Fig. 1). IGFBP-3 protein but not mRNA abundance was increased in liver tissue of fetuses of nutrient-restricted mothers compared with fetuses of CTR mothers (Figs. 1 and 4 and Table 2).
Ki67, caspase-3, and AKT immunoreactivity
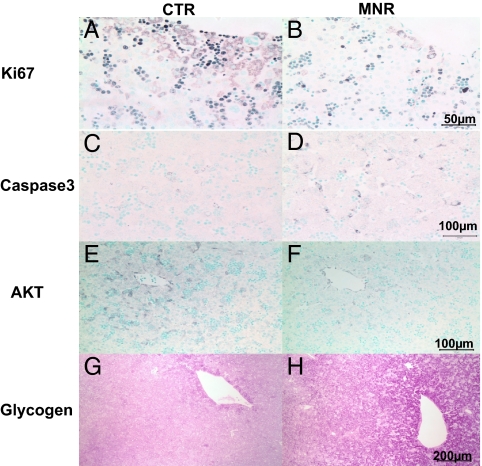
Ki67 was found predominantly in red and white blood cell nuclei in the liver sinuses but occasionally could be seen in the cytoplasm of hepatic cells predominantly around the central vein (Fig. 5). Because the major staining Ki67 was in the red cells, it was not possible to quantify Ki67 separately in hepatic parenchymal cells and red cells. However, it is of interest that MNR fetal livers showed decreased immunostaining expressed as fraction (CTR: 19.9 ± 2.0% vs. MNR: 5.1 ± 0.9%, P < 0.05), which may reflect an effect of MNR on hepatic hematopiesis. Caspase-3 was distributed in the cytoplasm of hepatocytes throughout the liver lobule and showed an increase in MNR fetuses (Fig. 5). Fraction stain was 0.05 ± 0.01 and 0.15 ± 0.04% in CTR and MNR fetuses, respectively (P < 0.05). AKT was distributed in the cytoplasm of hepatocytes around the central vein, and fraction was decreased in MNR (1.0 ± 0.2 vs. 5.0 ± 1.2 in CTR, P < 0.01).
Figure 5.
Photomicrographs of staining in liver sections from fetuses whose mothers were fed ad libitum (CTR; A, C, E, and G) or whose mothers were nutrient restricted (MNR; fed 70% CTR diet from 30 to 90 d gestation; B, D, F, and H). Immunostaining included: Ki67 (A and B); Caspase3 (C and D); AKT (E and F); and periodic acid Schiff staining for glycogen (G and H). Bar (right panel) applies to same-row panels.
Liver glycogen
In both CTR and MNR fetal livers, glycogen staining was strongest around the central vein of the liver lobule. Staining for liver glycogen showed an increase in fraction stained in the livers of MNR group fetuses (Fig. 5). For the CTR group, the density of staining and the percentage area stained were 13.5 × 107 ± 16.3 × 106 arbitrary units and 39.7 ± 5.2%, respectively, whereas for the MNR group, they were 21.1 × 107 ± 5.7 × 106 and 67.5 ± 2.9% (both values differed between the groups; P < 0.01).
Relationship of stain intensity to measures of maternal and fetal serum and amniotic fluid IGF-I and IGF-II concentrations
Using data published previously (15), we evaluated the relationship of serum and amniotic fluid peptide values to the intensity of both IHC and in situ staining for all measures made. A significant positive correlation was noted between maternal plasma IGF-I and fetal hepatic (but not plasma) IGF-I and a negative correlation between fetal hepatic IGF-I protein by IHC and amniotic IGF-II and fetal hepatic IGF-II and amniotic IGF-II (P < 0.05).
Discussion
Despite contrary opinion, MNR is a major problem in developed societies. The number of hungry people in the United States is greater now than it was when international leaders set hunger-cutting goals at the 1996 World Food Summit. U.S. government leaders’ pledges to cut the number of Americans living in hunger, from 30.4 to 15.2 million by 2010, are unfulfilled (16). In addition to decreased maternal nutrient intake, poor fetal nutrition can accompany maternal vascular disease, teenage pregnancy, and other situations in which intrauterine growth retardation (IUGR) occurs. Poor delivery of nutrition during development has major effects on the fetus and newborn. The physiological state of the fetus differs greatly from the postnatal mammal, e.g. a lower fetal arterial PO2 (about half neonatal values) and lower plasma glucose concentrations compared with postnatal mammals (17). The fetus is totally dependent on the quality, quantity, and timing of maternal nutrient supply. There have been extensive experimental studies on both normal and challenged GH-IGF axis function in fetal rodents and sheep, but information in fetal nonhuman primates is sparse (18,19). In sheep global nutrient restriction (50% reduction from conception to 0.64 of gestation with normal nutrition until term, reduced fetal liver but not body weight, whereas hepatic IGF-I and IGF-II mRNA were not affected (20). Rodent IGF-I and binding protein levels at birth can be elevated or reduced according to the nutritional challenge (21).
Our previous publications on the placenta (15) and fetal kidney (8) in this model demonstrate that 30% global nutrient restriction in the nonhuman primate results in marked developmental changes. We now show that alterations in several components of the fetal liver IGF system in the absence of a significant change in overall fetal weight or liver weight. In general, the changes in mRNA abundance were about 2-fold whereas protein changes were much greater. Several other investigators have shown decreases in IGF-I at the mRNA and protein levels, but to our knowledge, this is the first study to evaluate the effect of maternal nutrient restriction on fetal hepatic IGF-II at both the mRNA and protein levels simultaneously in any species. In the present study, maternal nutrient restriction resulted in a fall in both IGF-I and IGF-II protein and mRNA in fetal hepatocytes by more than 50%, indicating a decreased production of these two key growth-promoting factors. The only discrepancy in mRNA measurement between QRT-PCR and ISH was in IGF-II in which both ISH and IHC were consistent with a fall. QRT-PCR is an extremely precise method of measuring mRNA in a heterogeneous cell homogenate, whereas ISH is semiquantitative but measures signal for individual cells. Although decreasing maternal intake to 60% of controls for the first half of gestation in sheep also decreased fetal hepatic IGF-I mRNA, IGF-II mRNA abundance was increased, suggesting differences between primates and ruminants (22). Differences may be due to the duration of the nutritional challenges in the various different studies in the two species as well as developmental stage at which fetal nutrition is challenged.
The liver is the major site of IGF-I and -II synthesis in adult rodents (1), and hepatic production of IGF-I is sufficient to maintain the relatively large amount of IGF (∼50% of total body content) present in the circulation (23). In addition to quantification, the use of IHC in our study provides new information on the cellular distribution of several IGF system components in the fetal primate liver at midgestation. Whereas mRNA and protein for IGF-I and -II, IGF-IIR, and IGFBP-1 and -3 mRNA and protein were widely present throughout the liver, IGF-IR was restricted to a small number of cells lining hepatic sinuses.
To our knowledge the distribution of fetal hepatic IGF-IR has not been reported. Judging by their position and available evidence from cell separation methods, these cells are likely to be either hepatic stellate cells (24) or Kupfer cells. To determine whether these are Kupfer cells rather than hepatic stellate cells, in vivo fetal vascular injection of carbon granules would be required to demonstrate uptake of carbon particles into IGF-IR immunopositive cells. Kupfer cells are suggested to be the source of production of cytokines and hence may be of importance when the fetus has been challenged by abnormal levels of certain dietary compounds such as lipids and possibly also in infection. The functions of both Kupfer and hepatic stellate cells at this early stage of gestation remain to be determined. Hepatic IGF-IR mRNA is reported present in fetal sheep liver in which it remains unchanged after IUGR or IGF-I treatment (25), human placenta using ligand binding techniques (26) and human fetal lung (27). IGF-I increases DNA synthesis in rat hepatic stellate cells (28).
The view that IGF signaling occurs through IGF-IR and that the IGF-IIR is a clearance receptor has been challenged in placental studies, which show that IGF-II stimulates extravillous trophoblast cell migration by signaling through IGF-IIR by a pathway involving G protein inhibition and activation of the MAPK pathway (4). The finding of very low levels of IGF-IR in hepatocytes suggests either that IGF-I and -II may not be major trophic factors for fetal liver growth and function at this stage of development in primates or that they act through the IGF-IIR or another receptor such as the insulin receptor to which IGF-I binds (29) and may be a mechanism by which IGF-I alters fetal liver function and growth at this stage of development. In contrast to IGF-IR, IGF-IIR was present in hepatocytes throughout the liver. If IGF-IIR is solely a clearance receptor, the high levels of IGF-IIR in the fetal hepatic parenchyma would tend to increase clearance of IGF-II, thereby lessening its potency within the fetal liver, and the fall in IGF-IIR in MNR may help to maintain liver growth.
IGFBP-1 mRNA and protein were present in abundance in fetal hepatocytes. Because IGFBP-1 binds IGFs and decreases their growth-promoting activity, high levels of IGFBP-1 will likely also decrease growth promotion by IGF-I and -II (30). ISH studies are required to show that IGFBP-3 is synthesized by hepatic cells because it is clear that immunoreactive hepatic IGFBP-3 protein was present, mostly associated with red blood cells in the plentiful blood sinuses that comprise the hematopoietic portions of the developing liver. The presence of IGFBP-3 protein on fetal erythrocytes likely represents binding of circulating IGFBP-3 rather than synthesis within the red cell, even though the fetal erythrocyte does contain an active nucleus at this stage of development. These considerations mean that the increase in fetal hepatic IGFBP-3 protein in the absence of any change in message at either the RT-PCR or ISH level must be interpreted with caution. Whereas the finding may represent possible translational regulation, the possibility that more IGFBP-3 protein is synthesized elsewhere and transported to the liver in the plasma rather than synthesized by hepatic cells cannot be excluded.
It is clear that poor fetal nutrition can lead to IUGR associated with increased rates of perinatal and neonatal mortality and morbidity. However, moderate degrees of nutrient restriction can also have organ-specific effects on the development of important fetal organs (31). This will increase the risk of abnormal postnatal renal (32), metabolic (33), and cardiovascular function (34) without a decrease in absolute fetal or newborn weight or even organ weight changes. Experimental protocols that differ little have produced varying outcomes in fetal body and organ weights. Similar protein restriction models report both change and no change in birth weight (31). The importance of the findings presented here is that they support the view that, in the primate fetus, as in other species, changes in cellular composition precede changes in the gross end point provided by overall weight. Change in gross weight is a late consequence of fetal insult and thus an insensitive measure of functional deficits. The decreased abundance of several factors in the IGF system would clearly act to impair development of key organs. Detailed studies of cellular structure, function, and content are required to determine the nature of deficits produced in the fetus as a result of maternal challenges like nutrient restriction and the initiatory mechanisms responsible.
Increased liver glycogen in fetuses of the nutrient restricted mothers suggests energy conservation by reduction of glycolysis or increased gluconeogenesis in response to reduced fetal nutrients. We have reported changes in metabolic factors and circulating concentrations of key components of the fetal and maternal IGF system with this reduced maternal nutrition paradigm. Maternal plasma glucose was maintained and even showed a tendency to rise, which would help maintain fetal glucose (15). Further studies on effects on key hepatic enzymes such as phosphoenolpyruvate carboxykinase are necessary to determine underlying mechanisms. A similar rise in fetal glycogen occurs during chronic maternal hypoglycemia in sheep (35) and in IUGR fetuses of mothers exposed to hyperthermia (36). Decreased fetal Akt protein indicates that fetal hepatic cells sense lowered nutrient availability, responding accordingly. The positive correlations between maternal plasma and fetal hepatic IGF-I in the absence of any correlation between the plasma levels supports the view that both mother and fetus respond to the reduced nutrient availability but that changes in factors such as binding proteins and differential clearance rates in the various tissues that control absolute plasma levels may differ between mother and fetus.
The moderate level of nutrient challenge was also associated with decreased maternal serum total and free IGF-I in nutrient-restricted mothers compared with controls and decreased fetal IGF-I, which did not quite reach significance (P = 0.057). IGF-I to IGFBP3 ratio decreased significantly in both the maternal and fetal circulation (15). IGFPB-3 binds 90% of circulating IGF and is the major blood binding protein. Although IGFBP-3 is generally considered to inhibit IGF function, there are indications it may also stimulate its activity (37).
IGFs exert an antiapoptotic and growth-promoting effect and an overall decrease in abundance of key factors in the system is compatible with the observed increase in caspase-3. In addition, Ki67 staining was reduced, indicating an overall decrease in cell proliferation. El Khattabi et al. (38) investigated the effect of isocaloric maternal low-protein diet during rat pregnancy on proliferative capacity of cultured fetal hepatocytes. Fetal liver weight and fetal plasma IGF-I were reduced and IGFBP-1 in fetal plasma and liver were increased. Liver IGF-II mRNA was unchanged after the maternal low-protein diet. In primary culture, hepatocytes from fetuses of mothers fed a low protein diet produced less DNA and IGF-I and more 29- to 32-kDa IGFBPs in vitro than hepatocytes from control fetuses (38). These findings are consistent with our observation of increased apoptosis in livers of MNR fetuses. Although difficult to interpret because of the masking by red cell staining, our Ki67 data indicate that mitotic rate in hepatic cells also decreases. The distribution of Ki67 in hepatocyte cytoplasm is an interesting observation that we cannot explain. When this antibody is used on fetal kidney, staining is nuclear (our unpublished data), suggesting that trafficking of the protein after synthesis in the cytoplasm may be altered in fetal hepatic cells.
In conclusion, our data show both similarities and differences from published effects of various MNR models in nonprimate species, highlighting the importance of studies on primate as well as nonprimate species. They also sustain the view that weight, either whole body or individual organ weight, is a poor measure of compromised fetal development, particularly in the early stages of impairment. Moderately reduced maternal nutrition was accompanied by a decrease in available mRNA and/or protein of the three factors we evaluated, which increase function of the IGF system: IGF-I, IGF-II, and IGF-IR. Further studies are needed to address the relative roles of macronutrient and micronutrient delivery in this decrease in the fetal liver IGF system.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grant HD 21350.
Disclosure Summary: P.W.N., L.A.C., N.E.S.-L., and T.J.M. received a research grant from the National Institutes of Health (PO1 HD 21350) for this research. All remaining authors have nothing to declare.
First Published Online July 2, 2009
Abbreviations: Ct, Threshold cycle; CTR, control; dG, days of gestation; IGFBP, IGF binding protein; IGF-IR, IGF-I receptor; IGF-IIR, IGF-II receptor; IHC, immunohistochemistry; ISH, in situ hybridization; IUGR, intrauterine growth retardation; KPBS, potassium PBS; MNR, maternal nutrient restriction; QRT-PCR, quantitative real-time PCR.
References
- Han VKM, Hill DJ 1994 Growth factors in fetal growth. In: Thorburn GD, Harding R, eds. Textbook of fetal physiology. Oxford, UK: Oxford University Press; 48–69 [Google Scholar]
- Fowden AL 2003 The insulin-like growth factors and feto-placental growth. Placenta 24:803–812 [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Efstratiadis A, Robertsen EJ 1990 A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 345:78–80 [DOI] [PubMed] [Google Scholar]
- McKinnon T, Chakraborty C, Gleeson LM, Chidiac P, Lala PK 2001 Stimulation of human extravillous trophoblast migration by IGF-II is mediated by IGF type 2 receptor involving inhibitory G protein(s) and phosphorylation of MAPK. J Clin Endocrinol Metab 86:3665–3674 [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A 1993 Mice carrying null mutations of the genes encoding insulin-like growth factor 1 (lgf-1) and type 1 IGF receptor (lgf1r). Cell 75:59–72 [PubMed] [Google Scholar]
- Yakar S, Kim H, Zhao H, Toyoshima Y, Pennisi P, Gavrilova O, LeRoith D 2005 The growth hormone-insulin like growth factor axis revisited: lessons from IGF-1 and IGF-1 receptor gene targeting. Pediatr Nephrol 20:251–254 [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Gargosky SE 1995 Characterization of the insulin-like growth factor (IGF) axis in the serum of maternal and fetal macaques (Macaca mulatta and Macaca fascicularis). Growth Regul 5:190–198 [PubMed] [Google Scholar]
- Nijland MJ, Schlabritz-Loutsevitch N, Hubbard GB, Nathanielsz PW, Cox LA 2007 Nonhuman primate fetal kidney transcriptome analysis indicates mTOR is a central nutrient responsive pathway. J Physiol 579(Pt 3):643–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch N, Ballesteros B, Dudley C, Jenkins S, Hubbard G, Burton GJ, Nathanielsz P 2007 Moderate maternal nutrient restriction, but not glucocorticoid administration, leads to placental morphological changes in the baboon (Papio sp.). Placenta 28:783–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Bill Cummins L, Frost PA, McDonald TJ, Nathanielsz PW 2004 Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol 33:117–126 [DOI] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch NE, Hubbard GB, Dammann MJ, Jenkins SL, Frost PA, McDonald TJ, Nathanielsz PW 2004 Normal concentrations of essential and toxic elements in pregnant baboon and fetuses (Papio species). J Med Primatol 33:152–162 [DOI] [PubMed] [Google Scholar]
- Grieves JL, Dick Jr EJ, Schlabritz-Loutsevich NE, Butler SD, Leland MM, Price SE, Schmidt CR, Nathanielsz PW, Hubbard GB 2008 Barbiturate euthanasia solution-induced tissue artifact in nonhuman primates. J Med Primatol 37:154–161 [DOI] [PubMed] [Google Scholar]
- Han VKM, Carter AM 2000 Spatial and temporal patterns of expression of messenger RNA for insulin-like growth factors and their binding proteins in the placenta of man and laboratory animals. Placenta 21:289–305 [DOI] [PubMed] [Google Scholar]
- Han VK, Basset N, Walton J, Challis JR 1996 The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. J Clin Endocrinol 81:2680–2693 [DOI] [PubMed] [Google Scholar]
- Li C, Levitz M, Hubbard GB, Jenkins SL, Han V, Ferry Jr J, McDonald TJ, Nathanielsz PW, Schlabritz-Loutsevitch NE 2007 The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet. Placenta 28:1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord M, Andrews M, Carlson S 2003 Household food security in the United States (2002): food assistance and nutrition research report no. 35. Washington DC: U.S. Department of Agriculture [Google Scholar]
- Koenen SV, Mecenas CA, Smith GS, Jenkins S, Nathanielsz PW 2002 Effects of maternal betamethasone administration on fetal and maternal blood pressure and heart rate in the baboon at 0.7 of gestation. Am J Obstet Gynecol 186:812–817 [DOI] [PubMed] [Google Scholar]
- Putney DJ, Henson MC, Pepe GJ, Albrecht ED 1991 Influence of the fetus and estrogen on maternal serum growth hormone, insulin-like growth factor-II, and epidermal growth factor concentrations during baboon pregnancy. Endocrinology 129:3109–3117 [DOI] [PubMed] [Google Scholar]
- Zollers Jr WG, Babischkin JS, Pepe GJ, Albrecht ED 2001 Developmental regulation of placental insulin-like growth factor (IGF)-II and IGF-binding protein-1 and -2 messenger RNA expression during primate pregnancy. Biol Reprod 65:1208–1214 [DOI] [PubMed] [Google Scholar]
- Hyatt MA, Gopalakrishnan GS, Bispham J, Gentili S, McMillen IC, Rhind SM, Rae MT, Kyle CE, Brooks AN, Jones C, Budge H, Walker D, Stephenson T, Symonds ME 2007 Maternal nutrient restriction in early pregnancy programs hepatic mRNA expression of growth-related genes and liver size in adult male sheep. J Endocrinol 192:87–97 [DOI] [PubMed] [Google Scholar]
- Remacle C, Bieswal F, Reusens B 2004 Programming of obesity and cardiovascular disease. Int J Obes Relat Metab Disord 28(Suppl 3):S46–S53 [DOI] [PubMed] [Google Scholar]
- Brameld JM, Mostyn A, Dandrea J, Stephenson TJ, Dawson JM, Buttery PJ, Symonds ME 2000 Maternal nutrition alters the expression of insulin-like growth factors in fetal sheep liver and skeletal muscle. J Endocrinol 167:429–437 [DOI] [PubMed] [Google Scholar]
- Schwander JC, Hauri C, Zapf J, Froesch ER 1983 Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone status. Endocrinology 113:297–305 [DOI] [PubMed] [Google Scholar]
- Sanz S, Pucilowska JB, Liu S, Rodríguez-Ortigosa CM, Lund PK, Brenner DA, Fuller CR, Simmons JG, Pardo A, Martínez-Chantar ML, Fagin JA, Prieto J 2005 Expression of insulin-like growth factor I by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut 54:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh S, Bloomfield FH, Bauer MK, Phua HH, Gilmour RS, Harding JE 2005 Amniotic IGF-I supplementation of growth-restricted fetal sheep alters IGF-I and IGF receptor type 1 mRNA and protein levels in placental and fetal tissues. J Endocrinol 186:145–155 [DOI] [PubMed] [Google Scholar]
- Diaz E, Cardenas M, Ariza AC, Larrea F, Halhali A 2005 Placental insulin and insulin-like growth factor I receptors in normal and preeclamptic pregnancies. Clin Biochem 38:243–247 [DOI] [PubMed] [Google Scholar]
- Chetty A, Andersson S, Lassus P, Nielsen HC 2004 Insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) expression in human lung in RDS and BPD. Pediatr Pulmonol 37:128–136 [DOI] [PubMed] [Google Scholar]
- Saile B, DiRocco P, Dudas J, El-Armouche H, Sebb H, Eisenbach C, Neubauer K, Ramadori G 2004 IGF-I induces DNA synthesis and apoptosis in rat liver hepatic stellate cells (HSC) but DNA synthesis and proliferation in rat liver myofibroblasts (rMF). Lab Invest 84:1037–1049 [DOI] [PubMed] [Google Scholar]
- Rechler MM, Nissley SP 1985 The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol 47:425–442 [DOI] [PubMed] [Google Scholar]
- Watson CS, Bialek P, Anzo M, Khosravi J, Yee SP, Han VKM 2006 Elevated circulating insulin-like growth factor binding protein-1 is sufficient to cause fetal growth restriction. Endocrinology 147:1175–1186 [DOI] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L 2004 Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol (Lond) 561:355–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LA, Nijland MJ, Gilbert JS, Schlabritz-Loutsevitch NE, Hubbard GB, McDonald TJ, Shade RE, Nathanielsz PW 2006 Effect of thirty per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. J Physiol 572:67–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz P 2005 Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol 566(Pt 1):225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley SC, Jackson AA 1994 Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci 86:217–222 [DOI] [PubMed] [Google Scholar]
- Rozance PJ, Limesand SW, Barry JS, Brown LD, Thorn SR, LoTurco D, Regnault T, Friedman JE, Hay Jr WWW 2008 Chronic late gestation hypoglycemia up-regulates hepatic PEPCK associated with increased PGC1α mRNA and pCREB in fetal Sheep. Am J Physiol Endocrinol Metab 294:E365–E370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault TRH, Keng J, Roper M, Wilkening RB, Hay W, Friedman JE 2006 IUGR is associated with loss of stress kinase sensing and dissociation of AKT-MTOR signaling in liver and skeletal muscle. Early Hum Dev 82:498 [Google Scholar]
- Martin JL, Baxter RC 1992 Inhibition of human fibroblast insulin-like growth factors binding protein (IGFBP) production by IGFBP-3. Endocrinology 131:1568–1570 [DOI] [PubMed] [Google Scholar]
- El Khattabi I, Grégoire F, Remacle C, Reusens B 2003 Isocaloric maternal low-protein diet alters IGF-I, IGFBPs, and hepatocyte proliferation in the fetal rat. Am J Physiol Endocrinol Metab 285:E991–E1000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.