Abstract
Type 1 diabetes (T1D) mellitus is characterized by progressive autoimmune destruction of insulin producing β-cells of the pancreatic islets of Langerhans. Cure of the disease will require control of autoimmunity to halt the destruction of β-cells in the pancreas and restoration of β-cell mass. We have built on the success of preclinical and clinical trials of anti-CD3 antibody treatment in modulating the immune response of T1D by the induction of tolerance and combined this treatment, using the nonobese diabetic mouse model, with a transplantation approach using fetal pancreatic anlagen as a source of β-cell precursor or progenitor cells. Here we report that transplantation of pancreatic anlagen into diabetic nonobese diabetic mice rendered tolerant to the autoimmune process by treatment with anti-CD3 antibody resulted in long-term recovery from diabetes with restored metabolic control. Using a green fluorescent protein marker that made it possible to unequivocally identify the cells derived from the transplanted tissue, we show that the transplanted anlagen cells migrate to the host pancreas and provide a major source of insulin leading to restoration of normal glucose tolerance. Our results contrast with other studies that showed restoration of endogenous islets after infusion of spleen cells in mice treated with Freund’s complete adjuvant and suggest that pancreatic fetal tissue has a tropism for the pancreatic site. This study suggests a novel mechanism of β-cell restoration by the migration of precursor cells or their progeny to the host pancreas and highlights the feasibility of using pancreatic precursors in combination with immune modulation as a treatment to effect long-term remission of T1D.
Long-term remission of type 1 diabetes can be attained by controlling autoimmunity and supplying pancreatic progenitor cells by transplantation of fetal tissue housing the pancreas.
Type 1 diabetes (T1D) mellitus is caused by genetic and environmental factors (1,2) and characterized by autoimmune destruction of insulin producing β-cells of the pancreatic islets of Langerhans. T1D is a chronic and debilitating disease that primarily affects children and young adults. Daily administration of insulin in appropriate doses is necessary to control blood glucose level of T1D patients, which in practice is not an easy task because of the risk of hypoglycemia. On the other hand, chronic hyperglycemia is associated with severe complications such as retinopathy, nephropathy, neuropathy, and cardiopathy. Thus, finding an effective, convenient, and safer treatment for T1D is of high priority.
In searching for an improved treatment for T1D, two complementary goals must be attained: control of autoimmunity to halt the destruction of β-cells in the pancreas and restoration of β-cell function to levels sufficient to control blood glucose, by either expansion of residual β-cells or replacement of β-cells from an exogenous source. One promising approach to suppression of the autoimmune response is the application of anti-CD3 antibody. This treatment is presumed to induce tolerance by induction of adaptive regulatory T cells, which is a more acceptable, less problematic immune modulation than chronic immune suppression. In two independent trials in patients with recent-onset diabetes, there was a slowing of the progressive decline in C-peptide levels over 12–18 months after a single course of anti-CD3 antibody (3,4,5). However, despite the immunological effects, a full recovery of lost β-cell function and insulin independence was not achieved, presumably due to the lack of sufficient residual β-cell mass. The main approach to the restoration of β-cell function in the treatment of T1D has been transplantation of adult pancreatic islets isolated from cadavers. Islet transplantation in combination with anti-CD3 antibody treatment has been shown effective in reversal of diabetes in a limited clinical application (6). However, because of the limited supply of suitable tissue, there has been avid interest in using embryonic stem cells or other types of progenitor cells as an alternative source of insulin-producing cells for engraftment.
Some success has been achieved in the control of streptozotocin-induced diabetes in mice by the transplantation of in vitro differentiated human embryonic stem cells (7) or cultured human embryonic pancreatic cells (8). In both cases, there was evidence that the grafted cells were responsible for improved blood glucose concentration. In the first study, however, teratomas also developed at the graft site. In another study, rat embryonic pancreatic anlagen were used as a source of β-cell precursors and were transplanted to the fold of the peritoneal mesentery in streptozotocin-treated rats, resulting in an improvement in blood glucose, although no assessment of the endogenous pancreas was made (9).
In the nonobese diabetic (NOD) mouse, which spontaneously develops T1D, modulation of the immune response coupled with transplantation of adult mouse islets and infusion of adult spleen cells has been reported to result in long-term remission of diabetes through the recovery of β-cell function in the endogenous pancreas (10,11,12,13,14). Each of these studies reported recovery of endogenous β-cell function and long-term metabolic control, even after removal of the islet graft, although recovery rates varied considerably and were generally quite low. The authors attributed recovery to the restoration of endogenous β-cell mass; however, the studies differ in whether progeny of the infused spleen cells could be detected in the endogenous pancreas. Faustman et al. (15) used fluorescence in situ hybridization to detect the Y chromosome in cells within the islets of the female host pancreas and concluded that failure to detect a low level of spleen-derived cells in the other studies could be attributed to technical differences in the efficiency of the detection methods used. Thus, questions remain as to the mechanism of this treatment regime in restoring β-cell mass and whether it is through recovery of endogenous β-cell function or a contribution of the transplanted cells. Furthermore, the immune system modulation in these studies involved the use of Freund’s complete adjuvant, which has failed in human trials (16,17).
In the present study, we have built on the partial success of clinical trials of anti-CD3 antibody treatment in modulating the immune response by the induction of tolerance and combined this treatment, using the NOD mouse model, with a transplantation approach using fetal pancreatic anlagen as a source of β-cell precursors or progenitor cells. Here we report that transplantation of pancreatic anlagen into diabetic NOD mice rendered tolerant to the autoimmune process by treatment with anti-CD3 antibody resulted in long-term recovery from diabetes with restored metabolic control. We used a green fluorescent protein (GFP) transgenic marker to unequivocally identify the cells derived from the transplanted tissue. The transplanted cells migrate to the host pancreas and provide a major source of insulin, suggesting that pancreatic fetal tissue has a tropism for the pancreatic site. These findings have important implications for the use of stem cells or progenitor cells in the treatment of T1D.
Materials and Methods
Animals
All mice were maintained under specific pathogen-free conditions. Random bred ICR mice were obtained from Taconic Farms (Germantown, NY). NOD/ShiLtJ female and NOR/LtJ male mice were purchased from Jackson Laboratory (Bar Harbor, ME). Nonobesese, diabetes-resistant (NOR) mice were chosen as the source of transplanted tissue because of their histocompatibility with and close relationship to NOD mice but without the complication of the T1D phenotype (18). NOD and NOR mice share the major histocompatibility complex (MHC) class I (H-2Db and Kd) and class II (H2-I-Ag7) molecules. To provide a source of histocompatible, fluorescently marked tissue for transplantation of pancreatic anlagen, NOR/LtJ mice were crossed with CAG::H2B-EGFP mice, which have a ubiquitously expressed, histone-enhanced GFP fusion transgene (19), and then backcrossed with selection for fluorescence and the NOR MHC class I and class II types. Offspring were phenotyped for GFP fluorescence of an ear punch under UV light and screened by typing of peripheral blood mononuclear cells. Peripheral blood mononuclear cells were stained with antimouse MHC I (H-2Kd, phycoerythrin conjugated; BD Biosciences, Franklin Lakes, NJ) and MHC II antibodies (I-Ak, cross-reacts with I-Ag7, allophycocyanin conjugated; BD Biosciences) and analyzed by flow cytometry. For this and subsequent generations, females positive for GFP and the NOR MHC I and II types were selected for backcrossing to male NOR mice to produce a congenic NOR-CAG::H2B-EGFP strain. GFP expression in both exocrine and endocrine tissues of the pancreas was confirmed by immunofluorescence with anti-GFP and antiinsulin antibodies (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Although this GFP is usually localized to the nucleus, we observed primarily cytoplasmic localization in the transplanted tissue (see Fig. 4A). Embryos of the N2-N8 backcross generations were used for transplantation.
Figure 4.
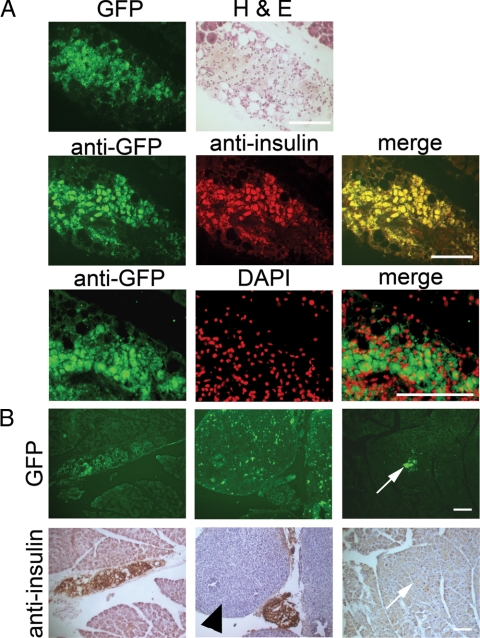
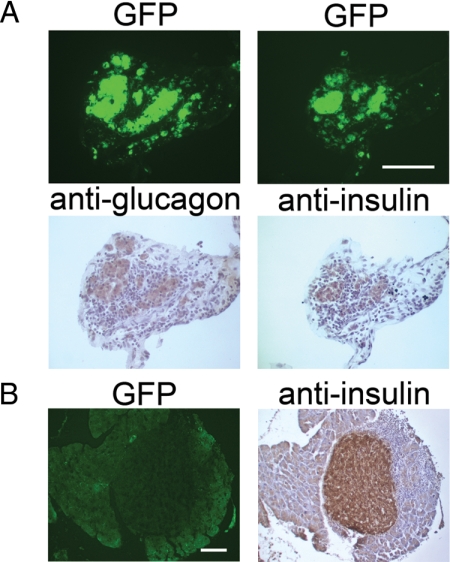
Embryonic pancreatic precursor-derived cells colonize the host pancreas and form insulin-producing β-cells. A, Frozen sections through the pancreas of a diabetic NOD mouse that received the combination treatment and was unilaterally nephrectomized at 45 d. Top row, GFP fluorescence and H&E staining on the same section. Exocrine tissue is evident in the top corners. Middle row, Colocalization of GFP and insulin as evidenced by two-color immunohistochemistry with a merged image. Bottom row, Dual staining with anti-GFP and 4′,6-diamidine-2-phenylindole dihydrochloride (DAPI; false colored) with a merged image. Bar, 100 μm. B, GFP fluorescence (upper panels) and immunohistochemical analysis with antiinsulin (lower panels) was carried out on frozen sections of pancreas from diabetic NOD mice that received the combination treatment and were unilaterally nephrectomized at 45 d. Panels show several areas with different morphology of GFP-positive, insulin-positive clumps of cells in the interlobular spaces or attached to the endogenous pancreas. Additionally, the middle and right pairs of panels show examples of GFP-positive, insulin-negative cells in a large clump in the interlobular space (arrowhead) and a small clump integrated within the exocrine pancreas (arrows). Bar, 100 μm.
Disease monitoring, antibody and insulin treatment, and transplantation of anlagen
Blood glucose levels in NOD mice were monitored twice weekly from 12 wk of age using a Free Style blood glucose monitoring system (Therasense, Alameda, CA), and mice were considered diabetic when blood glucose concentration was greater than 300 mg/dl for at least 2 consecutive days. Blood glucose levels greater than 500 mg/dl (top of the scale on monitor) were considered severely diabetic. Onset of diabetes ranged from 13 to 52 wk. The age of onset did not differ significantly between diabetic and severely diabetic mice (Mann-Whitney U test, P > 0.05). Within 5 d of diabetes onset, mice received a 5-d course of anti-CD3 monoclonal antibody, followed within 1 d by surgical transplantation of pancreatic anlagen and sc implantation of an insulin pellet or some combination of these treatments as controls. Control mice that did not receive antibody treatment, were subjected to anlagen transplantation within 1 d of diabetes diagnosis. Anti-CD3 antibody 145-2C11 F(ab)2 fragments or whole anti-CD3 antibody (Taconic, Hudson, NY; produced from hybridoma clone 145-2C11) at a dose of 50 or 10 μg/d, respectively, was administered by tail vein injection as indicated in the figure legends. Efficacy of these two anti-CD3 regimens has been shown to be comparable (20,21). Furthermore, we observed no systematic difference in the results from animals receiving either type of antibody, and thus, the results have been combined.
Embryonic pancreatic anlagen from NOR-CAG::H2B-EGFP mice were dissected in cold PBS on embryonic day (E) 12.5 under a dissecting microscope and cultured overnight using culture plate inserts (Millipore Corp., Billerica, MA) in advanced DMEM/F12 reduced serum medium (1:1) containing B-27 supplement (Invitrogen, Carlsbad, CA), 5 mm HEPES, 3 mm Na2HCO3, 2 mm l-glutamine, 20 μg/ml heparin, 100 U/ml penicillin, and 100 μg/ml streptomycin. Groups of two to 10 anlagen were transplanted beneath the left kidney capsule of diabetic NOD hosts under tribromoethanol anesthesia (0.2 ml per 10 g body weight). At the same time, a slow-release insulin pellet (Linshin Canada Inc., Ontario, Canada) was implanted sc near the cervical region. The release rate of insulin is estimated as 0.1 U/d per implant for more than 30 d. After treatment, blood glucose levels were monitored twice a week and animals were killed if three to four consecutive readings were greater than 500 mg/dl. After 3 wk, the insulin pellet was removed if blood glucose level had dropped to normal for 3–4 consecutive days.
Unilateral nephrectomy
Unilateral nephrectomy was performed on some animals 20, 45, or 120 d after transplantation of pancreatic anlagen to remove the graft-containing kidney. Under anesthesia, the left renal artery, vein, and ureter were ligated and the kidney was resected. After surgery mice were treated with bupivacaine (Henry Schein, Melville, NY) locally after surgery and ketoprofen (Sigma, St. Louis, MO) ip (5 mg/kg body weight) after surgery and 24 h later. Blood glucose levels were monitored as before.
Intraperitoneal glucose tolerance test (IPGTT) and insulin secretion assay
An IPGTT was performed on some animals with measurement of insulin and glucose. Age-matched ICR (n = 4) and NOR-CAG::H2B-EGFP females (n = 3) were used as controls. After a 14-h fast, blood was drawn from the tail vein before and 15, 30, 60, and 90 min after an ip injection of d-glucose (1 mg/g body weight), and blood glucose levels were measured. Blood serum was separated from blood by centrifugation and stored at −20 C for approximately 1 month. Glucose-stimulated insulin secretion in the serum samples was measured with a mouse insulin ELISA kit (ALPCO Diagnostics, Salem, NH) according to the manufacturer’s instruction.
Immunohistochemistry and immunofluorescence
Kidney, pancreas, liver, lung, and spleen samples were fixed in 4% paraformaldehyde for 3 h, washed overnight in 30% sucrose, embedded in Tissue Tek optimum cutting temperature compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan), snap frozen on dry ice in ethanol, and stored at −80 C until use. Frozen sections (10 μm thick) were quenched with 0.3% H2O2 and blocked with serum. Immunohistochemistry was performed using a standard avidin-biotin peroxidase staining method using peroxidase substrate kit diaminobenzidine (Vector Laboratories, Burlingame, CA) and hematoxylin counterstain. For immunofluorescence, sections were stained with primary antibodies overnight at 4 C. After secondary antibody staining, sections were mounted with GEL/MOUNT (Biomeda Corp., Foster City, CA).
The antibodies used were guinea pig antiswine insulin (Dako Cytomation, Dako, Denmark), guinea pig antihuman insulin, guinea pig antiglucagon (both from Linco Research, St. Charles, MO), rabbit antipancreatic duodenal homeobox-1 (Pdx1; a gift from Dr. Christopher Wright, Vanderbilt University, Nashville, TN), rabbit anti-GFP, Alexa fluor donkey antirabbit (both from Molecular Probes Inc., Eugene, OR), biotinylated guinea pig Vectastain ABC kit and diaminobenzidine kit (Vector Laboratories), rabbit anti-α-amylase (Sigma), horseradish peroxidase-conjugated antirabbit, horseradish peroxidase-conjugated anti-guinea pig, TX red-conjugated anti-guinea pig and Cy3-conjugated antirabbit (Jackson ImmunoResearch, West Grove, PA), fluorescein isothiocyanate-conjugated anti-CD3 antibody specific for CD3 (clone145-2C11; BD PharMingen, San Diego, CA).
Some pancreas sections were stained with hematoxylin and eosin (H&E) for morphological study and others were stained with 4′, 6′-diamidine-2-phenylindole dihydrochloride for nuclear visualization. Images of kidney grafts were taken under GFP fluorescence and bright field on an SMZ1500 microscope (Nikon, Tokyo, Japan). Sections of grafts and host organs were examined with a Nikon MICROPHOT-FXA microscope, and images were captured using Magna Fire 2.1C software (Optronics, Goleta, CA).
Semiquantitation of insulin-producing area and β-cell mass in pancreas
Pancreas from three of the treated, recovered NOD mice that were unilaterally nephrectomized at 45 d were harvested about 80 d after nephrectomy and serially sectioned for quantification of insulin-producing tissue. To determine the relative contribution of donor- and host-derived β-cells, all GFP-positive and insulin-positive regions were identified in every 40th section (400 μm intervals) throughout the pancreas by first locating and photographing GFP-positive regions by fluorescence and then visualizing insulin by immunohistochemistry. Regions were classified as donor origin (GFP positive), host origin (GFP negative), or a mixture of donor and host tissue. Finally, the area of insulin-positive regions in each section was approximated by measuring and multiplying two diameters. Some neighboring sections were stained with H&E for verification of tissue morphology. To determine the total β-cell mass, the β-cell and total pancreatic areas were measured using five sections from each pancreas. The relative ratio of β-cell area compared with entire pancreatic area was determined. Finally, β-cell mass was calculated by multiplying the relative ratio of the β-cell area by the total weight of the pancreas.
Survey of other organs for graft-derived cells
The peritoneal cavity of all animals was examined under UV fluorescence at autopsy to detect GFP-positive cells. Tissue samples of liver, lung, right kidney, and spleen were harvested from three treated mice that underwent unilateral nephrectomy at 45 d, with a prediabetic NOD mouse providing control tissue. Organs were sectioned at 10 μm and six to nine sections (100 μm interval) from each organ were stained with antiinsulin and anti-GFP antibodies.
Results
Mature islets develop from transplanted fetal pancreatic anlagen
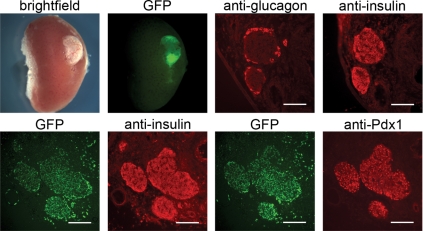
Preliminary experiments were done to determine the developmental potential of embryonic pancreatic anlagen in an ectopic site. Pancreatic anlagen were isolated from E12.5 NOR-CAG::H2B-EGFP embryos, which carry a ubiquitously expressed GFP transgene; cultured overnight; and transplanted beneath the kidney capsule of prediabetic NOD mice. Grafts were harvested after 20 d (n = 3) and verified by GFP fluorescence (Fig. 1). The grafts showed mature islet formation with glucagon-positive α-cells surrounding insulin-positive, Pdx1-positive β-cells (Fig. 1). No exocrine tissue was detected, as evidenced by the lack of amylase expression (data not shown), consistent with published work showing a selective differentiation of islets compared with acinar components in pancreatic anlagen transplanted to ectopic sites (9,22,23).
Figure 1.
Mature islets develop from transplanted fetal pancreatic anlagen. E12.5 fetal pancreatic anlagen transplanted under the kidney capsule of a prediabetic NOD mouse and recovered after 20 d develop into mature islets. The transplanted tissue is evident under the kidney capsule as shown in the bright-field and GFP fluorescence images at the upper left (not all of the anlagen transplanted were GFP-positive). Adjacent frozen sections of the graft site stained with antiglucagon and antiinsulin antibodies show insulin-positive β-cells surrounded by glucagon-positive α-cells in mature islets, shown in the right two images in the upper panel. Adjacent frozen sections showing GFP fluorescence and either insulin or Pdx1 expression are in the lower panels. The GFP marks cells of graft origin, whereas the localization of insulin and Pdx1 indicate that a subset of the GFP-positive cells have differentiated as β-cells. Bar, 100 μm.
Anti-CD3 antibody and ectopic transplantation of pancreatic anlagen reverse diabetes
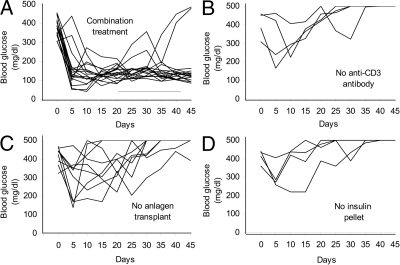
To test the potential of pancreatic anlagen to restore β-cell function in diabetic mice rendered tolerant to autoimmunity, we treated diabetic NOD mice with anti-CD3 antibody for 5 consecutive days and then transplanted histocompatible E12.5 pancreatic anlagen under the kidney capsule. An insulin pellet was implanted sc to control blood glucose in the short term. In preliminary experiments, we determined that transplantation of two to three pancreatic anlagen did not lead to reversal of diabetes (n = 4; supplemental Fig. 2A). Similarly, mice with initial extreme hyperglycemia (>500 mg/dl; n = 11) failed to recover normoglycemia (supplemental Fig. 2B). However, mice with initial blood glucose levels of 305–455 mg/dl that received anti-CD3 antibody in combination with a transplant of six to 10 pancreatic anlagen and an insulin pellet showed a dramatic improvement in blood glucose level. In most cases, blood glucose dropped to the normal range within 1–2 d of surgery. Fifteen of 17 mice maintained blood glucose levels within the normal range, even after the insulin pellet was removed 3–4 wk after anlagen transplantation, whereas only two of 17 mice returned to a diabetic state (>300 mg/dl for 2 consecutive days) within 45 d (Fig. 2A). These two animals received seven or eight pancreatic anlagen but showed no evidence of the grafted tissue in the kidney at autopsy.
Figure 2.
Long-term remission of diabetes after a combination of anti-CD3 antibody, pancreatic anlagen transplantation, and an insulin pellet. A, Blood glucose levels in newly diagnosed diabetic NOD mice, treated with a combination of 5 d of anti-CD3 antibody injections (two received whole antibody, 15 received F(ab′)2 antibody), transplantation of 6–10 pancreatic anlagen and an insulin pellet, decreased to normal levels within 1–2 days of treatment and 15/17 maintained normal blood glucose, even after removal of the insulin pellet (the range of time of removal of the pellet is indicated by the horizontal bar above the x-axis). The two animals that did not recover received F(ab′)2 anti-CD3 antibody and seven or eight pancreatic anlagen but showed no evidence of the grafted tissue in the kidney at autopsy. B–D, Control experiments with omission of either anti-CD3 antibody (n = 4) (B), pancreatic anlagen transplantation (n = 9; two received whole antibody, nine received F(ab′)2 antibody) (C), or the insulin pellet (n = 4; all received F(ab′)2 antibody) (D) resulted in a return to diabetic blood glucose levels within 2 wk. The insulin pellet was not removed from the mice in B and C. the x-axis represents days after anlagen transplantation (A, B, and D) or after insulin pellet transplantation in the case of animals that did not receive anlagen transplants (C).
In control experiments in which the anti-CD3 antibody treatment (n = 4) (Fig. 2B), anlagen transplantation (n = 9) (Fig. 2C), or insulin treatment (n = 4) (Fig. 2D) was omitted, none of the diabetic mice regained long-term metabolic control. Blood glucose levels of these control groups usually dropped within 2–3 d after treatment but increased again to diabetic levels within 2 wk and did not return to the normal range. At autopsy, two of the animals that were transplanted with anlagen and an insulin pellet but not treated with anti-CD3 antibody had large cystic grafts on the kidney, whereas two showed no evidence of the persistence of the grafted tissue. Animals that were transplanted with anlagen and treated with anti-CD3 antibody but did not receive an insulin pellet had little (two of four) or no (two of four) grafted tissue remaining at the transplant site.
Maintenance of grafted tissue in the ectopic site is not necessary for long-term reversal of diabetes
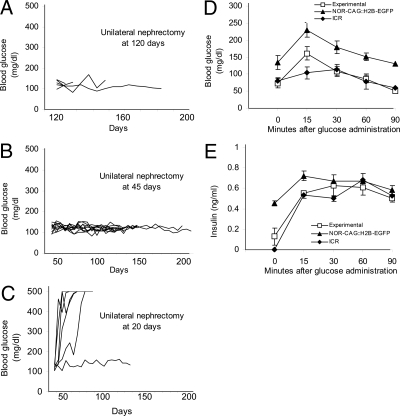
To determine whether recovery from diabetes in treated mice was due to insulin production by cells derived from the transplanted pancreatic anlagen or regenerated endogenous β-cells, we removed the kidney containing the graft site from the 15 mice that had attained long-term diabetes control either 120 d (n = 5) or 45 d (n = 10) after transplantation (the insulin pellet had been removed previously) and monitored blood glucose levels. All of the mice continued to maintain normoglycemia after unilateral nephrectomy (Fig. 3, A and B). Furthermore, compared with age-matched, nondiabetic controls, an IPGTT (n = 7) and glucose-stimulated insulin secretion assay (n = 6) were normal 2 wk after the unilateral nephrectomy at 45 d (Fig. 3, D and E).
Figure 3.
Unilateral nephrectomy reveals that, after 45 d, the graft site is not necessary for maintenance of normal blood glucose levels or metabolic control of blood glucose. A–C, Blood glucose levels of the diabetic NOD mice receiving the combination treatment of anti-CD3 antibody, six to 10 pancreatic anlagen, and an insulin pellet and undergoing a unilateral nephrectomy to remove the pancreatic anlagen graft site at 120 d[n = 5; F(ab′)2 antibody] (A), 45 d [n = 10; three received whole antibody, seven received F(ab′)2 antibody] (B), or 20 d [n = 6; four received whole antibody, two received F(ab′)2 antibody] (C). The insulin pellet was removed before unilateral nephrectomy at 120 or 45 d but remained in place in the mice nephrectomized at 20 d. With removal of the graft site at 120 or 45 d, all the mice maintained normoglycemia (A and B), whereas removal of the graft site after 20 d resulted in five of the six mice failing to maintain normoglycemia (C). Two weeks after nephrectomy at 45 d, seven mice were given an IPGTT (D), and glucose stimulated insulin secretion was measured in six of these by ELISA (E) and compared with nondiabetic, age-matched NOR-CAG::H2B-EGFP (n = 3) and ICR (n = 4) mice. Blood glucose and insulin secretion were measured on blood or serum samples, respectively, collected at intervals after an ip injection of d-glucose after a 14-h fast. The pattern of response, an initial increase followed by gradual decline, was similar in all three groups.
Analysis of the kidney graft sites after unilateral nephrectomy at 120 or 45 d revealed little or no grafted GFP-positive pancreatic tissue remaining in most animals. From the group that had a unilateral nephrectomy after 45 d, three graft sites containing grafted tissue were sectioned for immunohistochemical analysis, but no mature islets were found; only a few scattered insulin-positive cells were present, indicating that the grafted tissue remaining at the original transplantation site was not a major source of insulin by 45 d after transplantation.
Because our control experiments indicated that anlagen transplants were essential for long-term recovery from diabetes (Fig. 2C), we investigated the fate of transplanted tissue at an earlier time point in an additional group of six diabetic mice that received anti-CD3 antibody, pancreatic anlagen transplants, and an insulin pellet. Similar to most of the mice in the previous group receiving combination treatment, blood glucose dropped to normal levels soon after treatment (data not shown). A unilateral nephrectomy was done 20 d after treatment to remove the graft site, without removal of the insulin pellet. Within 1 wk, blood glucose levels started to rise and reached diabetic levels in five of the six mice (Fig. 3C). At autopsy, grafted tissue was evident at the kidney graft site. Immunohistochemical analysis of the grafts revealed the presence of scattered cells and clusters of cells positive for insulin, glucagon, or Pdx1. Examination of adjacent sections revealed that all grafts contained Pdx1-positive, insulin-negative cells as well as double-positive cells (supplemental Fig. 3). Mature islets, as observed in control grafts in prediabetic mice (Fig. 1), were extremely rare (data not shown).
Embryonic pancreatic precursor cells colonize the host pancreas and differentiate into insulin-producing β-cells
The failure of transplanted pancreatic anlagen to maintain insulin production in the ectopic graft site of treated diabetic host mice led us to consider several alternative explanations for the long-term recovery from diabetes. First, it could be that the transplanted anlagen differentiate into islets, produce insulin, and establish a normoglycemic environment for a sufficient time to allow regeneration of endogenous β-cells. Alternatively, the embryonic precursor or stem cells could migrate to other sites in the host in which they either produce insulin or stimulate endogenous β-cells to differentiate. To distinguish between these alternatives, we analyzed the pancreas and other tissues from seven mice that maintained normal blood glucose after unilateral nephrectomy at 45 d. Visual inspection of the peritoneum under UV light revealed no GFP-positive cells. Six to nine sections at 100-μm intervals were made throughout the parenchyma of the liver, lung, right kidney, and spleen and stained with antiinsulin and anti-GFP antibodies. No evidence of GFP-positive, graft-derived cells was found in these sections, nor were insulin-positive cells observed. However, fluorescent GFP-positive cells, confirmed by anti-GFP antibody staining (Fig. 4A), were found throughout the endogenous pancreas as single cells or in clusters sometimes associated with GFP-negative host cells and usually located in the interlobular spaces. These structures did not resemble conventional islets, but immunohistochemical analysis revealed that the vast majority of GFP-positive cells expressed insulin (Fig. 4, A and B), as did some of the associated host cells. GFP-positive cells that were insulin negative were of two types: cells in large, amorphous clumps in the interlobular spaces (Fig. 4B, arrowhead) and small groups of cells within the exocrine tissue of the pancreas (Fig. 4B, arrows). Antiglucagon antibody staining showed that some GFP-positive cells express glucagon indicating the presence of graft-derived α-cells (Fig. 5A). In addition, a very few endogenous, insulin-producing islets were seen within the host pancreas (Fig. 5B).
Figure 5.
A, GFP-positive graft-derived cells in the host pancreas express glucagon and insulin. Serial pancreatic sections from diabetic NOD mice that received the combination treatment and were unilaterally nephrectomized at 45 d were scored for GFP fluorescence (upper panels) and then stained with antiglucagon and antiinsulin antibodies (lower panels). Bar, 100 μm. B, Endogenous islet (GFP negative, insulin positive) from the pancreas of a diabetic NOD mouse that received the combination treatment and was unilaterally nephrectomized at 45 d. Bar, 100 μm.
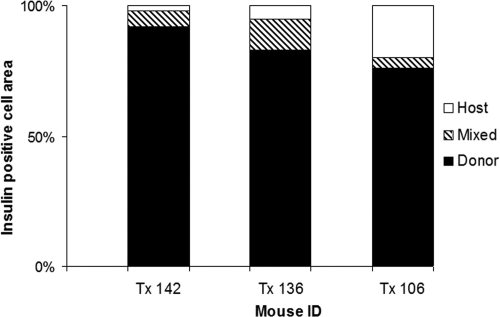
The pancreases from three of the treated diabetic mice that maintained normal blood glucose after unilateral nephrectomy at 45 d were analyzed in depth. The relative contribution of graft-derived cells to total insulin production was estimated by measuring the insulin-positive areas in a sampling of the entire pancreas and determining whether they were donor (GFP positive) or host (GFP negative) derived. In all three animals, more than 75% of the insulin-positive area was derived from the transplanted donor embryonic pancreatic anlagen, with only a small contribution from areas of mixed donor and host tissue and less than 20% in host-derived, endogenous islets (Fig. 6). We cannot rule out the possibility that the transplanted cells have stimulated insulin production in the endogenous pancreatic cells. However, the fact that the insulin-positive areas are overwhelmingly of donor origin indicates that endogenous cells play a minor role in recovery from diabetes. Measurements of total β-cell mass from the three mice that maintained normal blood glucose levels (2.56 ± 0.04 mg) were comparable with the total β-cell mass in normal, nondiabetic mice (24).
Figure 6.
Semiquantitation of GFP-positive, insulin-expressing areas sampled throughout the entire pancreas in diabetic NOD mice that received the combination treatment and were unilaterally nephrectomized at 45 d. Stacked histograms showing the percentage of insulin-producing area derived from donor-derived tissue, host tissue, or areas of mixed host and graft cells. In all three animals, more than 75% of the insulin-positive area was donor derived, a small percentage was mixed donor and host, and less than 20% was host derived.
The presence of periinsulitis was examined by detecting the presence of CD3-positive T cells around the GFP-positive cell clusters as well as around the few remaining endogenous islets of the pancreas. Results show a range in the number of T cells around graft derived tissue, indicating that the transplanted insulin-producing cells and endogenous islets are not entirely free from periinsulitis (supplemental Fig. 4).
Discussion
In this study, we developed a combination immune modulation, transplantation treatment for T1D that results in long-term remission from the symptoms of diabetes as well as the restoration of metabolic control with normal glucose tolerance. We made use of the tolerance-inducing effects of anti-CD3 antibody to ameliorate the autoimmune destruction of endogenous β-cells (20,25) in combination with transplantation of embryonic pancreatic precursors as a source of β-cell progenitors to restore β-cell mass and insulin production. Making use of a GFP transgene, we were able to unequivocally identify engrafted cells and determine that they migrated to the endogenous pancreas and became established as insulin-producing cells. In the treated animals, progeny cells of the grafted tissue accounted for the vast majority of the insulin-producing tissue in the pancreas. How did this come about?
Control experiments demonstrated that short-term control of blood glucose in newly diagnosed diabetes, accomplished by the implantation of an insulin pellet, is essential for the recovery of the mice receiving anti-CD3 antibody and pancreatic anlagen transplants. This presumably allows sufficient time for the embryonic precursors to differentiate into insulin-producing cells and/or migrate to the host pancreas. Without the induction of immune tolerance, the embryonic pancreatic anlagen are unable to stop the progression of diabetes even with short-term insulin treatment. In this study, treatment with anti-CD3 antibody did not have a long-term effect on blood glucose levels in the absence of transplanted embryonic pancreatic precursors. This is a somewhat different result from other studies in which more long-term effects of antibody alone were observed. However, in the earlier studies, initial blood glucose levels at the time of treatment were much lower than in our study, representing an earlier stage of β-cell destruction (20,21,26). It is worth pointing out that mice receiving combination treatment in our study regained metabolic control, whereas in previous studies with anti-CD3 antibody alone, glucose tolerance was not normal (27).
The most intriguing aspect of this study is the fate of the transplanted tissue. By performing unilateral nephrectomy to remove the site of the graft at different times after treatment, we were able to show that the grafted tissue in the kidney is necessary for continued blood glucose control for up to 20 d, even though the insulin pellet is still in place, but after 45 d, animals survive without the graft site or the insulin pellet. This result suggests that insulin production from the grafted tissue is necessary initially for maintenance of normal blood glucose as the effectiveness of the insulin pellet declines but that at later time points, insulin production is restored elsewhere in the body, by either recovery of endogenous islets or migration of the grafted tissue. Unequivocal identification of progeny cells of the grafted embryonic pancreatic anlagen using the GFP marker and the colocalization of insulin in these cells in the endogenous pancreas shows that the cells have migrated to the pancreas. Examination of the graft site after its removal at 20, 45, or 120 d after treatment supports this conclusion by revealing insulin-producing graft-derived cells at the early time point that have largely disappeared at later time points. Why the grafted tissue is not maintained in the ectopic site and how it migrates to the endogenous pancreas is not known, although the tissue was grafted to the left kidney, which is in close proximity to the pancreas. We can speculate that the local microenvironment is important in allowing the survival of insulin-producing cells and that pancreatic precursor or stem cells find the pancreas a more permissive environment than the kidney capsule.
Our results differ from another study in which embryonic pancreatic anlagen were transplanted to the fold of the peritoneal mesentery in streptozotocin-treated rats and remained in that ectopic site for up to 15 wk, although there was no assessment of the endogenous pancreas in that study to determine whether any cells had migrated (9). Similarly, in transplantation studies using adult islets for short-term control of diabetes coupled with transplantation of splenocytes, no assessment was made regarding whether the cells migrated from the adult islets to the endogenous pancreas (10,11,12,13,14). Because of a lack of suitable markers, it is conceivable that some of the insulin-producing cells in the host pancreas were actually derived from the transplanted islets used for short-term blood glucose control. We have recently shown that adult β-cells promote the differentiation of β-cells from embryonic precursors in vitro (28), and the differential survival of insulin-producing precursors in the endogenous pancreas may be a reflection of this potential interaction between the transplanted precursor cells and the endogenous islets. Whatever the route of dispersal of cells from the graft site, through either the circulation or the peritoneal cavity, the only site at which GFP-positive cells were found was the pancreas, indicating either active homing or preferential survival in the pancreas environment.
These studies present a novel treatment paradigm combining the induction of immune tolerance with the restoration of β-cell function by the transplantation of pancreatic precursor cells. Although it is not envisioned that direct transplantation of fetal pancreatic anlagen would be a viable treatment in human T1D patients, this study points to the possibility of using pancreatic precursors for therapy. Recent reports on the differentiation of pancreatic endoderm from human embryonic stem cells, which can generate glucose-responsive insulin-secreting cells in vivo (7), and the reprogramming of adult pancreatic exocrine cells (29) are just two lines of research that point to possible sources of tissue for such treatment. Together these approaches could lead to new, long-term treatment for T1D.
Supplementary Material
Acknowledgments
We thank Dr. Christopher Wright (Vanderbilt University, Nashville, TN) for his generous gift of anti-Pdx1 antibody and Dr. Lori Sussel (Columbia University, New York, NY) for helpful criticism of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants RO1 DK068661 (to V.E.P.) and R01 DK068678 (to K.C.H.), Juvenile Diabetes Research Foundation Award 1-2007-234 (to K.C.H.), and a gift from the Russel Berrie Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 9, 2009
Abbreviations: E, Embryonic day; GFP, green fluorescent protein; H&E, hematoxylin and eosin; IPGTT, ip glucose tolerance test; MHC, major histocompatibility complex; NOD, nonobese diabetic; NOR, nonobese, diabetes-resistant; Pdx1, pancreatic duodenal homeobox-1; T1D, type 1 diabetes.
References
- von Herrath M 2005 Insulin trigger for diabetes. Nature 435:151–152 [DOI] [PubMed] [Google Scholar]
- Street CN, Sipione S, Helms L, Binette T, Rajotte RV, Bleackley RC, Korbutt GS 2004 Stem cell-based approaches to solving the problem of tissue supply for islet transplantation in type 1 diabetes. Int J Biochem Cell Biol 36:667–683 [DOI] [PubMed] [Google Scholar]
- Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA 2005 A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA 2002 Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346:1692–1698 [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L 2005 Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352:2598–2608 [DOI] [PubMed] [Google Scholar]
- Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, Paraskevas S, Eckman PM, Sageshima J, Nakano M, Sawada T, Matsumoto I, Zhang HJ, Sutherland DE, Bluestone JA 2004 Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 4:390–401 [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE 2008 Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452 [DOI] [PubMed] [Google Scholar]
- Wu F, Jagir M, Powell JS 2004 Long-term correction of hyperglycemia in diabetic mice after implantation of cultured human cells derived from fetal pancreas. Pancreas 29:e23–e29 [DOI] [PubMed] [Google Scholar]
- Rogers SA, Liapis H, Hammerman MR 2003 Intraperitoneal transplantation of pancreatic anlagen. ASAIO J 49:527–532 [DOI] [PubMed] [Google Scholar]
- Chong AS, Shen J, Tao J, Yin D, Kuznetsov A, Hara M, Philipson LH 2006 Reversal of diabetes in non-obese diabetic mice without spleen cell-derived β cell regeneration. Science 311:1774–1775 [DOI] [PubMed] [Google Scholar]
- Kodama S, Kühtreiber W, Fujimura S, Dale EA, Faustman DL 2003 Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science 302:1223–1227 [DOI] [PubMed] [Google Scholar]
- Nishio J, Gaglia JL, Turvey SE, Campbell C, Benoist C, Mathis D 2006 Islet recovery and reversal of murine type 1 diabetes in the absence of any infused spleen cell contribution. Science 311:1775–1778 [DOI] [PubMed] [Google Scholar]
- Ryu S, Kodama S, Ryu K, Schoenfeld DA, Faustman DL 2001 Reversal of established autoimmune diabetes by restoration of endogenous β cell function. J Clin Invest 108:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri A, Calderon B, Esparza TJ, Frederick K, Bittner P, Unanue ER 2006 Immunological reversal of autoimmune diabetes without hematopoietic replacement of β cells. Science 311:1778–1780 [DOI] [PubMed] [Google Scholar]
- Faustman DL, Tran SD, Kodama S, Lodde BM, Szalayova I, Key S, Toth ZE, Mezey E 2006 Comment on papers by Chong et al., Nishio et al., and Suri et al. on diabetes reversal in NOD mice. Science 314:1243 [DOI] [PubMed] [Google Scholar]
- Powell HC, Mizisin AP, Wiley CA, Morey MK, Hughes RA 1987 Relationship of adjuvants and swine influenza vaccine to experimental neuropathy in rabbits. Acta Neuropathol 73:12–18 [DOI] [PubMed] [Google Scholar]
- Theil DJ, Tsunoda I, Rodriguez F, Whitton JL, Fujinami RS 2001 Viruses can silently prime for and trigger central nervous system autoimmune disease. J Neurovirol 7:220–227 [DOI] [PubMed] [Google Scholar]
- Prochazka M, Serreze DV, Frankel WN, Leiter EH 1992 NOR/Lt mice: MHC-matched diabetes-resistant control strain for NOD mice. Diabetes 41:98–106 [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Papaioannou VE 2004 Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol 4:33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L, Thervet E, Primo J, Bach JF 1994 Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA 91:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC 2006 Effects of autoimmunity and immune therapy on β-cell turnover in type 1 diabetes. Diabetes 55:3238–3245 [DOI] [PubMed] [Google Scholar]
- Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debas HT 1996 Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development 122:439–447 [DOI] [PubMed] [Google Scholar]
- Hammerman MR 2004 Organogenesis of endocrine pancreas from transplanted embryonic anlagen. Transplant Immunol 12:249–258 [DOI] [PubMed] [Google Scholar]
- Yin D, Tao J, Lee DD, Shen J, Hara M, Lopez J, Kuznetsov A, Philipson LH, Chong AS 2006 Recovery of islet β-cell function in streptozotocin-induced diabetic mice: an indirect role for the spleen. Diabetes 55:3256–3263 [DOI] [PubMed] [Google Scholar]
- Bression D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M 2006 Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest 116:1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L, Primo J, Bach JF 1997 CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol 158:2947–2954 [PubMed] [Google Scholar]
- Ablamunits V, Sherry NA, Kushner JA, Herold KC 2007 Autoimmunity and β cell regeneration in mouse and human type 1 diabetes: the peace is not enough. Ann NY Acad Sci 1103:19–32 [DOI] [PubMed] [Google Scholar]
- Chen W, Begum S, Opare-Addo L, Garyu J, Gibson TF, Bothwell AL, Papaioannou VE, Herold KC 2009 Promotion of β-cell differentiation in pancreatic precursor cells by adult islet cells. Endocrinology 150:570–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA 2008 In vivo reprogramming of adult pancreatic exocrine cells to ß-cells. Nature 455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.