Abstract
Insulin receptor substrate-2 (Irs2) integrates insulin-like signals with glucose and cAMP agonists to regulate β-cell growth, function, and survival. This study investigated whether increased Irs2 concentration in β-cells could reduce β-cell destruction and the incidence of type 1 diabetes in nonobese diabetic (NOD) mice. NOD mice were intercrossed with C57BL/6 mice overexpressing Irs2 specifically in β-cells to create NODIrs2 mice. After backcrossing NODIrs2 mice for 12 generations, glucose homeostasis and diabetes incidence were compared against NOD littermates. Compared with 12-wk-old NOD mice, the progression of severe insulitis was reduced and islet mass was increased in NODIrs2 mice. Moreover, the risk of diabetes decreased 50% in NODIrs2 mice until the experiment was terminated at 40 wk of age. Nondiabetic NODIrs2 mice displayed better glucose tolerance than nondiabetic NOD mice throughout the duration of the study and up to the age of 18 months. The effect of Irs2 to increase islet mass and improve glucose tolerance raised the possibility that NODIrs2 mice might have an increased capacity to respond to anti-CD3 antibody, which can induce remission of overt diabetes in some NOD mice. Anti-CD3 antibody injections restored glucose tolerance in newly diabetic NOD and NODIrs2 mice; however, anti-CD3-treated NODIrs2 mice were less likely than NOD mice to relapse during the experimental period because they displayed 10-fold greater β-cell mass and mitogenesis. In conclusion, increased Irs2 attenuated the progression of β-cell destruction, promoted β-cell mitogenesis, and reduced diabetes incidence in NODIrs2 mice.
Increasing expression of Irs2 in the pancreas attenuates the progression of β-celdestruction, promotes β-cell mitogenesis, and reduces diabetes incidence in NODIrs2-mice.
Diabetes mellitus is a complex disorder that arises from various causes, including dysregulated glucose sensing and impaired insulin secretion (maturity-onset diabetes of the young); autoimmune-mediated β-cell destruction (type 1); or insufficient β-cell insulin secretory capacity to compensate for peripheral insulin resistance (type 2) (1). Regardless of the underlying etiology, dysregulated insulin signaling exacerbated by chronic hyperglycemia promotes a cohort of acute and chronic sequela (2,3). Type 1 diabetes is an autoimmune disease caused by a dysregulated immune system that produces circulating autoantibodies against proteins expressed by pancreatic β-cells (4,5). Insulin is thought to be a principal autoantigen in the pathogenesis of type 1 diabetes in nonobese diabetic (NOD) mice and possibly humans (6,7). Type 1 diabetes progresses toward life-threatening hyperglycemia after infiltration of islets by leukocytes that eventually destroy most of the β-cells (5). Less than 1% of islet β-cell mass remains in most humans with type 1 diabetes (8). Because new β-cell formation occurs slowly during disease progression, it might be possible to retard the progression of or even cure the disease by accelerating the rate of β-cell regeneration (9).
Much of our information on the etiology of type 1 diabetes comes from analysis of inbred NOD mice or BioBreeding (BB) rats that spontaneously develop the disease (10). Between 4 and 12 wk of age, leukocytes surround pancreatic islets (insulitis) of NOD mice and destroy the β-cells between 13 and 40 wk of age (4). Life-threatening hyperglycemia and ketoacidosis occurs after more than 80% of the β-cell mass is destroyed in 60–80% of female and 20–30% of male NOD mice (4). Strategies to reduce the loss of β-cells or increase β-cell regeneration to offset the autoimmune destruction are difficult to establish once severe hyperglycemia develops (9,11). β-Cell replication increases during the progression of insulitis but is usually insufficient to maintain glucose tolerance (12,13,14). Nonetheless, NOD mice can recover from type 1 diabetes when immunosuppression is initiated at the onset of mild hyperglycemia (15,16,17).
The attenuation of chronic autoimmune destruction of islets is critical for sustained recovery; however, understanding the molecular basis of β-cell regeneration, whether through neogenesis from progenitors or replication of viable β-cells, appears to be essential for the cure type 1 diabetes (11). Multiple signaling cascades and nuclear regulatory factors coordinate β-cell differentiation, growth, and survival (18). Circulating glucose concentration is an important regulator of β-cell mass because it promotes an increase in the number of β-cells until sufficient insulin is secreted to restore the circulating glucose to a normal concentration (19,20,21). In β-cells, glucose metabolism stimulates Ca2+ and cAMP signaling cascades that have many effects on β-cells, including the acute secretion of insulin and the increased expression of insulin receptor substrate (Irs) (22).
Most, if not all, insulin signals are produced or modulated through tyrosine phosphorylation of Irs1 or Irs2. Irs2 is especially important because it promotes β-cell growth, function, and survival (23). The deletion of Irs2 in mouse β-cells completely blocks the effect of glucose to stimulate β-cell growth (24). Moreover, the growth-promoting effects of stable glucagon-like pepetide-1 receptor agonists that activate cAMP signaling fail to promote β-cell growth in the absence of Irs2 (25). Thus, Irs2 links cAMP agonists and the circulating glucose concentration to the growth-promoting action of the insulin-like signaling cascade (22,26). Mice expressing transgenic Irs2 only in β-cells are resistant to apoptosis after low-dose streptozotocin treatment that induces acute insulitis (27). Because Irs2 signaling is essential for β-cell growth, we generated NODIrs2 mice to investigate whether overexpression of Irs2 in β-cells could attenuate the progression of type 1 diabetes and promote β-cell growth in NOD mice challenged by chronic insulitis.
Materials and Methods
Generation and validation of congenic NODIrs2 mice
Irs2tg mice overexpress a flag-tagged Irs2 transgene specifically in β-cells under control of the rat insulin II promoter (RIPIrs2) (27). Irs2tg mice were backcrossed to NOD mice for 12 generations. NODIrs2 mice were verified as a congenic NOD strain through single nucleotide polymorphism (SNP) analysis (Jackson Laboratory, Bar Harbor, ME). Genotyping was previously described for Irs2tg mice (27).
Metabolic studies and mouse diet
Mice were fed ad libitum with standard chow [21.6% kcal from fat; PicoLab Mouse Diet 5058 (Research Diets Inc., New Brunswick, NJ)] and maintained under a 12-h light, 12-h dark cycle. All procedures were performed with female mice in accordance with the policies of the Institutional Animal Care and Use Committee of the Harvard School of Public Health (Boston, MA). Blood glucose levels were measured from tail bleeds between 0900 and 1100 h using an Ascencia glucometer elite (Elite XL; Bayer, Elkhart, IN). Diabetes was confirmed by 2 consecutive weeks of blood glucose concentrations greater than 200 mg/dl, with the first week of hyperglycemia considered as the age of disease onset. Onset data were analyzed in life tables and Kaplan-Meier survival curves, with relative risk assessed using Cox regression (version 16; SPSS, Chicago, IL). Serum insulin levels were measured by competitive rat ELISA (CrystalChem Inc., Chicago, IL). Glucose tolerance tests (GTTs) followed an overnight 16-h fast. Mice were injected ip with d-glucose (2 g/kg), and blood glucose concentrations were measured at indicated time points. Glucose-stimulated insulin release was measured from serum samples during the GTTs. For insulin tolerance tests, mice were fasted for a 4-h period in the light cycle before ip injections of insulin (Humulin R, 0.8 U/kg; Eli Lilly and Co., Indianapolis, IN) diluted in sterile saline. Blood glucose concentrations were measured at indicated time points. The homeostasis model assessment (HOMA) version 2.2 calculator (http//www.dtu.ox.ac.uk/homa) was used to estimate insulin sensitivity. For anti-CD3 treatment, mice were injected ip with 10 μg of anti-CD3 antibody on the day hyperglycemia was detected (d 1) and for 4 subsequent days.
Semiquantitative real-time RT-PCR and immunoblotting
Islets were isolated from nondiabetic NODIrs2 and NOD mice by intraductal collagenase digestion (28). After islet isolation, RNA was extracted (TRIZOL; Invitrogen, Carlsbad, CA) and purified (RNeasy; QIAGEN, Valencia, CA), cDNA was synthesized (RETROscript; Ambion Inc., Austin, TX), and semiquantitative real-time RT-PCR was performed (iQ SYBRGreen; Bio-Rad, Hercules, CA). For each reaction, the equivalent of 12.5 ng of purified islet RNA was used, with an annealing temperature of 60 C, and a 15-sec extension/data collection period. Primer sets were as follows: Irs2 forward, GTCCAGGCACTGGAGCTTT, Irs2 reverse, GCTGGTAGCGCTTCACTCTT; RIPIrs2 transgene forward, CGCCACCCATGACTACAAAG, RIPIrs2transgene reverse, CACGCTGTGGTTGTTGTTGT; and β-actin forward, CCCTAAGGCCAACCGTGAA, β-actin reverse, CAGCCTGGATGGCTACGTACA.
For relative quantitation, a pooled reference sample of cDNAs was used to construct a standard curve for each primer set. Using standard curves, the starting quantity (Sq) of each sample was calculated and normalized to the β-actin Sq. For immunoblotting, islet protein lysates were run on SDS-PAGE, transferred to nitrocellulose membrane (Bio-Rad), and blotted with rabbit anti-Irs2 (Upstate, Lake Placid, NY), rabbit anti-α-actinin (Santa Cruz Biotechnology, Santa Cruz, CA), or rabbit anti-β-actin (Cell Signaling, Beverly, MA). Densitometry was performed using Imagequant (Amersham Biosciences, Piscataway, NJ) on scanned films. Expression levels are normalized to α-actinin or β-actin as internal standards. The mean normalized Irs2 protein concentration from NODIrs2 mice at 1 month of age was set as 100% for each blot, and values were corrected by this mean to enable the pooling of densitometry data from multiple blots.
Histological analysis
Mice were injected with the thymidine analog 5-bromo-2′-deoxyuridine (BrdU) (0.1 mg/g body weight) 6 h before the animals were killed. Pancreata were harvested and fixed with 4% paraformaldehyde overnight at 4 C. Paraffin embedding and sectioning (5 μm) was done at the Joslin Histology Core (Joslin Diabetes Center, Boston, MA). To assess insulitis, islets from hematoxylin/eosin sections were scored using standard methods and placed into one of four categories: 1) no insulitis; 2) periinsulitis (leukocytes in the periphery of the islet); 3) invasive insulitis (25–50% coverage of the islet); and 4) severe insulitis (>50% infiltration) (29). For islet morphometrics and β-cell mitogenesis, pancreas sections were rehydrated using xylene and decreasing concentrations of EtOH, permeabilized using 0.2% Triton X-100/PBS, and antigen unmasking was performed in a boiling solution of 0.01 m sodium citrate (pH 6.0) for 20 min. Sections were blocked with normal goat serum for 1 h and incubated overnight (4 C) with primary antisera at the following concentrations: guinea pig antiinsulin (1:100; Zymed Laboratories, San Francisco, CA) and rat anti-BrdU (1:250, BU1/75 ICR1; Abcam, Cambridge, MA). Alexa-Flour (Molecular Probes, Eugene, OR) secondary antibodies were used (1:200). Mounting media included 4′,6′-diamino-2-phenylindole (DAPI) to stain nuclei (ProLong Gold; Molecular Probes). Islet area, islet density, and islet size were quantified by acquiring adjacent, nonoverlapping images of the pancreas area with a Axiovert microscope (Zeiss, New York, NY). Images were analyzed using Open Lab software (Improvision, Coventry, UK). Islets (50–100 per mouse) were scored for the presence of infiltration and double-labeled insulin/BrdU cells to assess mitogenesis.
Statistical analysis
The incidence of diabetes in NOD and NODIrs2 mice was determined from life table analysis using 2-wk time intervals. Time to diabetes was determined by Kaplan-Meier analysis, and the risk of diabetes was determined by Cox proportional regression (SPSS, version 16). The risk of hyperglycemia in diabetic NOD and NODIrs2 mice treated with anti-CD3 antibody was determined by multinomial logistic regression using Irs2 expression as a predictor and the glucose concentration at diagnosis (quartile) as a covariant (SPSS, version 16).
Results
Irs2 expression in NODIrs2 mice
We previously described Irs2tg mice on a C57BL/6 genetic background, which overexpress a flag-tagged RIPIrs2 specifically in pancreatic β-cells under the control of the RIPIrs2 (27). Irs2tg mice were intercrossed with NOD mice for 12 generations to generate NOD mice that express the RIPIrs2 transgene in β-cells (NODIrs2 mice). NODIrs2 mice were verified as a congenic NOD strain through SNP analysis of 150 characteristic markers (Jackson Laboratories). In the 11th generation of the backcross, NODIrs2 mice displayed 96.7–99.3% of the NOD-specific SNPs, including all 24 SNPs associated with insulin-dependent diabetes (data not shown). NODIrs2 mice were maintained as heterozygotes to reduce the potential for phenotypic problems related to the transgene insertion site as well as facilitate comparison of littermates that carry the transgene (NODIrs2 mice) against those that do not (NOD mice).
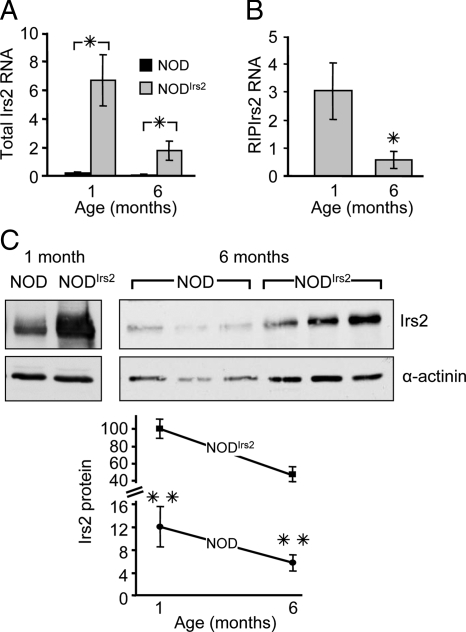
To establish whether transgenic Irs2 was expressed in β-cells, islets were isolated from nondiabetic NODIrs2 or NOD mice at 1 month and 6 months of age. RNA was purified from the islets, and PCR primers specific for both endogenous and transgenic Irs2 (total Irs2) or only transgenic Irs2 were used to quantify expression. Compared with NOD islets, the concentration of total Irs2 RNA in NODIrs2 islets was 25-fold greater at 1 month (NOD 0.26 ± 0.04; NODIrs2 6.68 ± 1.79) and 16-fold greater at 6 months of age (NOD 0.11 ± 0.02; NODIrs2 1.78 ± 0.64) (Fig. 1A). Primers specific for transgenic Irs2 revealed a 5-fold decrease in transgenic Irs2 expression between 1 and 6 months of age (3.04 ± 1.0 and 0.58 ± 0.31, respectively) (Fig. 1B).
Figure 1.
Characterization of Irs2 overexpression in NODIrs2 mice. A and B, Irs2 gene expression in isolated islets from 1- and 6-month-old NOD and NODIrs2 mice was analyzed by semiquantitative real-time RT-PCR. Results from each primer set are normalized to β-actin and expressed as the mean Sq ± sem (n = 4–5/group). *, P < 0.05. C, Irs2 protein concentration was assessed in isolated islet lysates by immunoblotting with Irs2 antibody. Expression is normalized to α-actinin as an internal standard. Representative Irs2 and α-actinin immunoblots are shown. The mean normalized Irs2 protein concentration from NODIrs2 mice at 1 month of age was set as 100% for each blot, and values were corrected by this mean to enable the pooling of densitometry data from multiple blots (n = 8). **, P < 0.01 (comparison of NOD to NODIrs2).
Irs2 protein concentrations were assessed by immunoblotting islet extracts with specific Irs2 antibodies. At 1 month of age, the Irs2 protein concentration was 8-fold greater in NODIrs2 islets than NOD-islets (NODIrs2: 100 ± 11.6%; NOD: 12.1 ± 3.6%) (Fig. 1C). By 6 months, the Irs2 concentration decreased about 50%, but NODIrs2 mice maintained an 8-fold higher concentration of Irs2 compared with NOD mice (NODIrs2 47.2 ± 9.4%; NOD 5.8 ± 1.5%) (Fig. 1C).
The progression of immune infiltration in NODIrs and NOD mice
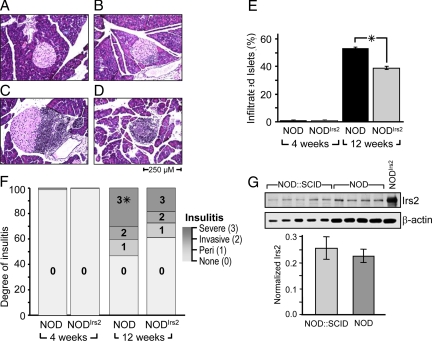
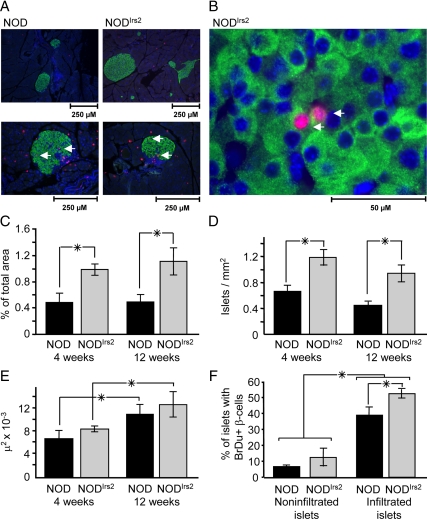
Insulitis begins shortly after weaning in the pancreas of all NOD mice (4). The pancreas was isolated from 4- or 12-wk-old NODIrs2 and NOD mice to determine whether Irs2 overexpression in β-cells changed immune cell infiltration. Fixed and paraffin-embedded sections were stained with hematoxylin/eosin and islets were sorted into four categories based on the relative degree of immune infiltration as previously described (29): no insulitis; periinsulitis; invasive insulitis; or severe insulitis (Fig. 2, A–D). Insulitis was rarely detected in 4-wk-old mice but readily detected in 12-wk-old NOD and NODIrs2 mice (Fig. 2E). By 12 wk, periinsulitis and invasive insulitis increased equally in NOD and NODIrs2 mice (Fig. 2F). However, at 12 wk more islets in the NOD mice displayed severe insulitis compared with NODIrs2 mice [odds ratio 1.86, 95% confidence interval (CI) 1.49–2.18, P < 0.001]. Thus, Irs2 did not prevent insulitis in NODIrs2 mice but reduced the overall percentage of islets that displayed the most severe form of insulitis (Fig. 2F).
Figure 2.
Immune infiltration in NOD and NODIrs2 mice. A–D, Sections (5 μm) of pancreas from 4- and 12-wk-old NOD and NODIrs2 mice were formaldehyde fixed, paraffin embedded, and stained with hematoxylin/eosin (n = 7 mice/group). Islets were sorted into four categories based on the relative degree of immune infiltration: no insulitis (A), periinsulitis (B), invasive insulitis (C) or severe insulitis (D). E, The percentage of islets showing any degree of infiltration is reported as estimated marginal means (percent ± se) determined by binary logistic regression (SPSS; version 16.0). *, P = 0.016. F, Islets were characterized by the relative degree of leukocyte infiltration: 0, no insulitis; 1, periinsulitis, in which leukocytes remained largely in the periphery of the islet; 2, invasive insulitis, corresponding to 25–50% coverage of the islet; and 3, severe insulitis, in which greater than 50% of the islet was covered with infiltrate. The significance of the distribution was determined by multivariate regression in SPSS (version 16.0). Severe insulitis was significantly greater in NOD mice (*, odds ratio 1.86, 95% CI 1.49–2.18, P < 0.001.) G, Irs2 protein concentrations in isolated islet lysates from NOD and NOD-SCID mice. Immunoblots were probed with Irs2 antibody and normalized to β-actin as an internal standard.
To investigate whether insulitis suppressed Irs2 protein concentration, islets were isolated from nondiabetic NOD mice and compared against islets from NOD::severe combined immunodeficiency (SCID) mice that lack T cell infiltration (30,31,32). At 6 months of age, there was no significant difference in the concentration of Irs2 between these groups of mice (Fig. 2G). Thus, leukocyte infiltration and the associated proinflammatory cytokines did not reduce the concentration of Irs2 protein in islets.
The effect of Irs2 on the incidence of diabetes
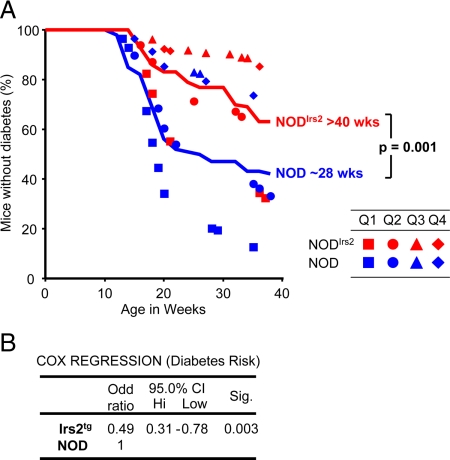
To investigate the effect of Irs2 on the incidence of diabetes, nonfasted blood glucose concentrations were measured weekly in NODIrs2 and NOD mice up to 40 wk of age. Diabetes was diagnosed by 2 consecutive weeks of blood glucose concentrations greater than 200 mg/dl with the first week of hyperglycemia considered as the age of disease onset. The mean nonfasted blood glucose concentrations in nondiabetic NOD and NODIrs2 mice were 108 ± 32 and 112 ± 25 mg/dl, respectively. Blood glucose concentrations greater than 200 mg/dl always indicated the onset of diabetes and a spontaneous reversal to less than 200 mg/dl was never observed in our colony without intervention (n = 72). Assuming that all the mice entering this study at different times behaved similarly, a life table was constructed to compare the occurrence of diabetes in NOD and NODIrs2 mice. By 40 wk of age, 36% of NODIrs2 mice and 58% of NOD mice developed diabetes, which was significantly different by the Wilcoxon test (P = 0.001) (Fig. 3). Kaplan-Meier analysis revealed that the average onset of diabetes occurred at 28 wk (95% CI 26.6–31.5) for NOD mice and longer than 40 wk for NODIrs2 mice. Finally, we used Cox regression to verify that Irs2 reduced the incidence of diabetes in NODIrs2 mice, regardless of when during the year the mice entered the study (33). Although the date of entry into the study had a significant effect on the incidence of diabetes in the colony (P < 0.001), Cox regression confirmed that the odds of developing diabetes decreased 2-fold in NODIrs2 mice (odds ratio 0.49, 95% CI 0.31–0.78, P = 0.003) compared with NOD mice at all time points (Fig. 3B). Thus, increased Irs2 in β-cells reduced the incidence of diabetes in NODIrs2 mice.
Figure 3.
The effect of transgenic Irs2 on development of type 1 diabetes. A, Blood glucose concentrations in NOD and NODIrs2 mice were monitored weekly until 40 wk of age. Sustained hyperglycemia for 2 consecutive weeks (>200 mg/dl) marked the onset of disease, which was used to create a life table to determine the incidence of diabetes (SPSS; version 16.0). Solid lines, red, NODIrs2 mice; blue, NOD mice. The median age of diabetes onset in NOD mice was 28 wk (n = 49), with an overall incidence of 58%. By 40 wk of age, 37% of the NODIrs2 mice (n = 58) developed diabetes. The difference between NOD and NODIrs2 mice was significant by the Wilcoxon (Gehan) statistic (P = 0.001). B, The results in A were analyzed by Cox regression (SPSS; version 16.0) using transgenic Irs2 and the birth date quartile (Q1, solid squares, March 25, 2005, to December 1, 2005; Q2, solid circles, December 3, 2005, to March 8, 2006; Q3, solid triangles, March 8, 2006, to June 7, 2006; Q4, solid diamonds, June 8, 2006, to August 4, 2006) as predictor variables. The incidence of diabetes for each mouse in each quartile is plotted in A.
Glucose metabolism in NOD and NODIrs2 mice
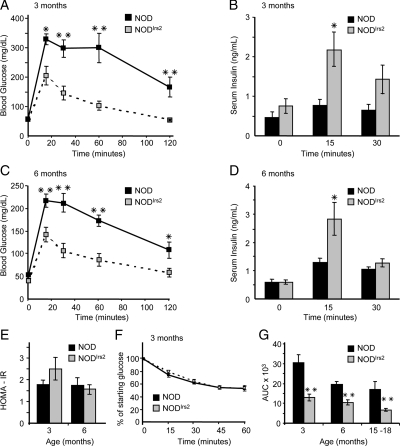
We restricted our attention to nondiabetic mice to establish the effect of increased β-cell Irs2 concentration on glucose homeostasis. Fasting blood glucose was indistinguishable in NOD and NODIrs2 mice; however, during the ip glucose tolerance test, young and old NODIrs2 mice displayed significantly lower blood glucose concentrations compared with NOD mice (Fig. 4, A and C). The greater glucose tolerance in NODIrs2 mice could be attributed, at least in part, to greater glucose-induced insulin secretion (Fig. 4, B and D). Moreover, the role of insulin secretion was validated by the equal insulin sensitivity of NODIrs2 and NOD mice estimated by the HOMA2 approximation and insulin tolerance tests (Fig. 4, E and F). Enhanced glucose tolerance of nondiabetic NODIrs2 mice was maintained in mice aged 15–18 months (Fig. 4G).
Figure 4.
Glucose sensitivity and insulin secretion. A and C, Glucose tolerance test after a 16-h fast at 3 and 6 months of age in NODIrs2 and NOD mice (n = 4–7 mice/group). B and D, Serum insulin concentrations during the GTT were determined by ELISA at indicated time points. E, Estimation of insulin resistance by the HOMA2 approximation. F, Insulin tolerance tests. G, Area under the curve for GTT at 3, 6, and 15–18 months of age. *, P < 0.05; **, P < 0.01. Statistical significance was determined using unpaired Student t tests.
The effect of Irs2 on β-cell mass and mitogenesis
To determine the effect of Irs2 on islet mass, the pancreas was isolated from 4- or 12-wk-old nondiabetic NOD and NODIrs2 mice. At both ages, the β-cell area estimated from multiple 5-μm pancreas sections was 2-fold greater in NODIrs2 mice compared with NOD mice (Fig. 5, A and C). This increase in NODIrs2 mice was largely due to more islets in the NODIrs2 pancreas (Fig. 5D). Although islet size increased between 4 and 12 wk of age, Irs2 had no significant effect on this parameter (Fig. 5E). Thus, Irs2 increased β-cell mass by increasing the number of islets without an effect on islet size.
Figure 5.
β-Cell mass and mitogenesis in NOD and NODIrs2 mice. Pancreas sections were formaldehyde fixed, paraffin embedded, and stained for with antibodies against insulin (green) and BrdU incorporation (red). Nuclei were labeled with DAPI (blue). A, Representative sections from 8-wk-old mice showing replicating β-cells double labeled with insulin and BrdU. B, High-magnification image demonstrating BrdU staining in the nuclei of insulin-positive β-cells. C, The percentage of islet area per pancreas. D, The number of islets per square millimeter of pancreas area. E, Mean islet size. F, The percentage of the infiltrated and noninfiltrated islets containing replicating β-cells (n = 6 mice/group). All results are expressed as the mean ± sem (n = 5–7 mice/group). *, P < 0.05.
Next, β-cell mitogenesis was determined in pancreas sections from 8-wk-old NODIrs2 and NOD mice. This age was selected because both leukocyte-infiltrated and noninfiltrated islets could be evaluated within the same pancreas sections. Islets were categorized for infiltration, and the rate of β-cell replication was determined by the percentage of islets containing cells that were colabeled with insulin and BrdU (Fig. 5B). Infiltrated islets displayed significantly greater β-cell replication than noninfiltrated islets in both genotypes (Fig. 5F). Moreover, leukocyte infiltration was associated with a slight but significant increase in β-cell mitogenesis in NODIrs2 islets (1.3-fold, P < 0.05) compared with infiltrated NOD islets.
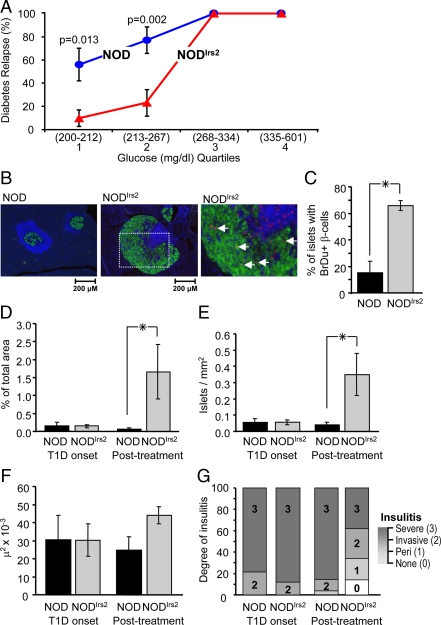
Anti-CD3 therapy of NODIrs2 and NOD mice
Anti-CD3 antibody can induce the remission of overt diabetes in some NOD mice, at least in part, by suppressing the immunological stress on β-cells (15). The effect of Irs2 to increase β-cell mass/mitogenesis and improve glucose tolerance raised the possibility that NODIrs2 mice might have an increased capacity to respond to anti-CD3 antibody. To test this hypothesis, NODIrs2 and NOD mice were monitored weekly for the appearance of diabetes (glucose >200 mg/dl). Diabetic NOD (n = 11) and NODIrs2 mice (n = 11) received five consecutive daily ip injections of anti-CD3 antibody (10 μg/d) (34). The blood glucose concentration of each mouse was followed up for 16 d after the fifth injection, and an average concentration greater than 200 mg/dl during this interval was used to diagnose the recurrence of diabetes. By these criteria, five NODIrs2 mice and two NOD mice remained nondiabetic, whereas diabetes recurred in the other mice. The recurrence of diabetes after anti-CD3 therapy is related to the glucose concentration when diabetes was initially diagnosed (34). To establish whether Irs2 had a significant effect, the recurrence of diabetes was analyzed by logistic regression using the diagnostic glucose concentration quartile and the presence of transgenic Irs2 as predictors (Fig. 6A). When the glucose concentration was between 200 and 267 mg/dl at diagnosis, Irs2 significantly reduced the recurrence of diabetes (Fig. 6A). However, Irs2 had no effect when the glucose concentration was above 270 mg/dl at the initial diagnosis of diabetes. At diagnosis, the β-cell mass was very low in all of the mice; however, 16 d after anti-CD3 treatment, β-cell mass increased 10-fold in NODIrs2 mice, whereas β-cell mass was not increased in NOD mice (Fig. 6D). As observed in nondiabetic NODIrs2 mice, these results were largely due to an increase in islet number (Fig. 6E) and not an increase in islet size (Fig. 6F). Consistent with these results, β-cell mitogenesis was significantly greater in NODIrs2 islets compared with NOD islets (Fig. 6C). Moreover, the severity of immune cell infiltration in the pancreas of NODIrs2 mice at this time point (16 d after anti-CD3 treatment) was significantly reduced compared with treated NOD mice (Fig. 6G).
Figure 6.
Anti-CD3 treatment of diabetic NOD (n = 11) and NODIrs2 mice (n = 11). Recent-onset diabetic mice (glucose >200 mg/dl) were injected daily with anti-CD3 antibody (10 μg/d) for 5 d. Remission of diabetes was defined as an average blood glucose concentration less than 200 mg/dl for 16 d after the last anti-CD3 antibody injection. A, The predicted fraction of NOD and NODIrs2 mice without diabetes was determined by binary logistic regression using the initial diagnostic glucose quartile and the presence or absence of transgenic Irs2 as covariates. The likelihood ratio test confirmed that the diagnostic glucose quartile and transgenic Irs2 expression were significant determinants for the recurrence of diabetes 16 d after the last injection of anti-CD3 antibody (glucose quartile, P < 0.0003; Irs2, P = 0.014). The estimated marginal means ± se were determined using SPSS (version 16.0). B, Representative images of pancreas sections from NODIrs2 and NOD mice that remained nondiabetic 16 d after the last anti-CD3 injection. The pancreas sections (5 μm) were formaldehyde fixed, paraffin embedded, and immunostained for insulin (green) and BrdU incorporation (red); nuclei were labeled with DAPI (blue). A higher-magnification image demonstrating BrdU staining in the nuclei of insulin-positive β-cells is shown for NODIrs2. C, Pancreas sections from NOD and NODIrs2 mice after anti-CD3 treatment were scored for the presence of replicating β-cells (double labeled insulin positive/BrdU positive cells). Results are expressed as the percentage of the total islets containing replicating β-cells. *, P < 0.01. D, Average percentage islet area in NODIrs2 and NOD mice. Type 1 diabetes-onset (T1D onset) mice are mice with recent onset of hyperglycemia. Posttreatment mice are animals with glucose less than 250 mg/dl 16 d after the last injection of anti-CD3 antibody. *, P < 0.01. E, The number of islets per square millimeter of pancreas area. F, Mean islet size. G, Islets from the same sections were also scored for the relative degree of leukocyte infiltration: 0, no insulitis; 1, periinsulitis (Peri), in which leukocytes remained largely in the periphery of the islet; 2, invasive insulitis, corresponding to 25–50% coverage of the islet; and 3, severe insulitis, in which greater than 50% of the islet was covered with infiltrate. Results are expressed as the percentage of the total islets in each group.
Discussion
Our results show that increased expression of Irs2 in β-cells of NOD mice can postpone the onset of hyperglycemia and reduce the number of mice that develop diabetes. Irs2 does not prevent immune cell infiltration into pancreatic islets; however, by 12 wk of age, the average number of islets displaying the most severe infiltration is reduced significantly in NODIrs2 mice. In NOD and NODIrs2 mice, average islet size is indistinguishable and increases equally between 4 and 12 wk of age. However, total β-cell mass is greater by 2-fold in the pancreas of NODIrs2 mice compared with NOD mice, owing to an increased number of islets. Transgenic Irs2 has a similar effect on islet density in C57BL/6 mice (27). In addition, during the progression of islet infiltration, Irs2 promotes β-cell mitogenesis above the normal response associated with autoimmunity. Together, these effects of Irs2 are associated with a reduced the incidence of diabetes in NODIrs2 mice.
Signaling cascades coordinated by Irs2 are essential for the regulation of β-cell growth, function, and survival (35). The complete deletion of Irs2 in mice causes peripheral insulin resistance and glucose intolerance; however, fatal diabetes starts to develop between 10 and 15 wk of age owing to a near complete loss of pancreatic β-cells (36). Moreover, mice lacking Irs2 in β-cells develop glucose intolerance at 2 months of age and progress to diabetes with reduced β-cell mass by 4 months age; however, diabetes resolves spontaneously between 6 and 8 months owing to the complete repopulation of the pancreas with functional β-cells expressing Irs2 (24). Although the exact source of cells and the regulatory mechanisms controlling β-cell regeneration are unknown in these mice, the results show that Irs2 signaling cascades are absolutely required for the process.
Many experiments suggest that Irs2 is a signaling gatekeeper that integrates β-cell plasticity and function with the systemic requirement for insulin-regulated glucose homeostasis. Irs2 is required for a compensatory increase in β-cell mass in response to chronic nutrient excess due to high-fat diet feeding (22). This mechanism depends on the phosphorylation of glucose by glucokinase to set in motion a cascade of cAMP and Ca2+ signaling that up-regulates Irs2 (22,26). Irs2 is also a principal activator of the phosphatidylinositol 3-kinase (PI3K)→AKT cascade in β-cells, playing a key role to inactivate Forkhead box O1 (Foxo1), glycogen synthase kinase (GSK)-3β, and p27kip. Inactivation of Foxo1 increases nuclear pancreatic duodenal homeobox-1 (Pdx-1), which inhibits apoptosis and promotes β-cell function and insulin secretion (27,35,37). Because GSK3β stabilizes p27kip1, genetically reducing GSK3β or p27kip1 promotes β-cell expansion (38,39). Other canonical components of the Irs2→PI3K →AKT cascade also modulate β-cell function, growth, and survival, including protein tyrosine phosphatase (PTP)-1b, phosphatase and tensin homolog deleted from chromosome 10 (PTEN), cyc lins, and cyclin-dependent kinase (CDK)-4 (40,41,42,43). Together, these Irs2-dependent pathways in β-cells regulate the expansion of β-cell mass required to maintain euglycemia, especially during metabolic stress.
Both type 1 and type 2 diabetes are characterized by relatively insufficient β-cell mass to maintain glucose homeostasis: too little β-cell mass expansion to compensate for peripheral insulin resistance in type 2 diabetes or an inability to maintain or restore β-cell mass in type 1 diabetes owing to destructive autoimmunity. The prevailing view posits that the pancreas of type 1 diabetics completely lacks functional β-cell mass (11,44). Whether regeneration of β-cell mass is possible in type 1 diabetics is difficult to establish. The extent of β-cell growth in humans is significantly less than what is observed in rodent models and compared with children β-cell replication diminishes significantly in adults (45,46). However, recent work with cadaver donors suggests that β-cell regeneration can be detected years after the initial diagnosis of type 1 diabetes (9). In addition, inflammatory infiltrate can induce β-cell replication in rodent models (13). In NODIrs2 mice, an enhanced response to infiltration-induced β-cell replication was observed compared with NOD mice. Thus, modulating Irs2 might establish a favorable balance between β-cell destruction and regeneration that is essential to treat type 1 diabetes. Consistent with this, although peri- and invasive insulitis were unaffected by Irs2 overexpression, the transition to severe insulitis, in which β-cell destruction overtakes regeneration, was significantly reduced.
The deficient β-cell mass in humans and NOD mice with type 1 diabetes is thought to be caused by autoimmune-mediated β-cell apoptosis (11,44,47). Suppression of insulitis is therefore an obvious strategy to correct the underlying cause of β-cell destruction. More than 100 immune interventions have been described that can prevent the onset of diabetes in NOD mice. Few of these approaches have been explored in human trials (48,49). However, anti-CD3 antibody has been used successfully in both diabetic NOD mice and humans. Experiments with NOD mice reveal a complex relationship between the severity of diabetes, the timing of treatment, and the dose of antibody (48,50,51). Successful therapy with anti-CD3 antibody appears to be limited to recent onset diabetes before hyperglycemia exceeds 350 mg/dl (49). Apparently, sufficient functional β-cell mass is required to mediate biologically relevant regeneration (48,49).
Our results suggest that Irs2 signaling promotes β-cell regeneration during suppression of autoimmune destruction with anti-CD3 antibody. Although our experiments were limited to 11 NOD and 11 NODIrs2 mice, 5 d of anti-CD3 antibody treatment was more effective in curing hyperglycemia in diabetic NODIrs2 mice (glucose between 200 and 267 mg/dl) than in NOD mice. However, transgenic Irs2 had no effect on the recurrence of diabetes when the anti-CD3 antibody treatment was initiated in severely diabetic mice (glucose >270 mg/dl), suggesting that perhaps a longer treatment period is needed for more severe diabetes. The mechanism by which Irs2 prevents diabetes in antibody-treated mice involves the promotion of islet regeneration. Anti-CD3 antibody clears immune cells from pancreatic islets, but insulitis recurs about 10 d after the treatment ends (15,52). The rate of mitogenesis in NOD mice after anti-CD3 treatment was significantly lower than what was observed in nondiabetic NOD mice. Others have also reported a resistance to regaining the immune-mediated increase in β-cell replication after anti-CD3 treatment (13). By contrast, diabetic NODIrs2 mice treated and cured for at least 2 wk with anti-CD3 antibody display significantly more β-cell mitogenesis than NOD mice. In addition, NODIrs2 mice display 5-fold more β-cell mass, which is indistinguishable from the β-cell mass of 3-month-old nondiabetic NODIrs2 mice. Because a difference in islet mass was not observed between NODIrs2 and NOD mice at the onset of hyperglycemia, these results suggest that the increased rate of mitogenesis in β-cells of NODIrs2 mice contributes to the successful treatment of early onset type 1 diabetes.
In summary, Irs2 signaling postpones and reduces the onset of hyperglycemia in NOD mice. Moreover, Irs2 increases the efficacy of anti-CD3 treatment in newly diagnosed and mildly diabetic NODIrs2 mice. We posit that these outcomes arise from the effect of Irs2 on β-cell mitogenesis that increases the number of islets in the pancreas during immune cell infiltration. Strategies to augment the activity of the Irs2 signaling cascade in β-cells might be a rational approach to attenuate β-cell destruction and prevent the progression of newly diagnosed type 1 diabetes.
Footnotes
This work was supported by Grant R01-DK55326 from the National Institutes of Health and Grant 1-2005-937 from the Juvenile Diabetes Research Foundation.
Disclosure Summary: L.D.N., K.E.D., L.M.O.-A., A.K., M.H., and M.F.W. have nothing to declare. J.A.B. holds a patent on anti-CD3 and is a stockholder of Macrogenetics.
First Published Online July 2, 2009
Abbreviations: BrdU, 5-Bromo-2′-deoxyuridine; CI, confidence interval; DAPI, 4′,6′-diamino-2-phenylindole; GSK, glycogen synthase kinase; GTT, glucose tolerance test; HOMA, homeostasis model assessment; Irs2, insulin receptor substrate-2; NOD, nonobese diabetic; RIPIrs2, rat insulin II promoter; SNP, single-nucleotide polymorphism; Sq, starting quantity.
References
- Stumvoll M, Goldstein BJ, van Haeften TW 2005 Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365:1333–1346 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA 2004 Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 88:787–835, ix [DOI] [PubMed] [Google Scholar]
- Reaven GM 1995 Pathophysiology of insulin resistance in human disease. Physiol Rev 75:473–486 [DOI] [PubMed] [Google Scholar]
- Anderson MS, Bluestone JA 2005 The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 23:447–485 [DOI] [PubMed] [Google Scholar]
- Eisenbarth GS, Jeffrey J 2008 The natural history of type 1A diabetes. Arq Bras Endocrinol Metab 52:146–155 [DOI] [PubMed] [Google Scholar]
- Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS 2005 Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435:220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Nakayama M, Eisenbarth GS 2008 Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol 20:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC 2007 Modestly increased β cell apoptosis but no increased β cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia 50:2323–2331 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC 2005 Sustained β cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 48:2221–2228 [DOI] [PubMed] [Google Scholar]
- Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y 1980 Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 29:1–13 [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Rhodes CJ 2005 Pancreatic regeneration in type 1 diabetes: dreams on a deserted islet? Diabetologia 48:2200–2202 [DOI] [PubMed] [Google Scholar]
- Ablamunits V, Sherry NA, Kushner JA, Herold KC 2007 Autoimmunity and β cell regeneration in mouse and human type 1 diabetes: the peace is not enough. Ann NY Acad Sci 1103:19–32 [DOI] [PubMed] [Google Scholar]
- Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC 2006 Effects of autoimmunity and immune therapy on β-cell turnover in type 1 diabetes. Diabetes 55:3238–3245 [DOI] [PubMed] [Google Scholar]
- Sreenan S, Pick AJ, Levisetti M, Baldwin AC, Pugh W, Polonsky KS 1999 Increased β-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 48:989–996 [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Thervet E, Primo J, Bach JF 1994 Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA 91:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlouf L, Grey ST, Dong V, Csizmadia E, Arvelo MB, Auchincloss Jr H, Ferran C, Sayegh MH 2004 Depleting anti-CD4 monoclonal antibody cures new-onset diabetes, prevents recurrent autoimmune diabetes, and delays allograft rejection in nonobese diabetic mice. Transplantation 77:990–997 [DOI] [PubMed] [Google Scholar]
- Maki T, Ichikawa T, Blanco R, Porter J 1992 Long-term abrogation of autoimmune diabetes in nonobese diabetic mice by immunotherapy with anti-lymphocyte serum. Proc Natl Acad Sci USA 89:3434–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann AM, Gannon M 2007 Molecular regulation of pancreatic β-cell mass development, maintenance, and expansion. J Mol Endocrinol 38:193–206 [DOI] [PubMed] [Google Scholar]
- Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocaña A 2007 Glucose infusion in mice: a new model to induce β-cell replication. Diabetes 56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Deery D, Leahy JL, Weir GC 1989 Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes 38:49–53 [DOI] [PubMed] [Google Scholar]
- Steil GM, Trivedi N, Jonas JC, Hasenkamp WM, Sharma A, Bonner-Weir S, Weir GC 2001 Adaptation of β-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab 280:E788–E796 [DOI] [PubMed] [Google Scholar]
- Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T 2007 Glucokinase and IRS-2 are required for compensatory β cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest 117:246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF 2003 Insulin signaling in health and disease. Science 302:1710–1711 [DOI] [PubMed] [Google Scholar]
- Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF 2004 Dysregulation of insulin receptor substrate 2 in β cells and brain causes obesity and diabetes. J Clin Invest 114:908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Dong X, Fisher TL, Dunn S, Omer AK, Weir G, White MF 2006 Exendin-4 uses irs2 signaling to mediate pancreatic β cell growth and function. J Biol Chem 281:1159–1168 [DOI] [PubMed] [Google Scholar]
- Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M 2003 cAMP promotes pancreatic β-cell survival via CREB-mediated induction of IRS2. Genes Dev 17:1575–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennige AM, Burks DJ, Ozcan U, Kulkarni RN, Ye J, Park S, Schubert M, Fisher TL, Dow MA, Leshan R, Zakaria M, Mossa-Basha M, White MF 2003 Upregulation of insulin receptor substrate-2 in pancreatic β cells prevents diabetes. J Clin Invest 112:1521–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybutt DR, Hawkins YC, Lock J, Lebet J, Sharma A, Bonner-Weir S, Weir GC 2007 Influence of diabetes on the loss of β cell differentiation after islet transplantation in rats. Diabetologia 50:2117–2125 [DOI] [PubMed] [Google Scholar]
- Fox CJ, Danska JS 1998 Independent genetic regulation of T-cell and antigen-presenting cell participation in autoimmune islet inflammation. Diabetes 47:331–338 [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, Baba S, Koga H, Kumashiro R, Ueno T, Ogata H, Yoshimura A, Sata M 2004 Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol 165:1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Tzameli I, Bjørbaek C, Flier JS 2004 Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J Biol Chem 279:34733–34740 [DOI] [PubMed] [Google Scholar]
- Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL 1995 Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol 154:180–191 [PubMed] [Google Scholar]
- Allison PD 1995 Survival analysis using SAS: a practical guide. Cary, NC: SAS Press [Google Scholar]
- Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, Santamaria P, Bluestone JA, Brillantes AM, Herold KC 2007 Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of β-cells. Endocrinology 148:5136–5144 [DOI] [PubMed] [Google Scholar]
- White MF 2006 Regulating insulin signaling and β-cell function through IRS proteins. Can J Physiol Pharmacol 84:725–737 [DOI] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF 1998 Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs 3rd WH, Wright CV, White MF, Arden KC, Accili D 2002 The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest 110:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Liu Z, Patel S, Doble BW, Li L, Cras-Méneur C, Martinez SC, Welling CM, White MF, Bernal-Mizrachi E, Woodgett JR, Permutt MA 2008 Genetic deficiency of glycogen synthase kinase-3β corrects diabetes in mouse models of insulin resistance. PLoS Biol 6:e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KI, White MF, Kasuga M 2005 Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 11:175–182 [DOI] [PubMed] [Google Scholar]
- Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M 1999 Loss of cdk4 expression causes insulin-deficient diabetes and cdk4 activation results in β-islet cell hyperplasia. Nat Genet 22:44–52 [DOI] [PubMed] [Google Scholar]
- Kushner JA, Simpson L, Wartschow LM, Guo S, Rankin MM, Parsons R, White MF 2005 Phosphatase and tensin homolog regulation of islet growth and glucose homeostasis. J Biol Chem 280:39388–39393 [DOI] [PubMed] [Google Scholar]
- Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF 2005 Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol Cell Biol 25:3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner JA, Haj FG, Klaman LD, Dow MA, Kahn BB, Neel BG, White MF 2004 Islet-sparing effects of protein tyrosine phosphatase-1b deficiency delays onset of diabetes in IRS2 knockout mice. Diabetes 53:61–66 [DOI] [PubMed] [Google Scholar]
- Atkinson MA 2005 ADA Outstanding Scientific Achievement Lecture 2004. Thirty years of investigating the autoimmune basis for type 1 diabetes: why can’t we prevent or reverse this disease? Diabetes 54:1253–1263 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC 2008 β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PC, Meier JJ, Butler AE, Bhushan A 2007 The replication of β cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab 3:758–768 [DOI] [PubMed] [Google Scholar]
- Mathis D, Vence L, Benoist C 2001 β-Cell death during progression to diabetes. Nature 414:792–798 [DOI] [PubMed] [Google Scholar]
- Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, Mathis D, Rossini AA, Campbell SE, Kahn R, Kreuwel HT 2005 A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity 23:115–126 [DOI] [PubMed] [Google Scholar]
- Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, Zhu S, Weiss A, Bluestone JA 2008 Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA 105:18895–18900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA 2005 A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L 2005 Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352:2598–2608 [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Primo J, Bach JF 1997 CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol 158:2947–2954 [PubMed] [Google Scholar]