Abstract
Uterine leiomyoma are the most common benign tumors of the myometrium. We previously identified endothelin (ET)-1 as a proliferative and antiapoptotic factor in Eker rat-derived leiomyoma (ELT3) cells. A major role of ETB receptor in the prosurvival effect was revealed. Here we investigated, in ELT3 and myometrial cells, the respective contribution of ETA and ETB in the proliferative effect of ET-1. In myometrial cells, binding experiments show that ETA is almost exclusively expressed and stimulates phospholipase C (PLC) activity and ERK1/2 phosphorylation and proliferation. In ELT3 cells, ETB is expressed at about the same level as ETA, and the two receptors are differently coupled to Gi protein. The ETB agonist, sarafotoxin S6c, stimulates PLC activity 60% less than ET-1 but is as potent as ET-1 to increase ERK1/2 phosphorylation and induce proliferation. However, the ability of ETA to activate ERK1/2 is observed after ETB desensitization. Although ETA and ETB antagonists partially reduce ET-1 stimulated PLC activity, they are without effect on ET-1-induced ERK1/2 phosphorylation and proliferation. Only the simultaneous use of ETA and ETB antagonists reduces ET-1-triggered ERK1/2 activation. These unconventional properties of ETRs may reveal the existence of functional ETA-ETB heterodimers. Finally, treatment of ELT3 cells with ETB but not ETA-directed small interfering RNA reduces the proliferative effect of ET-1. All the data obtained in ELT3 cells strengthen the relation between ETB overexpression, which decreases the ETA to ETB ratio, and the ability of leiomyoma cells to highly proliferate and resist apoptosis.
The capacity of rat leiomyoma ELT3 cells to highly proliferate in response to endothelin-1 is associated with the overexpression of the endothelin B receptor.
The endothelins (ETs) constitute a family of three 21-amino acid peptides (ET-1, ET-2, and ET-3), which exert their effects by binding to two different membrane receptors (ETRs), ETA and ETB, belonging to the family of the seven-transmembrane domain receptors coupled to G proteins (1). ETB binds the three peptide isotypes with equal affinity. In contrast, ETA exhibits a higher affinity for ET-1 than for ET-3. The ETRs modulate the generation of multiple second messengers, including inositol-1,4,5 triphosphate, Ca2+, diacylglycerol, arachidonic acid, and cAMP (2). The role of ET-1, as a potent endogenous vasoconstrictor and a mediator of cardiovascular and renal disorders, is well established (3). Nevertheless ET-1, considered the major effector of the ET axis, has emerged as an important peptide in many physiological functions such as proliferation, apoptosis, and development (4). ET-1, which is produced at the level of uterus (5), is also one of the most potent contractile agonist in rat and human myometrium (6,7,8). In rat myometrial cells, ET-1 also stimulates DNA synthesis through the sequential activation of phospholipase C (PLC), protein kinase C, Src kinases, and Ras, leading to the activation of MAPKs of the ERK1/2 type (9).
Recent data have underlined the role of ET axis in cancer (10,11,12). Indeed, ET-1 stimulates DNA synthesis, cell proliferation, and survival in various cancers, including steroid-dependent tumors such as prostate, ovary, and breast cancers. The growth of these tumors was reduced in the presence of ETA but not ETB antagonist, demonstrating that ET-1 acts only through ETA (10,12,13). Uterine leiomyoma are the most common benign tumor of myometrium clinically diagnosed in more than 30% of the women in reproductive age. It is well established that the growth of these tumors is controlled by growth factors (14) and ovarian steroid hormones (15,16). The steroid hormones control transcription of genes, but recently 17β-estradiol has been described to rapidly initiate cytoplasmic signaling pathways, which contribute to the proliferation of fibroids (17). Nevertheless, very few data are available with regard to the possible implication of the ET axis in this disease. In human leiomyoma, both ETA and ETB mRNAs are detected (18,19), but only ETA is coupled to PLC pathway (20) and overexpressed (21).
Eker rats, which derived from Long Evans rats, are heterozygous for a germline mutation in the tuberous sclerosis 2 (TSC2) gene encoding tuberin. They develop leiomyoma with high frequency and were used to investigate the hormonal modulation in association with this pathology. These rat leiomyoma share many characteristics of the human disease, including frequency, pathologic presentation, hormone responsiveness, and molecular alterations such as loss of tuberin expression associated with aberrant expression of high mobility group A2 protein (22,23,24,25). ELT3 uterine leiomyoma cells have been established from Eker rat leiomyoma and recapitulate many properties of the tumors (26). Using ELT3 cells, we recently demonstrated that ET-1 is a potent mitogenic factor acting via phospholipase D and ERK1/2 signaling pathways (27). Moreover, we showed that in ELT3 cells but not in normal myometrial cells, ET-1 possesses a potent antiapoptotic effect that involves sphingolipid metabolism through sphingosine kinase 1 but that is not dependent on phospholipase D and ERK1/2 activations. This antiapoptotic effect was fully reproduced by ETB agonists indicating a major role of ETB in ET-1-mediated survival (28).
In the present work, using complementary approaches, we investigated the relative contribution of ETA and ETB in ET-1-induced cell proliferation and activation of the signaling enzymes, PLC and ERK1/2 MAPKs. This study was performed on both ELT3 and corresponding normal cells prepared from Long Evans rat myometrium.
Materials and Methods
Cell culture
The Eker rat uterine leiomyoma (ELT3) cells have been previously described (26) and were kindly provided by Dr. C. Walker (Anderson Cancer Center, University of Texas, Smithville, TX). ELT3 cells were maintained in DF8 medium supplemented with 10% fetal calf serum, as described previously (27).
Prepubertal Long Evans female rats (Elevage Janvier, Genest-Saint-Isle, France), 31 d old, were killed by 1 min of carbon dioxide inhalation. All the treatments were performed in accordance with the principles and procedures outlined in the European guidelines for the care and use of experimental animals. Primary cultures of myometrial cells were prepared by collagenase digestion as previously described (9) and cultured in MEM supplemented with 10% fetal calf serum for the first 2 d and then in DF8 medium supplemented with 10% fetal calf serum as for ELT3 cells.
Cells were serum starved for 14 h before experiments.
RNA interference
ELT3 cells were harvested by trypsinization and electroporated with an Amaxa Nucleofector device (program T 030) at a density of 106 cells in 100 μl Nucleofector buffer V containing 5 μm of ETA- or ETB-directed small interfering RNA (siRNA; Dharmacon si GENOME ON-TARGET plus SMARTpool L-089698-01 and L-089829-01, respectively; Dharmacon, Lafayette, CO) or control siRNA, previously described (28).
Quantitative RT-PCR
Total cellular RNA from myometrial and ELT3 cells were isolated with TRIzol (Promega, Madison, WI) according to the manufacturer’s protocol, and 1 μg of total RNA was reverse transcribed into cDNA using 200 U Moloney murine leukemia virus-reverse transcriptase, 0.2 mm deoxynucleoside triphosphates, and 10 μm random hexamer primers.
Quantitative real-time PCR was performed on a Lightcycler 2.0 (Roche, Neuilly sur Seine, France). PCR was carried out using 1:240 of the reverse transcriptase products as template and the Fast Start DNA Master SYBR Green I reagent (Roche) according to the manufacturer’s instructions under the following conditions: 95 C (10 sec), 57 C (10 sec), and 72 C (20 sec) for 40 cycles.
The primer sets used are described in supplemental Table S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Binding assay
Equilibrium binding studies were performed for 14 h on confluent serum-starved cells seeded in 24-well plates. All experiments were performed at 4 C to avoid receptor internalization. Briefly, cells were incubated in 2 ml of 0.1% BSA in MEM in the presence of [125I]ET-1 or [125I]ET-3 (2000 Ci/mmol, GE Healthcare, Indianapolis, IN) concentrations ranging from 0.1 to 8 nm (saturation analysis) or 1 nm (competition studies). After the incubation, the cells were washed three times with 0.1% BSA in MEM and solubilized in 500 μl of 0.5 n NaOH. The radioactivity of the samples was then counted. The specific binding was defined as the difference between the amounts of ET bound in the absence (total binding) and the presence (nonspecific binding) of 200 nm unlabeled ET. In each experiment two wells of the plate, identically treated, were used for protein assay (using bicinchoninic assay kit).
Measurement of PLC activity
Confluent cells seeded in 24-well plates were serum starved in the presence of 5 μCi/ml Myo-[2-3H]inositol (16 Ci/mmol; PerkinElmer, Les Ulis, France). The cells were washed three times with MEM and then incubated at 37 C for 30 min in 1 ml of MEM in the absence or presence of antagonists and then exposed to the agonists tested for 30 min. LiCl (10 mm) was added 10 min before the agonists. Total inositol phosphates (IPs) produced by PLC were quantified as previously described (29).
Western blot analysis of phosphorylated ERK1/2
Serum-starved confluent cells seeded in 12-well plates were treated as described in figure legends and then lysed in 50 μl lysis buffer [50 mm HEPES (pH 7.4), 150 mm NaCl, 100 mm NaF, 10% glycerol, 10 mm Na4P2O7, 200 μm Na3VO4, 10 mm EDTA, 1% Triton X-100, 10 μg/ml aprotinin and leupeptin]. Detergent-extracted proteins were analyzed by Western blot technique using antiactive phosphorylated ERK1/2 (Promega) or anti-ERK2 (Santa Cruz Biotechnology, Tebu-Bio, Le Perray en Yvelines, France) antibodies (1:6000 each) as previously described (29).
[3H]thymidine incorporation
Serum-starved cells (about 75% confluent) seeded on 24-well plates were incubated for 48 h with the different agonists to be tested. [3H]thymidine (2 μCi/ml) was added to each well for the last 24 h. Reactions were stopped by aspiration of the incubation medium and addition of 0.5 ml cold 10% trichloroacetic acid. Radioactivity of trichloroacetic acid-precipitable material was quantified.
Statistical analysis
Results are expressed as the mean ± sem of at least three independent experiments performed in duplicate. Statistical analysis was performed using one- or two-way ANOVA followed by post hoc comparisons with the Fisher’s least significant differences test. Values with P < 0.05 were considered as statistically significant.
Results
Differential expression and function of ETRs
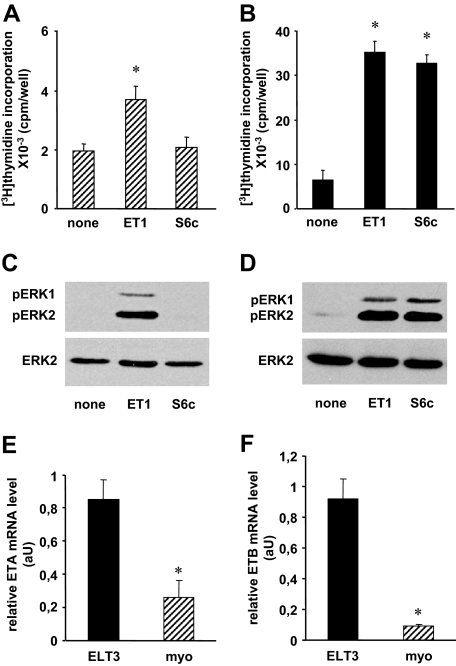
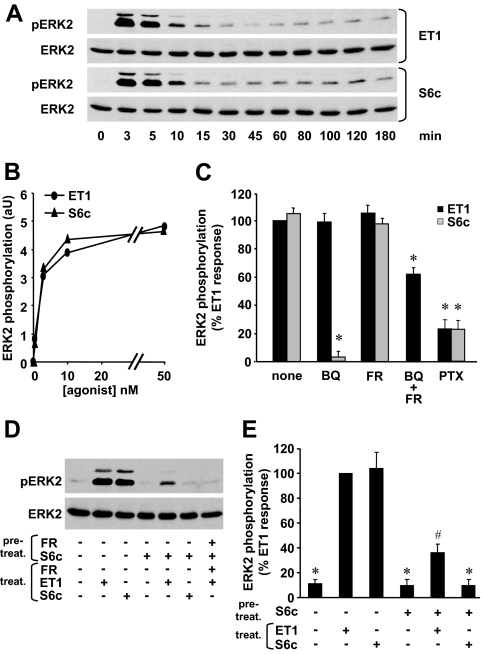
We analyzed the relative contribution of ETA and ETB in both long- and short-term effects of ET-1, cell proliferation, and activation of the ERK1/2 MAPKs previously shown to be involved in this process (9,27). Figure 1 (A–D) shows that ET-1 stimulated DNA synthesis and ERK1/2 phosphorylation in both normal myometrial and tumoral ELT3 cells. However, [3H]thymidine incorporation was more important in ELT3 cells. By contrast to ET-1, the highly specific ETB agonist S6c (1,30) failed to enhance [3H]thymidine incorporation and ERK1/2 activation in myometrial cells. However, in ELT3 cells, S6c was as potent as ET-1 to trigger these responses. These results suggesting a differential expression of ETRs in the two cell models, ETA and ETB mRNAs, were quantified by quantitative RT-PCR. The results shown in Fig. 1 (E and F) indicate that ETA and ETB mRNAs are 3.3- and 10.2-fold more abundant in ELT3 than in myometrial cells, respectively.
Figure 1.
Differential expression and function of ETA and ETB in myometrial and ELT3 cells. A and B, [3H]thymidine incorporation. Myometrial (A) and ELT3 (B) cells were treated or not for 48 h with or without 50 nm ET-1 or 50 nm S6c. [3H]thymidine (2 μCi/ml) was added 24 h before the end of the incubation, and [3H]thymidine incorporation was measured as described in Materials and Methods. Values are the mean ± sem of four (myometrial cells) or six (ELT3 cells) separate experiments performed in duplicate. *, P < 0.05 vs. untreated cells. C and D, ERK1/2 activation. Myometrial (C) and ELT3 (D) cells were incubated at 37 C for 5 min with or without 10 nm ET-1 or 10 nm S6c. Cells were lysed and detergent-extracted proteins were analyzed by 10% SDS-PAGE followed by immunoblotting with antiactive ERK1/2 antibody. After stripping, the blots were reprobed with antitotal ERK2 antibody (ERK2). Result of a typical experiment is among three performed. E and F, ETA (E) and ETB (F) mRNAs in myometrial (myo) and ELT3 cells were quantified by quantitative RT-PCR as described in Materials and Methods. Values, expressed as relative mRNA amounts normalized to total RNA, are the mean ± sem of three separate experiments performed in duplicate. *, P < 0.05 vs. ELT3 cells. aU, Arbitrary units; pERK1, phosphorylated ERK1.
Binding of [125I]ET-1 and [125I]ET-3
Because we observed a difference in ETR mRNA expression in the two cell types, we tested the presence of ETRs at the cell surface by binding experiments with [125I]ETs.
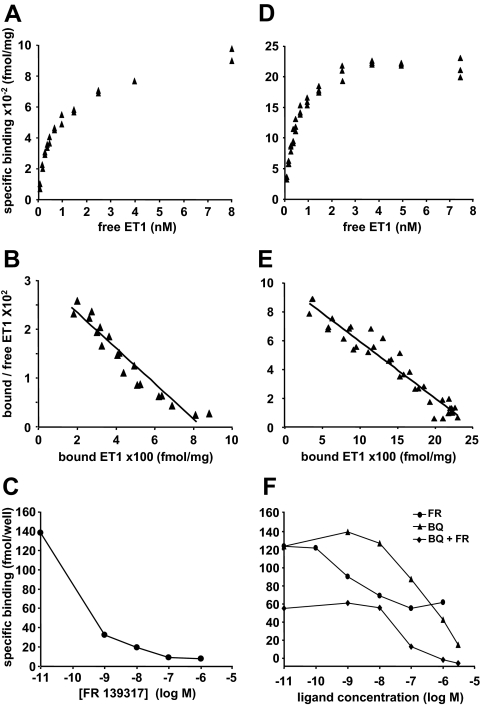
In myometrial cells, binding of [125I]ET-1 on whole cells was saturable (Fig. 2A). Scatchard plot revealed a typical linear plot (Fig. 2B), indicating one site with an apparent affinity constant (KD) of about 0.8 nm and a maximal binding capacity (Bmax) of 740 fmol/mg protein (Table 1). [125I]ET-3, which binds only to ETB at the concentrations used, exhibited a very low binding on these cells. The estimated Bmax value (Table 1) represented only 13% of that determined for ET-1 binding. The saturation curve was unworkable for KD measurement due to the low number of binding sites. Competition experiments performed with 1 nm [125I]ET-1 showed that the ETA highly specific antagonist FR 139317 (1,31), at 100 nm, displaced 94% ± 5% (n = 3) of bound [125I]ET-1 (Fig. 2C). Because ET-1 binds to both ETA and ETB, but ET-3 only to ETB, our data indicate that in myometrial cells the proportion of ETB able to bind ET-1 can be estimated at about 10% only of the total ETRs.
Figure 2.
Equilibrium binding of [125I]ET-1 on myometrial and ELT3 cells. Myometrial (A) or ELT3 (D) cells were treated as described in Materials and Methods in the presence of [125I]ET-1 concentrations ranging from 0.1 to 8 nm. For each concentration, duplicate or triplicate determinations were performed. Each of the saturation binding curves represents a typical experiment among three or four performed for myometrial and ELT3 cells, respectively. B and E, Scatchard plots obtained from the data shown in A and D, respectively. C, Competition binding experiments on myometrial cells: 1 nm [125I]ET-1 was used in the presence of increasing concentrations of FR 139317. Each point represents the mean value of duplicate measurements. Result of a typical experiment among three performed. F, Competition binding experiment performed on ELT3 cells: 1 nm [125I]ET-1 was used in the presence of increasing concentrations of FR 139317 (FR) or BQ 788 alone (BQ) or BQ788 in the presence of 100 nm FR 139317 (BQ + FR). Each point represents the mean value of duplicate determinations. Result of a typical experiment among three performed.
Table 1.
Binding parameters obtained from [125I]ET-1 and [125I]ET-3 binding experiments performed on myometrial and ELT3 cells
| Myometrial cells
|
ELT3 cells
|
|||
|---|---|---|---|---|
| [125I]ET-1 | [125I]ET-3 | [125I]ET-1 | [125I]ET-3 | |
| KD (nm) | 0.77 ± 0.11 (n = 3) | nd | 0.61 ± 0.07 (n = 4) | 0.72 ± 0.15 (n = 3) |
| Bmax (fmol/mg) | 740 ± 140 (n = 5) | 100 ± 60 (n = 3) | 2310 ± 480 (n = 6) | 1020 ± 230 (n = 3) |
| ETA (fmol/mg) | 640 | 1290 | ||
| ETB (fmol/mg) | 100 | 1020 | ||
Binding experiments were performed as described in Fig. 2. Results are expressed as mean ± sem of parameters obtained from independent Scatchard plots (n indicated in the table). For ET-3 binding, no precise KD was measurable for myometrial cells (nd). Bmax values were expressed in femtomoles of bound ET per milligram of total cellular proteins. ETA and ETB amounts were deduced from [125I]ET-1 and [125I]ET-3 Bmax values. nd, Not determined.
In ELT3 cells, [125I]ET-1 binding was also saturable (Fig. 2D) and Scatchard plot was linear (Fig. 2E), indicating one site with an apparent Kd value of 0.6 nm (Table 1). [125I]ET-3 binding was also saturable and much higher than in myometrial cells (data not shown), indicating that ELT3 cells express a high amount of ETB. Scatchard plot of [125I]ET-3 binding data was linear in the concentration range tested and the Kd value was not significantly different from that obtained with ET-1 (Table 1). However, the Bmax values determined with the two hormones were different (Table 1) and indicated that ETB and ETA represent 44 and 56% of the ETRs, respectively. Competition experiments showed that 100 nm FR 139317 displaced 53% ± 2% (n = 3) of bound [125I]ET-1, and for higher concentrations, the displacement curve plateaued (Fig. 2F). This result is in good correlation with the percentage of ETA deduced from the Bmax values (Table 1). The results of the competition binding experiments performed with BQ 788, a peptide described as a specific ETB antagonist (1,32), were unexpected because at 3 μm nearly all the bound [125I]ET-1 was displaced, indicating that in our cells, this antagonist, used at concentrations higher than 1 μm, displays lower selectivity for ETB as previously reported (1,33). These data indicated that myometrial cells express mainly ETA, whereas ELT3 cells express a high level of ETB in addition to ETA.
Comparison of the Bmax values reported in Table 1 also indicates that ETA and ETB are twice and 10 times more expressed in leiomyoma cells than in normal cells, respectively. These data fully agree with the results of quantitative RT-PCR described above (Fig. 1).
ET-mediated PLC activation
Because myometrial and ELT3 cells present differential expression and binding properties of ETA and ETB, we investigated the impact of this differential expression on subsequent signaling pathways. We thus analyzed the activity of PLC in the two cell models.
In myometrial cells ET-1-induced PLC activation plateaued for concentrations greater than 10 nm (supplemental Fig. S1A). S6c, the ETB agonist, induced a production of IPs less than 5% of ET-1 effect (Fig. 3A and supplemental Fig. S1A). Moreover, 100 nm BQ 788 had no effect on ET-1 response, whereas 1 μm FR 139317 decreased by about 87% the effect of ET-1 (Fig. 3A). These results show that in Long Evans rat myometrial cells, PLC activation by ET-1 is dependent on ETA.
Figure 3.
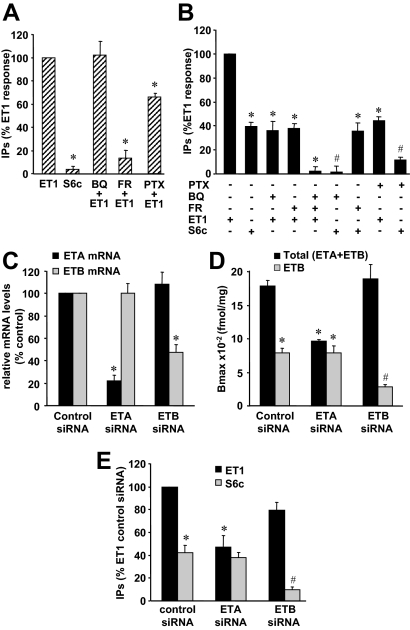
Role of ETA and ETB in ET-1-induced PLC activation in myometrial and ELT3 cells. A, [3H]inositol-labeled myometrial cells were preincubated or not for 30 min with 1 μm FR 139317 (FR) or 100 nm BQ 788 (BQ) or for 14 h (during the labeling) with 100 ng/ml PTX before a 30 min-stimulation with 50 nm ET-1 or 50 nm S6c. Values, expressed as percentages of the ET-1 value, are the mean ± sem of three separate experiments performed in duplicate. *, P < 0.05 vs. ET1-treated cells. B, [3H]inositol-labeled ELT3 cells were preincubated or not with 1 μm FR 139317 (FR), 1 μm BQ 788 (BQ), or both 1 μm FR 139317 + 1 μm BQ 788 for 30 min or with 100 ng/ml PTX for 14 h (during the labeling) before a 30-min stimulation with 10 nm ET-1 or 10 nm S6c. Values, expressed as percentages of the ET-1 value, are the mean ± sem of four separate experiments performed in duplicate. *, P < 0.05 vs. ET1-treated cells; #, P < 0.05 vs. S6c-treated cells. C, Twenty-four hours after nucleofection of ELT3 cells with control or ETA- or ETB-targeted siRNA, the expression of ETR subtype was examined by PCR quantitative as described in Materials and Methods. Values, expressed as relative mRNA amounts, are the mean ± sem of three separate experiments performed in duplicate. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript was used as a standard. *, P < 0.05 vs. corresponding mRNA in cells transfected with control siRNA. D, Twenty-four hours after nucleofection with control or ETA- or ETB-targeted siRNA, ELT3 cells were preincubated or not with 1 μm FR 139317 and treated at 4 C in the presence of 10 nm [125I]ET-1 as described in Materials and Methods. ETA was determined as the FR139317-sensitive [125I]ET-1 binding and ETB as the FR139317-insensitive [125I]ET-1 binding. Values are the mean ± sem of three separate experiments performed in triplicate. *, P < 0.05 vs. control siRNA-nucleofected cells treated without FR 139317; #, P < 0.05 vs. control siRNA-nucleofected cells treated with FR 139317. E, Twenty-four hours after nucleofection with control or ETA- or ETB-targeted siRNA, ELT3 cells were labeled with [3H]inositol for 14 h before stimulation with 10 nm ET-1 or 10 nm S6c for 30 min. Values, expressed as percentages of the ET-1 value obtained with the control siRNA, are the mean ± sem of three separate experiments performed in duplicate. *, P < 0.05 vs. control siRNA-nucleofected cells treated with ET1; #, P < 0.05 vs. control siRNA-nucleofected cells treated with S6c.
In ELT3 cells, ET-1 and S6c induced a production of IPs that plateaued for concentrations greater than 10 nm (supplemental data Fig. S1B). Maximal S6c effect was 40% of the maximal ET-1 response (Fig. 3B and supplemental data Fig. S1B). FR 139317 (1 μm) had no effect on the S6c response, confirming its high specificity toward ETA, and reduced the ET-1-induced IP production by about 60% (Fig. 3B). BQ 788 (1 μm) fully inhibited the effect of S6c on IP production and decreased the effect of ET-1 by about 64% (Fig. 3B). As expected, coincubation of cells with 1 μm of both BQ788 and FR 139317 led to a total inhibition of ET-1 response (Fig. 3B).
To confirm the results obtained by pharmacological approaches, we tested the effect of ETA and ETB down-regulation by RNA interference technique using specific siRNAs. In the experimental conditions used, the levels of ETA and ETB mRNAs were reduced by 78 and 52%, respectively (Fig. 3C). The efficiency and specificity of ETA- and ETB-directed siRNAs were determined by [125I]ET-1 binding experiments (Fig. 3D). In control siRNA-treated cells, ETB-dependent [125I]ET-1 binding was evaluated to 44% of total binding (Fig. 3D) as observed in untransfected cells (Fig. 2F). Treatment with ETA siRNA reduced the total [125I]ET-1 binding but not the ETB-dependent binding, indicating a strong down-regulation of ETA (82%). Treatment with ETB siRNA reduced the ETB-dependent binding by 64% compared with the ETB-dependent binding in control siRNA-treated cells. Unexpectedly, treatment with ETB-targeted siRNA did not affect total [125I]ET-1 binding, suggesting a possible negative cross-regulation of ETB on ETA binding. Results in Fig. 3E show that ETA siRNA reduced ET-1-induced IP production by 53% without significantly reducing S6c response. ETB siRNA did not fully reduce S6c response and reduced ET-1 response by only 20%. These results are in perfect correlation with the binding results (Fig. 3D).
Taken together, our results show that in myometrial cells PLC activation is fully dependent on ETA, whereas in ELT3 cells, ETA and ETB contribute almost equally to the ET-1 response.
ET-1 responses have been shown to involve different G proteins including Gi. The potential coupling of ETA and ETB with protein Gi was investigated using pertussis toxin (PTX). Figure 3A shows that in myometrial cells, IP amount produced by ET-1 was reduced by 35% after PTX treatment. This result suggests that ETA is mainly coupled to PTX-insensitive G protein to activate PLC, which is consistent with previously published studies performed in rat myometrium (8). In PTX-pretreated ELT3 cells, S6c and ET-1 responses were reduced by 71 and 48%, respectively (Fig. 3B). These results indicate that in these cells, activation of PLC by ETB involves Gi protein for about 70% of the response.
ET-induced ERK1/2 activation
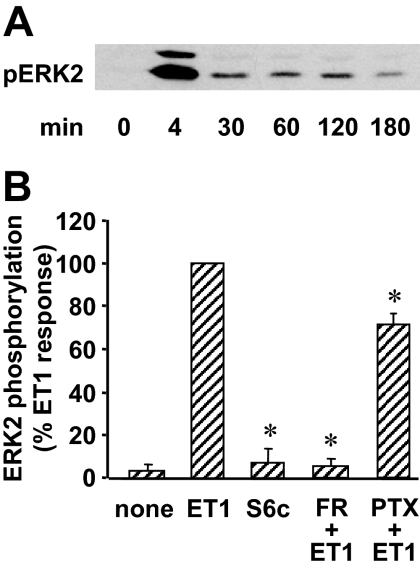
In myometrial cells, ET-1 stimulated a rapid and transient ERK1/2 phosphorylation that peaked at about 4 min and then declined to a low level that persisted for at least 3 h (Fig. 4A). FR 139317 (1 μm) fully abolished this ET-1-induced phosphorylation, and S6c was unable to trigger any significant ERK1/2 phosphorylation (Fig. 4B). PTX treatment reduced ET-1 effect by about 29% (Fig. 4B). This result indicates that ETA-Gi complex is responsible for about one third of the ET-1 response as already observed for PLC activation.
Figure 4.
Role of ETA in ET-1-induced ERK1/2 activation in myometrial cells. A, Myometrial cells were incubated for indicated times in the presence of 10 nm ET-1. Phosphorylated ERK1/2 (pERK1/2) was detected as described in Fig. 1. Result of a typical experiment is among three performed. B, Myometrial cells were preincubated or not with 1 μm FR 139317 (FR) for 30 min or 100 ng/ml PTX for 14 h before a 5-min stimulation with 10 nm ET-1 or 10 nm S6c. Immunoblotting was performed as described above, and phosphorylated ERK2 and total ERK2 bands were quantified. The levels of ERK2 phosphorylation were normalized with respect to total ERK2 amount in each sample, and the results are expressed as percent of ET-1 response. Values are the mean ± sem of three separate experiments performed in duplicate. *, P < 0.05 vs. ET1-treated cells.
In ELT3 cells, ET-1 and S6c presented identical kinetics of ERK1/2 activation with a maximal effect at 3–5 min of stimulation and a low but significant activation for the following 3 h (Fig. 5A). Moreover, at identical concentrations, the two agonists gave the same response, suggesting that a full ERK1/2 phosphorylation can occur via ETB activation alone (Fig. 5, B and C). To confirm these results, we tested the effect of FR 139317 or BQ 788 on ET-1 stimulation (Fig. 5C). As expected, 1 μm BQ 788 decreased by about 95% the level of ERK1/2 phosphorylation induced by S6c, whereas 1 μm FR 139317 had no effect on S6c response (Fig. 5C). Unexpectedly, the effect of ET-1 was reduced by neither 1 μm BQ 788 nor 1 μm FR 139317 (Fig. 5C). Surprisingly, ERK1/2 phosphorylation was reduced by only 40% when 1 μm BQ 788 and 1 μm FR 139317 were used together (Fig. 5C). When 3 μm BQ 788 and 1 μm FR 139317 were combined, the inhibition of ERK1/2 phosphorylation reached 60% (data not shown).
Figure 5.
Role of ETA and ETB in ET-1-induced ERK1/2 activation in ELT3 cells. A, ELT3 cells were incubated for the indicated times in the presence of 10 nm ET-1 or 10 nm S6c. Phosphorylated ERK1/2 (pERK1/2) and total ERK2 were detected as described in Fig. 1. Result is of a typical experiment among three performed. B, ELT3 cells were incubated for 5 min with ET-1 or S6c concentrations ranging from 0.5 to 50 nm. Phosphorylated ERK2 and total ERK2 bands were quantified. The levels of ERK2 phosphorylation were normalized with respect to total ERK2 amount in each sample and expressed in arbitrary units (aU). Each point represents the mean value of duplicate measurements. Result is of a typical experiment among three performed. C, ELT3 cells were preincubated either for 30 min with 1 μm BQ788 (BQ) or 1 μm FR 139317 (FR) or 1 μm BQ788 + 1 μm FR 139317 (BQ + FR) or for 14 h with 100 ng/ml PTX before a 5-min stimulation with 10 nm ET-1 or 10 nm S6c. The levels of ERK2 phosphorylation were calculated as in B and expressed as percent of ET-1 response. Values are the mean ± sem of three to six separate experiments performed in duplicate. *, P < 0.05 vs. ET1-treated cells. D, ELT3 cells were desensitized or not for 30 min by a pretreatment with 50 nm S6c with or without 1 μm FR 139317 (FR) during this pretreatment (pretreat.). The media were changed and the cells were then stimulated or not for 5 min by 50 nm ET-1 or 50 nm S6c in the presence or absence of 1 μm FR 139317 (treat.). Result is of a typical experiment among four performed. E, ELT3 cells were pretreated or not for 30 min with 50 nm S6c (pretreat.). The media were changed and the cells were then stimulated or not for 5 min by 50 nm ET-1 or 50 nm S6c (treat.). The levels of ERK2 phosphorylation were calculated as above and expressed as percent of ET-1 response. Values are the mean ± sem of four separate experiments performed in duplicate. *, P < 0.05 vs. ET1-treated cells; #, P < 0.05 vs. untreated S6c-desensitized cells.
From all these results, it appears that ETB alone is able to fully activate ERK1/2, raising the question of ETA function. We then tested the effect of ETB desensitization, induced by a 30-min incubation with S6c, on ERK1/2 stimulation by ET-1. Figure 5, D and E, shows that after S6c-induced desensitization, S6c effect was abolished, whereas ET-1 was still able to induce a phosphorylation of ERK1/2. The effect of ET-1 after desensitization by S6c was only 30% of the full effect of ET-1. This residual response was mediated by ETA because it was fully abolished by 1 μm FR 139317 (Fig. 5D). This result indicates that in ELT3 cells, ETA is coupled to a pathway leading to ERK1/2 phosphorylation but that this coupling is detectable only when ETB signaling is abolished.
Finally, we tested the effect of PTX on ERK1/2 phosphorylation. Figure 5C shows that 78% of both ET-1 and S6c responses were reduced by PTX.
From all these results, it seems that, in ELT3 cells, ERK1/2 activation by ET-1 is mainly dependent on ETB coupled to Gi protein.
ET-stimulated DNA synthesis
To analyze in ELT3 cells the respective role of ETA and ETB in the ET-1-mediated DNA synthesis, we performed the experiments in the presence of the antagonists. Unexpectedly, neither BQ 788 (1 or 3 μm) nor 1 μm FR 139317 nor both antagonists together were able to reduce [3H]thymidine incorporation triggered by 50 or 10 nm ET-1 (data not shown). This may indicate that the low level of ERK1/2 activation observed in these conditions (see above) is sufficient to induce a maximal effect on DNA synthesis. This may also result from the low reversibility of ET-1 binding compared with the higher reversibility of antagonist binding as already described in several other models (34,35,36).
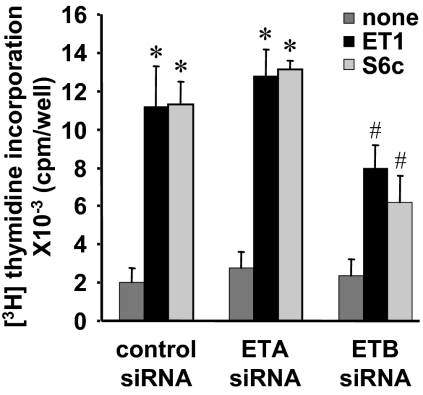
To overcome the failure of the pharmacological approach, we tested the effect of ETA and ETB down-regulation by RNA interference technique in the same experimental conditions as those described in Fig. 3. In these conditions, serum-induced proliferation was modified by neither ETA siRNA nor ETB siRNA (data not shown). As shown in Fig. 6, ETA siRNA, which strongly down-regulated ETA (Fig. 3D), did not reduce ET-1-triggered [3H]thymidine incorporation. In contrast, ETB siRNA reduced S6c-triggered [3H]thymidine incorporation by 45% but also ET-1 effect by 30%. These results indicate that the stimulation of DNA synthesis by ET-1 is mainly dependent on ETB.
Figure 6.
Role of ETA and ETB in ET-1-induced DNA synthesis in ELT3 cells. ELT3 cells, 18 h after nucleofection with control or ETA- or ETB-targeted siRNA, were serum starved and then treated for 48 h with or without 50 nm ET-1 or 50 nm S6c. [3H]thymidine (2 μCi/ml) was added 24 h before the end of the incubation, and [3H]thymidine incorporation was quantified as described in Materials and Methods. Values are the mean ± sem of three separate experiments performed in duplicate. *, P < 0.05 vs. control siRNA-nucleofected untreated cells; #, P < 0.05 vs. control siRNA-nucleofected cells treated with ET1.
Discussion
In the present study, we analyzed the respective contribution of ETA and ETB on different responses triggered by ET-1 stimulation in Long Evans rat myometrial cells and ELT3 leiomyoma cells.
In normal rat myometrial cells, even if both ETA and ETB mRNAs are detected, ETB protein is very weakly expressed. Identical results were previously reported on human myometrial cells (18,19,37). Consistent with previous results obtained in Wistar rat myometrium and myometrial cells (8,9), we found that PLC and ERK1/2 are activated by ET-1, but only via ETA as S6c was quasi-inefficient. Furthermore, we showed that S6c was unable to trigger DNA synthesis. Taken together, those results indicate that ETA is the receptor predominantly expressed and functional in Long Evans rat myometrial cells. This result agrees with previous data reported in porcine and human myometrial cells (9,37,38,39), showing that ET-1 responses are mainly dependent on ETA.
In ELT3 leiomyoma cells, the situation is more complex. Both ETA and ETB mRNAs are also expressed, but ETA and ETB mRNA levels are about 3 times and 10 times higher, respectively, than those found in myometrial cells. In contrast to what we observed with myometrial cells, both [125I]ET-1 and [125I]ET-3 bind to ELT3 cells. These binding experiments clearly show that ETA is overexpressed in ELT3 cells compared with myometrial cells, which is consistent with previous reports demonstrating ETA overexpression in human leiomyoma (19,20,21). Such up-regulation of ETA has also been reported in prostate, ovary, and breast cancers (10,12,13), which are steroid-dependent tumors as leiomyoma (15). In these cancers a relationship between steroid hormones and ETR expression has been established; however, nothing is known in leiomyoma. This point would merit to be investigated. Our results also revealed a marked increase of ETB expression in ELT3 cells, leading to a decrease in the ETA to ETB ratio (1.3 in ELT3 cells compared with 6.4 in myometrial cells).
Concerning the signaling pathways triggered by ETRs in ELT3 cells, we showed that PLC is activated by ET-1 via both receptors. Indeed, S6c or ET-1 in the presence of FR 139317 or ET-1 in cells transfected with ETA siRNA resulted in an IP production 60% lower than that triggered by ET-1, indicating that ETB contribute to about 40% in PLC activation. This result is consistent with the results of the binding experiments, indicating an ETA to ETB ratio of 1.3, and it indicates that the level of IPs produced via stimulation of ETB is additive to ETA-mediated IP response.
Regarding the activation of ERK1/2, we showed that S6c, and thus ETB activation, fully reproduced the ET-1 response. Moreover, FR 139317 had no inhibitory effect on ET-1-induced ERK1/2 activation. These first data may suggest that ETA does not participate in the ERK1/2 activation process, which would be fully dependent on ETB. Surprisingly, BQ 788 was also unable to reduce ERK1/2 phosphorylation induced by ET-1, whereas when used in combination with FR 139317, it reduced ERK1/2 activation, suggesting nonetheless a role for ETA in this response. The need of both ETA and ETB antagonists to reduce different ET-1-triggered responses has been reported in other models (40,41). To confirm the involvement of ETA, we tested the ability of ET-1 to stimulate ERK1/2 phosphorylation after desensitization of ETB by S6c. We showed that in these conditions, S6c response was abolished, but ET-1 was still able to induce a phosphorylation of ERK1/2 in an ETA-dependent manner. All these results show that each ETR is able to induce ERK1/2 activation. Furthermore, in contrast to what we observed for the activation of PLC, a pharmacological approach failed to reveal an additive effect of ETA and ETB in ERK1/2 activation.
Although these results may appear surprising, comparable atypical results were obtained in other cells coexpressing ETA and ETB, e.g. astrocytes or epithelial cells of the anterior pituitary gland, and it was suggested that the two receptor subtypes form heterodimers (42,43). More recently it has been confirmed by fluorescence resonance energy transfer and coimmunoprecipitation techniques that the coexpression of recombinant ETA and ETB in HEK 293 cells leads to the formation of functional heterodimers (44,45,46). These heterodimers behave differently from monomers (or homodimers) in terms of binding properties, intracellular trafficking, and coupling to signal transduction pathways (44,45,46). Based on this background, it is tempting to speculate that our data fit well with the presence of ETA-ETB heterodimers in ELT3 cells. This hypothesis is supported by the high level of expression in a quasi-stoechiometric ratio of the two receptors. The existence of ETA-ETB heterodimers may account for the results obtained for ERK1/2 activation. Indeed, ET-1 and S6c give the same response, and the antagonists FR139317 or BQ788 used alone have no effect, whereas used together they reduce the ET-1 response. However, heterodimers may not be responsible for 100% of the ERK1/2 response. Indeed, in the case of the desensitization of ETB by S6c, ETA is still functional and able to stimulate ERK1/2. The interpretation of the desensitization experiment is not easy because it has been shown that prolonged exposure of cells expressing ETA-ETB heterodimers to an ETB agonist induced the sequestration of ETB inside the cell, whereas ETA was recycled back to the plasma membrane (46). This phenomenon should increase the amount of ETA, monomers or homodimers, at the cell surface and therefore possibly lead to an over estimation of the ETA-dependent response after the desensitization of ETB. Conversely, the results obtained for PLC activation, obtained with pharmacological and RNA interference approaches, are compatible with the involvement of ETA and ETB monomers and /or homodimers in this pathway.
Heterodimerization of G protein-coupled receptors may also alter their coupling to signaling pathways (47). Among the partners that may interact differentially with the ETR monomers and dimers are the trimeric G proteins. In the myometrial cells, in which ETA is the main functional ETR, ET-1-triggered PLC and ERK activations were dependent on Gi protein for about 35 and 29% for IP production and ERK phosphorylation, respectively. In ELT3 cells, PTX reduced S6c-induced IP production by 71% and ERK phosphorylation by about 78%. Thus, we can conclude that ETA and ETB are mainly coupled to Gq/11 and Gi/o proteins, respectively. These results also suggest that in the hypothesis of the formation of functional ETA-ETB heterodimers, they would be mainly coupled to Gi/o protein as ETB. The attractive hypothesis, in which monomers or homodimers couple to PLC, whereas heterodimers couple to ERK1/2 activation, will need further work to be confirmed.
Finally, we observed that ET-1 triggers higher thymidine incorporation in ELT3 cells than in Long Evans myometrial cells. A similar result was already obtained when ELT3 cells were compared with myometrial cells from Wistar rats (27). In the present study, we clearly show that in addition to ETA, ELT3 cells express ETB. The high expression of this receptor in ELT3 cells, compared with myometrial cells, could be responsible for the higher proliferative effect of ET-1. Indeed, in ELT3 cells, S6c is as effective as ET-1 to stimulate DNA synthesis. Moreover, ETA siRNA treatment is without effect on ET-1-induced proliferation, whereas ETB siRNA reduced this response.
All these observations argue for a predominant role of ETB in the proliferation triggered by ET-1. Thus, it can be postulated that not only the level of ETB expression is important but also the ETA to ETB ratio is determinant. Indeed, in ELT3 cells the ETA to ETB ratio is 1.3 and the proliferation rate is high, whereas in ETB siRNA-treated ELT3 cells and in myometrial cells in which the ETA to ETB ratios are equivalently elevated (5.7 and 6.4, respectively), the proliferation due to ET-1 is lower. In addition, the higher proliferative effect of ET-1 may be related to the ability of ETB to inhibit apoptosis in ELT3 cells. Indeed, we previously reported (28) that ET-1, as well as S6c (and thus ETB), was able to rescue ELT3 cells from apoptotic death after serum starvation. This effect of ET-1 was not observed in myometrial cells, strengthening the hypothesis that ETB was responsible for the antiapoptotic effect of ET-1. Thus, in ELT3 cells, this prosurvival signal of ETB probably makes that more cells resist to serum starvation and remain competent for proliferation. By contrast, in myometrial cells, in the absence of the prosurvival effect of ETB, a larger proportion of cells may die before entering S phase and incorporating [3H]thymidine.
These results are consistent with previous reports describing an overexpression of ETB and its implication in the control of invasiveness and the balance proliferation/survival in some tumor cells such as choriocarcinoma (48), melanoma (49,50,51,52), and oligodendroglioma (53). Nevertheless, a possible interaction between ET-1 axis and other modulators of proliferation has to be considered. The development of diverse tumors and the proliferation of ELT3 cells have been shown to be associated with the loss of TSC2 gene function (54,55). Reexpression of TSC2 gene in TSC2−/− cells, including ELT3 cells, reduces proliferation (55). Thus, we cannot exclude that ET-1 proliferative effect could be potentiated by the loss of TSC2 gene function in these cells. ELT3 cell proliferation is also under the control of steroid hormones, which can cooperate with growth factors (56) or angiotensin (57). Because ET and angiotensin are peptidic hormones, which share many common properties, and because estrogens have been shown to modulate ET axis in steroid-dependent tumors (10,12,13), a modulation of ET proliferative effect by steroid hormones may occur and would merit to be investigated.
In conclusion, our data reveal the crucial role of ET axis and particularly ETB-dependent signaling in rat leiomyoma cell proliferation. If these findings are validated in in vivo models, ET axis would be confirmed as a potential target for novel therapeutic management of uterine leiomyoma.
Supplementary Material
Footnotes
This work was supported by grants from the Centre National de la Recherche Scientifique and the Université Paris Sud 11. F.D.Z. received an Erasmus fellowship from the European Union.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 23, 2009
Abbreviations: Bmax, Maximal binding capacity; ET, endothelin; ETR, ET receptor; IP, inositol phosphate; KD, affinity constant; PLC, phospholipase C; PTX, pertussis toxin; siRNA, small interfering RNA; TSC2, tuberous sclerosis 2.
References
- Davenport AP 2002 International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol Rev 54:219–226 [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Polokoff MA 1994 Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 46:325–415 [PubMed] [Google Scholar]
- Levin ER 1995 Endothelins. N Engl J Med 333:356–363 [DOI] [PubMed] [Google Scholar]
- Pla P, Larue L 2003 Involvement of endothelin receptors in normal and pathological development of neural crest cells. Int J Dev Biol 47:315–325 [PubMed] [Google Scholar]
- Cameron IT, Bacon CR, Collett GP, Davenport AP 1995 Endothelin expression in the uterus. J Steroid Biochem Mol Biol 53:209–214 [DOI] [PubMed] [Google Scholar]
- Bacon CR, Morrison JJ, O'Reilly G, Cameron IT, Davenport AP 1995 ETA and ETB endothelin receptors in human myometrium characterized by the subtype selective ligands BQ123, BQ3020, FR139317 and PD151242. J Endocrinol 144:127–134 [DOI] [PubMed] [Google Scholar]
- Héluy V, Germain G, Fournier T, Ferré F, Breuiller-Fouché M 1995 Endothelin ETA receptors mediate human uterine smooth muscle contraction. Eur J Pharmacol 285:89–94 [DOI] [PubMed] [Google Scholar]
- Khac LD, Naze S, Harbon S 1994 Endothelin receptor type A signals both the accumulation of inositol phosphates and the inhibition of cyclic AMP generation in rat myometrium: stimulation and desensitization. Mol Pharmacol 46:485–494 [PubMed] [Google Scholar]
- Robin P, Boulven I, Desmyter C, Harbon S, Leiber D 2002 ET-1 stimulates ERK signaling pathway through sequential activation of PKC and Src in rat myometrial cells. Am J Physiol Cell Physiol 283:C251–C260 [DOI] [PubMed] [Google Scholar]
- Bagnato A, Rosanò L 2008 The endothelin axis in cancer. Int J Biochem Cell Biol 40:1443–1451 [DOI] [PubMed] [Google Scholar]
- Nelson J, Bagnato A, Battistini B, Nisen P 2003 The endothelin axis: emerging role in cancer. Nat Rev Cancer 3:110–116 [DOI] [PubMed] [Google Scholar]
- Smollich M, Wülfing P 2007 The endothelin axis: a novel target for pharmacotherapy of female malignancies. Curr Vasc Pharmacol 5:239–248 [DOI] [PubMed] [Google Scholar]
- Carducci MA, Jimeno A 2006 Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. Clin Cancer Res 12:6296s–6300s [DOI] [PubMed] [Google Scholar]
- Sozen I, Arici A 2002 Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril 78:1–12 [DOI] [PubMed] [Google Scholar]
- Maruo T, Ohara N, Wang J, Matsuo H 2004 Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update 10:207–220 [DOI] [PubMed] [Google Scholar]
- Walker CL 2002 Role of hormonal and reproductive factors in the etiology and treatment of uterine leiomyoma. Recent Prog Horm Res 57:277–294 [DOI] [PubMed] [Google Scholar]
- Nierth-Simpson EN, Martin MM, Chiang TC, Melnik LI, Rhodes LV, Muir SE, Burow ME, McLachlan JA 2009 Human uterine smooth muscle and leiomyoma cells differ in their rapid 17β-estradiol signaling: implications for proliferation. Endocrinology 150:2436–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuiller-Fouché M, Honoré JC, Robert B, Fournier T, Vacher-Lavenu MC, Chapron C, Dubuisson JB, Ferré F 1999 [Endothelin receptors in benign human tumours of uterine muscle]. Bull Cancer 86:773–778 [PubMed] [Google Scholar]
- Pekonen F, Nyman T, Rutanen EM 1994 Differential expression of mRNAs for endothelin-related proteins in human endometrium, myometrium and leiomyoma. Mol Cell Endocrinol 103:165–170 [DOI] [PubMed] [Google Scholar]
- Honoré JC, Robert B, Vacher-Lavenu MC, Chapron C, Breuiller-Fouché M, Ferré F 2000 Expression of endothelin receptors in human myometrium during pregnancy and in uterine leiomyomas. J Cardiovasc Pharmacol 36:S386–S389 [DOI] [PubMed] [Google Scholar]
- Weston G, Trajstman AC, Gargett CE, Manuelpillai U, Vollenhoven BJ, Rogers PA 2003 Fibroids display an anti-angiogenic gene expression profile when compared with adjacent myometrium. Mol Hum Reprod 9:541–549 [DOI] [PubMed] [Google Scholar]
- Cesen-Cummings K, Houston KD, Copland JA, Moorman VJ, Walker CL, Davis BJ 2003 Uterine leiomyomas express myometrial contractile-associated proteins involved in pregnancy-related hormone signaling. J Soc Gynecol Investig 10:11–20 [PubMed] [Google Scholar]
- Hunter DS, Klotzbücher M, Kugoh H, Cai SL, Mullen JP, Manfioletti G, Fuhrman U, Walker CL 2002 Aberrant expression of HMGA2 in uterine leiomyoma associated with loss of TSC2 tumor suppressor gene function. Cancer Res 62:3766–3772 [PubMed] [Google Scholar]
- Wei J, Chiriboga L, Mizuguchi M, Yee H, Mittal K 2005 Expression profile of tuberin and some potential tumorigenic factors in 60 patients with uterine leiomyomata. Mod Pathol 18:179–188 [DOI] [PubMed] [Google Scholar]
- Wei JJ, Chiriboga L, Mittal K 2005 Expression profile of the tumorigenic factors associated with tumor size and sex steroid hormone status in uterine leiomyomata. Fertil Steril 84:474–484 [DOI] [PubMed] [Google Scholar]
- Howe SR, Gottardis MM, Everitt JI, Goldsworthy TL, Wolf DC, Walker C 1995 Rodent model of reproductive tract leiomyomata. Establishment and characterization of tumor-derived cell lines. Am J Pathol 146:1568–1579 [PMC free article] [PubMed] [Google Scholar]
- Robin P, Chouayekh S, Bole-Feysot C, Leiber D, Tanfin Z 2005 Contribution of phospholipase D in endothelin-1-mediated extracellular signal-regulated kinase activation and proliferation in rat uterine leiomyoma cells. Biol Reprod 72:69–77 [DOI] [PubMed] [Google Scholar]
- Raymond MN, Bole-Feysot C, Banno Y, Tanfin Z, Robin P 2006 Endothelin-1 inhibits apoptosis through a sphingosine kinase 1-dependent mechanism in uterine leiomyoma ELT3 cells. Endocrinology 147:5873–5882 [DOI] [PubMed] [Google Scholar]
- Boulven I, Palmier B, Robin P, Vacher M, Harbon S, Leiber D 2001 Platelet-derived growth factor stimulates phospholipase C-γ1, extracellular signal-regulated kinase, and arachidonic acid release in rat myometrial cells: contribution to cyclic 3′,5′-adenosine monophosphate production and effect on cell proliferation. Biol Reprod 65:496–506 [DOI] [PubMed] [Google Scholar]
- Williams Jr DL, Jones KL, Pettibone DJ, Lis EV, Clineschmidt BV 1991 Sarafotoxin S6c: an agonist which distinguishes between endothelin receptor subtypes. Biochem Biophys Res Commun 175:556–561 [DOI] [PubMed] [Google Scholar]
- Aramori I, Nirei H, Shoubo M, Sogabe K, Nakamura K, Kojo H, Notsu Y, Ono T, Nakanishi S 1993 Subtype selectivity of a novel endothelin antagonist, FR139317, for the two endothelin receptors in transfected Chinese hamster ovary cells. Mol Pharmacol 43:127–131 [PubMed] [Google Scholar]
- Ishikawa K, Ihara M, Noguchi K, Mase T, Mino N, Saeki T, Fukuroda T, Fukami T, Ozaki S, Nagase T, Nishikibe M, Yano M 1994 Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci USA 91:4892–4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BG, Phuong LL, Farhat H, Chevalier D 2003 Both endothelin-A and endothelin-B receptors are present on adult rat cardiac ventricular myocytes. Can J Physiol Pharmacol 81:95–104 [DOI] [PubMed] [Google Scholar]
- Henry PJ, King SH 1999 Typical endothelin ETA receptors mediate atypical endothelin-1-induced contractions in sheep isolated tracheal smooth muscle. J Pharmacol Exp Ther 289:1385–1390 [PubMed] [Google Scholar]
- Wu-Wong JR, Chiou WJ, Dixon DB, Opgenorth TJ 1995 Dissociation characteristics of endothelin ETA receptor agonists and antagonists. J Cardiovasc Pharmacol 26(Suppl 3):S380–S384 [PubMed] [Google Scholar]
- Wu-Wong JR, Chiou WJ, Magnuson SR, Opgenorth TJ 1994 Endothelin receptor agonists and antagonists exhibit different dissociation characteristics. Biochim Biophys Acta 1224:288–294 [DOI] [PubMed] [Google Scholar]
- Héluy V, Breuiller-Fouché M, Cavaillé F, Fournier T, Ferré F 1995 Characterization of type A endothelin receptors in cultured human myometrial cells. Am J Physiol 268:E825–E831 [DOI] [PubMed] [Google Scholar]
- Fomin VP, Cox BE, Word RA 1999 Effect of progesterone on intracellular Ca2+ homeostasis in human myometrial smooth muscle cells. Am J Physiol 276:C379–C385 [DOI] [PubMed] [Google Scholar]
- Isaka M, Takaoka K, Yamada Y, Abe Y, Kitazawa T, Taneike T 2000 Characterization of functional endothelin receptors in the porcine myometrium. Peptides 21:543–551 [DOI] [PubMed] [Google Scholar]
- Blomstrand F, Giaume C, Hansson E, Rönnback L 1999 Distinct pharmacological properties of ET-1 and ET-3 on astroglial gap junctions and Ca(2+) signaling. Am J Physiol 277:C616–C627 [DOI] [PubMed] [Google Scholar]
- Hasselblatt M, Kamrowski-Kruck H, Jensen N, Schilling L, Kratzin H, Sirén AL, Ehrenreich H 1998 ETA and ETB receptor antagonists synergistically increase extracellular endothelin-1 levels in primary rat astrocyte cultures. Brain Res 785:253–261 [DOI] [PubMed] [Google Scholar]
- Ehrenreich H 1999 The astrocytic endothelin system: toward solving a mystery focus on “distinct pharmacological properties of ET-1 and ET-3 on astroglial gap junctions and Ca(2+) signaling.” Am J Physiol 277:C614–C615 [DOI] [PubMed] [Google Scholar]
- Harada N, Himeno A, Shigematsu K, Sumikawa K, Niwa M 2002 Endothelin-1 binding to endothelin receptors in the rat anterior pituitary gland: possible formation of an ETA-ETB receptor heterodimer. Cell Mol Neurobiol 22:207–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans NJ, Walker JW 2008 Sustained Ca2+ signaling and delayed internalization associated with endothelin receptor heterodimers linked through a PDZ finger. Can J Physiol Pharmacol 86:526–535 [DOI] [PubMed] [Google Scholar]
- Evans NJ, Walker JW 2008 Endothelin receptor dimers evaluated by FRET, ligand binding, and calcium mobilization. Biophys J 95:483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregan B, Jürgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A 2004 Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem 279:27679–27687 [DOI] [PubMed] [Google Scholar]
- George SR, O'Dowd BF 2007 A novel dopamine receptor signaling unit in brain: heterooligomers of D1 and D2 dopamine receptors. ScientificWorldJournal 7:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh A, Windischhofer W, Kovacevic A, DeVaney T, Huber E, Semlitsch M, Leis HJ, Sattler W, Malle E 2008 Endothelin (ET)-1 and ET-3 promote expression of c-fos and c-jun in human choriocarcinoma via ET(B) receptor-mediated G(i)- and G(q)-pathways and MAP kinase activation. Br J Pharmacol 154:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato A, Rosanò L, Spinella F, Di Castro V, Tecce R, Natali PG 2004 Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Cancer Res 64:1436–1443 [DOI] [PubMed] [Google Scholar]
- Lahav R, Heffner G, Patterson PH 1999 An endothelin receptor B antagonist inhibits growth and induces cell death in human melanoma cells in vitro and in vivo. Proc Natl Acad Sci USA 96:11496–11500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav R, Suvà ML, Rimoldi D, Patterson PH, Stamenkovic I 2004 Endothelin receptor B inhibition triggers apoptosis and enhances angiogenesis in melanomas. Cancer Res 64:8945–8953 [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosanò L, Di Castro V, Decandia S, Nicotra MR, Natali PG, Bagnato A 2007 Endothelin-1 and endothelin-3 promote invasive behavior via hypoxia-inducible factor-1α in human melanoma cells. Cancer Res 67:1725–1734 [DOI] [PubMed] [Google Scholar]
- Anguelova E, Beuvon F, Leonard N, Chaverot N, Varlet P, Couraud PO, Daumas-Duport C, Cazaubon S 2005 Functional endothelin ET B receptors are selectively expressed in human oligodendrogliomas. Brain Res Mol Brain Res 137:77–88 [DOI] [PubMed] [Google Scholar]
- Curatolo P, Bombardieri R, Jozwiak S 2008 Tuberous sclerosis. Lancet 372:657–668 [DOI] [PubMed] [Google Scholar]
- Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, Panettieri Jr RA, Krymskaya VP 2002 Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. J Biol Chem 277:30958–30967 [DOI] [PubMed] [Google Scholar]
- Finlay GA, Hunter DS, Walker CL, Paulson KE, Fanburg BL 2003 Regulation of PDGF production and ERK activation by estrogen is associated with TSC2 gene expression. Am J Physiol Cell Physiol 285:C409–C418 [DOI] [PubMed] [Google Scholar]
- Isobe A, Takeda T, Sakata M, Miyake A, Yamamoto T, Minekawa R, Nishimoto F, Okamoto Y, Walker CL, Kimura T 2008 Dual repressive effect of angiotensin II-type 1 receptor blocker telmisartan on angiotensin II-induced and estradiol-induced uterine leiomyoma cell proliferation. Hum Reprod 23:440–446 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.