Abstract
Recent evidence suggests that hormones such as insulin and leptin act in the hypothalamus to regulate energy balance and glucose metabolism. Here we show that in leptin receptor-deficient Koletsky (fak/fak) rats, adenovirally induced expression of leptin receptors in the area of the hypothalamic arcuate nucleus improved peripheral insulin sensitivity via enhanced suppression of hepatic glucose production, with no change of insulin-stimulated glucose uptake or disposal. This effect was associated with increased insulin signal transduction via phosphatidylinositol-3-OH kinase (as measured by pY-insulin receptor substrate-1 and pS-PKB/Akt) in liver, but not skeletal muscle, and with reduced hepatic expression of the gluconeogenic genes, glucose-6-phosphatase and phosphoenolpyruvate kinase. Moreover, the beneficial effects of hypothalamic leptin signaling on hepatic insulin sensitivity were blocked by selective hepatic vagotomy. We conclude that hypothalamic leptin action increases peripheral insulin sensitivity primarily via effects on the liver and that the mechanism underlying this effect is dependent on the hepatic branch of the vagus nerve.
Hypothalamic leptin signaling improves hepatic insulin sensitivity via vagal input to the liver.
Growing evidence suggests that the brain receives input from both nutrient-related and hormonal signals such as insulin and leptin that convey information regarding levels of circulating energy substrates as well as fuel stored in the form of fat. In response to this input, key brain areas such as the hypothalamus activate pathways that regulate food intake, energy expenditure, autonomic function, and glucose metabolism to maintain both energy and glucose homeostasis (1). Consequently, conditions associated with reduced or defective adiposity signaling are predicted to favor both increased food intake and insulin resistance in peripheral tissues. Although several studies have investigated the control of glucose metabolism by hypothalamic insulin action, less is known about how leptin affects insulin sensitivity.
In addition to its well-known effects in peripheral tissues, recent studies support a role for brain insulin action in the regulation of glucose homeostasis. For example, neuron-specific insulin receptor (IR)- and insulin receptor substrate (IRS)-2-deficient mice are characterized by mild obesity and insulin resistance that is at least partially independent of increased body fat (2,3,4). Furthermore, inducible inactivation of the IR in both the brain and peripheral tissues in mice causes a more pronounced hyperglycemia compared with peripheral tissues alone (5). Consistent with these studies, both intrahypothalamic administration of antisense oligonucleotides to reduce IR signaling and intracerebroventricular (icv) infusion of an inhibitor of phosphatidylinositol-3-OH kinase (PI3K; a major intracellular mediator in insulin action) cause insulin resistance in rats (6), whereas conversely, infusion of insulin either into ventricular cerebrospinal fluid or directly into the hypothalamic arcuate nucleus (ARC) improved insulin sensitivity via enhanced insulin suppression of hepatic glucose production (HGP), rather than increased glucose uptake (7).
Like insulin, leptin signaling in the brain is also implicated in the control of insulin sensitivity in peripheral tissues. Models of genetic leptin deficiency (ob/ob or lipodystrophic mice) are characterized by severe insulin resistance and type 2 diabetes (8,9), and leptin treatment ameliorates these conditions via a mechanism that involves the central nervous system (CNS) and cannot be explained by changes of food intake (9,10,11). These observations are further supported by our recently published evidence that restoring leptin signaling to the hypothalamic ARC of leptin receptor-deficient Koletsky (fak/fak) rats improves their insulin sensitivity via a mechanism that is, at least in part, independent of food intake and body weight (12). Similarly, Coppari and colleagues (13) reported a dramatic improvement in glucose homeostasis in leptin receptor-deficient mice in which leptin signaling was selectively restored to the ARC. Thus, intact neuronal signaling by both insulin and leptin appear to be important for maintenance of normal insulin sensitivity.
One hypothesis forwarded to explain how these circulating signals act in the CNS to regulate insulin sensitivity proposes that key subsets of hypothalamic neurons regulate HGP via descending projections to hindbrain areas that control autonomic outflow to the liver via the vagus nerve. Consistent with this, the ability of hypothalamic insulin and free fatty acids (FFAs) to suppress HGP is blocked by hepatic branch vagotomy but unaffected by selective vagal deafferentiation (14,15).
Based on these observations, we hypothesized that the effect of ARC-directed leptin receptor gene therapy to increase insulin sensitivity in obese Koletsky rats is mediated via enhanced insulin-induced suppression of HGP, rather than by increased glucose uptake. To test this hypothesis, we directed expression of either the signaling form of the leptin receptor or a reporter gene to the ARC of obese Koletsky rats and measured insulin sensitivity using the euglycemic-hyperinsulinemic clamp technique. Our results demonstrate that restored hypothalamic leptin signaling enhanced insulin-induced suppression of HGP and that this effect was blocked by hepatic vagotomy (HV). The mechanism underlying this effect appears to involve increased hepatic insulin signal transduction via PI3K, resulting in reduced gluconeogenic gene expression. Taken together, our findings support the existence of a brain-liver neurocircuit that plays an important role in the regulation of glucose metabolism by leptin.
Materials and Methods
Experimental animals
Adult male obese (fak/fak) Koletsky rats (Vassar College, Poughkeepsie, NY) were generated from serial backcrosses (N10 equivalent) of the fak mutation to the inbred rat strain, LA/N. Additional obese Koletsky rats were obtained from Charles River Laboratories (Wilmington, MA). All animals were housed individually in a specific pathogen-free environment, maintained in a temperature-controlled room with a 12-h light, 12-h dark cycle and provided with ad libitum access to water and standard laboratory chow (PMI Nutrition International Inc., Brentwood, MO), unless otherwise stated. All study protocols were approved by the Animal Care and Use Committee at the University of Washington and conducted in accordance with National Institutes of Health guidelines for the care and use of laboratory animals.
Adenovirus microinjection
Adenovirus (Ad) expressing either green fluorescent protein (GFP; 2.7 × 1012 pfu/ml) or a construct that contains the mouse leprb and also expresses GFP (Ad-LEPR-B; 4.7 × 1012 pfu/ml) was microinjected bilaterally into the ARC of obese Koletsky rats under isoflurane anesthesia as previously described (n = 6–8/group) (12,16,17,18). Bilateral microinjection of adenovirus and implantation of an indwelling catheter in both the right internal jugular vein and the left carotid artery were performed during the same surgical session 7 d before testing via the euglycemic-hyperinsulinemic clamp technique. Buprenorphine hydrochloride (0.3 mg/kg; Reckett Colman Pharmaceuticals, Richmond, VA) was administered at the completion of the surgery. Upon study completion, anatomical distribution of adenoviral gene expression was assessed by visualization of GFP in coronal brain sections by fluorescent microscopy, which permits detection of either adenoviral construct (Ad-GFP or Ad-LEPR-B-GFP). Animals in which the ARC was not successfully targeted (<10%) were removed from the study.
Selective hepatic branch vagotomy
Before adenoviral injection and implantation of iv catheters, a subgroup of obese Koletsky rats were subjected to selective HV or a sham operation (Sham) to generate four treatment groups: 1) Ad-GFP-Sham, 2) Ad-GFP-HV, 3) Ad-LEPR-B-Sham, and 4) Ad-LEPR-B-HV (n = 4–6/group). Briefly, a laparotomy incision was made on the ventral midline and the abdominal muscle wall opened, revealing the gastrointestinal tract in the peritoneum. The gastrohepatic ligament was severed, and the stomach was gently retracted onto sterile saline soaked cotton gauze, revealing the descending ventral esophagus and the ventral subdiaphragmatic vagal trunk. The hepatic branch of this vagal trunk was visualized using a neurosurgical dissecting scope under ×10–20 magnification, and the hepatic branch of the vagus was ligated using two 6-0 silk ties. The hepatic nerve trunk was then transected by microcautery between the two sutures, severing and cauterizing the hepatic vagus, thereby minimizing the possibility of regeneration. The abdominal muscle wall incision was closed, and the skin incision was closed with stainless steel wound clips.
Body composition analysis
Determinations of body lean mass and fat mass were made in conscious rats both the day before adenoviral microinjection and euglycemic-hyperinsulinemic clamp studies using quantitative magnetic resonance (EchoMRI body composition analyzer; Echo Medical Systems, Houston, TX).
Euglycemic-hyperinsulinemic clamps
Six days after adenoviral microinjections, 24-wk-old obese Koletsky rats were provided with 6 g of food at dark cycle onset and then fasted overnight. This early time point for metabolic studies was selected because it preceded the effect of ARC-directed leptin receptor gene therapy to reduce food intake and body weight. Animals were placed into a clear animal enclosure with bedding and connected to a rat infusion system (Instech Solomon, Plymouth Meeting, PA) to allow simultaneous sampling from the artery and infusion into the vein in a conscious, unrestrained animal. The clamp protocol consisted of a 120-min tracer equilibration period (t = −120 to 0 min) followed by a 120-min experimental period (t = 0–120 min). A blood sample was obtained at t = −120 min for determination of fasting plasma glucose, insulin, leptin, and FFAs. A 24-μCi prime of [3-3H]glucose was given at t = −120 min for 3 min followed by a continuous 0.2-μCi/min infusion for 2 h. At t = −30, −20, −10, and 0 min, blood samples of 80 μl were taken for determination of basal glucose turnover. Two hours after the basal period, a primed continuous infusion of regular human insulin (60 mU/kg bolus followed by 5 mU/kg · min HumulinR; Eli Lilly, Indianapolis, IN) was administered at t = 0 min. In studies determining whether vagal outflow to the liver is required for the effect of hypothalamic leptin signaling on peripheral insulin sensitivity, a primed continuous infusion of human insulin at 2.5 mU/kg · min was used instead because these studies were performed in 12-wk, relatively more insulin-sensitive Koletsky rats (mean body weight ∼350 g). The [3-3H]glucose infusion was increased to 0.3 μCi/min (at t = 0 min) for the remainder of the experiment (t = 120 min) to keep specific activity constant. During the clamp, glucose levels were determined every 10 min using a handheld glucometer (Accu-Chek; Roche, Indianapolis, IN) and maintained at about 110 mg/dl by infusion of a 50% dextrose solution as needed. Plasma insulin levels and clamp glucose turnover rates were calculated from 80-μl blood samples drawn at 10-min intervals during the last 30 min of the clamp.
Processing of plasma samples
Plasma for [3-3H]glucose determinations was deproteinized with Ba(OH)2 and ZnSO4 and then dried overnight at 60 C. Plasma glucose levels were measured using a GM9D glucose direct analyzer (Analox Instruments, Ltd., London, UK). Plasma immunoreactive insulin and leptin levels were determined by ELISA (Crystal Chem, Chicago, IL). FFAs were measured using a colorimetric assay kit that relies on fatty acid as substrate for enzymatic acylation of CoA (WAKO Chemicals, Richmond, VI), whereas plasma corticosterone levels were measured using an enzyme immunoassay (Diagnostics Systems Laboratories, Webster, TX).
Tissue processing and biochemical analysis
We previously demonstrated that hypothalamic leptin signaling improves peripheral insulin sensitivity, as measured by an insulin tolerance test (12). In this same group of animals, 16 d after adenoviral microinjection, animals were fasted overnight and received an ip injection of either insulin (10 U/kg; HumulinR; Eli Lilly) or vehicle. Twenty minutes later, animals were euthanized, and liver and skeletal muscle (gastrocnemius) were immediately excised and snap frozen for subsequent analysis.
Muscle and liver were homogenized in T-Per lysis buffer (10 μl/mg tissue; Pierce, Rockford, IL) supplemented with protease and phosphatase inhibitor cocktails (Roche Diagnostics). Homogenates were centrifuged, pellets discarded, and supernatants retained for determination of protein content using a Micro BCA protein assay kit (Pierce), and equal amounts of protein were used for each condition in each assay. Tyrosine phosphorylation (pY) of IRS-1 was assessed by Western blot using a monoclonal antiphosphotyrosine antibody (Cell Signaling Technology, Beverly, MA) after immunoprecipitation with an anti-IRS-1 antibody (Cell Signaling Technology, Beverly, MA) and SDS-PAGE of liver and muscle extracts. The membranes were stripped and reprobed with an anti-IRS-1 antibody to verify equal amounts of total IRS-1 protein. IRS-1 tyrosine phosphorylation protein bands were quantified by densitometry using Image J software (National Institutes of Health, Bethesda, MD), whereas activation of PI3K signal transduction was assessed by measuring serine phosphorylation of Akt (residue 473), respectively, using an ELISA (Invitrogen, Camarillo, CA).
RT-PCR
Total RNA was extracted from liver and muscle using TRIzol B according to the manufacturers’ instructions (MRC, Cincinnati, OH), quantitated by spectrophotometry at 260 nm, reverse transcribed (1 μg) with avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI) and real-time PCR performed on a ABI Prism 7900 HT (Applied Biosystems, Foster City, CA) as previously described (16). Expression levels of each gene were normalized to a housekeeping gene (18S RNA) and expressed as a percentage of controls. Nontemplate controls were incorporated into each PCR run.
Triglyceride content
Liver and muscle tissue triglyceride content was measured in frozen tissues samples collected as described above using the method of Folch et al. (19) for lipid extraction followed by spectrophotometric measurement of triglyceride content (Thermo Electron, Louisville, CO). In some animals, liver triglyceride levels were also determined using the Echo 3-in-1 MRI analyzer (Echo Medical Systems, Houston, TX).
Calculations
After deproteinization with ZnSO4 and Ba(OH)2 and dried 12 h at 60 C, plasma [3-3H]glucose radioactivity was determined by liquid scintillation on a Tri-Carb 2810 (Beckman, Fullerton, CA) (20). Sample radioactivity divided by plasma glucose concentration gives the plasma glucose-specific activity. Glucose rate of appearance (Ra) and rate of disposal (Rd) were calculated by using (Steele’s) non-steady-state equations. Endogenous Ra was determined by subtracting the glucose infusion rate (GIR) from the Ra.
Statistical analysis
All results are expressed as mean ± sem. Statistical analyses were performed using Statistica (version 7.1; StatSoft, Inc., Tulsa, OK). A one-way ANOVA with a least significant differences post hoc test was used to compare mean values between multiple groups, and a two-sample unpaired student’s t test was used for two-group comparisons. In all instances, P < 0.05 was considered significant.
Results
Effect of ARC-directed leptin receptor gene therapy on insulin sensitivity
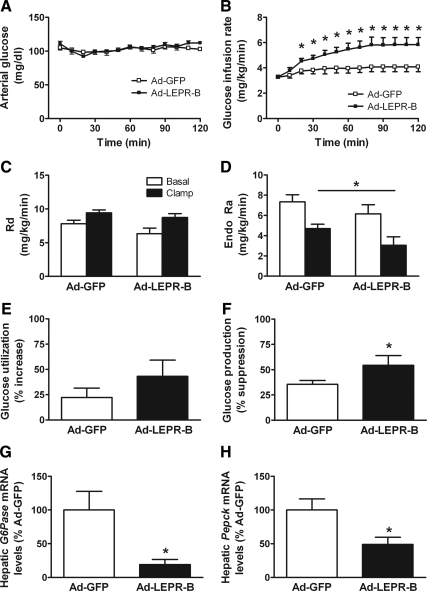
To determine the effect of hypothalamic leptin action on peripheral insulin sensitivity, we performed euglycemic-hyperinsulinemic clamp experiments in obese Koletsky rats (mean body weight 715 g) 7 d after bilateral microinjection of adenovirus expressing either GFP or LEPR-B directed to the ARC, at a time there was no significant differences between groups with respect to body weight, food intake, or body composition (Table 1). Consistent with our previously reported results, basal measures of plasma glucose, insulin, leptin, and FFAs were similar between groups after an overnight fast (Table 1). During the clamp procedure, arterial glucose (Fig. 1A) and plasma insulin levels were similar between the two groups (P = ns) (Table 1). However, the GIR required to maintain euglycemia was increased by 43% in animals that received Ad-LEPR-B compared with those receiving Ad-GFP (Fig. 1B). These data confirm our previous findings that restored hypothalamic leptin signaling improves peripheral insulin sensitivity (12).
Table 1.
Basal and clamp characteristics in 24-wk-old obese Koletsky rats 7 d after bilateral microinjection of an adenovirus expressing either Ad-LEPR-B or Ad-GFP to the area of the ARC
| Ad-GFP | Ad-LEPR-B | |
|---|---|---|
| n | 5 | 5 |
| Basal | ||
| Δ Body weight (g) | −18.9 ± 3.3 | −22.9 ± 5.2 |
| Food intake (g/d) | 18.2 ± 1.1 | 16.7 ± 1.1 |
| Fat (%) | 41.2 ± 1.0 | 42.2 ± 0.7 |
| Arterial plasma glucose (mg/dl) | 122.0 ± 3.9 | 120.1 ± 3.4 |
| Plasma insulin (ng/ml) | 14.2 ± 4.7 | 11.7 ± 2.3 |
| Plasma leptin (ng/ml) | 161.3 ± 11.5 | 144.7 ± 7.4 |
| Plasma FFAs (mmol/liter) | 1.00 ± 0.14 | 0.90 ± 0.10 |
| Plasma corticosterone (ng/ml) | 806 ± 163 | 677 ± 147 |
| Clamp | ||
| Arterial plasma glucose (mg/dl) | 114.9 ± 3.2 | 124.0 ± 4.7 |
| Plasma insulin (ng/ml) | 27.9 ± 4.5 | 22.9 ± 6.4 |
| Plasma FFA (mmol/liter) | 0.72 ± 0.10 | 0.51 ± 0.06 |
| Plasma corticosterone (ng/ml) | 619 ± 125 | 597 ± 89 |
| GIR (mg/kg · min) | 4.08 ± 0.38 | 5.84 ± 0.57a |
, P < 0.05 vs. Ad-GFP.
Figure 1.
ARC-directed leptin receptor gene therapy improves insulin sensitivity via increased suppression of endogenous glucose production. Arterial glucose (A) and GIR (B) required to maintain euglycemia during the euglycemic-hyperinsulinemic clamp in obese Koletsky rats that received Ad-GFP or Ad-LEPR-B directed to the ARC. Rd (C) and the endogenous rate of glucose appearance (Endo Ra) (D) in Ad-GFP- and Ad-LEPR-B-treated animals during the basal (open squares) and clamp (closed squares) period. Effect of ARC-directed leptin receptor gene therapy on percent increase in glucose uptake or disposal (E), percent suppression of hepatic glucose production (F), and hepatic mRNA levels of the gluconeogenic genes G6Pase (G) and Pepck (H) (n = 5/group). *, P < 0.05 vs. Ad-GFP.
During both the basal and the clamp periods, differences in the rate of glucose uptake or disposal measured using tracer dilution techniques were not detected between animals that received Ad-LEPR-B compared with Ad-GFP (Fig. 1C). In contrast, whereas basal levels of endogenous Ra were similar, the rate of endogenous Ra was significantly reduced during the clamp in animals that received Ad-LEPR-B compared with Ad-GFP (Fig. 1D), such that insulin-mediated suppression of glucose production (GP) was enhanced (P < 0.05) (Fig. 1F). These data suggest that restoration of leptin receptor signaling to the area of the ARC improves insulin sensitivity of Koletsky rats by increasing insulin-mediated suppression of HGP. To assess the extent to which clamp values were affected by stress associated with the procedure, we measured plasma corticosterone levels throughout the clamp. Because mean corticosterone levels were not different between the groups (Table 1), procedure-related stress was unlikely to have influenced outcomes.
To gain further insight into the mechanism by which leptin acts in the hypothalamus to suppress HGP, we measured expression of two key gluconeogenic genes, glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate kinase (Pepck), in liver samples obtained at the completion of the clamp. Relative to Ad-GFP-treated animals, the hepatic expression of G6Pase and Pepck were significantly lower (by 80 and 51%, respectively) in Ad-LEPR-B-treated compared with Ad-GFP-treated rats (P < 0.05 for each) (Fig. 1, G and H). Thus, the effect of restored hypothalamic leptin signaling in Koletsky rats to increase insulin-mediated suppression of HGP is associated with reduced gluconeogenic gene expression.
Role of vagal outflow in effect of ARC-directed leptin receptor gene therapy to suppress GP
We next sought to determine whether the effect of ARC-directed leptin receptor gene therapy to suppress GP involves vagal innervation of the liver. To accomplish this, groups of obese Koletsky rats (mean body weight 342 g) were subjected to either selective HV or a sham operation before ARC-directed microinjection of adenovirus expressing either GFP or LEPR-B, followed 7 d later by performance of clamps as described above. The baseline metabolic characteristics of the experimental rats were similar in each group (Table 2). During the clamp procedure, arterial glucose (Fig. 2A), plasma insulin, FFAs, and corticosterone concentrations were similar in all groups (Table 2). Consistent with our previous experiment, the GIR required to maintain euglycemia was significantly increased in sham animals that received Ad-LEPR-B compared with Ad-GFP directed to the ARC (Fig. 2B). Whereas HV had no effect on GIR in Ad-GFP-treated animals, HV blocked the increase of GIR in Ad-LEPR-B-treated sham controls (Fig. 2B). We next examined whether the vagus-dependent increase in GIR induced by hypothalamic leptin signaling was due to effects on glucose uptake or disposal or HGP. Consistent with our earlier observations, insulin-induced suppression of HGP was increased in sham-treated animals that received Ad-LEPR-B compared with Ad-GFP (P < 0.05) (Fig. 2F). Whereas the suppression of HGP was similar in Ad-GFP-sham and -HV-treated animals, the increase of HGP suppression induced by hypothalamic leptin receptor gene therapy was blocked by HV (P < 0.05) (Fig. 2F). In contrast, the rate of glucose uptake or disposal was not affected by either Ad-LEPR-B-treatment or HV (P = ns) (Fig. 2E). Moreover, hepatic expression of G6Pase and Pepck mRNA was reduced by hypothalamic leptin receptor gene therapy in sham-operated animals (P < 0.05 for each) but not in animals subjected to HV (Fig. 2, G and H).
Table 2.
Basal and clamp characteristics in 12-wk-old obese Koletsky rats 7 d after bilateral microinjection of an adenovirus expressing either Ad-LEPR-B or Ad-GFP to the area of the ARC
| Ad-GFP-SHAM | Ad-GFP-HV | Ad-LEPR-B-SHAM | Ad-LEPR-B-HV | |
|---|---|---|---|---|
| n | 5 | 5 | 5 | 6 |
| Basal | ||||
| Δ Body weight (g) | 3.2 ± 12.0 | 3.3 ± 6.2 | −8.7 ± 2.6 | 1.3 ± 2.5 |
| Food intake (g/d) | 28.0 ± 2.4 | 27.8 ± 0.8 | 24.5 ± 1.9 | 27.6 ± 0.9 |
| Fat (%) | 38.0 ± 1.1 | 36.8 ± 2.0 | 36.7 ± 2.9 | 37.7 ± 1.6 |
| Arterial plasma glucose (mg/dl) | 115.6 ± 8.1 | 110.3 ± 4.9 | 112.0 ± 9.1 | 121.0 ± 7.3 |
| Plasma insulin (ng/ml) | 11.6 ± 2.4 | 11.4 ± 1.5 | 9.7 ± 2.6 | 8.2 ± 1.3 |
| Plasma leptin (ng/ml) | 80.5 ± 6.5 | 67.6 ± 8.9 | 69.4 ± 20.5 | 60.1 ± 5.7 |
| Plasma FFAs (mmol/liter) | 1.45 ± 0.38 | 1.26 ± 0.08 | 1.36 ± 0.16 | 1.63 ± 0.24 |
| Plasma corticosterone (ng/ml) | 695 ± 102 | 807 ± 69 | 591 ± 182 | 773 ± 172 |
| Clamp | ||||
| Arterial plasma glucose (mg/dl) | 116.4 ± 3.9 | 115.4 ± 1.6 | 121.4 ± 5.5 | 122.4 ± 1.5 |
| Plasma insulin (ng/ml) | 14.7 ± 2.6 | 13.5 ± 1.4 | 13.3 ± 3.4 | 11.4 ± 1.3 |
| Plasma FFAs (mmol/liter) | 1.04 ± 0.38 | 0.87 ± 0.16 | 0.90 ± 0.17 | 1.01 ± 0.12 |
| Plasma corticosterone (ng/ml) | 636 ± 51 | 674 ± 254 | 673 ± 249 | 869 ± 210 |
| GIR (mg/kg · min) | 7.08 ± 0.34 | 6.57 ± 0.66 | 9.04 ± 0.32a | 6.25 ± 0.77 |
, P < 0.05 vs. Ad-GFP-SHAM.
Figure 2.
Restoring hypothalamic ARC leptin receptor signaling improves hepatic insulin action via the hepatic branch of the vagus nerve. Arterial glucose (A) and GIR (B) required to maintain euglycemia during the euglycemic-hyperinsulinemic clamp in obese Koletsky rats that underwent either sham surgery or selective HV and then received Ad-GFP or Ad-LEPR-B directed to the ARC. Rd (C) and the endogenous rate of glucose appearance (Endo Ra) (D) in Ad-GFP- and Ad-LEPR-B-treated animals during the basal (shaded squares) and the clamp period in animals that received sham surgery (open squares) or HV (closed squares). Effect of selective HV compared with sham surgery in animals that received either Ad-GFP or Ad-LEPR-B on percent increase in glucose use (E), percent suppression of hepatic glucose production (F), and hepatic mRNA levels of the gluconeogenic genes G6Pase (G) and Pepck (H) (n = 5–6/group). *, P < 0.05 vs. Ad-GFP-sham.
Effect of hypothalamic leptin signaling on hepatic insulin signal transduction
The results from the clamp studies led us to ask whether hypothalamic leptin signaling enhances insulin signal transduction selectively in liver and not skeletal muscle. This study was conducted in an experiment designed to examine whether indirect effects may contribute to the effect of hypothalamic leptin signaling to improve peripheral insulin sensitivity and therefore was carried out over a time frame during which restored hypothalamic leptin signaling reduces food intake and body weight. Consequently, an additional group of obese Koletsky rats was included that received Ad-GFP and were pair fed (Ad-GFP-PF) to the intake of Ad-LEPR-B-treated animals (n = 8), as previously described (12). After ip saline, there were no differences in levels of either pY-IRS-1 or serine-pS473-Akt (a downstream marker of PI3K activation) in liver tissue from animals that received Ad-LEPR-B or the reporter gene, regardless of whether they were fed ad libitum or pair fed (Fig. 3). As expected, systemic insulin injection significantly increased hepatic levels of both pY-IRS-1 and pS473-Akt in all groups compared with saline vehicle (P < 0.05) (Fig. 3, A and B). Importantly, the ability of insulin to increase hepatic levels of both pY-IRS-1 and pS473-Akt was significantly enhanced in animals that received Ad-LEPR-B compared with Ad-GFP (P < 0.05). This effect cannot be explained by reduced food intake or body weight because hepatic content of both pY-IRS-1 and pS473-Akt were also significantly greater in Ad-LEPR-B compared with Ad-GFP-PF-treated animals (Fig. 3A). In contrast, the effects of insulin to increase pY-IRS-1 and pS473-Akt (Fig. 3, C and D) in skeletal muscle were not affected by leptin receptor gene therapy.
Figure 3.
ARC-directed leptin receptor gene therapy increases hepatic insulin signal transduction. Effect of ip (10 U/kg) insulin (black bars)-induced activation of tyrosine phosphorylation of IRS-1 (A and C) and serine phosphorylation of PKB/Akt (B and D) compared with vehicle (white bars) in liver and muscle (gastrocnemius), respectively, in obese Koletsky rats 16 d after microinjection of an adenovirus expressing Ad-LEPR-B or the reporter gene, Ad-GFP, that were either fed ad libitum or pair fed to the Ad-LEPR-B-treated animals. Hepatic (E) and muscle (F) triglyceride content in this same group of animals is shown. *, P < 0.05 vs. Ad-GFP; #, P < 0.05 vs. Ad-GFP-PF.
To investigate whether changes of tissue lipid accumulation might contribute to centrally mediated effects of leptin on hepatic glucose metabolism, we measured triglyceride content in both liver and skeletal muscle in this same group of animals. In liver, triglyceride content was significantly reduced in Ad-LEPR-B-treated animals compared with those that received Ad-GFP (20.3 ± 1.3 vs. 25.8 ± 1.3 μmol/g wet tissue; P < 0.05). However, this effect was likely due to reduced food intake and body weight because Ad-GFP-PF animals exhibited similar decreases of liver triglyceride content (Fig. 3E). In contrast, neither Ad-LEPR-B treatment nor pair feeding had a significant affect on triglyceride content in muscle (Fig. 3F). Moreover, liver triglyceride levels were similar in Ad-GFP- and Ad-LEPR-B-treated animals that had either HV or a sham operation (data not shown).
Discussion
Recent studies suggest that hypothalamic input from humoral signals such as insulin and FFAs improve insulin sensitivity via increased suppression of HGP (7,14,15). We and others have provided evidence that leptin also plays an important role in the regulation of glucose metabolism through actions in the hypothalamus (12,13). To investigate the mechanisms mediating the insulin-sensitizing effect of leptin, we used the euglycemic-hyperinsulinemic clamp technique to determine the effect of restored hypothalamic leptin signaling on insulin sensitivity of muscle and liver in obese Koletsky rats. We found that the insulin-sensitizing effect of hypothalamic leptin receptor gene therapy in these animals is mediated by enhanced insulin-mediated inhibition of HGP via a mechanism associated with inhibition of liver gluconeogenic gene expression and that both effects were blocked by selective HV. Taken together, these findings suggest that like insulin, leptin activates a neural circuit that exists between the brain and liver that regulates hepatic insulin action.
Our finding that restoration of hypothalamic leptin signaling to obese Koletsky rats increases insulin inhibition of HGP would appear at odds with published evidence that in normal rats, icv administration of pharmacological doses of leptin fails to affect HGP (21,22). Rather than having no effect on hepatic glucose metabolism, however, central leptin administration stimulates gluconeogenesis (via activation of the central melanocortin pathway) and simultaneously decreases glycogenolysis (via a melanocortin independent pathway) (22), an effect that requires central signaling through signal transducer and activator of transcription-3 (23). Furthermore, icv leptin infusion reversed the insulin resistance induced by short-term exposure of rats to a high-fat diet by reducing both glycogenolysis and gluconeogenesis (24). Our rat model also differs in that our goal was to restore functional leptin signaling to discrete hypothalamic nuclei of animals that otherwise lack the leptin receptor, rather than inject a pharmacological dose of leptin delivered into the third ventricle. Thus, the source of the ligand in our model is circulating leptin, and the site of leptin action in our model is restricted to the area of the ARC, whereas icv leptin can simultaneously act at multiple leptin-sensitive sites within the hypothalamus (25,26) as well as extrahypothalamic sites including the ventral tegmental area (27,28) and the nucleus of the solitary tract (NTS) in the hindbrain (29).
Recent studies suggest that the effect of hypothalamic insulin and FFA signaling to suppress GP requires intact vagal input to the liver (14,15). Consistent with the hypothesis that a similar mechanism underlies the hepatic response to leptin action in the ARC, we found that the effect of restored hypothalamic leptin signaling to improve peripheral insulin sensitivity in obese Koletsky rats was attributable to increased insulin-induced suppression of HGP and that this effect was blocked by selective HV. Previously published data suggest that vagal efferent fibers that supply the liver are the critical vagal population because the hypothalamic effects of both insulin and FFAs to suppress HGP are blocked by selective HV but not selective vagal deafferentiation (14,15). By comparison, the rate of HGP was not affected by HV in Koletsky rats receiving the control adenovirus, suggesting that although the parasympathetic nervous system is a key mediator of hypothalamic leptin action on hepatic insulin sensitivity (as is also true for insulin and FFAs), it is not a key determinant in the absence of a leptin signal. One potential explanation for this observation is the possibility that vagal tone to the liver is reduced or absent in leptin receptor-deficient rats. Because previous studies have shown that selective HV in genetically normal rats is also without effect on hepatic glucose metabolism (14,15), however, vagal input to the liver may play a redundant role in the control of hepatic insulin action in the absence of a change of key CNS control systems. A relevant parallel may be found in the observation that whereas vagal afferents are well known to play a crucial role in the perception of satiety and hence control of meal size, total or selective vagotomies fail to affect overall energy balance (30), owing to compensatory responses elsewhere in the system. Additional studies are warranted to better understand the role of the hepatic vagus in the control of hepatic glucose metabolism.
The potential importance of the role played by vagal innervation of the liver in our studies is highlighted by our inability to find other, indirect mediators of the effect of hypothalamic leptin on insulin sensitivity. Previous studies suggest that increased central leptin signaling has wide-ranging effects in multiple tissues including activation of AMP-activated protein kinase in muscle (31), reduced triglyceride content in liver (10), increased mitochondrial activity in brown adipose tissue (32), and changes in plasma hormone levels that may, in turn, affect peripheral tissue insulin action. We previously reported that ARC-directed leptin receptor gene therapy does not reliably alter fasting plasma levels of leptin, FFAs, triglycerides, or corticosterone in Koletsky rats (12). Our findings are also unlikely to result from decreased liver triglyceride content because there was no difference in hepatic fat content between treatment groups at the completion of the clamps, and studies examining biochemical hepatic insulin sensitivity showed that the modest effect of hypothalamic leptin receptor gene therapy to reduce liver triglyceride content was likely explained by reduced food intake. Because altered hepatocellular fatty acid metabolism can clearly influence insulin signal transduction (33), however, it is interesting to speculate that changes in vagal outflow to the liver influence hepatic lipid handling and that this effect in turn explains our findings. Moreover, central leptin signaling stimulates fatty acid oxidation in white adipose tissue (34) and controls adipose tissue lipogenesis (35), an affect implicated in the ability of hypothalamic leptin to improve peripheral insulin sensitivity. However, recent data suggest that leptin regulation of peripheral lipid metabolism is predominantly explained by effects on food intake (36).
To identify cellular mechanisms whereby hypothalamic leptin signaling increases hepatic insulin sensitivity, we examined whether restored hypothalamic leptin signaling increases insulin receptor signal transduction in either liver or skeletal muscle. Here we found that hypothalamic leptin receptor gene therapy increased insulin signaling via the IRS-PI3K pathway selectively in liver, but not muscle, among obese Koletsky rats. Because insulin stimulation of PI3K signaling inhibits gluconeogenesis in hepatocytes, we also measured expression levels of PEPCK and G6Pase, in liver samples obtained at the completion of the hyperinsulinemic clamp and found them to be reduced in Ad-LEPR-B- compared with Ad-GFP-treated controls. However, the question of whether restored hypothalamic leptin signaling in our studies reduced gluconeogenesis, glycogenolysis, or both awaits further investigation.
One mechanism proposed to explain how leptin reduces food intake is by enhancing the response to gut-derived satiety peptides, e.g. cholecystokinin, that are released upon food ingestion and activate vagal afferent fibers that terminate in the NTS in the hindbrain (37,38,39). Specifically, leptin signaling in the ARC has been shown to activate a descending projection to the NTS that enhances the response to input from cholecystokinin (17,40). Our current findings raise the possibility that a parallel neurocircuit may link hypothalamic leptin signaling to control hepatic insulin sensitivity and that a leptin-sensitive neuronal pathway conveys input to the liver by modulating the activity of neurons in the hindbrain. Consistent with this hypothesis, inhibition of fat oxidation in the ARC increases hepatic insulin sensitivity via a mechanism that requires intact hepatic vagal signaling and also activates hindbrain neurons (15).
Because insulin resistance was only partially reversed by rescue of leptin receptor signaling in the area of the ARC, an important role for leptin action in other brain areas in the control of glucose homeostasis is suggested. Leptin receptors are expressed in several additional hypothalamic areas as well as extrahypothalamic sites (29,41,42), and several of these are also important in the control of glucose metabolism and involve signaling mechanisms that may differ from those involved in the ARC. For example, hypothalamic leptin action increases AMP-activated protein kinase activity in muscle (31), and this effect may be mediated by leptin action in the ventromedial hypothalamic nucleus because microinjection of leptin to this brain area increases glucose uptake in muscle via the sympathetic nervous system (43,44). Consistent with this, selective deletion of leptin receptors from steroidogenic factor 1 neurons in the ventromedial hypothalamic nucleus causes obesity as well as insulin resistance (45).
One limitation of these experiments is that because we are unable to direct the adenovirus to specific neuronal cell types, we cannot identify the neuronal cell groups that mediate the observed effects. In this regard, the recent finding that expression of insulin receptors by hypothalamic neuropeptide Y/agouti gene-related peptide neurons is required for the full effect of circulating insulin to suppress HGP (46), combined with evidence that leptin, like insulin, inhibits the firing of these neurons (47), suggests that this neuronal subset might contribute to the actions we observed after ARC-directed leptin receptor gene therapy. Indeed, we previously reported inhibition of hypothalamic neuropeptide Y gene expression after this intervention (16), but whether leptin signaling in these neurons is required for its hepatic effects awaits further study. Another caveat is that our approach does not selectively target neurons that normally express leptin receptors, nor can we verify that normal expression levels were attained in individual neurons; however, this is unlikely to affect experimental outcomes via a nonspecific mechanism because neither Ad-LEPR-B-GFP nor Ad-GFP has any detectable metabolic effect when injected into the ARC of wild-type animals (12,16,17).
In conclusion, we report that restoring leptin receptor signaling to the area of the ARC of leptin receptor-deficient Koletsky rats improves peripheral insulin sensitivity by enhanced suppression of HGP, rather than increased glucose uptake, and that these effects are blocked by selective HV. Taken together, these findings support the existence of a neurocircuit linking hypothalamic leptin signaling with autonomic innervation of the liver that plays a physiological role in the control of insulin sensitivity. Moreover, these data raise the possibility that pharmaceutical approaches to increase central leptin sensitivity in conditions of insulin resistance resulting from diet-induced obesity may improve both energy and glucose homeostasis.
Acknowledgments
We acknowledge the excellent technical assistance provided by Alex Cubelo, Loan Nguyen, and James Graham for performing the tissue triglyceride measurements.
Footnotes
This work was supported by National Institutes of Health Grants DK068384, DK052989, DK083042, DK047208, DK020541, and DK073878; the Skirball Institute for Nutrient Sensing (to G.J.S.); Clinical Nutrition Research Unit Grant DK035816; Diabetes Endocrine Research Center Grant P30 DK17047; Mouse Metabolic Phenotyping Center Grant U24 DK076126 at the University of Washington; research funding from AstraZeneca; and an American Heart Association Scientist development grant (to G.J.M.). G.J.M. is supported by a Naomi Berrie Investigator in Diabetes Research Award from Columbia University.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 2, 2009
Abbreviations: Ad, Adenovirus; ARC, arcuate nucleus; CNS, central nervous system; FFA, free fatty acid; GFP, green fluorescent protein; GIR, glucose infusion rate; GP, glucose production; G6Pase, glucose-6-phosphatase; HGP, hepatic glucose production; HV, hepatic vagotomy; icv, intracerebroventricular; IR, insulin receptor; IRS, IR substrate; LEPR, leprb; NTS, nucleus of the solitary tract; PEPCK, phosphoenolpyruvate kinase; PI3K, phosphatidylinositol-3-OH kinase; pY, phosphorylation; Ra, glucose rate of appearance; Rd, rate of disposal.
References
- Schwartz MW, Porte Jr D 2005 Diabetes, obesity, and the brain. Science 307:375–379 [DOI] [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K, Takamoto I, Satoh H, Maki T, Kubota T, Moroi M, Okada-Iwabu M, Ezaki O, Nagai R, Ueta Y, Kadowaki T, Noda T 2004 Insulin receptor substrate 2 plays a crucial role in β cells and the hypothalamus. J Clin Invest 114:917–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF 2004 Dysregulation of insulin receptor substrate 2 in β cells and brain causes obesity and diabetes. J Clin Invest 114:908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L, Wunderlich FT, Seibler J, Könner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Brüning JC 2008 Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest 118:2132–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L 2002 Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5:566–572 [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L 2002 Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8:1376–1382 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL 1999 Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401:73–76 [DOI] [PubMed] [Google Scholar]
- Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, Friedman JM 2004 Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest 113:414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte Jr DJ, Woods SC, Seeley RJ, Weigle DS 1996 Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 45:531–535 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW 2005 Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab 2:411–420 [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwing T, Chua Jr SC, Lowell BB, Elmquist JK 2005 The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metabolism 1:63–72 [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L 2005 Hypothalamic K(ATP) channels control hepatic glucose production. Nature 434:1026–1031 [DOI] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz GJ, Rossetti L 2005 A brain-liver circuit regulates glucose homeostasis. Cell Metab 1:53–61 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Niswender KD, Rhodes CJ, Myers Jr MG, Blevins JE, Baskin DG, Schwartz MW 2003 Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky [fa(k)/fa(k)] rats. Endocrinology 144:2016–2024 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW 2005 Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers Jr MG, Rhodes CJ, Schwartz MW 2006 Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab 3:67–73 [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH 1957 A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509 [PubMed] [Google Scholar]
- Wall JS, Steele R, De Bodo RC, Altszuler N 1957 Effect of insulin on utilization and production of circulating glucose. Am J Physiol 189:43–50 [DOI] [PubMed] [Google Scholar]
- Liu L, Karkanias GB, Morales JC, Hawkins M, Barzilai N, Wang J, Rossetti L 1998 Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem 273:31160–31167 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Juárez R, Obici S, Rossetti L 2004 Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem 279:49704–49715 [DOI] [PubMed] [Google Scholar]
- Buettner C, Pocai A, Muse ED, Etgen AM, Myers Jr MG, Rossetti L 2006 Critical role of STAT3 in leptin’s metabolic actions. Cell Metab 4:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L 2005 Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54:3182–3189 [DOI] [PubMed] [Google Scholar]
- Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, Elmquist JK 2000 Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol 423:261–281 [PubMed] [Google Scholar]
- Hübschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W 2001 Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci 21:2413–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS 2006 Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51:811–822 [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ 2006 Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG 2002 Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143:239–246 [DOI] [PubMed] [Google Scholar]
- Berthoud HR 2008 The vagus nerve, food intake and obesity. Regul Pept 149:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D, Kahn BB 2002 Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415:339–343 [DOI] [PubMed] [Google Scholar]
- Gullicksen PS, Flatt WP, Dean RG, Hartzell DL, Baile CA 2002 Energy metabolism and expression of uncoupling proteins 1, 2, and 3 after 21 days of recovery from intracerebroventricular mouse leptin in rats. Physiol Behav 75:473–482 [DOI] [PubMed] [Google Scholar]
- Angulo P 2002 Nonalcoholic fatty liver disease. N Engl J Med 346:1221–1231 [DOI] [PubMed] [Google Scholar]
- Plum L, Rother E, Münzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Könner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Brüning JC 2007 Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab 6:431–445 [DOI] [PubMed] [Google Scholar]
- Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L 2008 Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med 14:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur X, Tung YC, Griffin JL, Farooqi IS, O'Rahilly S, Coll AP 2008 Leptin regulates peripheral lipid metabolism primarily through central effects on food intake. Endocrinology 149:5432–5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrachina MD, Martínez V, Wang L, Wei JY, Taché Y 1997 Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA 94:10455–10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond M, Schwartz GJ, Ladenheim EE, Moran TH 1999 Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol 276:R1545–R1549 [DOI] [PubMed] [Google Scholar]
- Wang L, Martínez V, Barrachina MD, Taché Y 1998 Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res 791:157–166 [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG 2004 Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brainstem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287:R87–R96 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB 1998 Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547 [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG 2003 Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964:107–115 [DOI] [PubMed] [Google Scholar]
- Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T 1999 Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes 48:1706–1712 [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Haque MS, Shimazu T 1999 Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48:287–291 [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua Jr S, Elmquist JK, Lowell BB 2006 Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC 2007 Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5:438–449 [DOI] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D 2004 Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 7:493–494 [DOI] [PubMed] [Google Scholar]