Abstract
Normal reproductive functioning may require secretion of LH independently of FSH. Variation in GnRH pulse frequency and inhibin negative feedback are mechanisms for differential gonadotropin regulation; however, the first instance of differential regulation in rats is during fetal development, prior to the establishment of GnRH connections, when LH accumulates appreciably 2–4 d prior to FSH. Pituitary adenylate cyclase activating polypeptide (PACAP) can differentially regulate the gonadotropins in vitro by stimulating α-subunit transcription, lengthening LHβ transcripts and decreasing FSHβ mRNA levels, probably through stimulation of follistatin transcription. These experiments are the first to examine whether PACAP influences gonadotroph function in perinatal pituitaries. In vivo, pituitary PACAP mRNA and peptide levels were high at embryonic d 19 and declined by 94 and 85%, respectively, after parturition. This was accompanied by a decrease of 65 and 96% in total follistatin and follistatin-288 mRNAs. These changes were temporally associated with a 20- and 6.5-fold rise in FSHβ and GnRH receptor mRNAs, respectively, with no significant increase in LHβ mRNA. In pituitary cell cultures from fetal and postnatal male rats, PACAP mRNA levels were likewise highest in fetal cultures in which the PACAP 6-38 antagonist decreased α-subunit and increased FSHβ mRNA. PACAP 6-38 also reduced basal and GnRH-stimulated LH secretion with little effect on FSH. These data support the hypothesis that PACAP expressed at high levels in the fetal pituitary stimulates α-subunit expression and LH secretion and restrains FSH synthesis relative to LH and that a decline in PACAP allows for the neonatal rise in FSH and GnRH receptor because follistatin is decreased.
Findings support the hypothesis that pituitary adenylate cyclase activating polypeptide (PACAP) is involved in the suppression of FSH mRNA expression in the fetus, and that a pronounced perinatal decline in pituitary PACAP expression facilitates a dramatic rise in FSH expression.
The gonadotropin subunit genes are each up-regulated by GnRH; however, there are many normal and pathological conditions in which LH and FSH are disproportionately secreted. In the male rat, significant accumulation of LH peptide is evident by embryonic day (E) 17 (1,2), and low levels of the peptide are detectable as early as E12 (1,3), whereas FSH is not detected and accumulation is not observed until E19 or E21 (1,2). Lhb (LHβ) is also detected before Fshb (FSHβ) mRNA in the fetal mouse pituitary (4). This developmental example of differential regulation of the gonadotropins occurs before the establishment of GnRH neuronal connections within the median eminence (5). Interestingly, the onset of FSH synthesis in rodents coincides with the formation of GnRH neuronal connections (5). Regardless, gonadotropin synthesis can initiate without GnRH as demonstrated by the presence of gonadotropins in the GnRH-deficient hpg mouse (6). These observations imply that some other factor may be responsible for initiating gonadotropin synthesis in the fetal pituitary. However, the mechanism by which gonadotropin synthesis is initiated and how differential regulation is accomplished during fetal development has received little attention.
The neuropeptide, pituitary adenylate cyclase activating polypeptide (PACAP or ADCYAP1) has been shown to differentially regulate the gonadotropins. In cultures of pituitary cells, PACAP induces the immediate release of LH, FSH, and uncombined glycoprotein α-subunit and augments the gonadotroph response to GnRH (7). When PACAP is delivered to cultured rat pituitary cells in hourly pulses, there is a rapid but transient stimulation of gonadotropin secretion that declines with repeated stimulation (8). Continuous treatment of cultured pituitary cells with PACAP causes a dose-dependent increase in the sensitivity of gonadotrophs to pulsatile GnRH signaling and increases α-subunit (Cga or αGSU) mRNA levels, lengthens LHβ mRNA transcripts, but reduces FSHβ mRNA levels (9).
Although generally viewed as a hypothalamic hypophysiotropic neuropeptide, PACAP is also found in the pituitary. In the adult rat, PACAP mRNA and peptide are found within gonadotrophs and folliculostellate cells (10,11). A transient increase in PACAP occurs within the pituitary of female rats during the overnight hours between proestrus and estrus (12,13) when FSH levels are elevated and could contribute to termination of the secondary surge of FSH that occurs during the early hours of estrus. Moreover, there is a significant decline in pituitary PACAP mRNA in male rats between 17 and 21 d of age, a time when there is a predominant rise in FSHβ compared with LHβ mRNA (14). From these data we hypothesized that alterations in pituitary expression of PACAP affect the synthesis and secretion of the gonadotropins during development of the mammalian pituitary. The experiments described in this report were designed to evaluate PACAP expression as a mechanism for the differential regulation of the gonadotropin subunit genes in the fetal pituitary.
Materials and Methods
Animals
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals according to a protocol approved by the Animal Care and Use Committee of the University of Louisville. Timed-pregnant Sprague Dawley rats were delivered from the supplier (Charles Rivers Laboratories, Wilmington, MA) and housed for 1 wk with free access to rat chow and water. Pregnant females were killed on gestational d 19, and the pituitaries from the fetuses were collected using a stereoscopic microscope. Pituitaries were also collected from male rat pups killed on postnatal day (PN) 1, 5, and 15. The rats were sexed by evaluation of the urogenital-anal distance and confirmed by the presence of testes at the time of dissection. The pituitaries were processed for mRNA isolation, pituitary cell dispersal, or protein extraction.
Pituitary cell cultures
Anterior pituitaries were minced and treated with 0.33% collagenase (Roche Molecular Biochemicals, Mannheim, Germany) and 0.003% deoxyribonuclease (Sigma, St. Louis, MO). Cells were then treated with 0.25% pancreatin (Life Technologies, Inc., Grand Island, NY) for 8 min and washed with DMEM containing 5% charcoal-stripped fetal calf serum and 5% charcoal-stripped calf serum. Dispersed cells were cultured in DMEM with 10% charcoal-stripped fetal calf serum at a density of 5 × 105 cells/well in 24-well plates. The pituitary preparations were preincubated for 48 h and then treated with media containing 0, 0.01, 1.0, or 10 μm PACAP 6-38 antagonist. After 30 min, GnRH (1 nm) was added to several of the cultures treated with vehicle or 10 μm PACAP 6-38. After an additional 7.5 h, media were collected and analyzed for LH and FSH. Eight hours of treatment was chosen for optimization of simultaneous quantification of mRNA and hormone levels. The cells were quick frozen in 4 m guanidinium thiocyanate solution on an ethanol, dry ice slurry and stored at −80 C for RNA extraction.
Hormone determinations
Enzyme immunoassays developed by GE Healthcare Life Sciences (Piscataway, NJ) were used to measure FSH and LH protein in culture media. The within-assay coefficient of variation in the standard curves was 11.4% for FSH and 7.6% for LH. The range of the standards was 6.25–400 ng/ml for FSH and 0.41–100 ng/ml for LH.
Pituitary PACAP protein determination
Frozen pituitaries were pooled (five per sample) and extracted by boiling in 1.0 mol/liter acetic acid for 10 min, followed by homogenization for 1 min. The samples were then frozen at −80 C, lyophilized, and dissolved in 0.5 mol/liter Tris-phosphate buffer (pH 7.5), for measurement of PACAP using an enzyme immunoassay (EIA) kit purchased from Bachem (San Carlos, CA). The sensitivity (average IC50)of the PACAP EIA was 3 ng/ml (150 pg/well), and its intraassay coefficient of variation was less than 5%. All samples were analyzed in the same assay to eliminate interassay variability.
Quantitative real-time PCR
Pituitary and primary culture RNA was extracted according to the method of Chomczynski and Sacchi (15). The concentration of total RNA was determined by spectrophotometric analysis. Sample purity was determined by calculating the ratio of sample absorbance at 260:280 nm, and samples were rejected if less than 1.8. For quantitative real-time PCR (qRT-PCR), standard curves of known amounts of target mRNAs were prepared by the method of Fronhoffs et al. (16) as previously described (14). RNA (1 μg) isolated from pituitary samples was reverse transcribed in parallel with cRNA standards using an oligo dT(12–24) as the primer. Reverse-transcribed cRNA standards and samples were amplified in parallel by PCR with an MX4000 multiplex quantitative PCR system (Stratagene, La Jolla, CA) using the Brilliant SYBR Green QPCR master mix (Stratagene) and specific primers (14). Accumulation of PCR product was monitored in real time (Mx4000; Stratagene), and the crossing threshold was determined using Mx4000 software. For each set of primers, a no-template control and a no-reverse amplification control were included. Postamplification dissociation curves were performed to verify the presence of a single amplification product in the absence of DNA contamination. Concentrations of mRNAs were determined by interpolation using a standard curve from known starting mRNA concentrations. For control of RNA input, a qRT-PCR assay for glyceraldehyde-3-phosphate dehydrogenase was performed for each sample, and the result was used to normalize those of the other measured mRNA species. Glyceraldehyde-3-phosphate dehydrogenase was chosen because it was shown to be unaffected by the experiments conducted, as were 18S, 28S, and cyclophin RNAs.
Statistical analyses
For each data set, Levene’s test of equality of error variances was performed to examine the data for homoscedasticity. If Levene’s test revealed unequal variance, then Welch’s ANOVA followed by Tukey-Kramer-honestly significant difference post hoc t tests (TK-HSD≠) were performed when appropriate allowing for unequal sds. If variance was equal, then a Student’s t test or one- or two-way ANOVA was performed when appropriate.
Results
Perinatal expression of pituitary PACAP mRNA
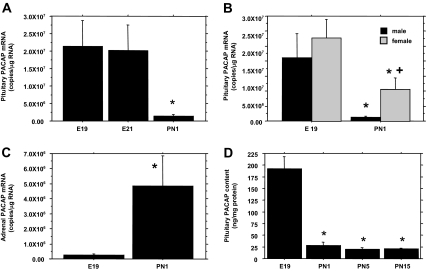
PACAP mRNA within the late fetal pituitary gland (E19) was present at a 48-fold higher concentration than previously observed in infantile male rats (14) and declined by 94% (P < 0.001, Welch’s ANOVA) by PN1 (Fig. 1A). A substantial decrease was also observed within the perinatal female pituitary (P < 0.01, TK-HSD≠). Although two-way ANOVA revealed no overall gender difference, correcting for unequal variance revealed that PACAP mRNA was significantly higher (P < 0.01, TK-HSD≠) in PN1 females than PN1 males (Fig. 1B). The perinatal decrease is specific to pituitary PACAP expression as PACAP mRNA in the adrenal (Fig. 1C) increased significantly (P < 0.05, TK-HSD≠) during the same period.
Figure 1.
Pituitary PACAP mRNA and protein levels decline at birth. qRT-PCR and EIA were used to determine the concentrations of PACAP mRNA (A) and protein (D) within the perinatal anterior pituitary gland of male rats. B, Differences in PACAP mRNA levels between male and female rats at E19 and PN1. Results are from the same assay to avoid between-assay differences. C, Adrenal PACAP mRNA concentrations in E19 and PN1 male rats by qRT-PCR. For each panel, the data points represent the mean ± sem of six to eight subjects per group. Statistical analyses were performed with Welch’s ANOVA (A and D) or two-way ANOVA followed by TK-HSD≠ (B), or Tukey-Kramer HSD t test (C). *, P < 0.05 vs. E19; +, P < 0.05 vs. PN1 males.
Perinatal concentrations of PACAP protein within the anterior pituitary
To verify that higher levels of PACAP mRNA within the fetal pituitary translate into higher concentrations of protein, PACAP peptide concentrations in pooled, perinatal male pituitaries were determined by EIA. Pituitary PACAP levels decreased by 85.5% (P < 0.05, Welch’s ANOVA) between E19 and PN1 (Fig. 1D), and no further decline was observed between PN5 and PN15.
Perinatal expression of the gonadotropin subunits and Gnrhr [GnRH receptor (GnRH-R)] mRNAs
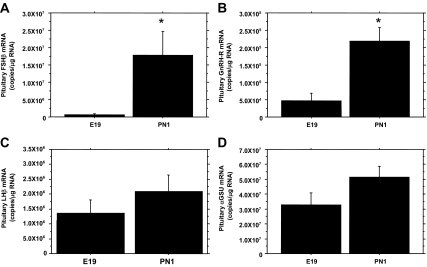
To evaluate whether a relationship exists between the expression of PACAP and the gonadotropin subunits and the GnRH-R, qRT-PCR was performed using pituitary RNA isolated from E19 and PN1 male rats. Similar to earlier reports of pituitary FSH content (1,2), FSHβ mRNA levels increased markedly (23-fold) between E19 and PN1 (Fig. 2A). GnRH-R mRNA also significantly increased by 4.6-fold between E19 and PN1 (Fig. 2B). LHβ mRNA concentrations increased by 2-fold (Fig. 2C) and αGSU mRNA rose 1.5-fold between E19 and PN1 (Fig. 2D), but these increases were not statistically significant. When the results for all 16 animals were combined, there was a significant negative correlation between FSHβ and PACAP mRNA expression levels (Pearson r = −0.6587, P < 0.05). The expression of Gnrhr (GnRH-R) mRNA was also reciprocal to PACAP mRNA expression (Pearson r = −0.6316, P < 0.05).
Figure 2.
Gonadotropin subunit and GnRH-R expression during the perinatal period in male rats. Pituitary RNA from E19 and PN1 male rats was used for qRT-PCR to determine the relative mRNA levels of FSHβ (A), GnRH-R (B), LHβ (C), and αGSU (D). The data are expressed as copies of specific RNA per microgram total RNA. Each value represents the mean ± sem of six subjects per group. Statistical analyses were performed with TK-HSD≠ (A) or Student’s t test (B-D). *, P < 0.05 vs. E19.
Perinatal expression of pituitary follistatin mRNAs
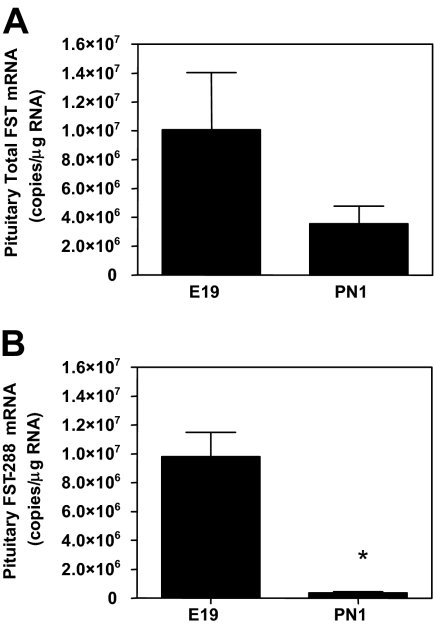
Follistatin (FST) is known to inhibit FSH and GnRH-R synthesis by binding activin and limiting activin signaling to gonadotrophs (17,18). Because PACAP stimulates follistatin synthesis (19), we evaluated whether follistatin mRNA expression during the rat perinatal period followed the same expression pattern as PACAP. qRT-PCR analysis revealed a 65% decrease in total Fst mRNA between E19 and PN1 (Fig. 3A). The mRNA species encoding FST-288 has a 10-fold greater FSH-suppressing activity than the Fst-315 isoform (20). Analysis of Fst-288 mRNA levels revealed a 96% decrease within the anterior pituitary between E19 and PN1 (Fig. 3B). Thus, Fst mRNA, particularly the 288-isoform, is decreased in parallel (Pearson r = 0.823, P < 0.01) with pituitary PACAP expression and is reciprocal to the rise in FSHβ and GnRH-R mRNAs.
Figure 3.
Pituitary follistatin mRNA levels decline significantly at the time of birth. Pituitary RNA from E19 and PN1 male rats were used in qRT-PCR to determine the relative mRNA levels of total FST (A) and the FST-288 isoform (B). The data are expressed as copies of specific RNA per microgram total RNA. Each value represents the mean ± sem of six subjects per group. Statistical analyses were performed with Student’s t test. *, P < 0.05 vs. E19.
Perinatal expression levels of PACAP mRNA are maintained in vitro
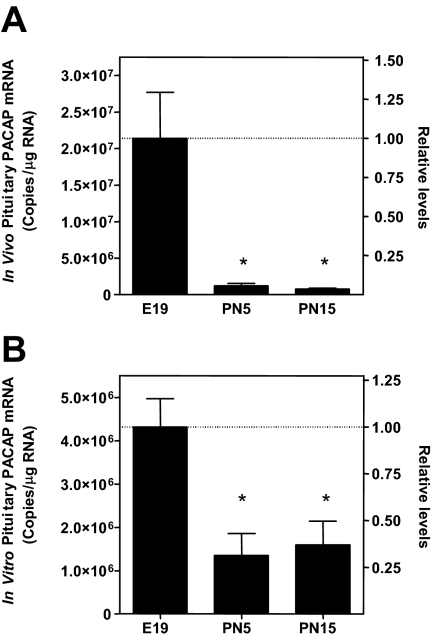
To further evaluate the role of PACAP in the synthesis and secretion of gonadotropins within the perinatal pituitary, we used cultures of primary pituitary cells from late embryonic and postnatal rats. Analysis of PACAP mRNA levels after 48 h of incubation (Fig. 4B) revealed a similar pattern of expression as observed in vivo (Fig. 4A). PACAP mRNA levels were significantly (P < 0.001) higher in pituitary cell cultures from E19 rats compared with PN5 and PN15 cell cultures, whereas there was no difference in PACAP mRNA levels between PN5 and PN15 in vitro or in vivo. Moreover, as in vivo, the levels of α-subunit, LHβ, and FSHβ mRNAs increased during development with the greatest change in FSHβ (Fig. 5C). Thus, primary cell cultures are a valid model to investigate how endogenous pituitary PACAP affects gonadotropin synthesis and secretion.
Figure 4.
Perinatal expression levels of pituitary PACAP mRNA are maintained in vitro. RNA from pituitaries (A) and 48-h pituitary cell cultures (B) from E19, PN5, and PN15 male rats were analyzed for PACAP mRNA content by qRT-PCR. For comparison of in vivo and in vitro data, the values are expressed as copies per microgram total RNA and levels relative to E19 values. Each value represents the mean ± sem of six subjects per group for whole pituitaries and three separate cell culture preparations performed in triplicate. Statistical analyses were performed with Student’s t test. *, P < 0.05 vs. E19.
Figure 5.
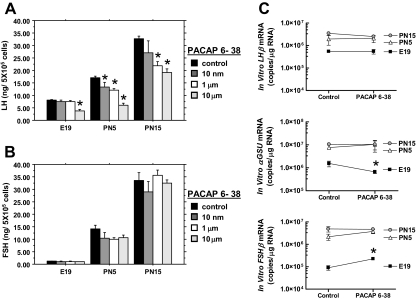
Endogenous pituitary PACAP contributes to basal gonadotropin secretion and subunit mRNA expression levels. Pituitary cell monolayer cultures from E19, PN5, and PN15 male rats were incubated with various concentrations of PACAP 6-38 for 8 h, and the media were evaluated for LH (A) and FSH (B) secretion. C, Pituitary cell monolayer cultures from E19, PN5, and PN15 male rats were incubated in the presence or absence of 10 nm PACAP 6-38 for 8 h. Pituitary cell RNA was isolated and processed for qRT-PCR analyses of the gonadotropin subunit mRNA levels. The data are expressed as copies of gonadotropin subunit mRNAs per microgram total RNA. Each value (n = 3) represents the mean ± sem of three independent experiments, each performed in triplicate. Statistical analyses were performed with two-way ANOVA followed by pairwise comparison using TK-HSD≠ (A and B) or (C) by Student’s t tests. *, P < 0.05 vs. control.
Effects of PACAP antagonist on LH and FSH secretion from perinatal pituitary cell cultures
Previous studies revealed that PACAP stimulates LH and FSH secretion when administered to adult pituitary cell cultures (8,9). To evaluate the potential role of endogenous PACAP on basal gonadotropin secretion from the perinatal pituitary, we treated cultures from E19, PN5, and PN15 male rats with increasing doses of the potent PACAP antagonist, PACAP 6-38 (22). Two-way ANOVA revealed a significant effect of PACAP antagonist concentration and age on LH secretion. One-way ANOVA at each age revealed that 10 μm PACAP antagonist significantly reduced basal LH secretion (Fig. 5A) but lower concentrations of PACAP 6-38 were less effective in E19 than in PN5 and PN15 cultures. In contrast, the PACAP antagonist had no statistically significant effect on the secretion of FSH (Fig. 5B).
Effects of PACAP antagonist on gonadotropin subunit mRNAs in perinatal pituitary cell cultures
PACAP increases αGSU mRNA, lengthens LHβ mRNA, and decreases FSHβ mRNA levels in adult pituitary cell cultures (9). To evaluate the influence of endogenous pituitary PACAP on gonadotropin subunit expression in the perinatal rat, pituitary cell cultures from E19, PN5, and PN15 male rats were treated with 10 μm PACAP 6-38 for 8 h, and gonadotropin mRNA levels were measured. These conditions were selected based on the rapid half-life of FSHβ mRNA in pituitary cultures (8,23,24). The PACAP antagonist had no significant effect on the levels of the gonadotropin subunits in either of the postnatal pituitary cell preparations (Fig. 5C). By contrast, PACAP 6-38 significantly decreased (P < 0.05) αGSU mRNA levels and increased (P < 0.005) the level of FSHβ mRNA in E19 pituitary cell cultures (Fig. 5C).
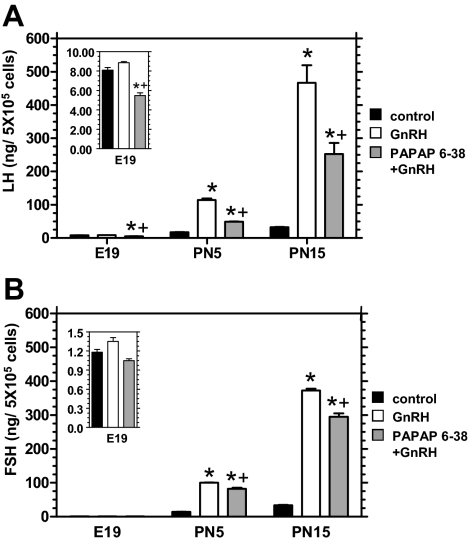
Effects of PACAP antagonist on GnRH-stimulated secretion of LH and FSH from perinatal pituitary cell cultures
In addition to directly stimulating LH and FSH release from adult pituitary cell cultures, PACAP has been shown to augment GnRH-stimulated gonadotropin release (8). To evaluate the effect of endogenous PACAP on the GnRH-stimulated secretion of FSH and LH from perinatal pituitary cell cultures, the cell preparations were exposed to 1 nm GnRH in the presence or absence of 10 μm PACAP 6-38. In pituitary cell cultures from E19 rats GnRH alone failed to stimulate either LH or FSH secretion, whereas PACAP 6-38 decreased LH secretion in the presence of GnRH (Fig. 6). In cultures from PN5 and PN15 rats, GnRH stimulated LH and FSH secretion, and stimulation was partially yet significantly reduced by the PACAP antagonist with a greater effect on LH than on FSH.
Figure 6.
Endogenous pituitary PACAP contributes to GnRH-stimulated gonadotropin secretion. Pituitary cell monolayer cultures from E19, PN5, and PN15 male rats were preincubated in the presence or absence (control) of 10 nm PACAP 6-38 for 30 min, and then 1 nm GnRH or medium alone (control) was added for 7.5 h of incubation. The media were collected and evaluated for LH (A) and FSH (B). The data are expressed as level of LH or FSH secreted (nanogram) from 5 × 105 pituitary cells during 8 h. Each value (n = 3) represents the mean ± sem of three independent experiments each performed in triplicate. The insert in each figure are the E19 values graphed on a lower value scale. Statistical analyses were performed with multivariate analysis of variance followed by pairwise comparison using TK-HSD≠. *, P < 0.05 vs. control; +, P < 0.05 vs. GnRH.
Discussion
This is the first study to describe the endogenous levels of PACAP peptide and mRNA within the anterior pituitary of the perinatal rat. These experiments identified a striking and abrupt decline in PACAP production within the pituitary at or near the time of birth. A decrease in pituitary FST expression during the perinatal period parallels the change in PACAP expression and is reciprocal to the dramatic neonatal rise in FSHβ and GnRH-R mRNAs. Furthermore, blockade of endogenous PACAP by the PACAP 6-38 antagonist in vitro decreased basal LH secretion in pre- and postnatal preparations and decreased αGSU mRNA levels but increased FSHβ mRNA only in prenatal cultures. Taken together, these data suggest that the high level of PACAP expression in the fetal pituitary stimulates LH secretion and inhibits the expression of FSH mRNA. In addition, the reciprocal relationship between PACAP mRNA and protein levels and the expression levels of FSHβ and GnRH-R mRNAs suggest that the dramatic decline in PACAP between late gestation and PN1 allows FSHβ and GnRH-R expression to increase.
Previous investigations using pituitary cell cultures from adult rats demonstrated that PACAP stimulates LH and FSH secretion but suppresses FSHβ mRNA levels (8), and further experiments suggested that the mechanism for this suppression is through stimulation of FST transcription (17,25). Because FST is an activin-binding protein, high levels of FST within the pituitary would block activin stimulation of FSHβ transcription (26). Thus, PACAP stimulation of pituitary FST expression is a mechanism for regulating activin signaling and the differential expression of the gonadotropin subunit genes.
There is also a preferential rise in FSH expression between d 20 and 30 in the male rat that is concurrent with a significant reduction in the level of pituitary PACAP mRNA (14). Together, these separate instances support the hypothesis that pituitary PACAP provides a paracrine or autocrine signaling mechanism that selectively inhibits the expression of FSHβ mRNA and enhances gonadotropin secretion. Further support for this idea derives from a comparison of expression levels of PACAP and the gonadotropin subunit genes within the perinatal pituitary of the male and female rat. In the perinatal female, the ontogeny of FSH expression is delayed when compared with males because immunoreactive FSH is first detectable on the day of birth (2,27). In this research we found that PACAP mRNA levels in the PN1 female pituitary, although significantly less than in E19 females, were significantly greater than in PN1 males. Therefore, the delay in activating FSH expression in neonatal females is associated with, and may be explained by, higher PACAP expression.
In addition to regulating the gonadotropin subunit genes, previous investigations using pituitary cell cultures from adult rats revealed that adding PACAP stimulates gonadotropin secretion (9). PACAP levels within the adult pituitary are very low, however, and no studies have investigated the effects of endogenous pituitary PACAP. In the current study, LH secretion was suppressed by the PACAP 6-38 antagonist, and suppression was less in E19 cultures in which endogenous PACAP is elevated when compared with the neonatal and infant pituitary. These findings imply a developmental role for pituitary PACAP in the regulation of LH secretion before significant GnRH stimulation in the fetus and in tandem with GnRH stimulation postnatally. The PACAP antagonist had no effect on the release of FSH from cultures of any age, however. One explanation for these results is that the antagonist induced rise in FSHβ mRNA expression, and presumptive increased FSH synthesis offset any measurable suppressive effect of PACAP 6-38 antagonist on FSH secretion. In this regard it is well established that FSH secretion is more directly coupled to hormone synthesis than is LH secretion (28).
GnRH-stimulated LH and FSH secretion by cultures from PN5 and PN15 rats was also reduced by the PACAP antagonist with a greater effect on LH than on FSH. Previous studies have shown that adding PACAP augments gonadotropin secretion by adult pituitary cell cultures stimulated with GnRH (8,9,29). Whereas the mechanism for this effect remains unknown, PACAP stimulates transcription of GnRH-Rs (30,31). However, earlier experiments with various PACAP doses revealed increased sensitivity to GnRH (29,32) rather than the heightened maximum response that would be expected to accompany an effect due to increased GnRH receptor expression. There is evidence for some GnRH production in the pituitary (33,34), and the suppressive effects of PACAP 6-38 on gonadotropin secretion in the absence of added GnRH could also partly involve PACAP control of GnRH-R signaling. Pituitary cell cultures from PN5 and PN15 male rats responded to GnRH-stimulation with a 6- and 14-fold increase in LH secretion, respectively, whereas E19 fetal cultures failed to respond to GnRH. Although the mechanisms for these developmental changes are unclear, pituitary cell cultures from infant rats are known to be more responsive to GnRH stimulation than are adult pituitary cultures (35,36,37), and others have been unable to detect LH in the culture medium when fetal pituitary cells are stimulated by GnRH (38). Low levels of GnRH-R mRNA in the fetal pituitary may explain the absence of a GnRH effect (2). Furthermore, the continuous increase in hypothalamic GnRH production during perinatal development (39) is known to increase the number of gonadotrophs (40,41) and results in the greater basal and GnRH-stimulated LH release in the postnatal cultures when PACAP appears to have less of a role in gonadotropin regulation.
This report is the first to disclose the significant decline in pituitary PACAP mRNA and peptide that occurs near the time of birth; however, it is not the first to demonstrate high levels of PACAP mRNA within the fetal pituitary. Jaworski and Proctor (42) published a manuscript that demonstrated PACAP mRNA within the embryonic pituitary. Two previous manuscripts (43,44) also show figures that appear to demonstrate pituitary expression of PACAP mRNA in embryos, but expression was not explicitly stated within the text. A more recent report demonstrated that the relative expression level of PACAP mRNA was significantly higher within the pituitaries of 16- to 20-wk-old human fetuses than in the adult human pituitary (45). Our data support those findings and demonstrate that changes in pituitary PACAP protein levels correspond to those of mRNA expression.
The mechanism by which pituitary PACAP expression is reduced near the time of birth is presently unknown but is currently under investigation. Also, in this investigation, we observed that PACAP expression within the adrenal gland markedly increases during the perinatal period. PACAP-deficient mice tend to die at birth partly from hypoglycemia (46) and respiratory failure (47), and PACAP is known to stimulate catecholamine secretion (21). The increase in PACAP expression at birth may prove to be essential for survival.
In conclusion, these data support the hypothesis that pituitary PACAP plays an autocrine or paracrine role in the regulation of gonadotropin synthesis and secretion. The data suggest that pituitary PACAP is involved in the suppression of FSH mRNA expression in the fetus and that a pronounced perinatal decline in pituitary PACAP expression facilitates the dramatic rise in FSH expression. Furthermore, PACAP influences the secretion of both gonadotropins probably through effects on GnRH-R. Each of these PACAP effects may be mediated through its regulation of follistatin transcription (19). The autocrine/paracrine effects of PACAP are developmentally regulated, and pituitary PACAP expression decreases during two important periods of sexual maturation in the male rat. Experiments in vivo are currently underway in an effort to evaluate the consequences of over expression or absence of pituitary PACAP on the development and maintenance of reproductive function.
Acknowledgments
The authors acknowledge the expert technical assistance of Mr. Dushan Ghooray and Mr. Alan Icard, both from the University of Louisville, Department of Medicine.
Footnotes
This work was supported by National Institutes of Health Grants R01 HD050571 (to J.P.M.) and P20 RR017702-SPID 0005 from the Center of Biological Research Excellence Program of the National Center for Research Resources and the Walter F. and Avis Jacobs Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 2, 2009
Abbreviations: E, Embryonic day; EIA, enzyme immunoassay; FST, follistatin; GnRH-R, GnRH receptor; αGSU, gonadotropin α-subunit; PACAP, pituitary adenylate cyclase activating polypeptide; PN, postnatal day; qRT-PCR, quantitative real-time PCR; TK-HSD≠, Tukey-Kramer-honestly significant difference post hoc t test.
References
- Nemeskéri A, Kurcz M, Halász B 1984 Changes in hypophyseal luteinizing hormone (LH) content during fetal and early postnatal life, and capacity of fetal and early postnatal pituitaries to synthesize and release LH in vitro. Neuroendocrinology 38:393–396 [DOI] [PubMed] [Google Scholar]
- Aubert ML, Begeot M, Winiger BP, Morel G, Sizonenko PC, Dubois PM 1985 Ontogeny of hypothalamic luteinizing hormone-releasing hormone (GnRH) and pituitary GnRH receptors in fetal and neonatal rats. Endocrinology 116:1565–1576 [DOI] [PubMed] [Google Scholar]
- Nemeskéri A, Detta A, Clayton RN 1986 Hypothalamic GnRH and pituitary gonadotroph relationships during rat fetal life. Exp Clin Endocrinol 88:275–284 [DOI] [PubMed] [Google Scholar]
- Japón MA, Rubinstein M, Low MJ 1994 In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem 42:1117–1125 [DOI] [PubMed] [Google Scholar]
- Jennes L 1989 Prenatal development of the gonadotropin-releasing hormone-containing systems in rat brain. Brain Res 482:97–108 [DOI] [PubMed] [Google Scholar]
- Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G 1977 Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269:338–340 [DOI] [PubMed] [Google Scholar]
- Hart GR, Gowing H, Burrin JM 1992 Effects of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, on pituitary hormone release in rats. J Endocrinol 134:33–41 [DOI] [PubMed] [Google Scholar]
- Tsujii T, Ishizaka K, Winters SJ 1994 Effects of pituitary adenylate cyclase-activating polypeptide on gonadotropin secretion and subunit messenger ribonucleic acids in perifused rat pituitary cells. Endocrinology 135:826–833 [DOI] [PubMed] [Google Scholar]
- Tsujii T, Winters SJ 1995 Effects of pulsatile pituitary adenylate cyclase activating polypeptide (PACAP) on gonadotropin secretion and subunit mRNA levels in perifused rat pituitary cells. Life Sci 56:1103–1111 [DOI] [PubMed] [Google Scholar]
- Köves K, Kántor O, Scammell JG, Arimura A 1998 PACAP colocalizes with luteinizing and follicle-stimulating hormone immunoreactivities in the anterior lobe of the pituitary gland. Peptides 19:1069–1072 [DOI] [PubMed] [Google Scholar]
- Jin L, Tsumanuma I, Ruebel KH, Bayliss JM, Lloyd RV 2001 Analysis of homogeneous populations of anterior pituitary folliculostellate cells by laser capture microdissection and reverse transcription-polymerase chain reaction. Endocrinology 142:1703–1709 [DOI] [PubMed] [Google Scholar]
- Moore Jr JP, Burger LL, Dalkin AC, Winters SJ 2005 Pituitary adenylate cyclase activating polypeptide messenger RNA in the paraventricular nucleus and anterior pituitary during the rat estrous cycle. Biol Reprod 73:491–499 [DOI] [PubMed] [Google Scholar]
- Heinzlmann A, Kirilly E, Meltzer K, Szabó E, Baba A, Hashimoto H, Köves K 2008 PACAP is transiently expressed in anterior pituitary gland of rats: in situ hybridization and cell immunoblot assay studies. Peptides 29:571–577 [DOI] [PubMed] [Google Scholar]
- Moore Jr JP, Winters SJ 2008 Weaning and the developmental changes in follicle-stimulating hormone, pituitary adenylate cyclase-activating polypeptide, and inhibin B in the male rat. Biol Reprod 78:752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- Fronhoffs S, Totzke G, Stier S, Wernert N, Rothe M, Bruning T, Koch B, Sachinidis A, Vetter H, Ko Y 2002 A method for the rapid construction of cRNA standard curves in quantitative real-time reverse transcription polymerase chain reaction. Mol Cell Probes 16:99–110 [DOI] [PubMed] [Google Scholar]
- Fujii Y, Okada Y, Moore Jr JP, Dalkin AC, Winters SJ 2002 Evidence that PACAP and GnRH down-regulate follicle-stimulating hormone-β mRNA levels by stimulating follistatin gene expression: effects on folliculostellate cells, gonadotrophs and LβT2 gonadotroph cells. Mol Cell Endocrinol 192:55–64 [DOI] [PubMed] [Google Scholar]
- Duval DL, Ellsworth BS, Clay CM 1999 Is gonadotrope expression of the gonadotropin releasing hormone receptor gene mediated by autocrine/paracrine stimulation of an activin response element? Endocrinology 140:1949–1952 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Dalkin AC, Tsujii T 1997 Evidence that pituitary adenylate cyclase activating polypeptide suppresses follicle-stimulating hormone-β messenger ribonucleic acid levels by stimulating follistatin gene transcription. Endocrinology 138:4324–4329 [DOI] [PubMed] [Google Scholar]
- Inouye S, Guo Y, DePaolo L, Shimonaka M, Ling N, Shimasaki S 1991 Recombinant expression of human follistatin with 315 and 288 amino acids: chemical and biological comparison with native porcine follistatin. Endocrinology 129:815–822 [DOI] [PubMed] [Google Scholar]
- Ghzili H, Grumolato L, Thouënnon E, Tanguy Y, Turquier V, Vaudry H, Anouar Y 2008 Role of PACAP in the physiology and pathology of the sympathoadrenal system. Front Neuroendocrinol 29:128–141 [DOI] [PubMed] [Google Scholar]
- Vandermeers A, Vandenborre S, Hou X, de Neef P, Robberecht P, Vandermeers-Piret MC, Christophe J 1992 Antagonistic properties are shifted back to agonistic properties by further N-terminal shortening of pituitary adenylate-cyclase-activating peptides in human neuroblastoma NB-OK-1 cell membranes. Eur J Biochem 208:815–819 [DOI] [PubMed] [Google Scholar]
- Weiss J, Harris PE, Halvorson LM, Crowley Jr WF, Jameson JL 1992 Dynamic regulation of follicle-stimulating hormone-beta messenger ribonucleic acid levels by activin and gonadotropin-releasing hormone in perifused rat pituitary cells. Endocrinology 131:1403–1408 [DOI] [PubMed] [Google Scholar]
- Carroll RS, Corrigan AZ, Gharib SD, Vale W, Chin WW 1989 Inhibin, activin, and follistatin: regulation of follicle-stimulating hormone messenger ribonucleic acid levels. Mol Endocrinol 3:1969–1976 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Ghooray D, Fujii Y, Moore Jr JP, Nevitt JR, Kakar SS 2007 Transcriptional regulation of follistatin expression by GnRH in mouse gonadotroph cell lines: evidence for a role for cAMP signaling. Mol Cell Endocrinol 271:45–54 [DOI] [PubMed] [Google Scholar]
- Kogawa K, Nakamura T, Sugino K, Takio K, Titani K, Sugino H 1991 Activin-binding protein is present in pituitary. Endocrinology 128:1434–1440 [DOI] [PubMed] [Google Scholar]
- Chowdhury M, Steinberger E 1976 Pituitary and plasma levels of gonadotrophins in foetal and newborn male and female rats. J Endocrinol 69:381–384 [DOI] [PubMed] [Google Scholar]
- Winters SJ 1996 Relationship between gonadotropin subunit messenger ribonucleic acid levels and plasma gonadotropin concentrations in intact and orchidectomized adult rats. Biol Reprod 55:828–832 [DOI] [PubMed] [Google Scholar]
- Culler MD, Paschall CS 1991 Pituitary adenylate cyclase-activating polypeptide (PACAP) potentiates the gonadotropin-releasing activity of luteinizing hormone-releasing hormone. Endocrinology 129:2260–2262 [DOI] [PubMed] [Google Scholar]
- Ngan ES, Leung PC, Chow BK 2001 Interplay of pituitary adenylate cyclase-activating polypeptide with a silencer element to regulate the upstream promoter of the human gonadotropin-releasing hormone receptor gene. Mol Cell Endocrinol 176:135–144 [DOI] [PubMed] [Google Scholar]
- Pincas H, Laverrière JN, Counis R 2001 Pituitary adenylate cyclase-activating polypeptide and cyclic adenosine 3′,5′-monophosphate stimulate the promoter activity of the rat gonadotropin-releasing hormone receptor gene via a bipartite response element in gonadotrope-derived cells. J Biol Chem 276:23562–23571 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Tsujii T, Attardi B 1996 Effects of GnRH and PACAP on gonadotropin secretion and subunit messenger RNAs. Ann NY Acad Sci 805:343–352; discussion 352–344 (Review) [DOI] [PubMed] [Google Scholar]
- Krsmanovic LZ, Martinez-Fuentes AJ, Arora KK, Mores N, Tomić M, Stojilkovic SS, Catt KJ 2000 Local regulation of gonadotroph function by pituitary gonadotropin-releasing hormone. Endocrinology 141:1187–1195 [DOI] [PubMed] [Google Scholar]
- Bauer TW, Moriarty CM, Childs GV 1981 Studies of immunoreactive gonadotropin releasing hormone (GnRH) in the rat anterior pituitary. J Histochem Cytochem 29:1171–1178 [DOI] [PubMed] [Google Scholar]
- Bello-Pineda J, Luna J, Romano MC, Mendoza ME 1999 Developmental changes in LH secretion by male pituitaries in vitro: from the infantile to adult period. J Endocrinol 160:333–341 [DOI] [PubMed] [Google Scholar]
- Nash SJ, Brawer J, Robaire B, Ruf KB 1986 Changes in the dynamics of luteinizing hormone-releasing hormone-stimulated secretion of luteinizing hormone during sexual maturation of female rats. Biol Reprod 34:549–557 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Jameson HE, McCann SM 1977 Developmental changes in pituitary responsiveness to luteinizing hormone-releasing hormone (LHRH) in the female rat: ovarian-adrenal influence during the infantile period. Endocrinology 100:440–451 [DOI] [PubMed] [Google Scholar]
- Schafer SJ, McShan WH 1974 Gonadotropic hormone release from fetal and adult rat pituitary glands after in vitro exposure to synthetic LH-FSH-RH. Neuroendocrinology 16:332–341 [DOI] [PubMed] [Google Scholar]
- Moore Jr JP, Wray S 2000 Luteinizing hormone-releasing hormone (LHRH) biosynthesis and secretion in embryonic LHRH. Endocrinology 141:4486–4495 [DOI] [PubMed] [Google Scholar]
- Héritier AG, Dubois PM 1994 Re-evaluation of gonadotropin-releasing hormone (GnRH) action on pituitary cell differentiation with special regard to its effect on LH and TSH cell types. J Neuroendocrinol 6:33–37 [DOI] [PubMed] [Google Scholar]
- Kudo A, Park MK, Kawashima S 1994 Effects of gonadotropin-releasing hormone (GnRH) on the cytodifferentiation of gonadotropes in rat adenohypophysial primordia in organ culture. Cell Tissue Res 276:35–43 [DOI] [PubMed] [Google Scholar]
- Jaworski DM, Proctor MD 2000 Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Res Dev Brain Res 120:27–39 [DOI] [PubMed] [Google Scholar]
- Skoglösa Y, Takei N, Lindholm D 1999 Distribution of pituitary adenylate cyclase activating polypeptide mRNA in the developing rat brain. Brain Res Mol Brain Res 65:1–13 [DOI] [PubMed] [Google Scholar]
- Lindholm D, Skoglösa Y, Takei N 1998 Developmental regulation of pituitary adenylate cyclase activating polypeptide (PACAP) and its receptor 1 in rat brain: function of PACAP as a neurotrophic factor. Ann NY Acad Sci 865:189–196 [DOI] [PubMed] [Google Scholar]
- Ma YY, Qi XF, Song SJ, Zhao ZY, Zhu ZD, Qi J, Zhang X, Xiao HS, Teng Y, Han ZG 2005 cDNA microarray reveals signaling pathways involved in hormones expression of human pituitary. Gen Comp Endocrinol 143:184–192 [DOI] [PubMed] [Google Scholar]
- Shintani N, Hashimoto H, Tanaka K, Kawaguchi C, Tomimoto S, Baba A 2004 Overexpression of PACAP in the pancreas failed to rescue early postnatal mortality in PACAP-null mice. Regul Pept 123:155–159 [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Pendlebury JD, Sherwood NM, Wilson RJ 2004 Sudden neonatal death in PACAP-deficient mice is associated with reduced respiratory chemoresponse and susceptibility to apnoea. J Physiol 555:15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]