Abstract
Corticotropin-releasing factor (CRF) exerts proinflammatory effects in peripheral tissues, whereas the intracellular pathways mediating these effects have not been completely characterized yet. We have previously shown that CRF induces nuclear factor-κB DNA-binding activity in mouse and human leukocytes. Here we demonstrate that in the human monocytic THP-1 cells, CRF activates the phosphatidylinositol 3-kinase (PI3K)/Akt and ERK1/2 pathways. These effects of CRF are mediated by corticotropin-releasing factor receptor 2 (CRF2), as suggested by their abolishment after treatment with the specific CRF2 antagonist, astressin 2B. The CRF-mediated PI3K/Akt activation induces cell survival as suggested by the stimulation of the antiapoptotic factor Bcl-2. ERK1/2 activation results in up-regulation of IL-8 expression, an effect inhibited by the CRF-induced activation of PI3K/Akt. These studies demonstrate novel effects of CRF in human monocytes mediated by the activation of PI3K/Akt. Moreover, they reveal pathway-specific effects of the CRF/CRF2 system in chemokine activation and cell survival that may be of importance for the development of novel therapeutics for inflammatory diseases.
Corticotropin-releasing factor may protect human monocytes from apoptosis and up-regulate proinflammatory cytokines.
Corticotropin-releasing factor (CRF), or otherwise CRH, originally identified by its ability to stimulate pituitary ACTH secretion (1), has since been characterized as the major neuroendocrine mediator of the mammalian stress responses. Recently a family of CRF-related peptides has emerged that includes urotensin, urocortin (Ucn), stresscopin, and stresscopin-related peptides (2). These peptides interact with specific seven-transmembrane, G protein-coupled receptors (3). To date two such receptors have been identified, CRF receptor type 1 (CRF1) and type 2 (CRF2) (4,5,6,7) expressed in central and peripheral distinct areas (8,9). Hypothalamic CRF exerts antiinflammatory effects through activation of the hypothalamus-pituitary-adrenal (HPA) axis (10) that were inhibited by antalarmin, a CRF1 inhibitor (11). Peripheral CRF is a potent proinflammatory factor in rodents (12), whereas its expression is very high in human inflamed tissues (13,14). The proinflammatory actions of CRF are mainly mediated by CRF2, with nuclear factor-κB (NF-κB)/inhibitory-κB (IκB) the main intracellular pathway demonstrated so far to mediate these effects of CRF in leukocytes (15).
Current evidence suggests that the activation of MAPKs by CRF involves tissue-specific intracellular proteins and signaling pathways. Activation of the CRF receptor has been shown to induce ERK1/2 phosphorylation in a cAMP-independent way (16). In another report, in human pregnant myometrium Ucn induced ERK1/2 activation, an effect inhibited by protein kinase A activation, primarily via the CRF1 (17). A potent cardioprotective effect against hypoxic insults through activation of both Akt and ERK1/2 has been proposed for Ucn2 and Ucn3 that bind exclusively CRF2 (18,19,20,21,22,23).
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is implicated in a great spectrum of tissue responses and cellular processes including cell division, regulation of cell growth, suppression of apoptosis, inactivation of cell cycle inhibitors, induction of cyclin, and cytokine gene expression (24,25,26). In particular, in T and B cells, Akt activation follows antigen receptor engagement (27) and is correlated with induction of NF-κB-mediated functions (28,29). Transgenic mouse models have confirmed the role of Akt in states of altered lymphocyte homeostasis and autoimmunity (24,28,30,31). Furthermore, Ucn as well as the other members of this family, stresscopin and stresscopin-related peptide, induce cardiac hypertrophy via a PI3K/Akt-dependent pathway (23,32). Finally, the PI3K pathway has been recently suggested to play a critical role in CRF1-mediated effects, more specifically those of the subtype CRF1α, including signaling selectivity and the associated cellular responses (33).
In the present study, we demonstrate activation of the CRF/CRF2 system in the human monocytic cell line, THP-1, leading in phosphorylation of Akt. We also show that the above effect of CRF is related to the induction of antiapoptotic factors as opposed to the ERK1/2-mediated regulation of the expression of proinflammatory cytokines. Finally, we provide evidence for a dynamic interaction between signaling pathways in human monocytes after activation by CRF.
Materials and Methods
Materials
Phospho-ERK1/2 (Thr202/Tyr204), ERK1/2, phospho-Akt (Ser473), and Akt primary antibodies were purchased from Cell Signaling Technology Inc. (Danvers, MA), and Bcl-2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primary antibodies and all secondary antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). TRI reagent was purchased from Sigma-Aldrich (St. Louis, MO), whereas astressin 2B was a kind gift from Dr. D. E. Grigoriadis (Noracrine, La Jolla, CA). The inhibitors LY294002 and PD98059 were obtained from Calbiochem (San Diego, CA). Pyrrolidinedithiocarbamic acid (PDTC) and SN50 were purchased from Alexis Biochemicals (Lausen, Switzerland). Urocortin II was obtained from Bachem California, Inc. (Torrance, CA). The annexin V-fluorescein isothiocyanate apoptosis kit was purchased from PharMingen, Inc. (San Diego, CA).
Cell culture
The THP-1 human monocytic cell line obtained from American Type Culture Collection (Manassas, VA) was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/ml streptomycin, and 100 U/ml penicillin (Invitrogen, Carlsbad, CA). Eight hours before the experiment, the cells were switched to serum-free media and further treated according to the specific experimental procedure as indicated in Results. At the end of each experiment, the cells were collected and subjected to either total mRNA or total and nuclear protein extraction.
Total RNA preparation
Total RNA from THP-1 cells was prepared using TRI reagent. Its concentration was assessed by spectrophotometry and 2 μg of total RNA were used for cDNA synthesis initiated with random hexamer primers.
Semiquantitative RT-PCR
Amplification of the cDNA was performed using the following conditions: 94 C for 3 min of denaturation, followed by 30 cycles for β-actin or 35 cycles for IL-8 and Bcl-2 of annealing at 58 C, 62 C or 60 C for β-actin, IL-8, or Bcl-2, respectively, for 1 min and extension at 72 C for 1 min. Reactions were completed with an additional 7-min extension at 72 C. PCR conditions previously described support amplification conditions in the exponential stage and were performed in a 25 μl volume. PCR products were subsequently analyzed by electrophoresis through 1% agarose gels. The sense and antisense sequences of the primers used are: IL-8 sense, 5′-TCTGCAGCTCTGTGTGAAGGT-3′; IL-8 antisense, 5′-CACAACCCTCTGCACCCAGT-3′ (product size 222 bp), Bcl-2 sense, 5′-AAGATTGATGGGATCGTTGC-3′; Bcl-2 antisense, 5′-GCGGAACACTTGATTCTGGT-3′ (product size 229 bp); and β-actin sense, 5′-ATGGATGACGATATCGCTGCGC-3′; β-actin antisense, 5′-TCTGTCAGGTCCCGGCCA-3′ (product size 559 bp).
Real-time PCR
Human IL-8 was amplified from cDNA (dilution 1:10) using TaqMan reagents and a predeveloped set of primers and probe (Applied Biosystems, Foster City, CA). Reactions were run in duplicate in a 7700 sequence detector system (Applied Biosystems), and the results were normalized by human TATA-binding protein expression using a predeveloped assay (Applied Biosystems).
Isolation of total protein extracts
Total protein extracts were prepared using ice-cold radioimmunoprecipitation assay (RIPA) buffer containing appropriate protease inhibitors (Roche Applied Science, Mannheim, Germany). Cells were allowed to stand on ice for 10 min; the lysates were then passed through a 22-gauge needle and finally subjected to centrifugation 5000 × g for 10 min. The protein content in each sample was evaluated using the bicinchoninic acid (BCA) protein assay.
BCA protein assay
A BSA (Pierce Biotechnology Inc., Rockford, IL) standard curve ranging 1–0.0156 mg/ml was prepared. All unknown samples were assayed 1:25 to the same final volume as the standards. All samples received 200 μl of 49:1 (vol/vol) ratio of A to B BCA reagents (Pierce); after a 30-min incubation at 37 C, they were read in a plate reader (MicroLumatPlus LB 96V; Berthold Technologies, Bad Wildbad, Germany) using SoftMax Pro 4.7.1 program at 562 nm. The protein content of each sample was calculated according to the BSA protein standard curve.
Western blot analysis
Equal amounts of cell extracts were separated by (7 or 10%) SDS-PAGE. Proteins were transferred onto a polyvinyl difluoride membrane (Bio-Rad, Hercules, CA) at 100 V for 1 h at +4 C. Membranes were blocked for 2 h in 5% nonfat dried milk and 50 mm Tris (pH 7.5), 0.15 m NaCl, and 0.05% Tween 20 and then incubated with the corresponding primary antibody overnight at +4 C. Membranes were washed three times with 50 mm Tris (pH 7.5), 0.15 m NaCl, and 0.05% Tween 20 and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Peroxidase activity was detected by the chemiluminescent substrate (Amersham Biosciences, Piscataway, NJ). Membranes were probed for the phosphoprotein first and then stripped and reprobed for the total protein signals. Band densities were quantified using National Institutes of Health Image J program (http://rsb.info.nih.gov/ij). Phosphoprotein densities were normalizes relative to those of respective total protein, and the ratio of phosphoprotein to total protein was determined in terms of arbitrary units and expressed as percentage of control (vehicle treatment).
Measurement of apoptosis using annexin V
Apoptosis was measured using the annexin V-fluorescein isothiocyanate apoptosis kit according to manufacturer’s instructions (PharMigen). Annexin V binding was analyzed in the FACSCalibur flow cytometer using CellQuest Pro software (Becton Dickinson, Franklin Lakes, NJ) on at least 10,000 events.
Data analysis
All experimental data are presented as the mean ± se of four to eight independent experiments and expressed as percentage of the respective control. Statistical evaluations were performed on raw data using GraphPad Prism 4 software (San Diego, CA) by one-way ANOVA, with Dunnett’s or Bonferroni’s post hoc analysis. P < 0.05 was accepted as significant in all experiments.
Results
CRF activates Akt in THP-1 cells via CRF2
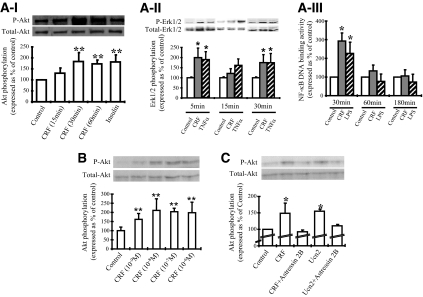
THP-1 cells were stimulated with increasing concentrations of CRF from 10−9 to 10−6 m. Akt phosphorylation was assessed by Western blot analysis using a specific antibody against phosphorylated Akt. Treatment lasted for 30 min, the point of maximal response as indicated by a time-course experiment (Fig. 1, A–I). CRF induced Akt phosphorylation by approximately 50% over the control values (Fig. 1B), at concentrations as low as 10−9 m, lower than the receptor affinity constant (ranging from 5.2 to 44 nm) (34). In all the experiments described below, we used CRF at 10−7 m due to the tightly reproducible responses compared with the other examined doses and the relevance to other published studies on the in vitro effects of CRF. To evaluate whether this effect was mediated by CRF2, the predominant receptor expressed in THP-1 cells, we pretreated the cells with the specific CRF2 receptor antagonist astressin 2B (10−6 m) for 30 min before challenge with CRF, the specific CRF2 agonist Ucn2, or vehicle for another 30 min before harvesting. CRF and Ucn2 induced Akt phosphorylation to a similar extent compared with control (Fig. 1C). Cotreatment with the specific CRF2 antagonist abolished the above response, indicating that CRF-induced phosphorylation of Akt in THP-1 cells is mediated by CRF2. Potential direct effects of PD98059 on Akt phosphorylation were excluded (data not shown). No effect of astressin 2B alone (data not shown) was detected in support of the specificity of these responses.
Figure 1.
A, Time-dependent effects of CRF on Akt phosphorylation (I). THP-1 cells were stimulated with CRF (10−7 m) for 15, 30. and 60 min, whereas insulin (10 μg/ml) was used as a positive control. Akt phosphorylation was examined by Western blot analysis. A representative Western blot of phosphorylated (P-Akt) and total Akt from one experiment, together with the quantitative results of four individual experiments are shown; phosphorylated Akt expression was standardized to total Akt and represented as percentage of the control. **, P < 0.01 relative to control. II, ERK1/2 phosphorylation. THP-1 cells were treated with CRF (10−7 m) for 5, 15, and 30 min, whereas TNFα (10 ng/ml) was used as a positive control. ERK1/2 phosphorylation was examined by Western blot analysis. A representative Western blot of phosphorylated (P-Erk1/2) and total ERK1/2 from one experiment, together with the quantitative results of four individual experiments are shown; phosphorylated ERK1/2 was standardized to total ERK1/2 and represented as percentage of the control. **, P < 0.01 relative to control. III. NF-κB DNA binding activity. THP-1 cells were treated with CRF (10−7 m) for 30, 60, or 180 min. The NF-κB DNA binding activity in the nuclear extracts was measured from six individual experiments. Results are represented as percentage of control. *, P < 0.05 relative to control. B, CRF stimulates Akt phosphorylation. THP-1 cells were stimulated with increasing concentrations of CRF (10−9, 10−8, 10−7, 10−6 m) for 30 min. Akt phosphorylation was examined by Western blot analysis. At the top a representative Western blot of phosphorylated and total Akt from one experiment are featured, whereas a graph of the quantitative results of the findings of four individual experiments are shown at the lower part of the figure; phosphorylated Akt expression was standardized to total Akt and represented as percentage of the control. **, P < 0.01 relative to control. C, CRF-induced Akt phosphorylation is mediated by CRF2. THP-1 cells were stimulated with CRF or Ucn2 (10−7 m) alone or in the presence of astressin 2B (10−6 m) for 30 min. Akt phosphorylation was examined by Western blot analysis. On the top, a representative Western blot of the phosphorylated and total Akt is presented, whereas below, a quantitative representation of findings of four individual experiments; phosphorylated Akt expression was standardized to total Akt and represented as percentage of the control. *, P < 0.05.
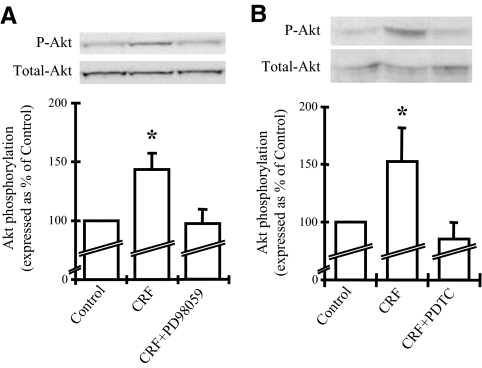
Inhibition of the ERK1/2 and NF-κB pathways abolishes the effects of CRF on Akt
Activation of ERK1/2 by CRF or other members of this family of peptides has been shown in different cell types (16,17,33). To define the role of ERK1/2 in the activation of the PI3K/Akt in THP-1 cells, ERK1/2 activation was blocked by using a specific inhibitor, PD98059 (IC50 20 μm) before assessing the CRF-induced phosphorylation of Akt. Thus, cells were pretreated with PD98059 (30 μm) for 30 min before adding CRF for an additional 30 min. Akt phosphorylation was evaluated in cellular extracts by Western blot analysis. CRF induced Akt phosphorylation, whereas pretreatment with PD98059 prevented this effect (Fig. 2A). This finding suggests that CRF-induced Akt phosphorylation in THP-1 cells is dependent on ERK1/2 activation. Induction of nuclear translocation of the p65 component of NF-κB or modulation of the NF-κB-induced cytokine expression by PI3K/Akt has been shown by several studies (35,36,37). We previously described CRF2-mediated induction of NF-κB along with degradation of IκB in mouse and human cells (38). To examine the interaction between NF-κB and PI3K/Akt we applied the NF-κB inhibitor PDTC (100 μm; IC50 17.5 μm) on THP-1 cells for 30 min before adding CRF for an additional 30 min. Akt phosphorylation assessed by Western blot analysis showed that pretreatment with PDTC completely prevented the CRF-induced phosphorylation of Akt (Fig. 2B). PDTC alone had no effect on Akt phosphorylation (data not shown). Similar results were obtained by treatment with the SN50 peptide, another potent inhibitor of NF-κB [final concentration in the well used was 36 μm (100 μg/ml), as indicated by the manufacturer for 85% inhibition in the particular cell line (http://www.alexis-biochemicals.com/ SN50.5+M544b62b0c17.0.html)] (data not shown).
Figure 2.
A, Inhibition of the ERK1/2 MAPK pathway diminishes the CRF-induced Akt phosphorylation. THP-1 cells were stimulated with CRF (10−7 m) alone or cochallenged with PD98059 (30 μm) for 30 min. Akt phosphorylation was examined by Western blot analysis. A representative Western blot of the phosphorylated (P-Akt) and total Akt is presented on the top, together with a graphical representation of the combined data of four individual experiments. Phosphorylated Akt expression was standardized to total Akt and represented as percentage of the control. *, P < 0.05 relative to control. B, Inhibition of the NF-κB pathway abolishes the CRF-induced Akt phosphorylation. THP-1 cells were stimulated with CRF (10−7 m) alone or cochallenged with PDTC (100 μm) for 30 min. Akt phosphorylation was examined by Western blot analysis. A representative Western blot of the phosphorylated and total Akt is presented on the top, together with a graphical representation of the combined data of four individual experiments. Phosphorylated Akt expression was standardized to total Akt and represented as percentage of the control. *, P < 0.05 relative to control.
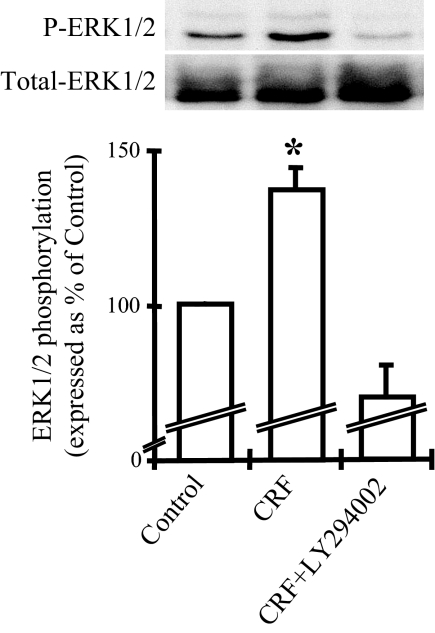
Inhibition of PI3K/Akt blocks the CRF-induced activation of ERK1/2
Inhibition of the PI3K/Akt pathway has been shown to prevent ERK1/2 activation, and in some experimental systems such as in T cell cultures, this was associated with decreased activation of Th1-mediated responses (39). To evaluate the relevance of this interaction in CRF-treated THP-1 cells, we pretreated the cells with the PI3K inhibitor LY294002 (30 μm; IC50 10 μm) for 30 min before adding CRF for an additional 5 min, sufficient time for maximal activation of ERK1/2. Selection of the particular time point was based on the time course of CRF (10−7 m)-induced ERK1/2 phosphorylation (Fig. 1A, II). ERK1/2 phosphorylation was evaluated by Western blot analysis in total protein extracts. As shown in Fig. 3, CRF induced ERK1/2 phosphorylation, an effect completely abolished by pretreatment with LY294002. No effect of LY294002 alone on ERK1/2 phosphorylation was found (data not shown).
Figure 3.
Inhibition of the PI3K/Akt pathway abolishes the CRF-induced ERK1/2 phosphorylation. THP-1 cells were stimulated with CRF (10−7 m) alone or costimulated with LY294002 (30 μm) for 5 min. ERK1/2 phosphorylation was examined by Western blot analysis. A representative Western blot of the phosphorylated (P-ERK1/2) and total ERK1/2 is presented on the top together with a graphical representation of the combined data of four individual experiments. Phosphorylated ERK1/2 expression was standardized to total ERK1/2 and represented as percentage of the control. *, P < 0.05 relative to control.
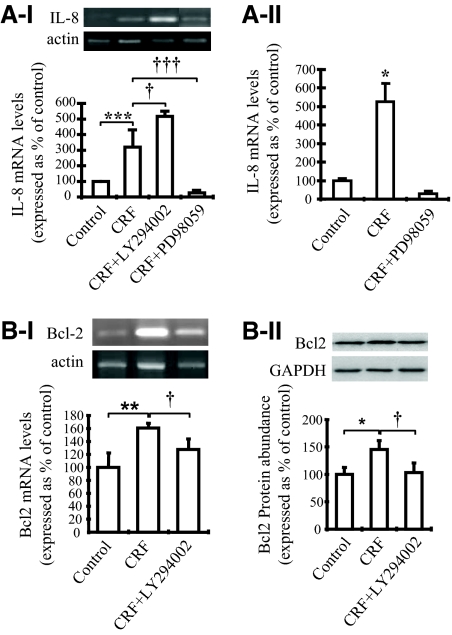
Downstream effects of the CRF-induced activation of ERK1/2 and Akt in THP-1 cells
To understand the significance of ERK1/2 and Akt activation by CRF in THP-1 cells, we assessed the expression of the IL-8 gene, a critical proinflammatory factor implicated in the pathogenesis of human inflammatory diseases. Cells were pretreated with the PI3K inhibitor LY294002 (30 μm) or the specific ERK1/2 inhibitor PD98059 (30 μm) or vehicle (control) for 30 min before addition of CRF for 2 h. As shown (Fig. 4A), CRF increased IL-8 mRNA levels by approximately 3-fold, an effect completely abolished by coaddition of CRF with PD98059. These results were also confirmed by real-time PCR (Fig. 4A, II). Interestingly, cotreatment with LY294002 and CRF resulted in further activation, by approximately 1.5-fold, of IL-8 expression. These findings suggest that in THP-1 cells, activation of Akt may serve to control the CRF-induced activation of IL-8.
Figure 4.
A, Intracellular signaling pathways involved in the CRF-mediated regulation of the IL-8 gene. I. Semiquantitative RT-PCR. THP-1 cells were treated with CRF (10−7 m) alone or cochallenged with LY294002 (30 μm) or PD98059 (30 μm) for 2 h. IL-8 mRNA levels were examined by PCR. On the top there is a representative picture of the IL-8 and actin expression. Below a graphical representation of the combined data of four individual experiments is presented. IL-8 expression levels were standardized using the respective expression of actin. *, P < 0.05; **, P < 0.01; ***, P < 0.001 relative to control; †, P < 0.05; †††, P < 0.001 relative to CRF-treated cells. II, Real-time PCR THP-1 cells were treated with CRF (10−7 m) alone or cochallenged with PD98059 (30 μm) for 2 h. IL-8 mRNA levels were examined by real-time PCR. A graphical representation of the combined data of four individual experiments is presented. IL-8 expression levels were normalized by human TATA-binding protein expression using a predeveloped assay. *, P < 0.05 relative to control. B, CRF induces Bcl-2 expression and protein levels via activating phosphorylation of Akt. I, Effect of CRF on Bcl-2 mRNA expression. THP-1 cells were treated with CRF (10−7 m) or CRF together with LY294002 (30 μm) for 2 h. Bcl-2 mRNA levels were examined by PCR. On the top, a representative picture of Bcl-2 and actin PCR products is presented. Below, a representation of the combined data of four individual experiments is shown. Bcl-2 expression was standardized to actin. **, P < 0.01 relative to control; †, P < 0.05 relative to CRF-treated cells. II, Effects of CRF on Bcl-2 protein expression. THP-1 cells were treated with CRF (10−7 m) alone or cochallenged with LY294002 (30 μm) for 48 h. Bcl-2 protein levels were examined by Western blot analysis. On the top, Representative Western blot of Bcl-2 and GAPDH protein abundance is presented. Below, there is a representation of the combined data of four individual experiments. Bcl-2 protein abundance was standardized to GAPDH expression. *, P < 0.05 relative to control; †, P < 0.05 relative to CRF-treated cells.
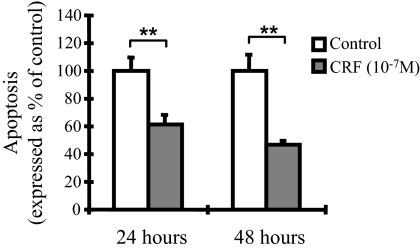
We next evaluated the possible contribution of CRF-induced Akt phosphorylation in cell survival, a well-described function of the activated PI3K/Akt pathway. For this reason, cells were first pretreated with the PI3K inhibitor LY294002 (30 μm) for 30 min, and then CRF was added for an additional 2 h. The inhibitor wortmannin (100 nm, IC50 15 nm) was also used and revealed effects similar to those shown by LY294002 (data not shown). Bcl-2 expression was assessed by evaluation of mRNA and protein abundance. As shown in Fig. 4B, CRF increased Bcl-2 mRNA (Fig. 4B, I) and protein (Fig. 4B, II) levels by approximately 50%, an effect abolished by cotreatment with LY294002. This finding suggests that in THP-1 cells, CRF induces prosurvival mechanisms via activation of the PI3K/Akt pathway. The CRF-mediated protection of THP-1 cells from apoptosis was also confirmed by annexin V binding after fluorescence-activated cell sorter (FACS) analysis. For this reason, THP-1 cells were cultured in serum-free medium for 24 or 48 h in the presence or absence of CRF (10−7 μm). CRF challenge for 24 or 48 h protected THP-1 cells from serum deprivation-induced apoptosis because it reduced annexin V-positive cells, by 39 or 53%, respectively, compared with untreated cells (Fig. 5).
Figure 5.
CRF-mediated protection of THP-1 cells from apoptosis. THP-1 cells cultured under serum-deprived conditions for 24 or 48 h in the presence or absence of CRF (10−7 m). Apoptosis was evaluated by annexin V binding, analyzed by FACScan. Result is a representation of the mean of triplicate experiments. **, P < 0.01 relative to control.
Discussion
In this study we investigated the intracellular signaling pathways engaged by CRF/CRF2 in the human mononuclear THP-1 cells and their downstream immunomodulatory effects. We report for the first time that in THP-1 cells CRF, acting via CRF2, activates PI3K/Akt and ERK1/2 MAPK together with its previously shown effects on NF-κB (40). Our results suggest that CRF may protect human monocytes from apoptosis and up-regulate proinflammatory cytokines.
PI3K/Akt activation is associated with processes related to cell division, growth, metabolism, and survival (24,25,26). CRF-mediated Akt activation can be seen in concentrations of CRF as low as 10−9 m (Fig. 1B), in support of the physiological relevance of this effect. Activation of Akt in THP-1 cells by CRF is mediated by CRF2 because administration of the specific CRF2 antagonist astressin 2B abolished this effect, whereas Ucn2, an exclusive ligand of CRF2 had similar effects (Fig. 1C). The ability of activated CRF2 to modulate phosphorylation of PI3K/Akt has been suggested by a recent study showing blockade of insulin-induced Akt and ERK1/2 phosphorylation in muscle cells by Ucn2 (41). In this study, unlike in ours, a CRF2 ligand modified the effect of insulin on Akt phosphorylation, whereas no direct effect of Ucn2 on Akt phosphorylation was shown. The differences between our findings and these results could be due to the experimental model used.
CRF is an immunomodulatory factor that, as we have previously shown, induces NF-κB DNA binding activity in parallel to IκB degradation in mouse and human endothelial cells via CRF2 (Zhao J. and K. Karalis, unpublished observation). We recently reported Ucn2-mediated phosphorylation of the p65 NF-κB subunit and parallel degradation of IκBα in human colonocytes (40). Akt has been shown to participate in NF-κB activation after lipopolysaccharide (LPS) treatment in some tissues (42,43,44). In human monocytes stimulated with Porphyromonas gingivalis LPS, activation of PI3K resulted in substantial reduction of IL-10 release, with concomitant increase in IL-12 levels (39). Interestingly, one study showed that inhibition of the PI3K/Akt pathway resulted in enhanced NF-κB p65 activation in LPS-stimulated monocytes (45), whereas another demonstrated that PI3K phosphorylation induces activation of p65 (46). Furthermore, Akt was shown to play a critical role in the activation of ERK1/2 and NF-κB after stimulation of Toll-like receptor-2 (TLR2) in mouse neutrophils (47). Interestingly, we found that CRF-induced Akt phosphorylation was blocked by the NF-κB inhibitor PDTC (Fig. 2B) and another specific NF-κB inhibitor (SN50). This is a novel effect of CRF; the time dependency and cell specificity of these findings will be examined in future studies. Other reports have shown opposite directions of activation of NF-κB and PI3K/Akt in related systems (48,49,50,51).
The CRF-related peptides activate ERK1/2 MAPK in various cell types. Initial findings from ischemia-activated cardiomyocytes treated with Ucn2 (21) suggested the possibility that Ucn contributes to the cardioprotective mechanisms activated during this process (18,22). Similar effects were also described for A7r5 and CATH cells (22). We have recently shown activation of ERK1/2 in response to Ucn2 and associated induction of the IL-8 gene in human colonocytes (34). Other studies in rat hippocampal cells (52) as well as the pituitary corticotrophs AtT20 demonstrated CRF1-mediated phosphorylation of ERK1/2 (22). In that study, Ucn-induced ERK1/2 phosphorylation was mediated by Gi via Gβγ-induced activation of PI3K (22). Finally, in a very recent study using embryonic kidney 293 cells, it was conclusively demonstrated that CRF1-associated ERK1/2 activation is dependent on PI3K phosphorylation (33). In our studies, blockade of PI3K activation abolished the CRF-induced ERK1/2 phosphorylation (Fig. 3). In agreement with our findings, it was shown that activation of ERK1/2 and p38, by factors acting through other G protein-coupled receptors, such as C5a or chemokine, was attenuated by inhibition of the PI3K activation (52,53). On the other hand, ERK-dependent PI3K/Akt activation has been shown in other tissues such as the liver (54).
Other reports have shown that in human monocytes, inhibition of PI3K abrogated ERK1/2 phosphorylation, with no particular effect in the activation of p38 and c-Jun N-terminal kinase (JNK; Ref. 48). Several studies described direct and indirect interactions between these two pathways. In our current study, we have also shown that in human macrophages, specific blockade of ERK1/2 phosphorylation diminished CRF-induced Akt phosphorylation (Fig. 2A). The above data suggest a dynamic interaction between these two signaling pathways in human monocytes after CRF exposure.
CRF stimulates the expression of proinflammatory cytokines in immune cells (55). As we have previously shown, leukocytes isolated from Crh-deficient mice have decreased TNFα and IL-1β expression after challenge with LPS, primarily via CRF2-mediated effects (56). Similarly, Ucn2-induced CRF2 activation in aortic smooth muscle cells resulted in increased IL-6 production (57). To assess the potential significance of ERK1/2 and Akt phosphorylation by CRF/CRF2 in THP-1 cells, we evaluated the effect of CRF on the expression of IL-8, a potent chemoattractant expressed in human cells. We found inhibition of CRF-mediated IL-8 expression after blockade of ERK1/2 activation (Fig. 4A). This is in agreement with our previous reports in HT-29 colonic adenocarcinoma epithelial cells showing increased expression of IL-8 and monocyte chemotactic protein-1 (MCP1) after treatment with Ucn2 (58). Our more recent findings demonstrated increased IL-8 expression in nontransformed human colonocytes after Ucn2 exposure via activation of both NF-κB and ERK1/2 (40). In other studies it was suggested that CRF2 mediated antiinflammatory effects because Ucn2 augmented IL-10 and decreased TNFα release from murine RAW264.7 macrophages infected with Listeria (59). In our current study, we found that cotreatment of THP-1 cells with CRF and the PI3K inhibitor, LY294002, resulted in further stimulation of IL-8 expression (Fig. 4A). These data suggest that CRF-induced Akt phosphorylation may serve as a brake to IL-8 stimulation. Consistent with this notion, PI3K/Akt has been reported to exert negative feedback effects in activated human monocytes (45) and dendritic cells (60).
CRF and Ucn have been implicated in regulation of either cell survival or apoptosis. Thus, it has been reported that they protect cardiomyocytes after ischemia and reperfusion (18,22) and neuronal cells against oxidative cell death (61). Furthermore, CRF induces proliferation and release of TNFα from rat microglia cells (62). However, induction of apoptosis by CRF has also been reported in PC12 cells and mouse macrophages, most likely via CRF1 (63,64). It is well established that growth factors or cytokines induce cell survival signals via PI3K/Akt-dependent signal transduction pathways. Activation of Akt has been shown to protect various cell types from programmed cell death and apoptosis, whereas it also modified caspase activity (65). We evaluated the effect of CRF-induced Akt phosphorylation in THP-1 cell survival, as indicated by regulation of Bcl-2 expression. Bcl-2 is a prototypic antagonist of caspase activation and acts to suppress apoptosis and delay cell cycle entry (66). As shown, CRF induces Bcl-2 mRNA expression and protein levels (Fig. 4B) via a PI3K/Akt-dependent pathway, as suggested by the abolishment of this effect by coaddition of LY294002. Similar findings have been previously described for other proinflammatory factors. Thus, TNFα and IL-1β activate a PI3K/Akt-mediated antiapoptotic pathway in human endothelial cells, independent of NF-κB induction (67). Another study described enhancement of apoptotic cell death by the PI3K/Akt inhibitor LY294002, a dominant-negative PI3K construct, or finally a kinase-dead Akt (68). Given the importance of Akt for promoting cell survival and the increased expression of CRF in inflammation, the implications of our findings (Fig. 6) may be of particular interest for tissue repair in states of inflammation.
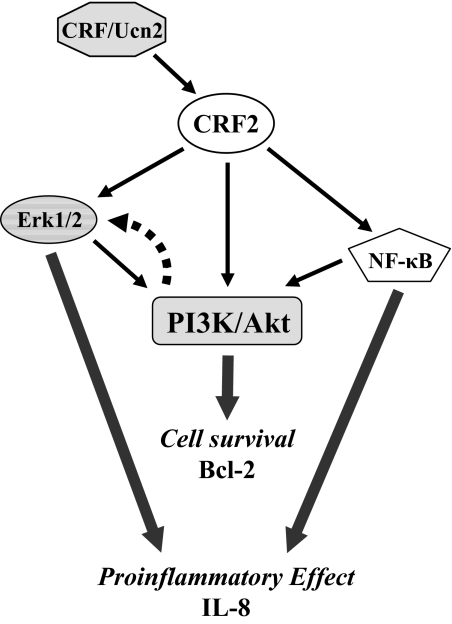
Figure 6.
Schematic representation of the intracellular signaling pathways mediating the effects of CRF on IL-8 and Bcl-2 expression in THP-1 cells.
In summary, data presented in this work define PI3K/Akt as a novel pathway mediating the effects of CRF via activation of CRF2 in human leukocytes. Our results also provide evidence for dynamic interaction among the signaling pathways mediating the immunomodulatory effects of CRF. Finally, we show the significance of CRF/CRF2 in human leukocytes for induction of chemoattractant genes as well as cell survival signals. Deciphering the contribution of the specific pathways mediating the effects of CRF/CRF1 or CRF/CRF2 during immune activation may provide new insights in the pathogenesis of inflammatory diseases such as arthritis, colitis, and endotoxemia and could open novel possibilities for specific therapeutics.
Footnotes
This work was supported by National Institutes of Health Grants RO1DK47977-05 (to K.P.K.) and 5RO1DK35506-18 (to C.P.).
Disclosure Summary: The authors C.C., Y.K., E.K., C.P., and K.P.K. have nothing to disclose.
First Published Online July 23, 2009
Abbreviations: BCA, Bicinchoninic acid; CRF, corticotropin-releasing factor; CRF1, CRF receptor type 1; CRF2, CRF receptor type 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IκB, inhibitory-κB; LPS, lipopolysaccharide; NF-κB, nuclear factor-κB; PDTC, pyrrolidinedithiocarbamic acid; PI3K, phosphatidylinositol 3-kinase; Ucn, urocortin.
References
- Vale W, Spiess J, Rivier C, Rivier J 1981 Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213:1394–1397 [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW 2004 CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557 [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Chrousos GP 2002 Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab 13:436–444 [DOI] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW 1993 Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci USA 90:8967–8971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W 1995 Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci USA 92:2969–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis DE, Liu XJ, Vaughn J, Palmer SF, True CD, Vale WW, Ling N, De Souza EB 1996 125I-Tyro-sauvagine: a novel high affinity radioligand for the pharmacological and biochemical study of human corticotropin-releasing factor 2α receptors. Mol Pharmacol 50:679–686 [PubMed] [Google Scholar]
- Liaw CW, Lovenberg TW, Barry G, Oltersdorf T, Grigoriadis DE, de Souza EB 1996 Cloning and characterization of the human corticotropin-releasing factor-2 receptor complementary deoxyribonucleic acid. Endocrinology 137:72–77 [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Pearse 2nd RV, Lin CR, Rosenfeld MG 1995 A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci USA 92:1108–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE 2000 Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428:191–212 [DOI] [PubMed] [Google Scholar]
- Webster EL, Torpy DJ, Elenkov IJ, Chrousos GP 1998 Corticotropin-releasing hormone and inflammation. Ann NY Acad Sci 840:21–32 [DOI] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP 1996 In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology 137:5747–5750 [DOI] [PubMed] [Google Scholar]
- Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP 1991 Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science 254:421–423 [DOI] [PubMed] [Google Scholar]
- Crofford LJ, Sano H, Karalis K, Webster EL, Goldmuntz EA, Chrousos GP, Wilder RL 1992 Local secretion of corticotropin-releasing hormone in the joints of Lewis rats with inflammatory arthritis. J Clin Invest 90:2555–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofford LJ, Sano Η, Karalis Κ, Friedman ΤC, Epps HR, Remmers EF, Mathern P, Chrousos GP, Wilder RL 1993 Corticotropin- releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J Immunol 151:1587–1596 [PubMed] [Google Scholar]
- Lu Y, Cuevas B, Gibson S, Khan H, LaPushin R, Imboden J, Mills GB 1998 Phosphatidylinositol 3-kinase is required for CD28 but not CD3 regulation of the TEC family tyrosine kinase EMT/ITK/TSK: functional and physical interaction of EMT with phosphatidylinositol 3-kinase. J Immunol 161:5404–5412 [PubMed] [Google Scholar]
- Rossant CJ, Pinnock RD, Hughes J, Hall MD, McNulty S 1999 Corticotropin-releasing factor type 1 and type 2α receptors regulate phosphorylation of calcium/cyclic adenosine 3′,5′-monophosphate response element-binding protein and activation of p42/p44 mitogen-activated protein kinase. Endocrinology 140:1525–1536 [DOI] [PubMed] [Google Scholar]
- Papadopoulou N, Chen J, Randeva HS, Levine MA, Hillhouse EW, Grammatopoulos DK 2004 Protein kinase A-induced negative regulation of the corticotropin-releasing hormone R1α receptor-extracellularly regulated kinase signal transduction pathway: the critical role of Ser301 for signaling switch and selectivity. Mol Endocrinol 18:624–639 [DOI] [PubMed] [Google Scholar]
- Chanalaris A, Lawrence KM, Stephanou A, Knight RD, Hsu SY, Hsueh AJ, Latchman DS 2003 Protective effects of the urocortin homologues stresscopin (SCP) and stresscopin-related peptide (SRP) against hypoxia/reoxygenation injury in rat neonatal cardiomyocytes. J Mol Cell Cardiol 35:1295–1305 [DOI] [PubMed] [Google Scholar]
- Brar BK, Stephanou A, Knight R, Latchman DS 2002 Activation of protein kinase B/Akt by urocortin is essential for its ability to protect cardiac cells against hypoxia/reoxygenation-induced cell death. J Mol Cell Cardiol 34:483–492 [DOI] [PubMed] [Google Scholar]
- Railson JE, Liao Z, Brar BK, Buddle JC, Pennica D, Stephanou A, Latchman DS 2002 Cardiotrophin-1 and urocortin cause protection by the same pathway and hypertrophy via distinct pathways in cardiac myocytes. Cytokine 17:243–253 [DOI] [PubMed] [Google Scholar]
- Brar BK, Jonassen AK, Stephanou A, Santilli G, Railson J, Knight RA, Yellon DM, Latchman DS 2000 Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J Biol Chem 275:8508–8514 [DOI] [PubMed] [Google Scholar]
- Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, Mjos OD, Latchman DS, Lee KF, Vale W 2004 Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology 145:24–35; discussion 21–23 [DOI] [PubMed] [Google Scholar]
- Brar BK, Railson J, Stephanou A, Knight RA, Latchman DS 2002 Urocortin increases the expression of heat shock protein 90 in rat cardiac myocytes in a MEK1/2-dependent manner. J Endocrinol 172:283–293 [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Waterfield MD 1999 Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res 253:239–254 [DOI] [PubMed] [Google Scholar]
- Cantrell DA 2001 Phosphoinositide 3-kinase signalling pathways. J Cell Sci 114:1439–1445 [DOI] [PubMed] [Google Scholar]
- Roymans D, Slegers H 2001 Phosphatidylinositol 3-kinases in tumor progression. Eur J Biochem 268:487–498 [DOI] [PubMed] [Google Scholar]
- Neri LM, Milani D, Bertolaso L, Stroscio M, Bertagnolo V, Capitani S 1994 Nuclear translocation of phosphatidylinositol 3-kinase in rat pheochromocytoma PC 12 cells after treatment with nerve growth factor. Cell Mol Biol 40:619–626 [PubMed] [Google Scholar]
- Kim KW, Cho ML, Park MK, Yoon CH, Park SH, Lee SH, Kim HY 2005 Increased interleukin-17 production via a phosphoinositide 3-kinase/Akt and nuclear factor κB-dependent pathway in patients with rheumatoid arthritis. Arthritis Res Ther 7:R139–R148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didichenko SA, Thelen M 2001 Phosphatidylinositol 3-kinase c2α contains a nuclear localization sequence and associates with nuclear speckles. J Biol Chem 276:48135–48142 [DOI] [PubMed] [Google Scholar]
- Metjian A, Roll RL, Ma AD, Abrams CS 1999 Agonists cause nuclear translocation of phosphatidylinositol 3-kinase γ. A Gβγ-dependent pathway that requires the p110γ amino terminus. J Biol Chem 274:27943–27947 [DOI] [PubMed] [Google Scholar]
- Bacqueville D, Déléris P, Mendre C, Pieraggi MT, Chap H, Guillon G, Perret B, Breton-Douillon M 2001 Characterization of a G protein-activated phosphoinositide 3-kinase in vascular smooth muscle cell nuclei. J Biol Chem 276:22170–22176 [DOI] [PubMed] [Google Scholar]
- Chanalaris A, Lawrence KM, Townsend PA, Davidson S, Jamshidi Y, Stephanou A, Knight RD, Hsu SY, Hsueh AJ, Latchman DS 2005 Hypertrophic effects of urocortin homologous peptides are mediated via activation of the Akt pathway. Biochem Biophys Res Commun 328:442–448 [DOI] [PubMed] [Google Scholar]
- Punn A, Levine MA, Grammatopoulos DK 2006 Identification of signaling molecules mediating corticotropin-releasing hormone-R1α-mitogen-activated protein kinase (MAPK) interactions: the critical role of phosphatidylinositol 3-kinase in regulating ERK1/2 but not p38 MAPK activation. Mol Endocrinol 20:3179–3195 [DOI] [PubMed] [Google Scholar]
- Perrin MH, Vale WW 1999 Corticotropin releasing factor receptors and their ligand family. Ann NY Acad Sci 885:312–328 [DOI] [PubMed] [Google Scholar]
- Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH 2003 Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem 278:37041–37051 [DOI] [PubMed] [Google Scholar]
- Li B, Cheung PY, Wang X, Tsao SW, Ling MT, Wong YC, Cheung AL 2007 Id-1 activation of PI3K/Akt/NFκB signaling pathway and its significance in promoting survival of esophageal cancer cells. Carcinogenesis 28:2313–2320 [DOI] [PubMed] [Google Scholar]
- Figueroa YG, Chan AK, Ibrahim R, Tang Y, Burow ME, Alam J, Scandurro AB, Beckman BS 2002 NF-κB plays a key role in hypoxia-inducible factor-1-regulated erythropoietin gene expression. Exp Hematol 30:1419–1427 [DOI] [PubMed] [Google Scholar]
- Zhao J, Karalis KP 2002 Regulation of nuclear factor-κB by corticotropin-releasing hormone in mouse thymocytes. Mol Endocrinol 16:2561–2570 [DOI] [PubMed] [Google Scholar]
- Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM 2003 Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol 171:717–725 [DOI] [PubMed] [Google Scholar]
- Moss AC, Anton P, Savidge T, Newman P, Cheifetz AS, Gay J, Paraschos S, Winter MW, Moyer MP, Karalis K, Kokkotou E, Pothoulakis C 2007 Urocortin II mediates pro-inflammatory effects in human colonocytes via corticotropin-releasing hormone receptor 2α. Gut 56:1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Brar B, Choi CS, Rousso D, Vaughan J, Kuperman Y, Kim SN, Donaldson C, Smith SM, Jamieson P, Li C, Nagy TR, Shulman GI, Lee KF, Vale W 2006 Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle. Proc Natl Acad Sci USA 103:16580–16585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna SK, Aggarwal BB 2000 Wortmannin inhibits activation of nuclear transcription factors NF-κB and activated protein-1 induced by lipopolysaccharide and phorbol ester. FEBS Lett 473:113–118 [DOI] [PubMed] [Google Scholar]
- Li X, Tupper JC, Bannerman DD, Winn RK, Rhodes CJ, Harlan JM 2003 Phosphoinositide 3 kinase mediates Toll-like receptor 4-induced activation of NF-κB in endothelial cells. Infect Immun 71:4414–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yum HK, Arcaroli J, Kupfner J, Shenkar R, Penninger JM, Sasaki T, Yang KY, Park JS, Abraham E 2001 Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol 167:6601–6608 [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N 2002 The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem 277:32124–32132 [DOI] [PubMed] [Google Scholar]
- Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG 2000 Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat Immunol 1:533–540 [DOI] [PubMed] [Google Scholar]
- Strassheim D, Asehnoune K, Park JS, Kim JY, He Q, Richter D, Kuhn K, Mitra S, Abraham E 2004 Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol 172:5727–5733 [DOI] [PubMed] [Google Scholar]
- Parsa KV, Ganesan LP, Rajaram MV, Gavrilin MA, Balagopal A, Mohapatra NP, Wewers MD, Schlesinger LS, Gunn JS, Tridandapani S 2006 Macrophage pro-inflammatory response to Francisella novicida infection is regulated by SHIP. PLoS Pathog 2:e71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Li F, Mahavadi S, Murthy KS 2009 Upregulation of RGS4 expression by IL-1β in colonic smooth muscle is enhanced by ERK1/2 and p38 MAPK and inhibited by the PI3K/Akt/GSK3β pathway. Am J Physiol Cell Physiol 296:C1310–C1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ramnath RD, Tamizhselvi R, Bhatia M 2009 Role of protein kinase C and phosphoinositide 3-kinase-Akt in substance P-induced proinflammatory pathways in mouse macrophages. FASEB J 23:997–1010 [DOI] [PubMed] [Google Scholar]
- Mostefai HA, Agouni A, Carusio N, Mastronardi ML, Heymes C, Henrion D, Andriantsitohaina R, Martinez MC 2008 Phosphatidylinositol 3-kinase and xanthine oxidase regulate nitric oxide and reactive oxygen species productions by apoptotic lymphocyte microparticles in endothelial cells. J Immunol 180:5028–5035 [DOI] [PubMed] [Google Scholar]
- Coxon PY, Rane MJ, Powell DW, Klein JB, McLeish KR 2000 Differential mitogen-activated protein kinase stimulation by Fcγ receptor IIa and Fc receptor IIIb determines the activation phenotype of human neutrophils. J Immunol 164:6530–6537 [DOI] [PubMed] [Google Scholar]
- Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Ping P, McLeish KR 2001 p38 kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem 276:3517–3523 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Matsui A, Inao M, Mochida S, Fujiwara K 2003 ERK/MAPK-dependent PI3K/Akt phosphorylation through VEGFR-1 after VEGF stimulation in activated hepatic stellate cells. Hepatol Res 26:232–236 [DOI] [PubMed] [Google Scholar]
- Karalis K, Muglia LJ, Bae D, Hilderbrand H, Majzoub JA 1997 CRH and the immune system. J Neuroimmunol 72:131–136 [DOI] [PubMed] [Google Scholar]
- Venihaki M, Zhao J, Karalis KP 2003 Corticotropin-releasing hormone deficiency results in impaired splenocyte response to lipopolysaccharide. J Neuroimmunol 141:3–9 [DOI] [PubMed] [Google Scholar]
- Kageyama K, Suda T 2003 Urocortin-related peptides increase interleukin-6 output via cyclic adenosine 5`-monophosphate-dependent pathways in A7r5 aortic smooth muscle cells. Endocrinology 144:2234–2241 [DOI] [PubMed] [Google Scholar]
- Kokkotou E, Torres D, Moss AC, O'Brien M, Grigoriadis DE, Karalis K, Pothoulakis C 2006 Corticotropin-releasing hormone receptor 2-deficient mice have reduced intestinal inflammatory responses. J Immunol 177:3355–3361 [DOI] [PubMed] [Google Scholar]
- Sashinami H, Kageyama K, Suda T, Nakane A 2005 Urocortin 2 suppresses host resistance to Listeria monocytogenes infection via up-regulation of interleukin-10. Endocrinology 146:5003–5011 [DOI] [PubMed] [Google Scholar]
- Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S 2002 PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol 3:875–881 [DOI] [PubMed] [Google Scholar]
- Lezoualc'h F, Engert S, Berning B, Behl C 2000 Corticotropin-releasing hormone-mediated neuroprotection against oxidative stress is associated with the increased release of non-amyloidogenic amyloid β precursor protein and with the suppression of nuclear factor-κB. Mol Endocrinol 14:147–159 [DOI] [PubMed] [Google Scholar]
- Wang W, Solc M, Ji P, Dow KE 2004 Corticotropin-releasing hormone potentiates neural injury induced by oxygen-glucose deprivation: a possible involvement of microglia. Neurosci Lett 371:133–137 [DOI] [PubMed] [Google Scholar]
- Dermitzaki E, Tsatsanis C, Gravanis A, Margioris AN 2002 Corticotropin-releasing hormone induces Fas ligand production and apoptosis in PC12 cells via activation of p38 mitogen-activated protein kinase. J Biol Chem 277:12280–12287 [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Dermitzaki E, Charalampopoulos I, Spiess J, Gravanis A, Margioris AN 2005 Urocortin 1 and urocortin 2 induce macrophage apoptosis via CRF2. FEBS Lett 579:4259–4264 [DOI] [PubMed] [Google Scholar]
- Marte BM, Downward J 1997 PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci 22:355–358 [DOI] [PubMed] [Google Scholar]
- Ranger AM, Malynn BA, Korsmeyer SJ 2001 Mouse models of cell death. Nat Genet 28:113–118 [DOI] [PubMed] [Google Scholar]
- Madge LA, Pober JS 2000 A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFκB in human endothelial cells. J Biol Chem 275:15458–15465 [DOI] [PubMed] [Google Scholar]
- Yang CH, Murti A, Pfeffer SR, Kim JG, Donner DB, Pfeffer LM 2001 Interferon α/β promotes cell survival by activating nuclear factor κB through phosphatidylinositol 3-kinase and Akt. J Biol Chem 276:13756–13661 [DOI] [PubMed] [Google Scholar]