Abstract
The medial basal hypothalamus, including the arcuate nucleus (ARC) and the ventromedial hypothalamic nucleus (VMH), integrates signals of energy status to modulate metabolism and energy balance. Leptin and feeding regulate the mammalian target of rapamycin complex 1 (mTORC1) in the hypothalamus, and hypothalamic mTORC1 contributes to the control of feeding and energy balance. To determine the mechanisms by which leptin modulates mTORC1 in specific hypothalamic neurons, we immunohistochemically assessed the mTORC1-dependent phosphorylation of ribosomal protein S6 (pS6). In addition to confirming the modulation of ARC mTORC1 activity by acute leptin treatment, this analysis revealed the robust activation of mTORC1-dependent ARC pS6 in response to fasting and leptin deficiency in leptin receptor-expressing Agouti-related protein neurons. In contrast, fasting and leptin deficiency suppress VMH mTORC1 signaling. The appropriate regulation of ARC mTORC1 by mutant leptin receptor isoforms correlated with their ability to suppress the activity of Agouti-related protein neurons, suggesting the potential stimulation of mTORC1 by the neuronal activity. Indeed, fasting- and leptin deficiency-induced pS6-immunoreactivity (IR) extensively colocalized with c-Fos-IR in ARC and VMH neurons. Furthermore, ghrelin, which activates orexigenic ARC neurons, increased ARC mTORC1 activity and induced colocalized pS6- and c-Fos-IR. Thus, neuronal activity promotes mTORC1/pS6 in response to signals of energy deficit. In contrast, insulin, which activates mTORC1 via the phosphatidylinositol 3-kinase pathway, increased ARC and VMH pS6-IR in the absence of neuronal activation. The regulation of mTORC1 in the basomedial hypothalamus thus varies by cell and stimulus type, as opposed to responding in a uniform manner to nutritional and hormonal perturbations.
The regulation of mTORC1 in the basomedial hypothalamus varies by cell and stimulus type, rather than responding uniformly to nutritional and hormonal perturbations.
The prevalence of obesity and its pathological sequelae, including type 2 diabetes and cardiovascular disease, has reached epidemic levels and continues to increase in developed countries (1,2,3,4). Understanding the mechanisms that regulate feeding and energy balance thus represents a crucial first step in identifying potential targets for the therapy of obesity and diabetes. The discovery of leptin and the elucidation of crucial leptin-regulated neurons in the brain have facilitated the study of these mechanisms. Adipocytes secrete leptin in approximate proportion to fat/energy storage so that circulating leptin levels reflect the status of long-term energy stores (5). Adequate leptin levels signal via a specific long-form leptin receptor (LepRb) on metabolic sensing neurons in the brain to modulate food intake, metabolism, and neuroendocrine energy use (6,7,8).
Numerous specialized neural populations in the brain express LepRb, including those in several basomedial hypothalamic nuclei important for energy balance, such as the arcuate nucleus (ARC) and the ventromedial hypothalamic nucleus (VMH). The ARC in particular contains a well-studied and well-understood circuit composed of two distinct neural populations (8,9,10). Proopiomelanocortin (POMC)-expressing neurons secrete peptides that act via central melanocortin (and perhaps other) receptors to suppress appetite and increase metabolic rate; leptin promotes the activity of these neurons (11). In contrast, leptin suppresses the activity of ARC neurons that express and secrete neuropeptide Y and Agouti-related protein (AgRP), both of which stimulate appetite and decrease energy use (12). In addition to responding to leptin, many ARC neurons sense and respond to other indicators of energy status, including insulin and a variety of nutrients (13,14,15).
Among the indicators of cellular energy status implicated in the regulation of ARC neural circuits are the AMP-dependent protein kinase (AMPK; which is activated in response to increased AMP to ATP ratios, among other signals of decreased cellular energy) (16,17), and the mammalian target of rapamycin (mTOR) (18,19). mTOR functions within two distinct multimolecular complexes; of these, mTOR complex 1 (mTORC1) responds to nutritional (especially amino acid) cues of energy repletion to promote macromolecular biosynthesis (20,21,22). Insulin similarly promotes anabolic mTORC1 action, as does signaling via protein kinase C, Ca+2, and other pathways. Among other downstream signals, stimulation of mTORC1 activates the S6 kinases (S6K1 and S6K2) to promote the phosphorylation of ribosomal protein S6 [phosphorylated S6(pS6)], an important regulator of protein translation (20,23). Acute rapamycin treatment specifically inhibits mTORC1 and mTORC1-dependent signals, such as S6K1/2 (21,22). Refeeding-, amino acid-, and leptin-stimulated activation of mTORC1-dependent signals in whole ARC and hypothalamic mTORC1 activity are important for the anorectic response to amino acids and leptin (18). In contrast, constitutive mTORC1 signaling in POMC neurons impairs their anorectic action and promotes obesity (24).
These data suggest the potential complexity, and neural cell type specificity, of mTOR regulation by signals of nutritional status and energy sufficiency. We thus sought to determine the mechanisms by which leptin and LepRb control mTORC1 activity in defined populations of ARC neurons. Consistent with the complexity of hypothalamic mTORC1 action, we report that whereas leptin modestly increases mTORC1 in the ARC, ARC mTORC1 is also stimulated by the activation of orexigenic AgRP neurons in response to leptin deficiency and other signals of negative energy balance. VMH neuronal activity and mTORC1 are conversely attenuated by signals of negative energy balance. Insulin activates mTORC1 more widely and by neural activity-independent mechanisms.
Materials and Methods
Materials
Leptin was from the National Hormone and Pituitary Program (Torrance, CA), insulin was from Novo Nordisk (Princeton, NJ), and ghrelin was obtained from Matthias Tschöp, M.D. (University of Cincinnati, Cincinnati, OH). Rabbit antiphospho-S6 (Ser 240/244) was purchased from Cell Signaling (Boston, MA), chicken anti-green fluorescent protein from Abcam (Cambridge, MA), goat anti-c-Fos was from Oncogene Sciences (Uniondale, NY), goat anti-β-galactosidase (β-gal) from Biogenesis (Poole, UK), and donkey serum was from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Donkey antichicken (or antirabbit) Alexa-488-conjugated and donkey antigoat Alexa-564 conjugated antibodies were purchased from Molecular Probes, Inc. (Eugene, OR). All other immunohistochemical supplies were purchased from Sigma-Aldrich (St. Louis, MO).
Animals
Animals for immunohistochemical analysis were bred in our colony in the Unit for Laboratory Animal Medicine at the University of Michigan, except for 10- to 12-wk-old male S6K1−/− animals (25), which were produced by heterozygote intercrosses at the University of Cincinnati Genome Research Institute animal facility. All procedures were in accordance with the guidelines of and with the approval of the respective University Committee on the Care and Use of Animals at the University of Michigan and University of Cincinnati.
To produce experimental animals, mice containing the coding sequence of Cre recombinase targeted into the 3′-untranslated region of the LepRb-specific exon 18b in the Lepr gene (LepRbcre mice) were bred with Gt-(ROSA)26Sortm2sho (Rosa26-EGFP) mice, in which a floxed transcription blocker precedes the enhanced green fluorescent protein (EGFP) reporter gene sequence. Cre-mediated recombination/removal of the transcription blocker results in EGFP expression in LepRb-expressing cells (LepRbEGFP reporter mice) (7). Heterozygote Leprdb (db/+), LeprTm1mgmj (s/+) (26), and LeprTm2mgmj (l/+) (27) mice were self-crossed to generate animals homozygous for each altered lepr allele. Heterozygous mice expressing LacZ from the AgRP locus (a/+) were produced as previously described (28).
Mice were housed in groups of two to four with ad libitum access to food and water. Some mice were fasted for 24 h (or as indicated) before they were killed between 0900 and 1200 h; access to water was not restricted. For immunohistochemical analysis, ad libitum-fed animals remained with food in the cage until the day the animals were killed between 0900 and 1200 h.
Intraperitoneal/intracerebroventricular (icv) injections
Ten to 12-wk-old male mice received an ip injection of leptin (5 mg/kg), insulin (400 mU/ml), or 1× sterile PBS for 1 h before the animals were killed. Stereotaxic implantation of cannulae was performed under isoflurane anesthesia into the left cerebral ventricle. After 7 d of recovery, 3 μl leptin (1 mg/ml), PBS, ghrelin (2 μg/μl), insulin (300 mU/ml), dimethylsulfoxide, or rapamycin (2 μg/μl) were injected icv 1 h before the animals were killed.
Perfusion and immunohistochemistry (IHC)
Handling of all animals was limited to less than 1 h before anesthesia and perfusion to minimize stress-related induction of c-Fos levels. Perfusion and IHC procedures were performed essentially as described previously (29). In brief, mice were deeply anesthetized with an overdose of pentobarbital (150 mg/kg, ip) and transcardially perfused with sterile PBS followed by 4% paraformaldehyde. Brains were removed, postfixed, and cryoprotected before sectioning into 30-μm coronal slices, which were collected into four representative series and stored at −20 C until further use.
For IHC, sections were pretreated in ice-cold methanol, 0.6% glycine, and 0.03% SDS and then blocked with donkey serum and incubated in the primary antibodies [rabbit anti-pS6 (1:100), chicken anti-green fluorescent protein (1:1000], goat anti-c-Fos (1:1000] and/or goat anti-β-Gal (1:3000)]. Detection of primary antibodies was done by immunofluorescence [donkey antichicken (or antirabbit)-Alexa 488 and antigoat-Alexa 564 (all 1:200 dilution)].
Quantification of IHC signals from hypothalamic sections and statistics
Regions of the hypothalamus denoted as ARC and VMH for counting purposes are depicted in supplemental Fig. 1. For quantification of pS6-positive neurons, LepRb (EGFP) neurons, AgRP neurons and pS6/EGFP, pS6/AgRP or pS6/c-Fos double-labeled neurons, pictures of matched brain areas were taken on channels for Alexa 488 and Alexa 564 from at least three sections containing the ARC of the hypothalamus for each brain between bregma −1.58 mm to −1.94 mm (according to the Paxinos and Franklin mouse brain atlas). All sections were arranged from rostral to caudal to examine the distribution of single- and double-labeled neurons. Using Photoshop software (Adobe, San Jose, CA), both channels (red and green) were combined in a Red-Green-Blue (RGB) picture and single and double-labeled neurons were counted and recorded digitally to prevent multiple counts. Total number of single- and double-labeled neurons was presented as means ± sem, and differences were analyzed by two-way ANOVA followed by a Fisher’s protected least significant difference test to determine significant differences between groups. Differences were accepted for P < 0.05, with an n value of 3 or greater.
Results
Activation of ARC mTORC1 and suppression of VMH mTORC1 by fasting and leptin deficiency
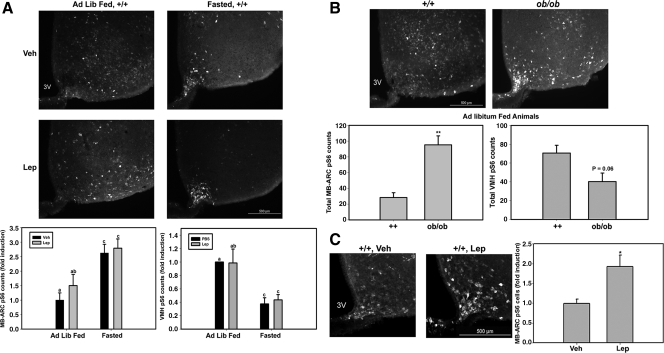
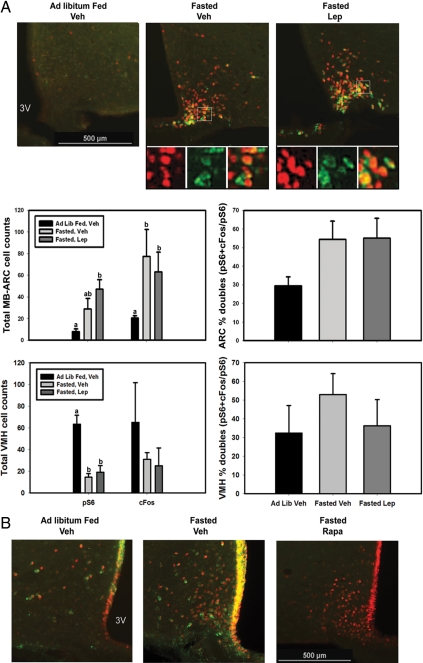
To more closely examine the molecular mechanisms and cellular specificity of the regulation of the mTORC1 pathway by leptin, we used the immunohistochemical detection of pS6-immunoreactivity (IR) as a marker for mTORC1 activity in the hypothalamus. Reasoning that decreased baseline levels of leptin would facilitate the detection of the leptin response, we examined pS6-IR in the hypothalamus of ad libitum-fed and 24-h fasted wild-type (+/+) mice after treatment with leptin (5 mg/kg, ip) or vehicle (Fig. 1A). Surprisingly, this analysis revealed an increased number of pS6-IR ARC neurons in fasted +/+ animals with and without leptin treatment but only a trend toward increased numbers of pS6-IR neurons with leptin in the ad libitum-fed animals. In contrast to the ARC, fasting suppressed pS6-IR in the VMH. We also compared ad libitum-fed +/+ mice to leptin-deficient (Lepob/ob) mice (Fig. 1B), revealing an increase in the number of pS6-IR ARC neurons and a trend toward decreased pS6-IR in VMH neurons (P = 0.06) in Lepob/ob animals relative to ad libitum-fed controls. The increase in the number of pS6-IR ARC neurons with fasting and leptin deficiency was especially pronounced in the medial basal ARC (MB-ARC) near the median eminence. These findings suggested that fasting and leptin deficiency mediate the phosphorylation of S6 in MB-ARC neurons and also that the conditions of leptin repletion or the increased MB-ARC pS6-IR at baseline with 24 h fasting impair our ability to examine leptin-mediated stimulation of pS6-IR. We thus reexamined the leptin effect in animals that had been fasted for a shorter period of time (overnight, ∼16 h) (Fig. 1C). This analysis revealed an increase in pS6-IR neurons with leptin treatment in the MB-ARC similar to that previously described in rats after a similarly modest fast (18).
Figure 1.
Phosphorylation of S6 in response to fasting and leptin in the MB-ARC and VMH. A, Wild-type (+/+) animals under ad libitum-fed (Ad Lib Fed) or 24-h-fasted conditions treated with leptin (Lep; 5 mg/kg, ip) or vehicle (Veh) for 1 h before the animals were killed. B, Ad libitum-fed Lepob/ob mice. C, Overnight (∼16-h) fasted +/+ mice (treated with leptin or vehicle, as indicated) that were processed for immunohistochemical analysis of pS6-IR. Top panels show representative images of immunofluorescent detection of pS6-IR, whereas the graphs below show the fold induction (or total counts) of the number of pS6 cells in the MB-ARC and VMH. Data were quantified and plotted as mean ± sem (n ≥ 3 per condition; bars with different letters were significantly different by ANOVA, P < 0.05). *, P < 0.01; **, P < 0.001 by Student’s t test; P value of trend as indicated in B. 3V, Third ventricle.
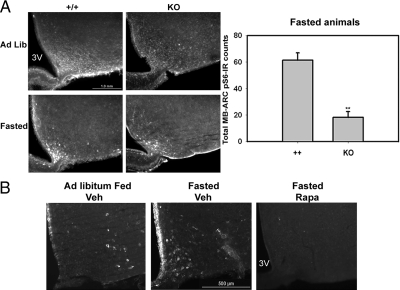
Because the finding of MB-ARC mTORC1 activation implied by the increased MB-ARC pS6-IR in fasted and Lepob/ob animals was unexpected, we examined the mTORC1 dependency of the fasting effect by examining pS6-IR in S6K1−/− animals (25) and animals treated with icv rapamycin (a specific mTORC1 inhibitor, when used acutely) (Fig. 2) (20,21,22). S6K1 and the related S6K2 interact with and are activated by mTORC1 to mediate the mTORC1-dependent phosphorylation of S6. Lack of S6K1 significantly decreased pS6-IR in the MB-ARC of knockout mice under ad libitum and fasted conditions compared with wild-type mice under similar conditions (Fig. 2A). S6K1−/− animals exhibited some residual pS6-IR in the fasted state, however, suggesting that whereas S6K1 mediates a portion of the basal and fasting-induced pS6-IR in the MB-ARC, other kinases (e.g. S6K2) also contribute. Intracerebroventricular rapamycin treatment abrogated MB-ARC pS6-IR (including during fasting), demonstrating the mTORC1 dependence of pS6-IR in the MB-ARC (Fig. 2B). Thus, fasting promotes increased mTORC1 activity in neurons of the MB-ARC in mice.
Figure 2.
S6K1/mTORC1-dependence of pS6-IR in the MB-ARC. A, Wild-type (+/+) or S6K1−/− (KO) animals under ad libitum-fed or 24-h-fasted conditions were perfused and processed for the immunofluorescent detection of pS6-IR. Top panel shows representative images. Numbers of pS6-IR neurons in the MB-ARC (mean ± sem) for each genotype are presented in the graph (n ≥ 3 per condition). **, P <.001 by Student’s t test. B, Wild-type animals under the indicated ad libitum (Ad Lib)-fed or fasted conditions were treated with icv vehicle or rapamycin (2 μg/μl) overnight and 2 h before the animals were killed and processing for the immunofluorescent detection of pS6-IR. Representative images under each condition are shown. In addition to the MB-ARC pS6-IR that is the focus of this analysis, note some inflammatory induction of pS6-IR in the ependymal cells that line the ventricle, which is also blocked by treatment with rapamycin. 3V, Third ventricle.
Neuronal populations demonstrating pS6-IR in the MB-ARC
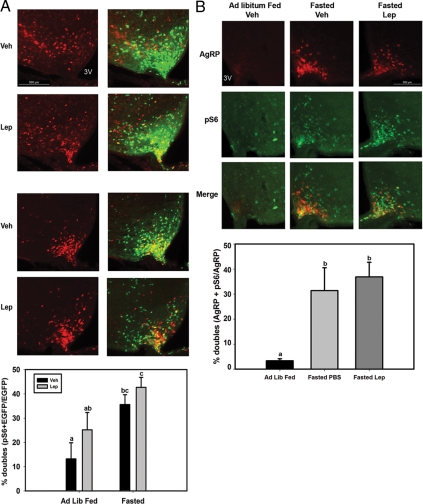
To determine whether the fasting-mediated activation of pS6 occurred in LepRb-expressing neurons, we examined pS6-IR and its colocalization with EGFP in LepRbEGFP mice (Fig. 3A), which demonstrate EGFP expression specifically in LepRb-expressing neurons throughout the brain (7,30,31). Because LepRb is expressed normally in the LepRbEGFP animals, they exhibit no detectable alterations in energy homeostasis (30). As previously, fasting increased pS6-IR in the MB-ARC of LepRbEGFP mice, and leptin treatment tended to increase the number of pS6-IR neurons (Fig. 3A). Furthermore, the fasting-stimulated pS6-IR neurons extensively colocalized with LepRb/EGFP neurons; approximately 40% of MB-ARC LepRb neurons demonstrated fasting-induced pS6-IR.
Figure 3.
Fasting induces pS6-IR in LepRb- and AgRP-expressing neurons in the MB-ARC. A, LepRbEGFP mice were allowed to feed ad libitum (Ad Lib Fed) or were fasted for 24 h before treatment with leptin (Lep; 5 mg/kg, ip) or vehicle (Veh) for 1 h before the animals were killed and processing for the immunofluorescent detection of LepRb/EGFP (green) expression and pS6-IR (red). Top panels show representative images of pS6-IR alone (left) and merged pS6-IR and EGFP-IR images (right). LepRb/EGFP neurons that contain pS6-IR are plotted as mean ± sem (n ≥ 3 per condition; bars with different letters were significantly different by ANOVA, P < 0.05). B, Animals heterozygous for AgrpLacZ were allowed to feed ad libitum or were fasted for 24 h before treatment with leptin (Lep; 5 mg/kg, ip) or vehicle (Veh) for 1 h before the animals were killed and processing for the immunofluorescent detection of AgRP/LacZ (red)-expression and pS6-IR (green). Top panels show representative images of AgRP (β-gal) alone (top), pS6-IR alone (middle), and merged β-gal and pS6-IR images (bottom). AgRP (β-gal) neurons that contain pS6-IR are plotted as mean ± sem (n ≥ 3 per condition; bars with different letters were significantly different by ANOVA, P < 0.05). 3V, Third ventricle.
The similarity of the distribution of MB-ARC neurons that demonstrated fasting (or leptin deficiency)-induced pS6-IR with the distribution of orexigenic neuropeptide Y/AgRP-expressing MB-ARC neurons suggested the possibility that these neurons might represent a major locus of fasting-induced pS6-IR. We thus used animals heterozygous for AgrpLacZ (in which the homologous targeting of the coding sequences for LacZ mediates the expression of β-gal by the native AgRP promoter and specifically in AgRP expressing neurons) (12,28) to examine the potential induction of pS6-IR in AgRP neurons during fasting (Fig. 3B and supplemental Fig. S2). These AgrpLacZ heterozygous animals have no detectable alteration in energy balance relative to control animals. Whereas little pS6-IR colocalized with the detectable AgRP neurons in fed animals, pS6-IR was extensively colocalized with β-gal/AgRP neurons in fasted animals (20–30% of the total pS6 neurons in the MB-ARC of fasted animals were AgRP positive). Thus, fasting promotes mTORC1 activity in LepRb- and AgRP-expressing neurons in the MB-ARC. Similar results were noted throughout the rostral-caudal axis of the ARC (supplemental Fig. 2).
Role of LepRb signaling in the regulation of pS6-IR by endogenous leptin in the MB-ARC
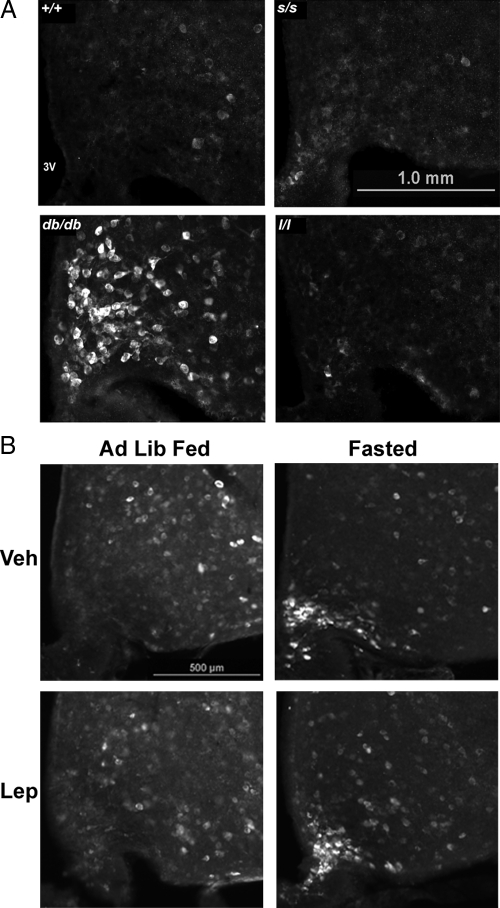
The finding of increased pS6-IR observed in the MB-ARC of Lepob/ob animals suggested that increased mTORC1 activity results from the absence of a LepRb-mediated signaling pathway. To determine the potential mechanism by which endogenous leptin might act via LepRb in wild-type animals to regulate MB-ARC pS6-IR, we examined pS6-IR in the ARC of mouse models containing specific alterations in LepRb signaling (Fig. 4). As in leptin-deficient Lepob/ob animals, Leprdb/db animals (in which LepRb is disrupted by a splicing defect) displayed a large number of pS6-IR MB-ARC neurons compared with controls, suggesting that the appropriate regulation of mTORC1 activity in MB-ARC neurons by endogenous leptin requires intact LepRb. We also examined pS6-IR in mice containing mutant LepRb molecules with substitution mutations of tyrosine residues that control specific signaling pathways. In contrast to the Lepob/ob and Leprdb/db animals, however, ad libitum-fed mice containing a mutation of LepRb Tyr985 [Leprtm2mgmj (l/l) mice] (27) or Tyr1138 [Leprtm1mgmj (s/s) mice] (26) demonstrated little pS6-IR in the MB-ARC (Fig. 4A), suggesting that the signals controlled by these residues (PTPN11(akaSHP2)/suppressor of cytokine signaling-3 and signal transducer and activator of transcription (STAT)-3, respectively) are not required for the normal modulation of mTORC1 activity by endogenous leptin in the MB-ARC. Whereas morbid obesity impairs the examination of the fasting response in s/s animals, we examined the effect of fasting in l/l mice, which revealed the fasting-mediated induction of pS6-IR in the MB-ARC in these animals (Fig. 4B), as in wild-type mice (Fig. 1A), ruling out an effect of Tyr985 in the regulation of the fasting-induced increase in MB-ARC mTORC1.
Figure 4.
pS6-IR in the medial basal hypothalamus of mouse models with specific alterations in LepRb signaling. A, Ad libitum-fed (Ad Lib Fed) wild-type (+/+), db/db, s/s, and l/l mice were perfused and processed for the immunofluorescent detection of pS6-IR. Representative images of pS6-IR from each genotype are shown. B, Ad libitum-fed and 24-h-fasted l/l mice were treated with leptin (Lep) or PBS, as indicated, perfused, and processed for the immunofluorescent detection of pS6-IR. Representative images are shown for each panel. Veh, vehicle; 3V, third ventricle.
Neuronal activation and S6 phosphorylation in the MB-ARC and VMH
As we sought to understand the mechanisms by which fasting and/or the absence of leptin action might activate mTORC1 in the MB-ARC and decrease mTORC1 in the VMH, we noted that the induction of MB-ARC pS6-IR correlated with the activation of orexigenic neurons, such as AgRP neurons. These neurons are activated by fasting in wild-type mice and also in the ad libitum-fed state in Lepob/ob and in Leprdb/db animals but not in l/l or s/s mice (8,9,10,12) (and supplemental Fig. S3).
To examine this correlation more closely, we examined the potential colocalization of pS6-IR and c-Fos-IR (a marker of neuronal activation) (32) in the MB-ARC and VMH during fasting and leptin treatment (Fig. 5A). In addition to demonstrating the expected induction of MB-ARC pS6 and c-Fos-IR by fasting, 54% of the pS6-IR neurons in fasted mice colocalized with c-Fos-IR, suggesting that the majority of the neurons of the MB-ARC that contain highly active mTORC1 represent actively firing neurons. Furthermore, fasting decreased VMH pS6-IR and tended to decreased VMH c-Fos-IR. Importantly, neither fasting nor leptin altered the proportion of MB-ARC or VMH pS6-IR neurons positive for c-Fos-IR (Fig. 5A, right-hand panels), consistent with the idea that the regulation of c-Fos/neuronal activation is tightly coupled to pS6/mTORC1.
Figure 5.
pS6-IR localization in activated neurons in the MB-ARC and VMH. A, Wild-type animals were allowed to feed ad libitum or were fasted for 24 h before treatment with leptin (Lep; 5 mg/kg, ip) or vehicle (Veh) for 1 h before the animals were killed and processing for the immunofluorescent detection of c-Fos-IR (red) and pS6-IR (green). Top panels show representative images of merged images at each condition. Smaller panels beneath show digital zoom of each channel and merged images from the boxed area in the larger panels above. Graphs: Total numbers of pS6-IR, c-Fos-IR, and percent double-labeled neurons in the MB-ARC and VMH are plotted as mean ± sem (n ≥ 3 per condition; bars with different letters were significantly different by ANOVA, P < 0.05). B, Wild-type animals under the indicated ad libitum-fed (Ad Lib Fed) or overnight (∼16 h) conditions were treated with icv vehicle or rapamycin ((Rapa) 2 μg/μl) via indwelling catheters overnight and for additional 2 h before the animals were killed and processing for the immunofluorescent detection of pS6-IR (green) and c-Fos-IR (red). Representative images under each condition are shown. In addition to the MB-ARC pS6/c-Fos-IR that is the focus of this analysis, note some inflammatory induction of pS6-IR in the ependymal cells that line the ventricle, which is also blocked by treatment with rapamycin. 3V, Third ventricle.
It is, of course, possible that mTORC1 activation might promote neuronal activation, rather than the converse. To address this issue, we treated fasted animals with icv rapamycin before killing them for the examination of pS6-IR and c-Fos-IR in the MB-ARC (Fig. 5B).Whereas, as expected, rapamycin treatment abrogated the mTORC1-dependent pS6-IR in the MB-ARC, c-Fos-IR remained high. Thus, whereas most pS6-IR neurons in the MB-ARC neurons of fasted mice are active by the criterion of c-Fos-IR, mTORC1 activity is not required for this neuronal activation, consistent with the notion that the depolarization of active neurons in the MB-ARC may induce mTORC1 activation.
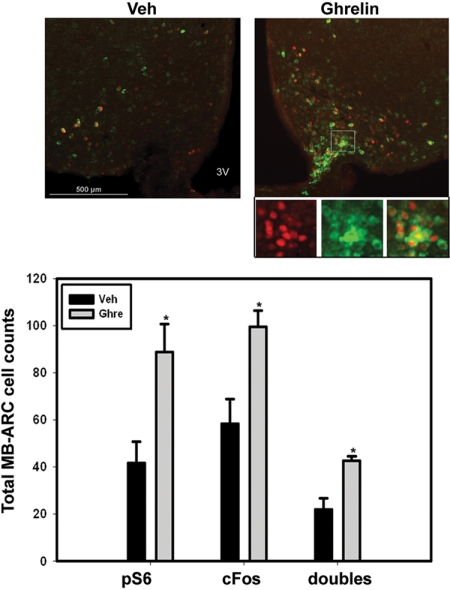
To further examine the idea that depolarization and neural firing may promote mTORC1 activity in the MB-ARC, we examined the induction of pS6- and c-Fos-IR by treatment with icv ghrelin, which rapidly and directly depolarizes orexigenic neurons in the MB-ARC (independently of any acute effects on circulating metabolites) (33) (Fig. 6). As for fasting, icv ghrelin treatment induced pS6-IR and c-Fos-IR and their colocalization in the MB-ARC, consistent with the activation of mTORC1 by depolarization of these neurons.
Figure 6.
Ghrelin-induced neuronal firing promotes mTORC1 activation in the MB-ARC. Ad libitum-fed wild-type animals with indwelling icv catheters were treated with vehicle (Veh) or ghrelin (2 μg/μl) for 1 h before the animals were killed and processing for the immunofluorescent analysis of pS6-IR (green) and c-Fos-IR (red). Top panels: Representative merged images. Panels beneath the image from the ghrelin-treated sample represent digital zoom of individual channels and merged images from the boxed area in the larger image. Graph: Total numbers of pS6-IR, c-Fos-IR, and double-labeled neurons are plotted as mean ± sem (n ≥ 3 per condition; *, P ≤ 0.01 by Student’s t test). 3V, Third ventricle.
Insulin and pS6-IR in the hypothalamus
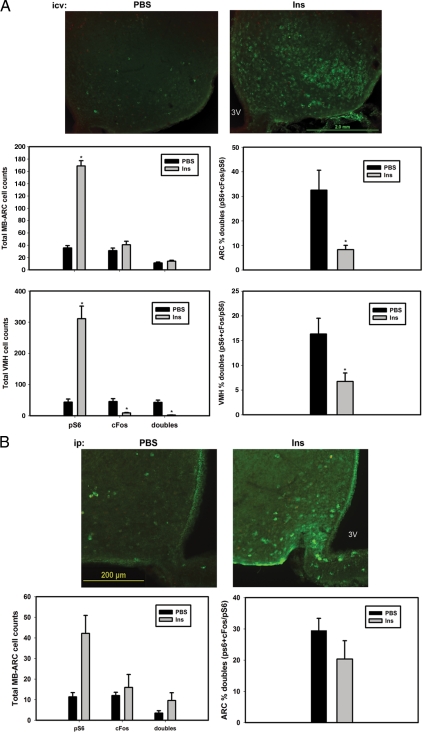
To understand the potential for additional mechanisms of hypothalamic mTORC1 activation, we examined pS6- and c-Fos-IR in the hypothalamus after insulin treatment (Fig. 7) because insulin hyperpolarizes all studied ARC neurons but activates mTORC1 via the phosphatidylinositol 3-kinase (PI3K) pathway in cultured cells (20,34).
Figure 7.
Insulin induces mTORC1 activation independently of c-Fos in the hypothalamus. A, Ad libitum-fed wild-type animals with indwelling icv catheters were treated with vehicle (Veh) or insulin (Ins) (300 mU/ml) for 1 h before the animals were killed and processing for the immunofluorescent analysis of pS6-IR (green) and c-Fos-IR (red). B, Ad libitum wild-type animals were treated with vehicle (Veh) or insulin (Ins) (400 mU/ml) for 1 h before the animals were killed and processing for the immunofluorescent analysis of pS6-IR (green) and c-Fos-IR (red). Top panels, Representative merged images. Graphs, Total numbers of pS6-IR, c-Fos-IR, and double-labeled neurons, along with percent double-labeled neurons, are plotted as mean ± sem (n ≥ 3 per condition; *, P ≤ 0.01 by Student’s t test). 3V, Third ventricle.
Indeed, whereas icv insulin treatment dramatically increased pS6-IR in the ARC and VMH, insulin did not change c-Fos-IR in the ARC and decreased c-Fos-IR in the VMH, with the result that the percent of pS6-IR neurons containing c-Fos decreased in each region with insulin treatment. Similarly, ip insulin injection increased c-Fos-IR in the MB-ARC (although not in the VMH or elsewhere in the brain) but without increasing c-Fos-IR or the percent of pS6-IR neurons containing c-Fos (Fig. 7B). These data are consistent with the notion that insulin activates mTORC1 widely and independently of neuronal activity. Thus, the regulation of mTORC1 in the basomedial hypothalamus varies by cell type/nucleus, nutritional status, and the particular stimulus used, as opposed to responding in a uniform manner to nutritional and hormonal perturbations.
Discussion
Leptin activates a variety of signals in hypothalamic LepRb neurons, such as STAT3, STAT5, ERK, PI3K, AMPK, and mTORC1 (17,18,19,35,36,37,38,39,40,41). Many of these pathways (including STAT3, PI3K, and mTORC1) are known to play important roles in anorexia and other leptin-dependent responses (6,11,18,24,39,42). Whereas the receptor motifs by which LepRb activates many signals (e.g. STAT3) have been revealed (19,40), the mechanisms by which LepRb modulates other signals, such as PI3K, AMPK, and mTORC1, remain obscure. The difficulty in dissecting these signals stems in large part from the inability to observe the regulation of these signals by LepRb in cultured cells, such as HEK293 cells, without the overexpression of multiple signaling proteins that likely result in the nonspecific activation of some signals. Additionally, the opposite regulation of some signals by leptin in distinct types of LepRb neurons complicates the analysis of their regulation. For instance, whereas leptin promotes the activation of PI3K in POMC neurons, it inhibits PI3K in AgRP neurons (19,37,43). Thus, the analysis of the mechanisms by which leptin regulates these signals requires further studies in vivo and presumably in a cell-type specific manner, as we have done here for the regulation of mTORC1.
Our data reveal the IHC detection of pS6-IR in the MB-ARC to be largely dependent on the major mTORC1 mediator, S6K1, and to be entirely dependent on mTORC1 itself. Using this IHC methodology to explore the regulation of mTORC1 by leptin and nutritional manipulation (i.e. fasting) not only confirmed the expected modest enhancement of mTORC1 signaling on leptin treatment but also revealed increased mTORC1 activity in LepRb- and AgRP-expressing neurons in the MB-ARC by fasting and leptin deficiency. In contrast, fasting and leptin deficiency decrease mTORC1 activity in the VMH.
The finding that endogenous leptin prevents mTORC1 activation in the MB-ARC suggested potential roles for specific LepRb signals in this process. We thus explored the signaling mechanisms by which LepRb might modulate the accumulation of pS6-IR in the MB-ARC. This analysis revealed that neither Tyr985 (the binding site for PTPN11 and suppressor of cytokine signaling-3) nor Tyr1138 (binding site for STAT3) participate in the regulation of MB-ARC pS6-IR under conditions of endogenous leptin (in contrast to the activation observed in the absence of leptin action in Lepob/ob and Leprdb/db mice).
Our data suggest that the mechanism by which fasting and leptin deficiency mediate this activation is mediated by the regulation of neural activity. This conclusion is supported not only by the correlation between the induction of pS6-IR in the MB-ARC by nutritional conditions and genetic environments (Leprdb/db mice) in which these orexigenic neurons are activated (12,44) but also by the induction of pS6-IR in neurons that also demonstrate IHC evidence of activation (c-Fos-IR), in both the MB-ARC and the oppositely regulated VMH. Furthermore, ghrelin, which acutely depolarizes the orexigenic neurons of the MB-ARC (33) without altering the overall nutritional milieu, also promotes pS6-IR coincident with c-Fos-IR in the MB-ARC. Note that whereas 1 h of stimulation, which generally represents the peak of mTORC1 activation/pS6 after an acute stimulus (and thus used here), precedes the peak of c-Fos-IR after neuronal depolarization; thus, the ultimate extent of c-Fos-IR colocalization in this experiment may be underrepresented.
Because leptin depolarizes and promotes the firing of some neurons within the MB-ARC (44,45), it is possible that the previously reported activation of mTORC1 during acute leptin treatment also takes place secondary to changes in neuronal activity. Another reasonable possibility is that leptin might promote mTORC1 signaling via the induction of PI3K, which is an important upstream activator of mTORC1 (20,21,22). Indeed, the observation that insulin [whose major signaling output is via PI3K (46)] promotes pS6-IR in the mediobasal hypothalamus without increasing c-Fos-IR suggests that robust PI3K activation in the hypothalamus stimulates mTORC1, as in cultured cells.
Overall, our present results demonstrate that leptin and nutritional cues do not modulate hypothalamic mTORC1 activity in a uniform manner but in many cases control mTORC1 via the regulation of neural activity (although PI3K signaling may also participate). In the case of many stimuli, such as fasting and leptin deficiency, mTORC1 may be regulated in opposite directions in different cell types and brain regions because the activity of many cell types are distinctly regulated as well.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of the core facilities of the Michigan Diabetes Research and Training Center (National Institutes of Health Grant DK20572) and the Michigan Comprehensive Cancer Center. We are grateful to Dr. Mark Sleeman and Regeneron, Inc. for the generous gift of AgrpLacZ mice, Dr. George Thomas and Dr. Sara Kozma (University of Cincinnati) for S6K1−/− animals, and Dr. Matthias Tschöp (University of Cincinnati) for the gift of ghrelin.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant R01 DK57768 (to M.G.M.), an American Diabetes Association grant (to M.G.M.), the American Heart Association (to H.M. and R.L.L.), and NIH Grant R01 DK078135 (to D.C.F.).
Disclosure Summary: E.C.V., H.M., D.C., R.L.L., K.K., R.I.-T., J.C.J., D.C.F., and M.G.M. have nothing to declare. R.J.S. has research grants from Johnson & Johnson and stock options in Zafgen and is a board member for Eli Lilly and Zafgen and on the speakers’ bureau for Merck and Amylin.
First Published Online July 23, 2009
Abbreviations: AgRP, Agouti-related protein; AMPK, AMP-dependent protein kinase; ARC, arcuate nucleus; EGFP, enhanced green fluorescent protein; β-gal, β-galactosidase; icv, intracerebroventricular; IHC, immunohistochemistry; IR, immunoreactivity; LepRb, long-form leptin receptor; MB-ARC, medial basal ARC; mTOR, mammalian target of rapamycin; mTORC1, mTOR complex 1; PI3K, phosphatidylinositol 3-kinase; POMC, proopiomelanocortin; pS6, phosphorylated S6; S6K, S6 kinase; STAT, signal transducer and activator of transcription; VMH, ventromedial hypothalamic nucleus.
References
- Tierney EF, Gregg EW, Narayan KM 2006 Leading causes of death in the United States. JAMA 295:383 [DOI] [PubMed] [Google Scholar]
- Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF 2003 Lifetime risk for diabetes mellitus in the United States. JAMA 290:1884–1890 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM 2003 Epidemiologic trends in overweight and obesity. Endocrinol Metab Clin North Am 32:741–760, vii [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM 2006 Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL 1998 Leptin and the regulation of body weight in mammals. Nature 395:763–770 [DOI] [PubMed] [Google Scholar]
- Bates SH, Myers Jr MG 2003 The role of leptin receptor signaling in feeding and neuroendocrine function. Trends Endocrinol Metab 14:447–452. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Bjornholm M, Munzberg H, Myers Jr MG 2006 Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 14(Suppl 5):208S–212S [DOI] [PubMed] [Google Scholar]
- Myers Jr MG 2004 Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 59:287–304. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB 2005 Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493:63–71 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C 2003 Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 144:2121–2131 [DOI] [PubMed] [Google Scholar]
- Münzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Björnholm M, Elmquist J, Sleeman M, Cowley MA, Myers Jr MG 2007 Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci 27:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Lam TK, Obici S, Rossetti L 2006 Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci 9:227–233 [DOI] [PubMed] [Google Scholar]
- Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L 2005 Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11:320–327 [DOI] [PubMed] [Google Scholar]
- Porte Jr D, Baskin DG, Schwartz MW 2005 Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes 54:1264–1276 [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG 2005 AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25 [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB 2004 AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574 [DOI] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ 2006 Hypothalamic mTOR signaling regulates food intake. Science 312:927–930 [DOI] [PubMed] [Google Scholar]
- Robertson SA, Leinninger GM, Myers Jr MG 2008 Molecular and neural mediators of leptin action. Physiol Behav 94:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Blenis J 2004 Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23:3151–3171 [DOI] [PubMed] [Google Scholar]
- Kim DH, Sabatini DM 2004 Raptor and mTOR: subunits of a nutrient-sensitive complex. Curr Top Microbiol Immunol 279:259–270 [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN 2006 TOR signaling in growth and metabolism. Cell 124:471–484 [DOI] [PubMed] [Google Scholar]
- Thomas G 2002 The S6 kinase signaling pathway in the control of development and growth. Biol Res 35:305–313 [DOI] [PubMed] [Google Scholar]
- Mori H, Inoki K, Münzberg H, Opland D, Faouzi M, Villanueva EC, Ikenoue T, Kwiatkowski D, MacDougald OA, Myers Jr MG, Guan KL 2009 Critical role for hypothalamic mTOR activity in energy balance. Cell Metab 9:362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC 1998 Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J 17:6649–6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers Jr MG 2003 STAT3 signaling is required for leptin regulation of energy balance but not reproduction. Nature 421:856–859 [DOI] [PubMed] [Google Scholar]
- Björnholm M, Münzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjørbaek C, Myers Jr MG 2007 Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest 117:1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, Yancopoulos GD, Wiegand SJ, Sleeman MW 2005 Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab 2:421–427 [DOI] [PubMed] [Google Scholar]
- Münzberg H, Flier JS, Bjorbaek C 2004 Region-specific leptin resistance within the hypothalamus of diet-induced-obese mice. Endocrinology 145:4880–4889 [DOI] [PubMed] [Google Scholar]
- Leshan RL, Louis GW, Jo YH, Rhodes CJ, Münzberg H, Myers Jr MG 2009 Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29:3138–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB 1998 Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547 [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG 1993 c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol 14:173–213 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL 2003 The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37:649–661 [DOI] [PubMed] [Google Scholar]
- Rother E, Könner AC, Brüning JC 2008 Neurocircuits integrating hormone and nutrient signaling in control of glucose metabolism. Am J Physiol Endocrinol Metab 294:E810–E816 [DOI] [PubMed] [Google Scholar]
- Banks AS, Davis SM, Bates SH, Myers Jr MG 2000 Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275:14563–14572 [DOI] [PubMed] [Google Scholar]
- Bjørbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers Jr MG, Flier JS 2001 Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem 276:4747–4755 [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS 2005 PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Münzberg H, Myers Jr MG 2007 The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem 282:31019–31027 [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers Jr MG, Schwartz MW 2001 Intracellular signalling: key enzyme in leptin-induced anorexia. Nature 413:794–795 [DOI] [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA 1996 The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA 93:8374–8378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørbaek C, Uotani S, da Silva B, Flier JS 1997 Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 272:32686–32695 [DOI] [PubMed] [Google Scholar]
- Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers Jr MG 2004 LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes 53:3067–3073 [DOI] [PubMed] [Google Scholar]
- Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW 2005 Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289:E1051–E1057 [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK 1999 Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23:775–786 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ 2001 Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- Myers Jr MG, White MF 2002 The molecular basis of insulin action. In: DeGroot LJ, Jameson JL, eds. Endocrinology. Philadelphia: Saunders; 712–727 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.