Abstract
Our objectives were to assess age differences in perceptual repetition priming and perceptual skill learning, and to determine whether they are mediated by cognitive resources and regional cerebral volume differences. Fragmented picture identification paradigm allows the study of both priming and learning within the same task. We presented this task to 169 adults (ages 18–80), assessed working memory and fluid intelligence, and measured brain volumes of regions that were deemed relevant to those cognitive skills. The data were analyzed within a hierarchical path modeling framework. In addition to finding age-related decrease in both perceptual priming and learning, we observed several dissociations with regards to their neural and cognitive mediators. Larger visual cortex volume was associated with greater repetition priming, but not perceptual skill learning, and neither process depended upon hippocampal volume. In contrast, the volumes of the prefrontal gray and white matter were differentially related to both processes via direct and indirect effects of cognitive resources. The results indicate that age-related differences in perceptual priming and skill learning have dissociable cognitive and neural correlates.
Keywords: aging, brain, MRI, perceptual repetition priming, perceptual skill learning, working memory, fluid intelligence, visual cortex, prefrontal cortex, prefrontal white matter
When applied to young people, the claim that skill is improved through practice is commonplace, and although the cognitive and neural mechanisms underlying the process of skill acquisition remain unclear, significant progress has been made toward their elucidation (Gilbert, Sigman, & Crist, 2001; Karni & Bertini 1997; Poldrack, et al., 1999; Salmon & Butters, 1995; Willingham 1998). It is unclear, however, whether the same can be said about older adults. In spite of the proverbial belief about old dogs and new tricks, the extant studies on age differences in skill acquisition do not converge on a simple conclusion. Some experiments reveal age-related declines in learning and maintenance of motor and perceptual-motor skills (Kennedy & Raz, 2005; Kennedy, et al., 2007; Raz et al, 2000; Rodrigue, et al., 2005), whereas others find that older adults can learn just as well as the young, albeit to an overall lower level of performance (Bock & Schneider, 2002; Seidler, 2007). The literature on age differences in perceptual learning is even less consistent (Gilbert & Rogers, 1996; Kotchouby et al., 2000).
According to current theories (e.g., Ulman, 2007), object recognition is a process characterized by progressive reduction of uncertainty, from encoding and recognition of components to knowledge-driven reconstruction of an object. The first, recognition-by-components stage of the process has been investigated in the framework of perceptual priming and learning paradigms such as the fragmented pictures identification task (Snodgrass et al., 1987). In that task, an observer views, in a descending order of fragmentation, line drawings of common objects. After repeated presentations of a set of stimuli, the observers are able to identify the object more quickly and/or at a greater level of fragmentation (stimulus uncertainty). The level of fragmentation at which they can be named correctly is the identification threshold (IT). If at the repeated presentations old stimuli are mixed with new ones, one can simultaneously assess at least two related cognitive processes: item-specific repetition priming and item-independent skill learning (Poldrack et al., 1999). Notably, identification of objects with reduced number of features can be acquired, and significantly improved even after a single trial (Maki et al., 1999).
Whereas perceptual identification is a skill that can be acquired by people of all ages, it remains unclear whether older observers improve at the same rate as their younger counterparts. The extant literature on age-related changes and differences in fragmented picture identification suggests that older adults may be especially vulnerable to reduced stimulus redundancy, decreased signal-to-noise ratio, and shortening of processing time (Frazier & Hoyer, 1992; Kennedy et al., 2007; Lindfield & Wingfield, 1999; Read, 1988). As a rule, reduction in the task information-processing demands by doubling the viewing time (Hashtroudi et al., 1991), improving stimulus signal-to-noise ratio (Hashtroudi, et al., 1991) and eliminating uncertainty in stimulus presentation (Lindfield et al., 1994) greatly attenuates, although does not entirely abolish age differences (but see Kotchouby et al., 2000).
Identifying and naming an object requires participation of multiple brain systems. Visual association cortices in the ventral (inferior temporal and fusiform) and dorsal (superior parietal) processing streams support the operations relevant to visual and spatial tasks, whereas tertiary association areas (prefrontal and inferior parietal) control integrative and executive aspects of performance (Constantinides & Wang, 2004; Martin, 2007; Passupathy, 2006). The neural substrates of object perception and identification, however, are not limited to association cortical circuitry. Whereas all accounts of object recognition and its brain mechanisms emphasize the importance of top-down, knowledge-based, acquired skills, converging evidence from lesion and imaging studies indicates that the primary visual cortex may play an important role in object recognition including its involvement in relevant high-level processes such as mental imagery (Li, 2008; Passupathy, 2006; Serre et al., 2007; Yotsumoto & Watanabe, 2008). Studies of patients with cortical lesions (Viggiano & Pitzalis, 1998) and degeneration of the striatum (Butters, 1984; Martone et al., 1984; Roncacci et al., 1996; Yamadori et al., 1996) suggest that cortico-striatal circuits are important for acquisition of perceptual skills. In contrast, skill acquisition is relatively preserved in amnesia caused by middle-temporal lesions and early (Maki, 1995) though not late (Viggiano et al., 2007) stages of degenerative diseases that are limited to the hippocampus and the entorhinal cortex (e.g., Alzheimer’s Disease). Thus, by implication, the medial temporal lobe structures are not generally viewed as important for perceptual learning and skill acquisition.
Repetition priming experiments with other stimuli show that measurable reductions in regional blood flow within the brain regions that participate in perceptual identification can be induced through a single exposure (Schacter & Buckner, 1998; 1999). Neuroimaging studies of related perceptual skills (e.g., mirror-reading) suggest that fronto-striatal circuits and cerebellum are active in acquisition (Cabeza & Nyberg, 2000; Grady, 2000; Poldrack, et al, 1998; Poldrack & Gabrieli, 2001), whereas prefrontal and inferior temporal and fusiform cortices are related to perception of the already primed objects (Koutstaal et al., 2001) or to the learned perceptual skill (Poldrack, 2002).
Little is known about the neural underpinnings of age differences in perceptual priming and learning. To date, age differences were examined only in neural correlates of verbal priming (word-stem completion), and three functional imaging investigations in that area yielded contradictory results. In two studies, the older participants demonstrated task-related reduction in regional activation similar to the younger adults (Bäckman et al., 1997; Lustig & Buckner, 2004). In the other, young participants evidenced greater reduction in activation to primed stimuli than did the old, albeit in similar regions (Daselaar et al., 2005).
In previous reports, we presented evidence that larger volumes in the relevant cortical regions may be associated with better procedural skills. For example, volumes of the putamen and the cerebellum (but not the neocortex or the hippocampus) are associated with higher proficiency in a perceptual-motor task (pursuit rotor), and in part mediate age-related differences in performance (Raz et al., 2000). Differences in the volumes of the caudate nucleus and the prefrontal cortex volumes partially mediate age-related performance differences in another procedural task with significant perceptual-motor requirements, mirror-drawing (Kennedy & Raz, 2005). In contrast, acquisition of a cognitive skill (solution of Tower of Hanoi puzzle) was dependent only upon the volume of the prefrontal cortex (Head et al, 2002). The observed age differences and longitudinal changes in learning may be mediated by differences in cognitive resources, as evident in significant contribution of working memory in all of the above-mentioned tasks (Ghisletta et al., submitted; Kennedy et al., 2008; Raz et al., 2000; Kennedy & Raz, 2005; Kennedy et al., 2008; Kennedy et al., 2007; Head et al., 2002). In addition, other cognitive characteristics such as speed of processing and mental flexibility may also mediate age-related changes perceptual skills (Rodrigue et al., 2005). Taken together, the reviewed findings suggest that specific neuroanatomical differences may mediate age-related variation in perceptual priming and learning either directly or via their effect on cognitive resources.
The current study was designed to examine the effects of age-related differences in brain structure and cognitive resources on perceptual priming and perceptual skill acquisition. To best accomplish this goal, we chose a well-established task in which we could gauge simultaneously the age, neural, and cognitive effects on both perceptual repetition priming and perceptual learning in the same task, thereby avoiding confounding effects of comparing across stimuli, presentation modalities, or tasks. Further, we used a large sample size so that we could make inferences about the entire adult agespan and so that we could implement hierarchical path analyses. This method of analysis is a powerful statistical technique, whose use is fairly novel in analyzing brain imaging data. This method allows us to test for dissociations in the cognitive and neural mediators of age-related differences in perceptual learning and perceptual priming by examining shared and unique variance among the variables of interest.
In these analyses, We hypothesized that improvement of object identification thresholds with repetition would be linked to greater cognitive resources (working memory, fluid intelligence) and larger volumes of the putative neuroanatomical substrates of priming (visual and prefrontal cortices), skill learning (caudate nucleus), and object recognition (fusiform gyrus). In contrast, we hypothesized that the hippocampus, because of its known involvement in declarative but not procedural memory (Squire, 1992), would predict neither perceptual priming nor skill acquisition. Because of the decreased availability of cognitive resources in older adults and the age-related shrinkage of the caudate nucleus, fusiform gyrus, and prefrontal cortex, we expected skill acquisition to be negatively associated with age, and that priming would be relatively age-invariant due to its reliance on age-stable primary visual areas.
Method
Participants
Participants were healthy adults recruited from a large MidSouth metropolitan area. They were administered a health questionnaire to screen for history of cardiovascular (e.g., stroke, heart attack, bypass surgery, congenital heart disease), neurological and psychiatric conditions, head trauma with loss of consciousness > 5 min, alcohol and drug abuse, thyroid problems, and diabetes. Controlled and uncomplicated essential hypertension was allowed. Participants were also screened for dementia and depression with a modified Blessed Information-Memory-Concentration Test (BIMC; Blessed et al, 1968) and a Geriatric Depression Questionnaire (CES-D; Radloff, 1977) with cut-offs of 30 and 15, respectively. All participants exhibited strong right-hand preference for basic manual activities (75% and above on the Edinburgh Handedness Questionnaire; Oldfield, 1971) and passed hearing (model MA27; Maico Diagnostics, Eden Prairie, Minn.) and vision (Optec 2000 vision tester, Stereo Optical Co., Inc., Chicago, Ill.) screenings administered before the cognitive tasks. Because the cognitive test used in this study (fragmented picture identification) is highly visual in nature, it was important to ensure sufficiently corrected near and far vision. The mean far (20/20) and near (24/20) visual acuity was excellent and did not differ with age (t < 1). Note the mean corrected visual acuity reported is almost 20/20 vision. An experienced neuroradiologist screened MRI scans for signs of space-occupying lesions and major cerebrovascular abnormalities. The sample consisted of 169 adults ranging in age from 18–80 years (mean age 46.33 ± 16.65 SD years) with 94 women and 75 men. Table 1 provides sample demographics. Participants had on average 16.03 ± .199 years of education, ranging from 12–25 years. Men and women did not differ significantly in age (p = .60), education (p = .08), BIMC scores (p = .36) or vocabulary scores (p = .44). Age was unrelated to education (r =−.09, ns) and Blessed scores (r =−.11, ns), but was positively associated with vocabulary scores (r = .36, p < .001).
Table 1.
Demographic information for participants, by sex and for the total sample: mean ± standard error of the mean
| N | Age | Edu | CES-D | BIMC | ITtrain | ITold | ITnew | |
|---|---|---|---|---|---|---|---|---|
| Men | 75 | 47.08±2.00 | 16.43±.03 | 4.44±.47 | 29.33±.15 | 4.60±.07 | 2.77±.08 | 4.18±.07 |
| Women | 94 | 45.72±1.66 | 15.71±.26 | 5.06±.42 | 29.15± (13 | 4.89±.07 | 3.26±.09 | 4.48±.07 |
| t | (167) | −0.53 | −1.79 | 0.99 | −0.92 | 2.88 | 3.80 | 2.93 |
| p | ns | ns | ns | ns | .004 | .001 | .004 | |
| Total | 169 | 46.33±1.28 | 16.03±.20 | 4.79±.31 | 29.23±.10 | 4.76±.05 | 3.04±.07 | 4.34±.05 |
Note. N – sample size; Edu – years of education; CES-D – center for epidemiology studies depression scale; BIMC – Blessed information, memory, concentration screening; ITtrain – identification thresholds for training set; ITold – identification threshold for repeated set; ITnew -- identification threshold for novel set.
MRI protocol
Images were acquired on a 1.5T Signa scanner (General Electric Co., Milwaukee, Wisconsin). The protocol is described in detail in our previous publications (Raz, et al., 2004). All volumetric measures were performed on the reformatted images acquired using T1-weighted 3-D spoiled gradient recalled acquisition sequence (SPGR; 124 contiguous axial slices, TE = 5 ms, and TR = 24 ms, FOV = 22 cm, acquisition matrix 256 × 192, slice thickness = 1.3 mm, and flip angle = 30°).
Volumetric image analysis
The MR images were processed and analyzed using BrainImage 2.3.3 software. Standard neuroanatomical landmarks were used to standardize the position of the brains and correct undesirable effects of head tilt, pitch, and rotation. To adjust for individual differences in head pitch (for all region of interest, ROIs, except the hippocampus) the brain volumes were rotated along the plane containing the anterior and posterior commissure (incorporating the AC-PC line). The differences in head rotation were corrected by aligning brain volumes along the interhemispheric fissure. Head tilt was corrected by rotating the brain volume into a position in which diameters of the left and the right orbits on a coronal projection were equal. To measure the hippocampus (HC), the brain volumes were aligned along the long axis of the right hippocampus instead of the AC-PC line. Reformatted images were partitioned into coronal sections 1.5 mm apart, and were .86 mm (one linear pixel) thick.
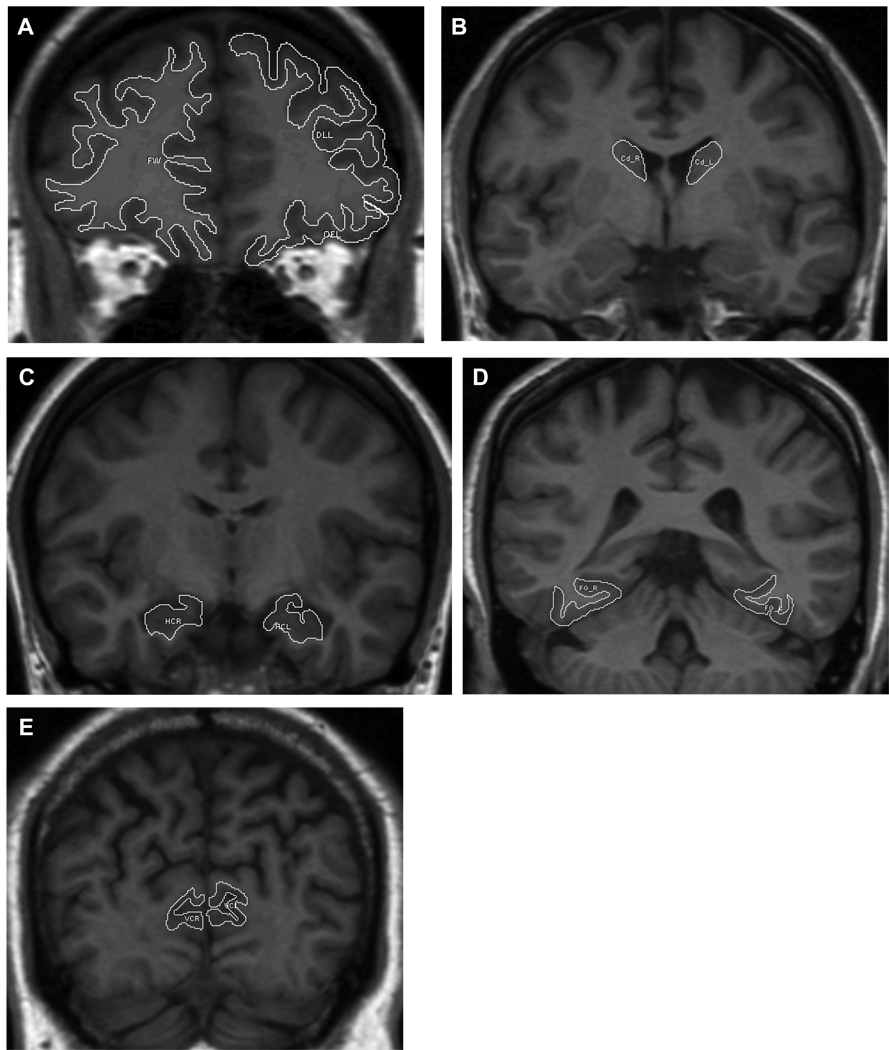
The ROI areas were measured with NIH Image software (Version 1.60). Images were displayed on a 21" monitor and each ROI was traced manually using a stylus and a digitizing tablet. The slices were divided into two equal groups at random, and each half-sample was manually traced by a different trained operator (blind to the participants' calendar age and sex). All reliability coefficients, intraclass correlations presuming random raters (ICC (2), Shrout & Fleiss, 1979), were above .90 among all raters for each ROI. Volumes were computed using the Cavalieri estimate (Rosen & Harry, 1990), and adjusted for body size (height) using a linear regression equation. All structures were measured separately for each hemisphere and after determining that the left and right hemispheres for each ROI did not differ significantly, the volumes of the bilateral regions were summed. The demarcation and tracing of the ROIs are illustrated in Figure 1.
Figure 1.
Illustration of regions of interest manually traced from structural MRI scans. A) Prefrontal cortex – dorsolateral, orbitofrontal, and white matter; B) Caudate nucleus; C) Hippocampus; D) Fusiform gyrus; E) primary visual cortex (calcarine).
Lateral Prefrontal Cortex (LPFC)
The volume of the lateral prefrontal cortex was computed from 10 to 13 coronal slices. To determine the range of inclusion, we calculated the number of slices from the frontal poles to the slice immediately rostral to the genu of the corpus callosum. The operators measured the LPFC on the caudal 40% of these slices. Only the continuous cortical ribbon was measured; gray matter was excluded if it was completely enclosed by white matter. The described ROI includes superior, middle, and inferior frontal gyri and covers Brodmann areas 8, 9, 10, 45, 46. The LPFC was defined as the gray matter located between the most dorsomedial point of the cortex and the orbital sulcus.
Orbitofrontal Cortex (OFC)
This ROI was measured on the same slices as the LPFC. The most lateral branch of the orbital sulcus that breaches the external aspect of the brain defines the lateral boundary. This branch appears on the ventrolateral surface of the brain. This is the same sulcus that served as the lower boundary for the LPFC. The medial boundary is defined by the olfactory sulcus, which appears on the ventromedial surface of the brain. The OFC ROI includes almost the entirety of BA 47 and a substantial part of BA 11.The entire cortex located between the medial and lateral boundaries across the 40% range constitutes the orbital-frontal cortex region of interest.
Prefrontal White Matter (Fwhite)
The white matter adjacent to the PFC was measured on the same slices as the gray matter ROIs described above. All white matter in this range was included.
Caudate Nucleus (Cd)
The volume of the head and the body of the Cd were estimated from 15 to 20 coronal slices. The most rostral slice was the one on which the Cd first appeared, usually lateral to the lateral ventricles. The Cd was traced on every other slice (inter-slice distance 3 mm) until no longer visible. The Cd was bordered by the lateral ventricle medially, by the internal capsule laterally, and by white matter dorsally. On the rostral slices the ventral boundary consisted of the stria terminalis. On more anterior slices of the Cd, the septal nucleus and nucleus accumbens served as the ventral border. The transition between the Cd and these nuclei was difficult to determine on some slices, so the ventral border was either defined as the line drawn from the most ventral part of the internal capsule to the most ventral part of the lateral ventricle, or on one or two slices, the nucleus accumbens was included in the caudate volume. Because of rarity of such inclusions (maximum on two slices) and the consistency of rule application across participants, the effect of this inclusion with Cd volume was deemed insignificant.
Hippocampal Formation (HC)
The HC was measured on a series of 19 to 25 slices aligned perpendicular to the long axis of the hippocampus. To avoid confounding age-related expansion of the inferior horns of the ventricles with demarcation of the amygdala-hippocampus border, we used the mammillary bodies as the rostral boundary of the HC. Thus, a small anterior portion of the structure was excluded. The slice showing the fornices rising from the fimbria marked its caudal boundary.
Fusiform gyrus (FG)
Because the FG spans the temporal and the occipital lobes of the cerebrum, two sets of rules were necessary to demarcate it reliably. Within the temporal lobe, the anterior boundary of the fusiform gyrus was determined on the most anterior slice that contained the anterior commissure. The measurement of FG proceeded until reaching the last slice on which the splenium of the corpus callosum was present. In the occipital lobe, the range of slices used in estimating the volume of the FG was determined by counting the number of slices between the occipital poles and the slice that was located caudally to the last slice on which the splenium was present. Thirty three percent of that distance covered the slices included in the range. Starting at the most anterior slice to the most caudal slice on which corpus callosum could be identified, the occipito-temporal sulcus served as the lateral boundary of the FG. Starting at the first slice after the splenium was no longer present, the lateral boundary was the most ventro-lateral sulcus, in most (but not all) cases, the occipito-temporal sulcus. The collateral sulcus constituted the medial boundary of the FG. In the posterior portion of the FG the collateral sulcus splits into two sulci: the lingual (more medial), and the collateral (lateral). The latter is the sulcus used in demarcation of the FG.
Visual (Pericalcarine) Cortex (VC)
The volume of the VC was estimated as the volume of the cortical ribbon lining the calcarine sulcus. This sulcus appeared as the most ventromedial sulcus in the temporal-occipital cortex at the coronal slice that is mid-vermis or immediately caudal to mid-vermis and was measured on the anterior 50% of the coronal slices between the mid-vermis slice and the occipital pole. The inferior and superior boundaries of this ROI were defined as the point at which the opening of the sulcus occurred. At this point a line was drawn horizontally so that no cortex (dorsal or ventral) outside of the calcarine sulcus was included. This ROI contained mainly the primary visual cortex (Brodmann area 17) and some of the secondary visual areas (Brodmann area 18) as well.
Cognitive measures
Working Memory (WM). Two verbal and two nonverbal working memory tests were administered. The verbal working memory tasks were Computation Span (CSPAN) and Listening Span (LSPAN) (Salthouse et al., 1990). Both measure the ability for simultaneous storage and processing of verbal information, and are very similar in structure, administration procedure, and scoring. In CSPAN, the participant is asked to solve simple arithmetic problems while simultaneously remembering the last digit in each problem. In LSPAN, the participants listen to simple sentences. After each sentence, they are asked to answer a question about its content and to report its final word. We used the absolute span (AS) as performance index in both tests. The AS is calculated by summing the number of correct items across all trials. In order to have the number of items added to their total, the participant must get all items in that trial correct. In comparison to other indices, such as simple span, AS has superior psychometric properties, its range is not too narrow and it is less prone to capitalization on chance than the total span that counts single successful items scored on the trials even when the participant fails the rest (Engle et al., 1992). The absolute span scores from LSPAN and CSPAN, (which correlated r = .64, p < .001; Cronbach’s alpha = .77), were standardized and then averaged to form the Verbal Working Memory (WMv) construct. See Table 2 for descriptive statistics.
Table 2.
Mean, standard deviations and Pearson correlation matrix of age, regional brain volumes and cognitive indices (N = 169).
| Age | LPFC | OFC | Fwh | HC | Cd | FG | VC | WMv | WMnv | Gf | Train | Repeated | Novel | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPFC | −.58*** | 1 | ||||||||||||
| OFC | −.35*** | .76*** | 1 | |||||||||||
| Fwh | −.33*** | .78*** | .79*** | 1 | ||||||||||
| HC | −.30*** | .53*** | .57*** | .60*** | 1 | |||||||||
| Cd | −.29*** | .39*** | .35*** | .34*** | .39*** | 1 | ||||||||
| FG | −.41*** | .61*** | .56*** | .63*** | .51*** | .43*** | 1 | |||||||
| VC | −.12 | .15 | .23** | .31*** | .36*** | .24** | .31*** | 1 | ||||||
| WMv | −.37*** | .25** | .22** | .25** | .18* | .15 | .18** | .15** | 1 | |||||
| WMnv | −.34*** | .29*** | .25** | .28*** | .25** | .15 | .23** | .16* | .55*** | 1 | ||||
| Gf | −.54*** | .38*** | .31*** | .33*** | .26** | .20** | .34*** | .20** | .52*** | .65*** | 1 | |||
| Train | .38*** | −.25** | −.12 | −.11 | −.15 | −.10 | −.25** | −.10 | −.29*** | −.44*** | −.54*** | 1 | ||
| Repeated | .50*** | −.31*** | −.12 | −.11 | −.16* | −.18* | −.25** | −.19* | −.32*** | −.46*** | −.55*** | .71*** | 1 | |
| Novel | .47*** | −.29*** | −.12 | −.14 | −.18* | −.09 | −.26** | −.16* | .20** | −.39*** | −.49*** | .72*** | .79*** | 1 |
| Mean | 46.33 | 18.74 | 9.64 | 39.96 | 6.78 | 9.55 | 19.42 | 5.45 | .004 | .000 | −.001 | 4.76 | 3.04 | 4.34 |
| St Dev | 16.65 | 3.31 | 1.79 | 7.49 | .90 | 1.28 | 2.71 | .95 | .89 | .83 | .92 | .66 | .86 | .68 |
Note. p < .05;
p < .01;
p < .001.
All regional brain volumes (cm3) corrected for height. WMv, WMnv, Gf are standardized scores with mean 0 and SD 1. Train, Repeated and Novel are identification thresholds. LPFC – lateral prefrontal cortex; OFC – orbitalfrontal cortex; Fwh – prefrontal white matter; HC – hippocampus; Cd – caudate; FG – fusiform gyrus, VC – primary visual cortex; WMv – standardized composite of verbal working memory tests; WMnv – standardized composite of nonverbal working memory tests; Gf – fluid intelligence composite; Repeated – repetition priming; Novel – skill learning.
Nonverbal Working Memory was measured by two tests. The first, Size Judgment Span, was modified after Cherry and Parks’ (1993) version. Participants were read aloud lists of objects and animals and asked to repeat each list with the objects arranged in order of their size from the smallest item to the largest. The first list was two items long (e.g., violin, ship). After successful completion of at least two out of three trials, the list was incremented by one item. The cumulative number of correct trials constituted the score on this test.
The second task used to measure nonverbal working memory was the Spatial Relations test (# 19) from the Woodcock-Johnson Psychoeducational Battery - Revised (Woodcock & Johnson, 1989). In this task, participants are shown a whole shape, and are required to choose a correct combination of components from a series of six disjointed shapes presented on the same board. The item difficulty increases across trials as the shapes become complex and abstract. The total number of correct responses is the score on this task. Although designated as a test of spatial abilities, the task exerts considerable demands on working memory, as the subject is required to mentally assemble the correct shape from its parts and to manipulate (rotate and translate) some of the components to the positions that would allow the assembly operation. Correlations between similar spatial tasks and measures of visual-spatial working memory are quite high (Shah & Miyake, 1996), and analogous tasks have been used for assessment of visual-spatial WM in young and older adults (Kirasic et al., 1996). The scores from SJS and Spatial Relations tests (which correlated r = .40, p < .001; Cronbach’s alpha = .55) were standardized and then averaged to form the Nonverbal Working Memory (WMnv) construct. See Table 2 for descriptive statistics.
Fluid Intelligence (Gf). We administered two tests of fluid reasoning known for their sensitivity to age, Cattell Culture Fair Intelligence Test (CFIT Tests 2 and 3; Cattell & Cattell, 1973) and Letter Sets Test (parts 1 and 2) from the Educational Testing Service Factor-Referenced Test Kit (Ekstrom et al. 1976). The CFIT is essentially a test of nonverbal reasoning composed of four subtests per form. The test is commonly used as a marker of fluid intelligence in studies of lifespan development aging and consists of nonverbal reasoning problems covering a wide range of difficulty (Rabbitt and Lowe 2000; Raz et al. 1998; Schretlen et al. 2000). Each subtest consists of 10–14 items tapping different nonverbal abstract reasoning domains, including detection of similarities in designs, completing matrices according to specific rules, and solving nonverbal syllogisms. In all problems, the participant has to derive the rule required to solve the problem. The second subtest calls for analyzing the stimulus, discovering the differences, and selecting the “”odd man out.’’ The third is similar to the first in calling for rule-based set completion, only in the framework of 2 ×3 ×2 and 3 × 3 ×3 matrices. The test is timed, with 2.5–4 min allowed for completion of each subtest. The index of performance is the number of total correct items across the subtests.
The Letter Sets Test served as an additional index of fluid reasoning. This test is composed of two forms containing 15 items each. Five sets of four letter series are presented in each item, and the participants’ task is to find the rule that relates four of the five sets to each other and to mark the one that does not fit this rule. Participants work for seven min on each form (for a total of 14 min). The score on this test is the total number of correct items minus 0.25 point for each incorrect item. Scores from CFIT and Letter Sets tests were standardized to z-scores and then averaged to form the construct Fluid Intelligence (Gf). Cronbach's Alpha for these two tests is .75. For mean and standard deviations see Table 2.
Perceptual Learning and Repetition Priming
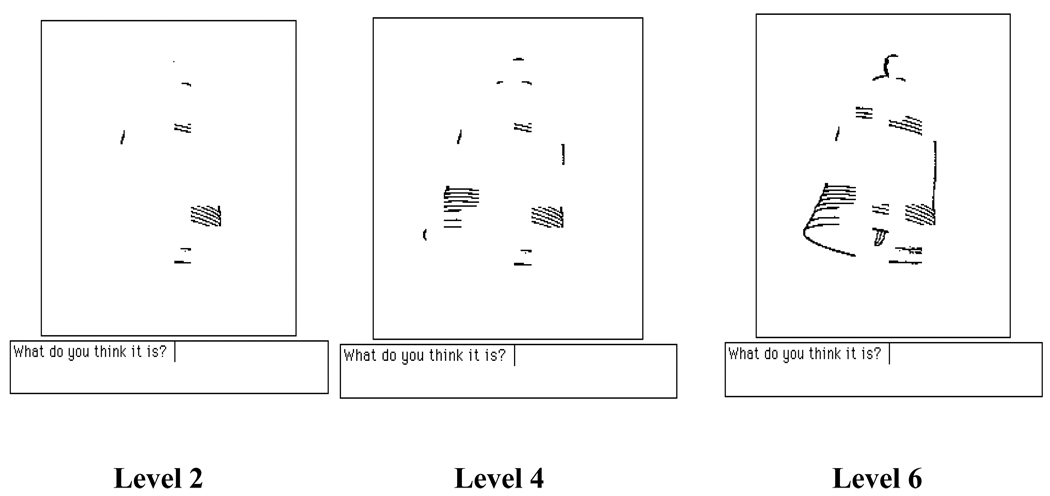
Fragmented Pictures Identification Task. The stimuli and software for the experiment were generously provided by Prof. Snodgrass (New York University). The software presents line drawings of objects from the pool of normed sets of objects from Snodgrass et al (1987), Snodgrass & Vanderwart (1980), and Snodgrass & Corwin (1988). Fifteen line drawings of common objects were used as a baseline set. The objects were: chair, doll, horse, kangaroo, kettle, ladder, leaf, lemon, mouse, nose, sailboat, saw, shirt, umbrella, and violin. Fifteen new line drawings were selected from the same pool of images and presented for identification. The objects were: belt, bottle, bowl, car, cat, desk, duck, gorilla, gun, lips, onion, sock, swing, telephone, and trumpet. On each trial, a stimulus was displayed on a 9-inch black-and-white computer monitor screen (Macintosh SE30, Apple Computer Corp., Cupertino, CA) at the highest level of degradation (level 1). Each object had eight potential levels of degradation, from total disintegration (level 1) to full view (level 8). For a sample line drawing at three levels of fragmentation, see Figure 2. The participants adjusted the degradation level in increments of 1, by pressing the spacebar on a computer keyboard until they could identify the object, at which point they entered the object name into the keyboard. See Appendix for verbatim instructions to the participants. The resulting level of degradation was assigned as the identification threshold (IT) for a given picture. The average IT across the 15 pictures was used as an index of performance for data analyses. Instructions for the participants were displayed on the monitor screen and were read aloud by the experimenter as well.
Figure 2.
Example of stimulus with various levels of fragmentation, ranging from Level 1 (very fragmented) to Level 8 (complete picture). Pictures are shown as they appeared to the participants.
The computer program presents fifteen line drawings of common objects, used as a training baseline. After the participants completed the baseline set, they were given a short pencil-and-paper test of nonverbal reasoning (CFIT 3A - test 4; Cattell & Cattell, 1973) which took 2.5 minutes. After that break, the participants proceeded to the test phase. The program then presented the set of fifteen new line drawings for identification interspersed with repeated presentation of the first set of fifteen line drawings, for a total of thirty stimuli (15 new and 15 old). Therefore, three scores were obtained from this task: baseline IT, old IT, and new IT. Reduction of identification threshold for previously observed pictures (“old”) was defined as perceptual repetition priming, whereas lowering of the identification threshold for the non-repeated (“new”) pictures was taken as evidence of perceptual skill acquisition. Note that higher identification thresholds indicate poorer performance.
Path analysis
To investigate the complex relations among age, regional brain volumes (lateral prefrontal cortex, LPFC; orbitofrontal cortex, OFC; prefrontal white, Fwhite; caudate nucleus, Cd; fusiform gyrus, FG; primary visual cortex, VC), mediating cognitive processes (verbal and nonverbal working memory, WMv, WMnv; fluid intelligence, Gf) and perceptual repetition priming and skill learning, we constructed path models to test these associations. Identification thresholds for the Training (baseline), Repeated (old) and Novel (new) conditions served as the picture identification constructs.
The flow of variance was determined by theoretical considerations outlined in the introduction. In short, we assumed that calendar age was the highest tier of the hierarchy, (i.e., it could have direct and indirect effects on all other variables, but the variance cannot flow in the opposite direction), that the regional brain volumes would comprise the second tier, (i.e., they could be affected by age, but no other variables included in the model). The potential cognitive mediators comprised the next tier and could not affect anything upstream, whereas the constructs of interest (perceptual priming and skill learning indices) that formed the final tier could be affected by any other variable in the model. The variables within the same tier that were known to be linked in theory or by empirical evidence were allowed to correlate. In the path diagram, correlations were represented graphically as curved arrows and hypothesized relations were represented by straight arrows and standardized regression weights. To test the hypotheses, we used maximum likelihood model fitting methods implemented in Mplus software (Muthèn & Muthèn, 2001).
In evaluating the path models, we used the following goodness-of-fit statistics criteria. For a model to fit well, the normal theory weighted χ2 should not be significant (i.e., p > .05), although with the current sample size, this is a strict criterion. In addition, the Comparative Fit Index (CFI) and Tucker-Lewis Index (TLI) should be at least .95 for a good fit and at least .90 for an adequate fit (Hu & Bentler, 1999). A Root Mean Squared Error of Approximation (RMSEA) index of ≤.05 would represent a good fitting model and an index of ≤.08 would indicate an adequate fit (Browne & Cudeck, 1993). Standardized Root Mean Residuals (SRMR) of <.05 is generally considered a good fit (Hu & Bentler, 1998) and is recommended for its sensitivity to model misspecification and robustness in face of deviations from distribution normality and small sample size. Additionally, as a measure of effect size, R2 (the proportion of variance explained) was computed for each variable. All figures on the path diagram are standardized, sample specific parameters.
Results
Descriptive statistics for the baseline, repeated, and novel objects are provided in Table 1 and Table 2. As indicated in Table 1, there were no sex differences on any of the demographic and screening parameters. However, men had lower (better) identification thresholds at baseline and repeated presentations.
Age and sex differences in perceptual repetition priming and perceptual skill learning
To examine the age and sex differences in perceptual repetition priming and perceptual skill acquisition, we used the General Linear Model (GLM) data-analytic approach. In each model, the identification threshold (IT) was the dependent variable; testing occasion (Baseline vs. Old pictures test or Baseline vs. New pictures test) was a repeated measure factor, whereas age and sex served as continuous and categorical independent variables. All interactions with the repeated measures were adjusted by the Huynh-Feldt ε coefficient to correct for violation of sphericity assumption.
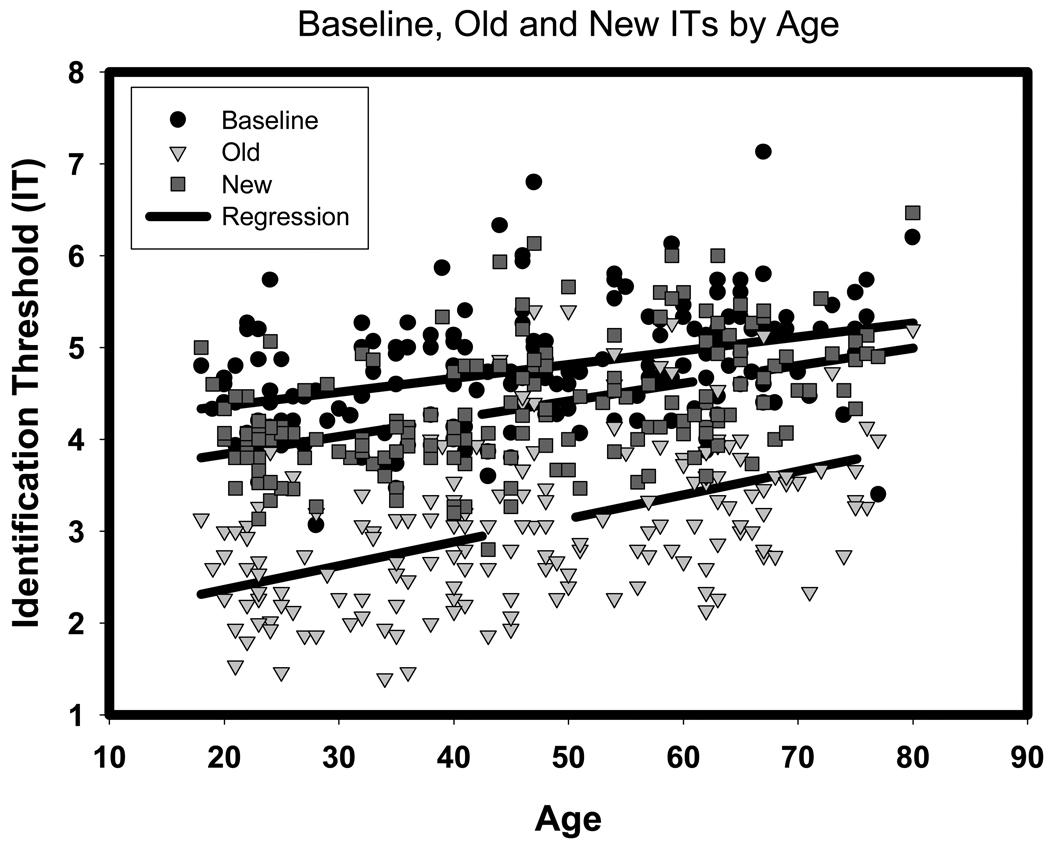
First, we tested the repetition priming effects by comparing thresholds obtained at baseline to those measured during exposure to the Repeated picture set. In this analysis, significant Age (F(1,165) = 59.54, p < .001) and Sex (F(1, 165) = 21.00, p < .001) main effects indicated that older adults had higher identification thresholds than younger adults (r = .50) and women (3.26 ± .09 SEM) had higher identification thresholds than men (2.77 ± .08 SEM). There was no significant Age × Sex interaction (F < 1). There was a strong main effect of Testing occasion, F(1,165) = 1546.78, p <.0001. The effect reflected a substantial and consistent reduction of identification threshold for the repeated exposure (from 4.76 to 3.04, a 36% drop) i.e., evidence of successful priming. However, a significant Age × Testing occasion interaction was also noted, F(1,165) = 16.79, p < .001. The association of age with the identification threshold increased from r = .38 at baseline to r = .50 at the second exposure, a significant difference, Steiger’s Z* = 3.44, p = .001 (Steiger, 1980). Moreover, there was a significant Sex × Testing occasion interaction, (F(1, 165) = 5.80, p = .02), which stemmed from women having higher IT then men both at baseline (4.89 vs 4.60, t = 2.88, p < .005), and at repetition (3.26 vs 2.77, t = 3.80, p < .001), but disproportionately more so at repetition. The regressions of identification thresholds on age for Training, Repeated, and Novel pictures are depicted in Figure
In the second linear model, we examined age and sex differences in acquisition of picture identification skill. We fitted to the data a model analogous to the one used in analysis of priming effects; the identification thresholds for the Novel set of pictures was the dependent variable instead of the Repeated pictures thresholds. A significant effect of Age was observed for the perceptual skill learning condition, F(1, 165) = 51.29, p < .001, with older adults having higher IT than their younger counterparts (r = .47), see Figure 3. There was also a main effect of Sex, F(1,165) = 15.00, p < .001, with women having higher IT (4.48 ± .07 SEM) than men (4.18 ± .07 SEM). Age × Sex interaction was not significant (F < 1). Within-subjects effects of this analysis revealed a significant reduction of Baseline-Novel identification thresholds from 4.76 to 4.34, or 9%, F (1,165) = 115.63, p < .001, albeit a much smaller gain than observed for priming. A trend for Age × Test occasion interaction did not reach significance, F(1,165) = 3.38, p = .07, although the association between age and IT at baseline was r = .38, compared to r = .47 for new pictures, a significant difference, Steiger’s Z* = 4.06, p < .001. There were no interactions with Sex (Fs < 1).
Figure 3.
Identification thresholds (IT) for baseline, old, and new line drawings across the lifespan. Note that lower IT reflects better performance. Old items & Age: t = 5.99, p < .001, b = .42; New items & Age: t = 2.19, p < .05, b = .17.
In the next analysis we compared the age-related effects on perceptual repetition priming to that on perceptual skill learning. To evaluate the magnitudes of age differences in priming and in skill acquisition, IT obtained at Baseline, Repetition, and Novel presentation were compared in a multivariate analysis. There was a significant main effect of Set, F(2, 330) = 974.91, p < .0001, that was qualified by a significant Age × Set interaction [F(2, 330) = 9.85, p < .001] indicating that the IT differences in picture recognition introduced by these two cognitive processes (learning and priming) differed, but that the magnitude of the difference depended on age. Simple effects analysis indicated that the reduction in IT (4.76 to 3.04) for priming (t[168] = 37.17, p < .001) was greater than the IT reduction (4.76 to 4.34) for skill learning, t(168) = 10.78, p = .001 (See Figure 3). The effect sizes for priming and learning were d = 2.84 vs. d = .84, respectively, and were significantly different as their 95% confidence interval limits did not overlap (priming 95%CI: 2.54 – 3.14; learning 95%CI: .62 - 1.06). Age correlated with Baseline IT r = .32, with Repeated pictures IT r = .50, and with Novel pictures IT r = .47, indicating that as age increases so does the threshold at which the fragmented pictures can be identified, as shown in Figure 3.
The role of neuroanatomical and cognitive processes on repetition priming and skill learning
The correlations among age, neuroanatomical volumes, and cognitive measures as well as sample means and standard deviations for the variables are presented in Table 2. As expected, age had a significant negative effect on all neural and cognitive variables (all p’s < .001) except primary visual cortex (r = −.12).
Neuroanatomical and Cognitive Mediators of Priming and Learning Gains: Path Analysis
Full (Baseline) Model (Model 0)
We first tested an initial Full (baseline) Model which estimated all direct effects from the highest variables in the hierarchy to those in the tiers below and, as expected, did not fit the data well (See Table 3 for fit indices). In this step we also determined the specific structure of the model. For example, the three regions of the prefrontal cortex were allowed to correlate, as were verbal and nonverbal working memory, and priming and learning thresholds. In addition to theoretical guidance, the order of the mediating cognitive variables was also determined empirically by testing different permutations of this structure (e.g., WMv and WMnv were specified to predict Gf). This hierarchy produced the best fitting baseline model listed in Table 3.
Table 3.
Goodness of Fit Indices of Path Models (N = 169).
| Model | χ2 | df | p | RMSEA (90%CI) |
CFI | TLI | SRMR | AIC | BIC (N adj) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 12.73 | 3 | .005 | .139 (.07–.22) | .990 | .735 | .036 | 9567 | 9839 (9564) |
| 1 | 23.46 | 12 | .02 | .075 (.03–.12) | .982 | .934 | .054 | 7780 | 7905 (7778) |
| 2* | 20.31 | 20 | .44 | <.01 (.0–.07) | .999 | .999 | .041 | 5517 | 5623 (5515) |
Notes. RMSEA = Root Mean Squared Error of Approximation; CFI = Comparative Fit Index;TLI = Tucker-Lewis Index; SRMR = Standardized Root Mean Residual; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; N adj = sample size adjusted BIC.Model 0 – test of initial Full (baseline) Model; Model 1-- test of Reduced Model I; Model 2 -- test of further reduced Model II. * Model 2 is the best fit to the data.
Reduced Model I
From the baseline model, we then specified Reduced Model I by reducing the number of estimated paths on the basis of theoretical and statistical considerations as follows. Age was specified to have a direct effect on all other variables except VC (effect of age on VC was nonsignificant, p = .12). Because they only evidenced age effects, but no significant associations existed between them and any of the cognitive variables, OFC, Cd, HC, and FG were dropped from the model at this stage. In addition to removing these variables, we specified the following associations: LPFC, Fwhite, and VC were estimated to predict each of the six cognitive variables; WMv and WMnv were each specified to predict Gf, Training, Priming, and Learning; Gf was estimated to predict Training, Priming, and Learning; Training was specified to predict Priming and Learning. Importantly, rather than specifying Priming and Learning to correlate with each other as we did in the Full baseline model, a directional path from Priming to Learning was estimated.
As expected, in spite of improving upon the Full baseline model (See Table 3 for model comparison), the initial Reduced Model I provided only an adequate fit to the data as it contained numerous nonsignificant paths: χ2 (12) = 23.46, p =.02; CFI = .98; TLI = .93; RMSEA = .08; SRMR = .05. It is important to note that estimating the directional path from Priming to Learning greatly improved the model fit and this path was significant (β = .56). When we reverse the direction of the specification of this path from Learning to Priming, the magnitude of the path coefficient is reduced (β = .47) and all goodness-of-fit statistics became considerably poorer (i.e., CLI and TLI reduced, RMSEA and SRMR increased, and Akaike Information Criterion and Bayesian Information Criterion indices increased). Thus, the difference in the goodness-of-fit supports the hypothesized effect of priming on learning, rather than the opposite.
Reduced Model II
The next step was to specify Reduced Model II by removing nonsignificant paths one-by-one based on t-values for the associations and theoretical considerations. The relations that did not have strong support from the literature were removed first, whereas those that had the greatest support in previous published research were removed last. The goal was to find the most parsimonious model that still fit the data (see specification of Model II in Figure 4). We therefore removed the following nonsignificant paths: from Age to Training and Age to Learning; from LPFC to WMv, to WMnv, and to Gf; from Fwhite to Gf, and to Learning; and from VC to all variables except Priming. We also removed nonsignificant paths from Gf to Priming and to Learning. We then removed the paths from WMv to Training, Priming, and Learning, and from WMnv to Learning, examining model fit as each path was eliminated. In all cases, path removal resulted in increased model fit.
Figure 4.
Path analysis model for Reduced Model II which provided the best fit for the data. All path parameters are standardized coefficients. Curved double-headed arrows represent correlations and straight directional arrows represent estimated paths. Age exerted a negative direct effect on perceptual priming, but all effects of age on perceptual learning were mediated by neural and cognitive factors. Priming had a strong effect on learning. LPFC – lateral prefrontal cortex volume; Fwhite – prefrontal white matter volume; VC – primary visual cortex; WMv – verbal working memory composite; WMnv – nonverbal working memory composite; Gf – fluid intelligence composite; Train – fragmented picture identification threshold for training set; Prime – fragmented picture identification threshold for repeated set; Learn – fragmented picture identification thereshold for novel picture set. See text and Table 3 for model fit indices and Table 2 for variable descriptive statistics.
After all nonsignificant paths were dropped, the Reduced Model II (see Figure 4 and Table 3) evidenced a remarkably good fit with 21 significant paths: χ2 (20) = 20.31, p = .44; CFI = .99; TLI = .99; RMSEA < .01; SRMR = .04. In this model, increasing Age exerted a direct negative effect on LPFC, Fwhite, verbal and nonverbal WM, Gf, and Priming threshold. Smaller LPFC volume, in turn, had a negative effect on Training and Priming thresholds. Smaller Fwhite volume had a negative effect on verbal and nonverbal WM scores. It also influenced Training and Priming, but in the opposite direction: Larger prefrontal white matter volume was associated with higher training and priming identification thresholds. The VC volume also had a direct (negative) effect on Priming, i.e. greater VC volume was associated with larger priming gains.
The variables deemed to reflect the availability of cognitive resources evidenced significant hierarchical relations and influenced the fragmented pictures thresholds. Verbal WM had a significant effect on Gf scores, as did nonverbal WM, however WMnv also had a significant direct effect selectively on Training and Priming thresholds, but not on Learning. In contrast, WMv had no direct effects on Training, Learning, or Priming, but influenced those indices via fluid intelligence. Training threshold had a stronger effect on thresholds obtained at repetition Priming (β = .55) than on those observed in a Learning set (β = .32). The model with all path coefficients is depicted in Figure 4.
It is important to note that all the effects of age and regional brain volumes on perceptual skill learning were indirect and mediated via cognitive variables such as fluid reasoning, and verbal working memory. In contrast, both age and regional brain volumes exerted both direct and indirect effects on priming. The indirect effects were mediated by the differences in fluid reasoning and nonverbal working memory. Notably, the relatively large path coefficient from Priming to Learning (β = .56, t = 8.94), indicates the important role of perceptual priming in skill learning (refer to Figure 4 for all path coefficients). Reduced Model II proved informative in describing the data as it explained 33% of the variance in Training thresholds, 61% of the variance in Priming thresholds, and 67% in Learning thresholds. It accounted for 16% of the variance in verbal working memory, 15% of the variance in nonverbal WM, and 55% of the variance in fluid intelligence. Further, age alone accounted for 34% and 11% of the variance in prefrontal gray and white matter, respectively.
Finally, to determine whether a more parsimonious fit could be established despite eliminating significant paths, we removed estimated paths one-by-one (based on strength of t values) until the model no longer fit. When removing paths to specify Reduced Model III, it was found that removal of any path led to a much poorer fit. Therefore, we conclude that Model II was the final reduced model as it was the most parsimonious fit for the data. We also verified the directionality of the cognitive variables by reversing their direction one at a time and re-estimated the model. In no case did the paths with reversed variance flow fit as well as those that were originally specified.
Regional white matter hyperintensity (WMH) burden is a significant predictor of age-related declines in many cognitive domains (Gunning-Dixon & Raz, 2000). It could, therefore, explain the association of smaller frontal white matter volume with greater object identification training and priming thresholds. If a significant part of the white matter was occupied by WMH, its volume would reflect a combination of viable axons and various pathological inclusions, thus making larger not necessarily better. The analysis of WMH measures (Gunning-Dixon & Raz, 2003) available for a part of this sample (participants older than 50 years of age) provided no support for such explanation. It is worth mentioning that in this older subsample (n = 68), the priming and learning effects were comparable to the whole sample results, with the exception of sex differences that failed to reach significance. The correlation between frontal WMH volume and priming was virtually nil (r = .08, ns). Parietal WMH volume was weakly correlated with priming, also in the expected direction (r = .19, ns). Because regional WMH for all lobes correlated positively with age (Frontal r = .29, p < .02; Parietal r = .27, ns; Temporal r = .38, p = .001; Occipital r = .22, p = .08) and were negatively correlated with the corresponding regional volumes (i.e., frontal WMH and frontal white matter volume r =−.22, p = .08), differential WMH burden also cannot explain the direction of the prefrontal white matter volume effect.
Finally, the differential effects of primary visual cortex on priming but not on learning are illustrated in the scatterplot in Figure 5. Regression analyses controlling for age and sex indicate the association of visual cortex volume and priming was significant (t = −2.06, p < .05, β = −.14), whereas its association with learning was not (t = −.95, ns, β = −.07), although the 95%CI are not entirely non-overlapping.
Figure 5.
Effect of primary visual cortex volume on identification of fragmented pictures. Regressions lines correspond to baseline set, repeated pictures, and novel pictures. Note that lower identification thresholds indicate better performance. Visual cortex volume is associated with repeated but not novel object identification thresholds. Old items & VC: t = −2.06, p < .05, β = −.14; New items & VC: t = −.95, ns, β = −.07.
Discussion
In a large sample of well-educated healthy adults covering a wide age range, we examined both perceptual repetition priming and perceptual learning in the same task, fragmented picture identification, thereby controlling for task-and stimulus-specific confounds. Through hierarchical path analysis, we observed several dissociations among neural and cognitive mediators of age-related differences in perceptual repetition priming and perceptual skill learning. First, whereas variance in the magnitude of perceptual priming was explained by both direct and indirect effects of calendar age, age differences in perceptual learning were fully mediated by neural and cognitive variables. Aging per se and undeclared variables that are subsumed under calendar age did not affect the magnitude of the learning gains. Thus, in acquisition of a perceptual skill by older adults the integrity of specific brain regions and specific cognitive resources appear more important than calendar age. Second, the volume of the primary visual cortex, independent of age, predicted perceptual priming, but not perceptual learning gains (or baseline threshold) in the same task. Third, we observed dissociation between working memory type and picture identification: nonverbal working memory directly predicted perceptual priming (and baseline threshold) but not perceptual learning, whereas verbal working memory did not directly predict either learning or priming. In addition, both indices of working memory influenced performance via their effects on fluid intelligence. Lastly, variance in perceptual skill learning gains was explained not only by indirect influence of regional brain volume via mediating cognitive processes, but also by the variance in perceptual repetition priming and its total combined effects. Notably, the effect of priming on learning was significant and substantial. Participants who showed greater priming gains were also more likely to exhibit better generalized skill learning.
One of the main objectives of this study was to test the hypothesis that volumes of specific brain regions would predict performance on fragmented picture identification and explain the age-related differences in performance. This hypothesis was partially sustained. Larger volume of the visual cortex, a region that showed no age-related reduction, reliably predicted greater priming (but not learning) gains. Priming gains were also reliably predicted by greater volume of the lateral prefrontal cortex, an age-sensitive region that has been linked not only to effortful, strategy-driven cognition (Miller, 2000) but also to repetition priming (e.g., Bunzeck et al., 2006) and identification of filtered objects under rTMS disruption to this area (Viggiano et al., 2008). By contrast, the volume of the prefrontal white matter, which also showed age-related declines, predicted reduction in cognitive resources (working memory, fluid intelligence), and via some of those factors, reduced perceptual priming and learning gains in this study.
It is possible that in addition to this direct involvement of the PFC, that finding also reflects the weakening in the white matter connections between the striatum and the prefrontal cortex, both age-sensitive regions (Raz et al., 2003; 2005). Studies of patients with neurodegenerative disease of the neostriatum implicated cortico-striatal circuits in procedural performance deficits (Saint-Cyr & Taylor, 1992). Although the lack of associations between caudate volume and performance in the current study does not provide support for that hypothesis, further research with direct indices of white mater integrity derived from diffusion tensor imaging may bring more clarity to the subject.
An unexpected finding was the link between larger prefrontal white matter volume and poorer priming and training identification thresholds. Although it may reflect a valid neurobiological phenomenon, we first discuss alternate explanations. First, the effect may reflect the nature of our sample, which, as in most similar studies, was a selected sample of convenience. In such a sample, the older participants with smaller prefrontal white matter, indicating worse state of brain health, could have been included because their relatively preserved functions were supported by cognitive reserve (Stern, 2002; Staff et al., 2004). Second, the finding may also reflect a statistical artifact known as a suppressor variable (Pedhazur, 1982). When a suppressor variable (i.e., age) is at play, the regression (semi-partial) coefficient is larger than the corresponding zero-order correlation between the variable (Prefrontal white volume) and the criterion (Training and Priming thresholds). Indeed, the prefrontal white volume does not correlate with priming or training threshold (r = −.11), whereas it correlates significantly with age (r = −.33). The standardized path (regression) coefficient for the frontal white matter and priming is .26, indicating worse priming in persons with larger prefrontal white matter volumes.
Alternately, if we assume that the observed association between larger prefrontal white matter volume and increased priming and training thresholds indeed reflects a real phenomenon, several speculations can be offered. For example, enlarged white matter volumes could be a sign of problematic developmental history resulting in incomplete pruning of irrelevant connections. Indeed, it has been found in adults 20–45 years old that white matter fiber length is negatively correlated with degree of connectivity (Lewis et al., 2008), suggesting that smaller volume of regional white matter may indicate more efficient neural conduction via shorter networks. We provided weak evidence that despite larger frontal white matter volume, presence of white matter hyperintensities (WMH) compromises the quality of that white matter. This may be driving the association between larger frontal white matter volume and reduced priming, as frontal (and parietal) WMH volume correlates positively with priming. Further, it has been shown by many studies that across the lifespan white matter volume evidences a nonlinear trajectory that consists of ascent, plateau, and decline (Bartzokis et al., 2001; Bartzokis et al., 2003; Courchesne et al., 2000; Fjell et al., 2005; Jernigan et al., 2001; Kennedy et al., 2008; Raz et al., 2005; see Raz & Rodrigue, 2006 for a review), whereas the gray matter regions are more likely to follow a linear age trajectory (see Raz & Rodrigue, 2006 for a review). Perhaps this inverted U-shaped trajectory could partially explain the current findings as our sample included adults from virtually the entire adult age span. A longitudinal design would be required to test this notion. The literature on the neuroanatomical foundations of cognitive aging has at least two other examples of negative association between regional volumes and cognitive performance. In one, the authors found negative correlations between local temporal lobe volumes and multiple memory indices in spite of replicating age-related reduction of volumes (Van Petten et al., 2004). In the other, a negative association between working memory scores and orbitofrontal volume was observed (Salat et al., 2002), although in that study an age-related increase in regional volume was also reported.
As hypothesized, perceptual priming and learning were unrelated to the hippocampal volume, adding support for the dissociation between declarative and nondeclarative memory processes in the literature. It is noteworthy, however, that according to the extant literature the implicit/explicit memory distinction is not clear-cut, and any study of “implicit” processes brings the concern of explicit contamination. It is more likely the true case that some explicit processes need to be brought online when carrying out “implicit” activities such as priming and perceptual learning (cf Poldrack, 2002). It also possible that the neural systems identified with nondeclarative (striatum) and declarative (medial temporal) processing interact or even compete during these procedural processes (Poldrack, 2002).
We did not find a significant effect for the fusiform gyrus volume, despite its known role in perceptual and visual identification and processing (e.g., Blondin & Lepage, 2005; Liu et al., 2006; Poldrack, 2002), nor did we find an effect of the caudate nucleus in this type of learning. It is unclear why we observed no such associations. It is possible that it was because the caudate (as well as the cerebellum) is involved in the multiple repetition aspect of priming, whereas prefrontal involvement is more stimulus-specific (Bunzeck et al., 2006). A functional imaging study would be needed to demonstrate that temporal distinction. For example, throughout its time course, perceptual learning is differentially related to visual cortex activation (Yotsumoto, Watanabe, & Sasaki, 2008).
In the fragmented picture identification task implemented in this study, we used the descending fragmentation method of stimuli presentation, in which presentation becomes incrementally more complete (i.e., less fragmented) until a correct identification is made. That approach is known to be more challenging to the observers than the ascending fragmentation order as it yields lower accuracy and longer latency of response for everybody, but it may be differentially more challenging for the elderly. In the extant literature, the differential effect of age has been explained by a difficulty to discard false hypotheses formed at the levels of stimulus degradation that precluded correct identification (Lindfield et al., 1994), and interpreted as support for the inhibition-deficit hypothesis (Hasher & Zacks, 1988). According to that hypothesis, efficient working memory relies upon successful inhibition of potential interference from task-irrelevant stimuli, an ability that is deficient in older adults (Salthouse, 1994). Lindfield and Wingfield (1999) suggested through a computational analysis that cognitive slowing might be the means by which inhibition deficits affect performance in identifying perceptually degraded objects. Our findings are in accord with this interpretation that relies on the working memory inhibition-deficit hypothesis (Hasher & Zacks, 1988). However, the results of our study leave room for more complex and nuanced explanations of a deceptively simple phenomenon.
The dissociation between the effects of nonverbal but not verbal working memory on perceptual priming and learning is informative. The role of compromised spatial working memory in age-related reduction of priming gains may reflect reduced availability of the resources for item-specific imagery (Kosslyn, 1994) in acquiring a skill. Such an explanation is in line with Anderson’s theory of skill acquisition (e.g., Anderson, 1982), as older adults may experience difficulty in automatizing the different portions of the skill. The findings from visual search experiments by Rogers and colleagues (Fisk & Rogers, 2000; Rogers, 1997) support the latter interpretation.
Finally, less specific factors than those discussed above may be summoned to explain the observed effects. For example, as Strayer and Kramer (1994) suggest, older adults might simply learn at a slower rate with the same (or better) accuracy as younger adults. Moreover, their apparent deficit might have been a result of adopting a conservative response bias. In a similar vein, some suggest that older adults might have handled the speed/accuracy tradeoff differently than younger adults and emphasize accuracy even in the absence of instructions to do so (Salthouse, 1979). Although we see no direct way to evaluate those propositions on the basis of the data available in this study, the results of a recent meta-analysis engenders skepticism about the inherent conservatisms of the older participants (Marquié & Baracat, 2000). Across multiple studies, there was no evidence of such a general trend, and we have to assume that it was unlikely to emerge in this study. The most general explanation of the observed working memory effects would be a reference to the fact that working memory tasks require commitment of attentional resources and that the association between working memory scores and identification threshold gains simply reflect that nonspecific cost of doing cognitive business (Maki et al., 1999). However, the observed dissociation between verbal and nonverbal working memory makes such a generalization difficult to sustain. Nonetheless, in light of the reported greater aging effects on visuo-spatial than on verbal tasks (Botwinick, 1977), more detailed direct comparisons of verbal and nonverbal skill acquisition and priming merit future research effort.
We observed that men were better than women in identifying fragmented pictures, with the effect being stronger for priming than for learning. The literature suggests that women perform better than men on verbal but worse than men on visuo-spatial tasks (Kimura, 1999), and the results reported here are certainly consistent with that trend. However, we cannot rule out the role of mediators that were not specified in the model. For example, identification of fragmented pictures by premenopausal women may be mediated by fluctuating levels of estrogen with women in the low estrogen phase identifying objects at a higher level of fragmentation (Hampson et al., 2005). In the current study, however, priming and learning performance were not affected by hormone replacement therapy1. Another possible explanation for the sex differences could be that because women are more proficient with living categories, and men are better with nonliving objects (Barbarotto et al., 2008; Gainotti, 2005), the inclusion of 2:1 more nonliving than living objects as stimuli in the task could have given men an advantage.
Our behavioral results are generally in accord with the extant priming and learning literature (Caggiano et al., 2006; Daaselaar et al., 2005; Fleischman et al. 2004; 2005; Koutstaal, 2003; Madden et al., 2005; Marczinski et al., 2003; Prull, 2004; Wiggs et al., 2006). A recent event-related potentials study using similar stimuli found that older adults were differentially affected by the repetition task at early and late components as compared to younger adults (Lawson et al., 2007). Thus far, functional studies of aging and (word-stem) priming contradict each other as they show both age-related differences in word priming (Daselaar, et al., 2005), and lack thereof (Lustig & Buckner, 2004). Thus, that matter remains unclear until additional data are available.
The results of this study should be interpreted in the context of its limitations. First, because in path analysis only the models that are specified can be tested, only one conceptualization of the true state-of-affairs is examined. Similarly, only those variables measured and included in the path analysis can be considered; there are certainly more variables at play than those that can be examined in one study.
Second, although we believe that the findings we present are largely due to procedural/nondeclarative processes, there is the possibility that they represent an admixture of both implicit and explicit processes and not “pure” perceptual priming and perceptual learning. However, several aspects of the study suggest that the likelihood of this possibility is relatively low. For example, explicit contamination is known to be reduced by encouraging the participants to respond quickly, which limits the time available to try to deliberately recall the practice session (Mitchell & Bruss, 2003). Another way to reduce explicit contamination is introduction of a demanding task during delay (here, fluid reasoning, CFIT) thus leaving little to no time for intentional encoding of the pictures (Mitchell & Bruss, 2003). In addition, as the participants were not informed of the delayed test, the incentive for intentional rehearsal is minimized. Further, research has shown that perceptual processes and identification responses are far less susceptible to explicit contamination than conceptual and semantic processes and production responses (Mitchell & Bruss, 2003). Indeed, some implicit tests are not susceptible to explicit contamination effects, particularly priming of pictures (Brown et al., 1991; Mitchell & Brown, 1988; Mitchell & Bruss, 2003). Moreover, we did not find a significant role of the hippocampal volume, usually associated with explicit memory processes, in either the perceptual learning or priming conditions.
Third, this study is limited by the shortcomings of a cross-sectional design (i.e., cohort effects, sample contamination by preclinical dementia cases as well as confounding aging with individual differences from other sources). Future projects should follow the individuals over time to investigate the existence and extent of the long-term effects of priming and skill retention. Longitudinal follow-up of a small subsample of this sample in our laboratory (Kennedy et al., 2007) is generally consistent with the current pattern of results. Specifically, in that study, poorer working memory was a strong predictor of a longitudinal age-related decline in both priming and skill learning. Additional tasks that measure both priming and skill acquisition are needed to compare patterns of performance in older adults across domains of priming and learning (see Perruchet & Baveux, 1989). We have preliminary evidence from a mirror-reading task that suggest similar age-related patterns in priming and skill learning, suggesting generalization across tasks (Raz, unpublished data). In addition, skill learning should also be investigated over a period of several days to fully understand the time course of learning to identify fragmented pictures. Perhaps spaced, rather than massed practice would provide differing results (but see Perruchet, 1989).
The implications of the current results are that age-related differences in perceptual priming and skill learning have dissociable cognitive and neural correlates, and the fragmented pictures identification task is more complex than it appears on the surface, resource-wise. Several design-features of this study allowed us to reach our conclusions. A particular strength of this study is the use of highly reliable manual tracings to measure regional brain volumes, which circumvents the problems inherent in automated and semi-automated methods (e.g., voxel-based morphometry, VBM). These problems range from tissue misclassification, errors in automated segmentation and registration, and drastic loss of resolution due to smoothing (Bookstein, 2001; Crum et al., 2003; Davatzikos, 2004; Jernigan et al., 2001). Despite our method’s strengths, it does have its limitations, namely that it is time-consuming and labor-intensive, and, in the hands of less than expertly competent operators, can suffer from low reliability. Most importantly, it requires hypothesized, a priori selection of the regions of interest involved in perceptual priming and skill learning, and it is possible that age-related differences in additional regions beyond those we measured are associated with these processes. A follow-up study using VBM, which allows for association of the variable of interest at each voxel in the brain, may suggest other brain regions of interest to explore; however those findings should be supplemented with manual tracing of the additional regions (see Kennedy et al., 2008; Tisserand et al., 2002 for a discussion of manual vs. VBM methodology in the study of aging). Second, a major strength of this study is the examination of regional brain volume and mediating cognitive processes in the same task, fragmented picture identification, thereby controlling for any unwanted task- or stimulus-specific variance between the perceptual priming and learning measures. Finally, in light of a relatively modest magnitude of the observed effects, the findings from the current study underscore the need for large sample sizes and the importance of multivariate approaches, especially when examining brain involvement. Using the general linear model approach alone would have missed these indirect effects of brain on priming and learning. Only through path analysis were we able to detect the mediating influence of the prefrontal white matter volume on fluid intelligence and working memory.
In sum, the results of this study indicate that although neither item-specific repetition priming nor more general skill learning of fragmented pictures identification are immune to aging, age effects on performance are largely mediated by multiple and dissociable neuroanatomical and cognitive factors. Moreover, availability of cognitive resources affect priming and learning in a differential fashion, and individuals who show stronger priming effects attained better learning gains. The fragmented pictures identification paradigm that allows examination of priming and learning effects within the same task framework merits further attention in cognitive aging research.
Acknowledgments
This study was supported in part by National Institutes of Health grant R37 AG-11230 and T32 HS-013819. A portion of an earlier version of this paper was presented at the Cognitive Neuroscience Society conference in April 2005.
Appendix
Instructions for the participants were displayed on the monitor screen and were read aloud by the experimenter as well, verbatim as follows:
“This is an experiment on how people identify pictures. In this experiment, you will be shown pictures of common objects and animals. The first presentation will always be the most fragmented. If you cannot identify the picture, press the spacebar and the computer will give you the next most complete version. When you think you know the name of the picture, type in its name. The computer will tell you whether or not you are correct. If you make a mistake in typing, use the backspace key to erase the mistake. Here are some hints for getting the name right. First, go with your first impulse. Second, use the shortest name that describes the object. You can use common abbreviations if you wish. And, for long names, you only need to type in the first four letters to be correct. You will see the message “Correct” if you name the picture correctly and you will see the message “Sorry” if you name it incorrectly. Sometimes your name will not match the name you are told, but if you received the “correct” message that means you got credit for your answer. If you think you did not get credit for a correct answer, notify the experimenter, or make a note of it on the sheet next to you.”
After the participants completed the training set, they were given a short test of nonverbal reasoning (Cattell & Cattell, 1973 CFIT 3A - test 4) which took 2.5 minutes. After that break, the participants proceeded to the test phase. The instructions appeared on the screen and the experimenter read them aloud as follows:
“In this phase of the experiment, we are going to show you some more incomplete pictures. As before, these will be pictures of common objects and animals. Some of these will be pictures you saw in the first phase. As before, you will be given several chances to identify each picture. The first presentation will always be the most fragmented. If you cannot identify the picture, press the spacebar and the computer will give you the next most complete version. When you think you know the name of the picture, type in its name. The computer will tell you whether or not you are correct. As in the first phase, there are several possible names for some of the pictures, so go with your first response. For long names, you only need to type in the first four letters in order to be correct. Press spacebar when you are ready to see the first picture.”
Footnotes
To investigate whether the sex effect found for priming and learning could be explained by hormone replacement therapy in the older women, we matched the 13 women who were receiving HRT to 13 who were not (50–80 years old), equated on age (mean age 59), education (mean 16 years), hypertension status, and ethnic origin. HRT status did not have a significant effect on either priming (p > .27) or learning (p > .65) scores.
References
- Anderson JR. Acquisition of cognitive skills. Psychological Review. 1982;89:369–406. [Google Scholar]
- Backman L, Almkvist O, Andersson J, Nordberg A. Brain activation in young and older adults during implicit and explicit retrieval. Journal of Cognitive Neuroscience. 1997;9:378–391. doi: 10.1162/jocn.1997.9.3.378. [DOI] [PubMed] [Google Scholar]
- Barbarotto R, Laiacona M, Capitani E. Does sex influence the age of acquisition of common names? A contrast of different semantic categories. Cortex. 2008;44:1161–1170. doi: 10.1016/j.cortex.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal volume in men: a magnetic resonance imaging study. Archives of General Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Archives of Neurology. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Blondin F, Lepage M. Decrease and increase in brain activity during visual perceptual priming: An fMRI study on similar but perceptually different complex visual scenes. Neuropsychologia. 2005;43:1887–1900. doi: 10.1016/j.neuropsychologia.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Bock O, Schneider S. Sensorimotor adaptation in young and elderly humans. Neuroscience and Biobehavioral Reviews. 2002;26:761–767. doi: 10.1016/s0149-7634(02)00063-5. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Voxel-based morphometry should not be used with imperfectly registered images. NeuroImage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Botwinick J. Intellectual abilities. In: Birren JE, Schaie KW, editors. Handbook of the Psychology of Aging. Van Nostrand Reinholt NY; 1977. pp. 580–605. [Google Scholar]
- Brown AS, Neblett DR, Jones TC, Mitchell DB. Transfer of processing in repetition priming: Some inappropriate findings. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1991;17:514–525. doi: 10.1037//0278-7393.17.3.514. [DOI] [PubMed] [Google Scholar]
- Browne M, Cudeck R. Alternate ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Sage, CA: 1993. pp. 136–162. [Google Scholar]
- Bunzeck N, Schütze H, Düzel E. Category-specific organization of prefrontal response-facilitation during priming. Neuropsychologia. 2006;44:1765–1776. doi: 10.1016/j.neuropsychologia.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Butters N. The clinical aspects of memory disorders: contributions from experimental studies of amnesia and dementia. Journal of Clinical Neuropsychology. 1984;6:17–36. doi: 10.1080/01688638408401193. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caggiano DM, Jiang Y, Parasuraman R. Aging and repetition priming for targets and distracters in a working memory task. Aging, Neuropsychology, and Cognition. 2006;13:552–573. doi: 10.1080/138255890969555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB, Cattell AKS. Handbook for the individual or group Culture-Fair Intelligence Test. Champagne, IL: Institute for Personality and Abilities Testing; 1973. [Google Scholar]
- Cherry K, Park D. Individual differences and contextual variables influence spatial memory in younger and older adults. Psychology and Aging. 1993;8:517–526. doi: 10.1037//0882-7974.8.4.517. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Wang XJ. A neural circuit basis for spatial working memory. Neuroscientist. 2004;10:553–565. doi: 10.1177/1073858404268742. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hind S, Press GA. Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Crum WR, Griffin LD, Hill DLG, Hawkes DJ. Zen and the art of medical image registration: correspondence, homology, and quality. NeuroImage. 2003;20:1425–1437. doi: 10.1016/j.neuroimage.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Aging affects both perceptual and lexical/semantic components of word stem priming: An event-related fMRI study. Neurobiology of Learning and Memory. 2005;83:251–262. doi: 10.1016/j.nlm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. NeuroImage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Dermen D. Manual for kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Services; 1976. [Google Scholar]
- Engle RW, Cantor J, Carullo JJ. Individual differences in working memory and comprehension: A test of four hypotheses. Journal of Experimental Psychology: Learning, Memory and Cognition. 1992;18:972–992. doi: 10.1037//0278-7393.18.5.972. [DOI] [PubMed] [Google Scholar]
- Fisk A, Rogers WA. Influence of training and experience on skill acquisition and maintenance in older adults. Journal of Aging and Physical Activity. 2000;8:373–378. [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Dale AM, Quinn BT, Makris N, Fischl B. Age does not increase rate of forgetting over weeks -neuroanatomical volumes and visual memory across the adult life-span. Journal of International Neuropsychological Society. 2005;11:2–15. doi: 10.1017/S1355617705050046. [DOI] [PubMed] [Google Scholar]
- Frasier L, Hoyer WJ. Object recognition by component features: Are there age differences? Experimental Aging Research. 1992;18:9–14. doi: 10.1080/03610739208253905. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Wilson RS, Gabrieli JDE, Bienias JL, Bennett DA. A longitudinal study of implicit and explicit memory in old persons. Psychology and Aging. 2004;19:617–625. doi: 10.1037/0882-7974.19.4.617. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Gabrieli JD, Wilson RS, Moro TT, Bennett DA. Repetition priming and recognition memory in younger and older persons: temporal stability and performance. Neuropsychology. 2005;19:750–759. doi: 10.1037/0894-4105.19.6.750. [DOI] [PubMed] [Google Scholar]
- Gainotti G. The influence of gender and lesion location on naming disorders for animals, plants and artefacts. Neuropsychologia. 2005;43:1633–1644. doi: 10.1016/j.neuropsychologia.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Kennedy KM, Rodrigue KM, Lindenberger U, Raz N. Effects of Age and Cognitive Resources on Acquisition of Perceptual-Motor Skill: Multilevel Latent Variable Analyses. (in revision) [Google Scholar]
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- Gilbert DK, Rogers WA. Age-related differences in perceptual learning. Human Factors. 1996;38:417–424. doi: 10.1518/001872096778701953. [DOI] [PubMed] [Google Scholar]
- Grady CL. Functional brain imaging and age-related changes in cognition. Biological Psychology. 2000;54:259–281. doi: 10.1016/s0301-0511(00)00059-4. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hampson E, Finestone JM, Levy N. Menstrual cycle effects on perceptual closure mediate changes in performance on a fragmented objects test of implicit memory. Brain and Cognition. 2005;57:107–110. doi: 10.1016/j.bandc.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks R. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The Psychology of Learning and Motivation. Vol. 22. San Diego, CA: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hashtroudi S, Chrosniak LD, Schwartz BL. Effects of aging on priming and skill learning. Psychology and Aging. 1991;6:605–615. doi: 10.1037//0882-7974.6.4.605. [DOI] [PubMed] [Google Scholar]
- Head D, Raz N, Gunning-Dixon F, Williamson A, Acker JD. Age-related differences in the course of cognitive skill acquisition: The role of regional cortical shrinkage and cognitive resources. Psychology and Aging. 2002;17:72–84. doi: 10.1037//0882-7974.17.1.72. [DOI] [PubMed] [Google Scholar]
- Hu L-T, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. [Google Scholar]
- Hu L-T, Bentler PM. Cutoff criteria for fit indices in covariance structural analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J. Effect of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Ostergaard AL, Fennema-Notestine C. Mesial temporal, diencephalic, and striatal contributions to deficits in single word reading, word priming, and recognition memory. Journal of International Neuropsychological Society. 2001;7:63–78. doi: 10.1017/s1355617701711071. [DOI] [PubMed] [Google Scholar]
- Karni A, Bertini G. Learning perceptual skills: behavioral probes into adult cortical plasticity. Current Opinion in Neurobiology. 1997;7:530–535. doi: 10.1016/s0959-4388(97)80033-5. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Age, sex, and regional brain volumes predict perceptual-motor skill acquisition in healthy adults. Cortex, Special Issue: Cognition and the Aging Brain. 2005;41:560–569. doi: 10.1016/s0010-9452(08)70196-5. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Partridge T, Raz N. Age-related differences in acquisition of perceptual-motor skills: Working memory as a mediator. Aging, Neuropsychology and Cognition. 2008;15:165–183. doi: 10.1080/13825580601186650. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Raz N. Fragmented pictures revisited: Long-term changes in repetition priming, relation to skill learning, and the role of cognitive resources. Gerontology. 2007;53:148–158. doi: 10.1159/000098029. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: A comparison of manual volumetry with optimized voxel-based morphometry. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Sex and Cognition. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- Kirasic KC, Allen GL, Dobson SH, Binder KS. Aging, cognitive resources, and declarative learning. Psychology and Aging. 1996;11:658–670. doi: 10.1037//0882-7974.11.4.658. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM. Image and Brain. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- Kotchoubey B, Haisst S, Daum I, Schugens M, Birbaumer N. Learning and self-regulation of slow cortical potentials in older adults. Experimental Aging Research. 2000;26:15–35. doi: 10.1080/036107300243669. [DOI] [PubMed] [Google Scholar]
- Koutstaal W. Older adults encode--but do not always use--perceptual details: intentional versus unintentional effects of detail on memory judgments. Psychological Science. 2003;4:189–193. doi: 10.1111/1467-9280.01441. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Lawson AL, Guo C, Jiang Y. Age effects on brain activity during repetition priming of targets and distracters. Neuropsychologia. 2007;45:1223–1231. doi: 10.1016/j.neuropsychologia.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Theilmann RJ, Sereno MI, Townsend J. The relation between connection length and degree of connectivity in young adults: A DTI analysis. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn105. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, DiCarlo JJ. Unsupervised natural experience rapidly alters invariant object representation in visual cortex. Science. 2008;321:1502–1507. doi: 10.1126/science.1160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindfield KC, Wingfield A. An experimental and computational analysis of age differences in the recognition of fragmented pictures: Inhibitory connections vs speed of processing. Experimental Aging Research. 1999;25:223–242. doi: 10.1080/036107399244002. [DOI] [PubMed] [Google Scholar]
- Lindfield KC, Wingfield A, Bowles N. Identification of fragmented pictures under ascending versus fixed presentation in young and elderly adults: Evidence for the inhibition-deficit hypothesis. Aging and Cognition. 1994;1:282–291. [Google Scholar]
- Liu LC, Plomp G, van Leeuwen C, Ioannides AA. Neural correlates of priming on occluded figure interpretation in human fusiform cortex. Neuroscience. 2006;141:1585–1597. doi: 10.1016/j.neuroscience.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42:865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Spaniol J, Bucur B. Adult age differences in the implicit and explicit components of top-down attentional guidance during visual search. Psychology and Aging. 2005;20:317–329. doi: 10.1037/0882-7974.20.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki P. Is implicit memory preserved in Alzheimer's disease? Implications for theories of implicit memory. Aging and Cognition. 1995;2:192–205. [Google Scholar]
- Maki P, Zonderman A, Weingartner H. Age differences in implicit memory: Fragmented object identification and category exemplar generation. Psychology and Aging. 1999;14:284–294. doi: 10.1037//0882-7974.14.2.284. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Milliken B, Nelson S. Aging and repetition effects: separate specific and nonspecific influences. Psychology and Aging. 2003;18:780–790. doi: 10.1037/0882-7974.18.4.780. [DOI] [PubMed] [Google Scholar]
- Marquié JC, Baracat B. Effects of age, education, and sex on response bias in a recognition task. Journal of Gerontology: Psychological Sciences. 2000;55B:P266–P272. doi: 10.1093/geronb/55.5.p266. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;8:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martone M, Butters N, Payne M, Becker JT, Sax DS. Dissociations between skill learning and verbal recognition in amnesia and dementia. Archives of Neurology. 1984;41:965–970. doi: 10.1001/archneur.1984.04050200071020. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Mitchell DB, Brown AS. Persistent repetition priming in picture naming and its dissociation from recognition memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1988;14:213–222. doi: 10.1037//0278-7393.14.2.213. [DOI] [PubMed] [Google Scholar]
- Mitchell DB, Bruss PJ. Age differences in implicit memory: Conceptual, perceptual, or methodological? Psychology and Aging. 2003;18:807–822. doi: 10.1037/0882-7974.18.4.807. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 2nd ed. Los Angeles, CA: Muthén & Muthén; 2001. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness. The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pasupathy A. Neural basis of shape representation in the primate brain. Progress in Brain Research. 2006;154:293–313. doi: 10.1016/S0079-6123(06)54016-6. [DOI] [PubMed] [Google Scholar]
- Pedhazur EJ. Multiple Regression in Behavioral Research. New York: Holt, Rinehart and Winston; 1982. [Google Scholar]
- Perruchet P. The effect of spaced practice on explicit and implicit memory. British Journal of Psychology. 1989;80:113–130. [Google Scholar]
- Perruchet P, Baveux P. Correlational analyses of explicit and implicit memory performance. Memory and Cognition. 1989;17:77–86. doi: 10.3758/bf03199559. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Neural systems for perceptual skill learning. Behavioral and Cognitive Neuroscience Reviews. 2002;1:76–83. doi: 10.1177/1534582302001001005. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JD. Characterizing the neural mechanisms of skill learning and repetition priming: evidence from mirror reading. Brain. 2001;124:67–82. doi: 10.1093/brain/124.1.67. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Desmond JE, Glover GH, Gabrieli JDE. The neural basis of visual skill learning: An fMRI study of mirror reading. Cerebral Cortex. 1998;8:1–10. doi: 10.1093/cercor/8.1.1. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Selco SL, Field JE, Cohen NJ. The relationship between skill learning and repetition priming: Experimental and computational analyses. Journal of Experimental Psychology: Learning, Memory and Cognition. 1999;25:208–235. doi: 10.1037//0278-7393.25.1.208. [DOI] [PubMed] [Google Scholar]
- Prull MW. Exploring the identification-production hypothesis of repetition priming in young and older adults. Psychology and Aging. 2004;19:108–124. doi: 10.1037/0882-7974.19.1.108. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Lowe C. Patterns of cognitive ageing. Psychological Research. 2000;63:308–316. doi: 10.1007/s004269900009. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Regional volumetry of the cerebral cortex in normal adults: Differential aging, sexual dimorphism, and hemispheric asymmetry. Neurobiology of Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences, and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, Acker JD. Aging of the human striatum: longitudinal evidence. American Journal of Neuroradiology. 2003;24:1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microscopy Research and Technique, special issue on Neuroimaging and Memory. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Read D. Age-related changes in performance on a visual-closure task. Journal of Clinical and Experimental Neuropsychology. 1988;10:451–466. doi: 10.1080/01688638808408252. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Raz N. Aging and longitudinal change in perceptual-motor skill acquisition in healthy adults. Journals of Gerontology: Psychological Sciences. 2005;60:174–181. doi: 10.1093/geronb/60.4.p174. [DOI] [PubMed] [Google Scholar]
- Rogers WA. Assessing age-related differences in the long-term retention of skills. In: Rogers WA, Fisk A, Walker N, editors. Aging and Skilled Performance: Advances in Theory and Applications. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 185–200. [Google Scholar]
- Roncacci S, Troisi E, Carlesimo GA, Nocentini U, Caltagirone C. Implicit memory in parkinsonian patients: evidence for deficient skill learning. European Neurology. 1996;36:154–159. doi: 10.1159/000117234. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Harry JD. Brain volume estimation from serial section measurements: a comparison of methodologies. Journal of Neuroscience Methods. 1990;35:115–124. doi: 10.1016/0165-0270(90)90101-k. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Taylor AE. The mobilization of procedural learning: The key signature of the basal ganglia. In: Squire LR, Butters N, editors. Neuropsychology of Memory. New York, NY: Guilford Press; 1992. pp. 188–202. [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Greater orbital prefrontal volume selectively predicts worse working memory performance in older adults. Cerebral Cortex. 2002;12:494–505. doi: 10.1093/cercor/12.5.494. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N. Neurobiology of skill and habit learning. Current Opinion in Neurobiology. 1995;5:184–190. doi: 10.1016/0959-4388(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Adult age and the speed accuracy tradeoff. Ergonomics. 1979;22:811–821. doi: 10.1080/00140137908924659. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging of working memory. Neuropsychology. 1994;8:535–543. [Google Scholar]
- Salthouse TA, Mitchell D, Skovronek E, Babcock R. Effects of adult age and working memory on reasoning and spatial abilities. Journal of Experimental Psychology: Learning, Memory and Cognition. 1990;15:507–516. doi: 10.1037//0278-7393.15.3.507. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. On the relations among priming, conscious recollection, and intentional retrieval: Evidence from neuroimaging research. Neurobiology of Learning and Memory. 1999;70:284–303. doi: 10.1006/nlme.1998.3854. [DOI] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. Journal of International Neuropsychological Society. 2000;6:52–61. doi: 10.1017/s1355617700611062. [DOI] [PubMed] [Google Scholar]
- Seidler RD. Aging affects motor learning but not savings at transfer of learning. Learning and Memory. 2007;14:17–21. doi: 10.1101/lm.394707. [DOI] [PubMed] [Google Scholar]
- Serre T, Kreiman G, Kouh M, Cadieu C, Knoblich U, Poggio T. A quantitative theory of immediate visual recognition. Progress and Brain Research. 2007;165C:33–56. doi: 10.1016/S0079-6123(06)65004-8. [DOI] [PubMed] [Google Scholar]
- Shah P, Miyake A. The separability of working memory resources for spatial thinking and language processing. Journal of Experimental Psychology: General. 1996;125:4–27. doi: 10.1037//0096-3445.125.1.4. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing raters reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Perceptual identification thresholds for 150 fragmented pictures from the Snodgrass and Vanderwart picture set. Perceptual and Motor Skills. 1988;67:3–36. doi: 10.2466/pms.1988.67.1.3. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Smith B, Feenan K, Corwin J. Fragmenting pictures on the Apple Macintosh computer for experimental and clinical applications. Behavioral Research Methods, Instruments and Computers. 1987;19:270–274. [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Deary IJ, Whalley LJ. What provides cerebral reserve? Brain. 2004;127:1191–1199. doi: 10.1093/brain/awh144. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Strayer DL, Kramer AF. Aging and skill acquisition: Learning-performance distinctions. Psychology and Aging. 1994;9:589–605. doi: 10.1037//0882-7974.9.4.589. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of International Neuropsychological Society. 2002;8:448–560. [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Arigita EJS, van Boxtel MJ, Evans AC, Jolles J, Uylings HBM. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. NeuroImage. 2002;17:657–669. [PubMed] [Google Scholar]
- Ullman S. Object recognition and segmentation by a fragment-based hierarchy. Trends in Cognitive Science. 2007;11:58–64. doi: 10.1016/j.tics.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PSR, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Viggiano MP, Pitzalis S. Identification of fragmented pictures in patients with brain damage. Applied Neuropsychology. 1998;5:93–99. doi: 10.1207/s15324826an0502_5. [DOI] [PubMed] [Google Scholar]
- Viggiano MP, Gori G, Zaccara G, Righi S, Vannucci M, Giovannelli F. Category-specific visual identification of filtered objects in Alzheimer's disease. Arch Gerontol Geriatr. 2007;44:125–139. doi: 10.1016/j.archger.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Viggiano MP, Giovannelli F, Borgheresi A, Feurra M, Berardi N, Pizzorusso T, Zaccara G, Cincotta M. Disruption of the prefrontal cortex function by rTMS produces a category-specific enhancement of the reaction times during visual object identification. Neuropsychologia. 2008;46:2725–2731. doi: 10.1016/j.neuropsychologia.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Weisberg J, Martin A. Repetition priming across the adult lifespan--the long and short of it. Aging, Neuropsychology and Cognition. 2006;13:308–325. doi: 10.1080/138255890968718. [DOI] [PubMed] [Google Scholar]
- Willingham DB. A neuropsychological theory of motor skill learning. Psychological Review. 1998;105:558–584. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Manual for Woodcock-Johnson Psychoeducational Battery - Revised. Allen, TX: DLM Teaching Resources; 1989. [Google Scholar]
- Yamadori A, Yoshida T, Mori E, Yamashita H. Neurological basis of skill learning. Brain Research: Cognitive Brain Research. 1996;5:49–54. doi: 10.1016/s0926-6410(96)00040-7. [DOI] [PubMed] [Google Scholar]
- Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron. 2008;57:827–833. doi: 10.1016/j.neuron.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]