Abstract
Objective
Many infants born with a facial port-wine (PW) birthmark will not develop brain involvement of Sturge-Weber syndrome (SWS). Previous studies have shown asymmetry in quantitative EEG (qEEG) correlates with degree of clinical impairment in children and adults with known SWS. We hope to determine if quantitative qEEG can be used as a method to predict which infants are most likely to develop SWS brain involvement on MRI. The current study looks at the ability of qEEG to differentiate between infants with radiographically-demonstrated SWS and those without.
Methods
We first performed an observational study of qEEG results on 8 infants with facial PW birthmark (4 had SWS brain involvement). We recorded standard clinical EEGs and then derived a measure of asymmetry. We subsequently validated this threshold through a study of an additional 9 infants with PW birthmark (5 with SWS brain involvement).
Results
Quantitative EEG correctly identified infants with SWS brain involvement in all cases in the validation cohort. This technique was at least as good as a pediatric electroencephalographer with extensive experience reading SWS EEGs.
Conclusions
This study demonstrates the ability for qEEG to discriminate between those infants with SWS brain involvement and those with neurologically asymptomatic PW birthmark.
Significance
This study represents an important step toward the development of a qEEG technique able to predict which infants with PW birthmark will develop SWS brain involvement.
Keywords: Sturge-Weber syndrome, electroencephalography
1. Introduction
Approximately 3 in 1000 infants are born with a port-wine (PW) birthmark (Jacobs and Walton, 1976). Of those babies who have a PW birthmark that involves the upper eyelid or forehead, roughly 15-52% (depending on size and extent of PW birthmark) will develop leptomeningeal angioma and the neurological and/or ophthalmic dysfunction that is characteristic of Sturge-Weber syndrome (SWS) (Mazereeuw-Hautier et al., 2006, Tallman et al., 1991); the rest remain neurologically asymptomatic. Clinical manifestations of SWS brain involvement include seizures, hemiparesis, visual field deficits and cognitive impairment. Glaucoma is also associated with SWS.
Infants who will go on to be diagnosed with SWS typically do not demonstrate neurological signs at birth. Of those infants who will ultimately be diagnosed with SWS, 75% develop seizures in the first year of life and 90% by the end of the second year (Sujansky and Conradi, 1995). With the advent of potential prophylactic strategies that are designed to interfere with the neurodegenerative processes of ischemia (Comi, 2007) and seizures (Ville et al., 2002), there is now a need for biomarkers that can assess risk of SWS presymptomatically in order to direct prophylactic interventions to those at the highest risk before seizures or hemiparesis would potentially occur.
Neonatal MRI is often used as a method to try to establish which babies with facial PW birthmark are most likely to develop SWS brain involvement. Unfortunately, MRIs done early are often negative and falsely reassuring (Comi, 2006). Serial MRI neuroimaging can be helpful in depicting a leptomeningeal angioma before the child displays clinical neurological involvement, but this technique would subject the child to the risk of recurrent sedation and gadolinium injection. The use of serial CT would expose children to potential effects due to repeated ionizing radiation and is less sensitive in depicting early changes. Experience in our center indicates that CT findings typically occur after MRI involvement is seen. Transcranial doppler studies have been looked at in Sturge-Weber syndrome (Jordan et al., 2008) but have not yet been investigated as a diagnostic modality.
Unlike MRI, electroencephalography (EEG) is a non-invasive neuroimaging modality that can be used serially with minimal risk and without the need for sedation. EEG abnormalities have long been noted in SWS (Brenner and Sharbrough, 1976). Routine clinical visual inspection in symptomatic individuals often demonstrates decreased amplitude of the EEG signal on the side of brain involvement in SWS (Figure 1), which is almost always the side of the PW birthmark. (In individuals with bilateral PW birthmark, the leptomeningeal angioma is typically maximally present on the side with the maximal skin involvement).
Figure 1.
This EEG in a patient is clearly asymmetrical, with decreased amplitude over the left (first and third groupings of 4 channels each).
Unfortunately, routine visual inspection of the EEG is subjective and heavily reliant on the experience and judgment of the reader. It is however possible to use mathematical techniques to quantify certain aspects of the EEG signal, an approach referred to as “quantitative EEG” (qEEG). Research on qEEG had its inception in the 1930’s, shortly after the discovery of the scalp human EEG (Niedermeyer, 2005). Although the EEG signal is complex, and although EEG interpretation has to a large extent resisted attempts at automation, nevertheless the specific abnormality in SWS (i.e., an asymmetry of amplitude) is easily quantified using fundamental and widely used signal processing techniques. Using a method based on work done on EEG monitoring of cerebral perfusion in carotid endarterectomy (van Putten et al., 2004), we demonstrated that EEG amplitude asymmetry correlates with the degree of clinical impairment of children and adults with known SWS (Hatfield et al., 2007).
We subsequently hypothesized that qEEG could stratify the risk for SWS in infants with facial PW birthmark. Our ultimate goal is to develop clinically useful qEEG techniques that can permit optimal timing of MRI and can therefore allow targeting of prophylactic interventions to the babies at greatest risk. As a step toward this goal, we report currently on an observational study of a qEEG threshold that separates infants with SWS brain involvement from those with neurologically asymptomatic facial PW birthmark. We also report on a study of a prospectively recruited cohort that validates this threshold.
2. Methods
This study was approved by our institutional review board. Parents were consented for the study.
2.1 Participants
All participants were recruited from the Hunter Nelson Sturge-Weber Center at the Kennedy Krieger Institute as a part of a prospective, longitudinal, multidisciplinary and multimodality study of the natural history of SWS. All subjects in this multidisciplinary study (and therefore all subjects in the current study) had a facial port-wine stain. Of all of the subjects in the multidisciplinary study, we included in the current study those individuals who (1) had a study EEG performed before 1 year of age, (2) had an MRI, and (3) met criteria for either the “SWS” group or the “NO-SWS” group.
The criterion for the “SWS” group was an MRI (at any point in time) that showed findings consistent with Sturge-Weber syndrome, specifically contrast-enhancing leptomeningeal lesions. A positive MRI therefore was the defining criterion for diagnosis of Sturge-Weber syndrome in this study. The criteria for the “NO-SWS” group were (1) at least 1 negative MRI, (2) absence of any clinic signs of neurological involvement over the course of clinical follow-up, and (3) clinical follow-up at least as far as 24 months of age. This age was selected because 90% of individuals who will go on to have brain involvement will do so by the second birthday (Sujansky and Conradi, 1995). No potential subject had clinical signs without also having had a positive MRI. Clinical and imaging characteristics of the subjects are listed in Tables 1, 2 and 3.
Table 1.
Clinical characteristics of the Initial Cohort. Neurological signs in the table refer to those at the maximum follow-up
| Subject | Group | Sex | Age at EEG |
Age at Follow- Up |
Neurological Signs | MRI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Seizures (onset) |
Dev Delay |
Stroke-Like Episodes |
Hemiparesis | Visual Field Cut | ||||||
| I-1 | NO-SWS | M | 2 mo. | 5.5 yrs. | No | No | No | No | No | Negative at 15 mos. |
| I-2 | NO-SWS | M | 5 mo. | 4 yrs. | No | No | No | No | No | Negative at 13 mos. |
| I-3 | NO-SWS | M | 3 mos. | 4 yrs. | No | No | No | No | No | Negative at 6 mos. |
| I-4 | NO-SWS | M | 2 mos. | 3.5 yrs. | No | No | No | No | No | Negative at birth |
| I-5 | SWS | M | 11 mos. | 6.5 yrs. | Yes (8 mos.) | Yes | Yes | No | No | Positive at 9 mos. |
| I-6 | SWS | F | 1 mo. | 4.5 yrs. | Yes (2 yrs.) | Yes | Yes | No | No | Negative at birth; Positive at 7 mos. |
| I-7 | SWS | M | 4 mos. | 3 yrs | Yes (3.5 mos.) | Yes | No | Yes | Yes | Positive at 1 mo. |
| I-8 | SWS | M | 3 mos. | 3 yrs | Yes (2 mos.) | Yes | Yes | Yes | Yes | Positive at birth |
Table 2.
Clinical characteristics of the Validation Cohort. Neurological signs in the table refer to those at the maximum follow-up
| Subject | Group | Sex | Age at EEG |
Age at Follow- Up |
Neurological Signs | MRI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Seizures (onset) |
Dev Delay |
Stroke-Like Episodes |
Hemiparesis | Visual Field Cut | ||||||
| V-1 | NO-SWS | F | 4 mos. | 40 mos. | No | No | No | No | No | Negative at 4 mos. |
| V-2 | NO-SWS | M | 5 mos. | 37 mos. | No | No | No | No | No | Negative at 6 mos. |
| V-3 | NO-SWS | M | 4 mos. | 26 mos. | No | No | No | No | No | Negative at 19 mos. |
| V-4 | NO-SWS | F | 1 wk. | 24 mos. | No | No | No | No | No | Negative at 8 mos. |
| V-5 | SWS | M | 8 mos. | 36 mos. | Yes (4 mos.) | No | No | No | No | Positive at 4 mos. |
| V-6 | SWS | M | 5 mos. | 17 mos. | Yes (10 wks.) | Yes | No | Yes | Yes | Positive at 3 mos. |
| V-7 | SWS | F | 9 mos. | 32 mos. | No | Yes | No | Yes | Yes | Positive at 8 mos. |
| V-8 | SWS | M | 8 mos. | 24 mos. | Yes (4 mos.) | Yes | No | Yes | Yes | Positive at 4 mos. |
| V-9 | SWS | F | 2 mos. | 16 mos. | No | No | No | No | No | Positive at 1 mo. |
Table 3.
MRI involvement for affected (SWS) subjects from both cohorts. MRIs reported are those performed closest in time to the qEEG recording. MRIs are graded per Jansen (2002), as modified in Hatfield (2007). Scores for each hemisphere range from 4-16 and reflect the sum of the scores in each of the four lobes. Lobar scoring ranges from 1 (no involvement) to 4 (severe involvement: angiomatosis + severe atrophy). Scores are reportedly for the hemisphere ipsilateral to the maximal extent of the PW birthmark and contralateral to the maximal PW birthmark
| Subject | Age at MRI | Bilateral MRI Involvement? |
MRI Involvement (ipsilateral) |
MRI Involvement (contralateral) |
|---|---|---|---|---|
| I-5 | 9 mos. | No | 7 | 4 |
| I-6 | Birth | No | 4 | 4 |
| I-6 | 7 mos. | No | 10 | 4 |
| I-7 | 1 mo. | No | 10 | 4 |
| I-7 | 8 mos | No | 11 | 4 |
| I-8 | Birth | No | 11 | 4 |
| V-5 | 4 mos. | No | 8 | 4 |
| V-5 | 21 mos. | No | 10 | 4 |
| V-6 | 3 mos. | Yes | 15 | 7 |
| V-7 | 8 mos. | No | 8 | 4 |
| V-8 | 4 mos. | Yes | 13 | 7 |
| V-9 | Birth | No | 7 | 4 |
| V-9 | 5 mos. | Yes | 15 | 13 |
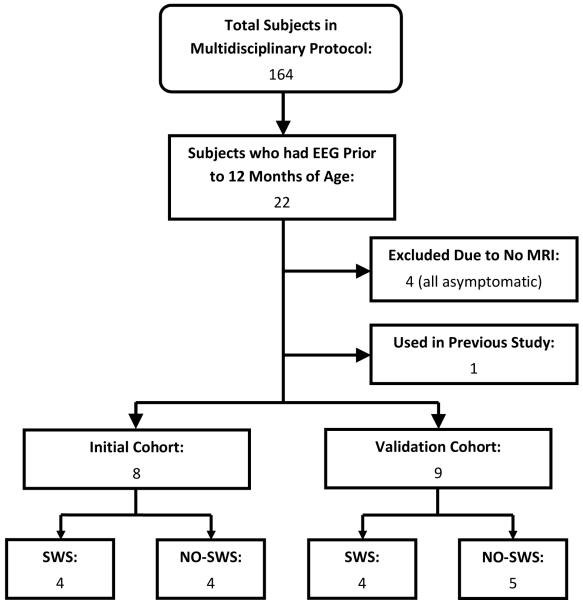
At a certain point in time, we reviewed the EEGs which had been collected to date (4 in meeting criteria for the SWS group and 4 meeting criteria for the NO-SWS group), in order to find a qEEG threshold that differentiates between the two groups. These 8 subjects are considered to be in the “Initial” cohort. Subjects whose EEGs were recorded after this threshold was developed (or who met inclusion criteria after the threshold was developed) were included in the “Validation” cohort. There were 5 SWS participants and 4 NO-SWS participants in the Validation cohort (Figure 2).
Figure 2.
Flow diagram of subject recruitment and categorization
The first technically adequate EEG from each subject was used for the analysis. In 2 cases (both in the Initial cohort), the second EEG collected had to be used for the analysis because the first EEG did not contain sufficient artifact-free recording to allow for selection of the required number of artifact-free epochs. The second EEG was collected 4 months after the first, inadequate EEG in 1 case and less than a month after the first EEG in the other case. MRIs were done per routine clinical care and not at predetermined ages.
2.2 Clinical Examinations
Clinical examinations were performed every 3 months. All examinations were performed by the pediatric neurologist who is the director of the Sturge-Weber Center (AMC). Her initial exam was performed before the collection of the EEG, but she was aware of the qEEG results and standard clinical EEG interpretation before the time of the subsequent examinations. History focused on seizures, developmental delay or stroke-like episodes. Physical exam focused on visual fields and signs of hemiparesis.
2.3 EEG Recording
Each subject had a 30-40 minute, 16-channel scalp EEG, collected in the standard clinical fashion, using a clinical EEG machine (Bio-logic Systems Corp., Mundelein, IL), with International 10-20 electrode placement and 256 Hz sampling rate. A pediatric electroencephalographer (EHK) produced a standard clinical visual interpretation for each EEG. This reader was aware of the study’s purpose, was not specifically blinded to either the side of PW birthmark or the patient’s group status and was in fact the attending epileptologist for some of the subjects. We therefore cannot rule out that this clinical knowledge influenced the standard visual EEG interpretation. Over the course of the study, we began to make an explicit effort to record sleep EEGs to reduce the burden of muscle, movement and electrode artifact.
2.4 qEEG Analysis
The EEG was reviewed in a bipolar longitudinal montage (display filters: high-pass time constant = 0.16 sec; low-pass = 35 Hz; notch = 60 Hz) by a research assistant. Research assistants were not specifically trained in EEG interpretation but were taught to look for several common artifacts. A research assistant selected 30 epochs of 2-second duration each from each record for analysis. The epoch length and number of epochs were chosen based on experience attempting to collect segments of artifact-free data, particularly from waking recordings of older infants. Initial analysis of the EEGs for both the Initial cohort and the Validation cohort employed several different research assistants who were not necessarily blinded to the patient’s group status or side of involvement. We subsequently repeated the qEEG analysis for the Validation cohort by using a single research assistant who was blinded to subjects’ group identity and side of PW birthmark.
Epochs were selected based on absence of visible artifact and transient phenomena (e.g., epileptiform spikes and vertex waves). The subject state (asleep vs. awake) varied from record to record: in the Initial cohort, 1 EEG was recorded during sleep while 7 were recorded during waking; in the Validation cohort, 8 were recorded during sleep, while 1 was recorded during waking. Channels with persistent artifact were excluded from the analysis (as was the symmetrically contralateral channel). A commercial analysis program (Prism:Spectrum, Persyst Development Corp, Prescott, Arizona) performed Fast Fourier Transform on unfiltered EEG data. This software first applies DC detrending to each epoch and calculates power values (power = square of the amplitude, in units of μV 2) for each bipolar channel × each epoch, with frequency resolution of 0.5 Hz. The frequency spectra were not smoothed.
For each epoch, power values from the 0.5 Hz bands were summed into classical EEG bands: delta (2.0 to 3.5 Hz), theta (4.0 to 7.5 Hz), alpha (8.0 to 12.5 Hz), beta (13.0 to 32.0 Hz) and total (2.0 to 32.0 Hz). These bands represent the classically accepted scalp EEG bandwidth.
We calculated a mean Laterality Score (LSb) (Hatfield et al., 2007) for each band, averaged over 30 epochs and included channel pairs by the equation
where b is the band (delta, theta, alpha, beta, total), e is the epoch (1 to 30) and c is the channel pair. Within the channel pair c, the index c-ipsi refers to the single channel that is ipsilateral to the PW birthmark, while c-contra refers to the channel from pair c that is contralateral to the side of the PW birthmark. N represents the total number of included channel pairs. In the “Whole Hemisphere” analysis, N=8 channel pairs were included (Fp1-F7/Fp2-F8, F7-T3/F8-T4, T3-T5/T4-T6, T5-O1/T6-O2, Fp1-F3/Fp2-F4, F3-C3/F4-C4, C3-P3/C4-P4, and P3-O1/P4-O2), minus those excluded due to artifact. In the “Occipital” analysis, N=2 channel pairs (T5-O1/T6-O2 and P3-O1/P4-O2), if both were artifact-free. In cases where both the T5-O1/T6-O2 and P3-O1/P4-O2 channel pairs were contaminated with persistent artifact, the “Occipital” analysis was not performed. The occipital analysis was proposed because SWS that includes facial port wine birthmark almost invariably involves the occipital lobes, whether or not the other lobes are affected.
Laterality Scores less than zero indicate lower power on the side ipsilateral to the PW birthmark. In subjects who had a bilateral PW birthmark, the side of maximal involvement was used (or the side of known involvement by MRI findings, where present).
We reviewed the qEEG data from the Initial cohort and empirically derived an optimal discriminative threshold that separated subjects in the SWS group (i.e., those diagnosed with SWS) from those in the NO-SWS group. We subsequently validated this threshold by demonstrating its ability to discriminate between those in SWS vs. NO-SWS groups in the separate Validation cohort. Additionally, we quantified the EEGs of the Initial cohort with the original BSI (van Putten et al., 2004), the “extended” sBSI (van Putten, 2006) and the revised r-sBSI (van Putten, 2007) to attempt to find a discriminative metric, in the spectral range of 2-15 Hz.
2.5 MRI Scoring
Clinical MRIs were reviewed by a single neuroradiologist (DDML) who was blinded to diagnosis. For individuals in the SWS group (in both Initial and Validation cohorts), the MRI obtained closest in time to the date of the EEG recording was also scored via the system recorded in Hatfield (2007), which was a modification of a previous system of scoring angiomatosis and atrophy on MRI (Jansen et al., 2002).
2.6 Statistics
Statistical significance was set a level of p < 0.05. Comparison of age between SWS and NO-SWS group for each cohort were done with a two-tailed Student’s t-test in SPSS version 15.0 (SPSS, Chicago, IL).
3. Results
3.1 Initial Cohort
3.1.1 Participants
A total of 8 subjects met criteria for either the SWS group or NO-SWS group at the time that the qEEG threshold was being developed. These participants were included in the Initial cohort: 4 in the SWS group and 4 in the NO-SWS group. Characteristics of the participants in the Initial cohort are listed in Table 1. There was no statistical difference in age at EEG between the two groups (mean ± S.D. age for NO-SWS = 3 ± 1.4 mos.; SWS = 4.75 ± 4.3 mos.; p = 0.47). No participant from the NO-SWS group has developed signs or symptoms consistent with SWS brain involvement to date since the Initial analysis (mean age at maximum follow-up = 4.25 years; range = 3.5-5.5 years). MRIs were performed prior to the EEG in 5 subjects (all 4 in the SWS group and 1 of 4 in the NO-SWS group). One SWS subject (#I-6) had a negative MRI prior to the EEG but subsequently had a positive MRI. All potential subjects who had neurological signs also had at least one positive MRI. One subject had an EEG collected for a previous study, and his qEEG were not reviewed for the development of the threshold.
Table 3 reports the degree of MRI involvement in the affected subjects, as taken from the MRIs performed closest in time to the qEEG recording. All subjects were mild or moderately involved and had only unilateral involvement. An example of a MRI of an affected child is shown in Figure 3. All SWS participants had partial seizures.
Figure 3.

Three month-old boy with bilateral intracranial involvement of SWS, right greater than left. Axial T2-weighted images (top panel) show moderate to marked right and mild left cerebral atrophy. Post-Gadolinium T1-weighted images (bottom panel) demonstrate leptomeningeal angiomatosis in all lobes of the right hemisphere as well as left temporal and occipital lobes (see arrows, imaging score R:15; L:7).
3.1.2 qEEG Analysis
The mean LS results for the Initial cohort are described in Table 4. Using these data, we found a threshold that provided 100% discrimination between the SWS and NO-SWS groups: If any two bands in the “Whole Hemisphere” analysis or any two bands in the “Occipital” analysis had a mean LS ≤ -0.2, then the EEG is considered positive for SWS. A total of 10 channel pairs were excluded from all 10 EEGs; the “Occipital” analysis was not performed (due to exclusion of both occipital channel pairs) in 3 of 8 EEGs. We found decreased accuracy when attempting to rely on any single band. For example, a cut-off of LS ≤ -0.2 in Whole Hemisphere delta would create 2 false negatives. The same cut-off in Whole Hemisphere theta would also yield 2 false negatives; in Whole Hemisphere alpha, 2 false negatives; in Whole Hemisphere beta, 1false negative; and Whole Hemisphere total band, 2 false negatives. In this cohort, the Occipital analysis could not be explored in this way, as it was excluded in 3 subjects’ EEGs. None of the 3 BSI measures produced a clear threshold that allowed for discrimination of the 2 groups.
Table 4.
Quantitative EEG results for the Initial Cohort. Laterality Scores ≤ -0.2 are in bold
| Subject | Group | Age at EEG |
State | Channels Excluded |
Whole Hemisphere Analysis | Occipital Analysis | qEEG Interpretation |
Clinical EEG |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta | Theta | Alpha | Beta | Total | Delta | Theta | Alpha | Beta | Total | |||||||
| I-1 | NO-SWS | 1 mo. | Awake | 1 | 0.045 | 0.056 | 0.039 | 0.095 | 0.046 | 0.093 | 0.072 | 0.14 | 0.46 | 0.096 | Symmetric | Normal |
| I-2 | NO-SWS | 5 mo. | Awake | None | -0.17 | -0.13 | -0.14 | -0.066 | -0.16 | -0.18 | -0.046 | -0.11 | 0.10 | -0.16 | Symmetric | Asymmetric |
| I-3 | NO-SWS | 3 mos. | Asleep | 2 | -0.017 | -0.034 | -0.019 | -0.080 | -0.018 | † | † | † | † | † | Symmetric | Asymmetric |
| I-4 | NO-SWS | 2 mos. | Awake | 3 | 0.015 | 0.10 | -0.0054 | -0.13 | 0.038 | † | † | † | † | † | Symmetric | Normal |
| I-5 | SWS | 11 mos. | Awake | 2 | -0.22 | -0.20 | -0.19 | -0.23 | -0.23 | † | † | † | † | † | Asymmetric | Normal |
| I-6 | SWS | 1 mo. | Awake | None | -0.10 | -0.11 | -0.082 | 0.13 | -0.11 | -0.51 | -0.55 | -0.42 | 0.041 | -0.53 | Asymmetric | Normal |
| I-7 | SWS | 4 mos. | Awake | None | -0.026 | -0.089 | -0.20 | -0.84 | -0.057 | -0.17 | -0.31 | -0.40 | -0.23 | -0.23 | Asymmetric | Normal |
| I-8 | SWS | 3 mos. | Awake | 2 | -0.21 | -0.35 | -0.20 | -0.34 | -0.25 | -0.29 | -0.64 | -0.55 | -0.50 | -0.36 | Asymmetric | Asymmetric |
indicates that the channels that constitute the Occipital Analysis were excluded.
3.1.3 Standard Visual EEG Interpretation Results
Standard clinical visual interpretation of EEG had a sensitivity of 25% (1/4) and a specificity of 50% (2/4) in the Initial cohort. One NO-SWS EEG was read as abnormal due to asymmetry of spindles; the other had asymmetry of hypnapompic hypersynchrony. The SWS EEG read as asymmetrical had ipsilaterally decreased voltage of the posterior basic rhythm and reduced normal background theta activity. No EEG in this study had epileptiform discharges (spikes or sharp waves).
3.2 Validation Cohort
3.2.1 Participants
A total of 9 eligible subjects were recruited into the Validation cohort. This cohort consisted of 5 subjects in the SWS group and 4 subjects in the NO-SWS group. After the threshold was developed, all subjects in the larger multidisciplinary study who subsequently met inclusion criteria for either the SWS or NO-SWS group were included in the Validation cohort. In 7 cases, the EEGs were collected after the threshold was developed. Two subjects (V-1 and V2) had EEGs collected prior to the development of the threshold, but they were not included in the Initial cohort or analyzed for the development of the threshold because they had not yet had sufficient follow-up to meet criteria for the NO-SWS group.
All 5 SWS subjects had positive MRIs prior to the recording of the EEG; all 4 NO-SWS subjects had MRIs performed after the EEG was recorded. Characteristics of the participants in the Validation cohort are listed in Table 2. Although there was a trend toward the babies in the SWS group being older than those in the NO-SWS group, we did not find a statistical difference (mean ± S.D. age for NO-SWS = 3.2 ± 2.2 mos.; SWS = 6.4 ± 2.9 mos.; p = 0.12). No participant from the NO-SWS group has developed signs/symptoms consistent with SWS since he/she was first entered into the NO-SWS group (mean age at maximum follow-up = 31.75 mos.; range = 24-40 mos.). Two subjects in the SWS group (Subjects #V-7, V-9) were asymptomatic at the time of the EEG, though both were already known to have a positive MRI. Table 3 reports the degree of MRI involvement in the affected subjects, as taken from the MRIs performed closest in time to the qEEG recording. Involvement ranged from mild to severe and was both unilateral and bilateral. All potential subjects who had neurological signs also had at least one positive MRI. All subjects who had seizures had partial seizures.
3.2.2 qEEG Analysis
The cut-off established in the Initial cohort was applied to the Validation cohort. Results are contained in Table 5. The threshold provided 100% discrimination between the two groups (Figure 4). When the selection of epochs was repeated by a single research assistant who was blinded to the subjects’ side of PW birthmark and group status (i.e., SWS vs. NO-SWS), there was only one EEG which was misclassified. When this subject’s qEEG analysis was initially done, the epochs were selected from waking, and no asymmetry was found. When the qEEG analysis was repeated, epochs were selected from the sleep portion from the record, and an asymmetry that was small in magnitude was found.
Table 5.
Quantitative EEG results for the Validation Cohort. Laterality Scores ≤ -0.2 are in bold
| Subject | Group | Age at EEG |
State | Channels Excluded |
Whole Hemisphere Analysis | Occipital Analysis | qEEG Interpretation |
Clinical EEG |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta | Theta | Alpha | Beta | Total | Delta | Theta | Alpha | Beta | Total | |||||||
| V-1 | NO-SWS | 4 mos. | Asleep | None | 0.082 | 0.071 | 0.055 | 0.043 | 0.082 | 0.20 | 0.16 | 0.16 | 0.18 | 0.20 | Symmetric | Normal |
| V-2 | NO-SWS | 5 mos. | Awake | None | -0.068 | -0.22 | 0.078 | 0.067 | -0.0008 | -0.096 | -0.20 | 0.18 | -0.11 | -0.15 | Symmetric* | Normal |
| V-3 | NO-SWS | 4 mos. | Asleep | None | -0.044 | -0.053 | 0.00093 | -0.029 | -0.042 | -0.11 | -0.19 | -0.13 | -0.11 | -0.13 | Symmetric | Normal |
| V-4 | NO-SWS | 1 wk. | Asleep | None | 0.027 | 0.011 | 0.027 | -0.026 | 0.026 | -0.016 | -0.092 | -0.092 | -0.078 | -0.049 | Symmetric | Normal |
| V-5 | SWS | 8 mos. | Asleep | None | -0.20 | -0.30 | -0.35 | -0.29 | -0.24 | -0.21 | -0.42 | -0.47 | -0.37 | -0.29 | Asymmetric | Asymmetric |
| V-6 | SWS | 5 mos. | Asleep | None | -0.19 | -0.16 | -0.18 | -0.18 | -0.19 | -0.24 | -0.14 | -0.21 | -0.38 | -0.22 | Asymmetric | Asymmetric |
| V-7 | SWS | 9 mos. | Asleep | 1 | -0.11 | -0.28 | -0.25 | -0.21 | -0.19 | -0.025 | -0.24 | -0.23 | -0.1 | -0.13 | Asymmetric | Asymmetric |
| V-8 | SWS | 7 mos. | Asleep | None | -0.28 | -0.41 | -0.44 | -0.34 | -0.34 | -0.15 | -0.2 | -0.19 | -0.14 | -0.17 | Asymmetric | Asymmetric |
| V-9 | SWS | 2 mo. | Asleep | None | -0.20 | -0.22 | -0.22 | -0.088 | -0.20 | 0.032 | -0.06 | -0.1 | 0.11 | 0.018 | Asymmetric | Normal |
indicates that this qEEG was interpreted as Asymmetrical when re-analyzed by a second researcher.
Figure 4.

This diagram shows the topographical distribution of power in each frequency band for subject V-5. Power is reduced on the left side. Note the different power scale for delta/theta vs. alpha/beta.
In the Validation cohort, there was a decrease in number of channels that needed to be excluded due to persistent artifact: only a single channel pair was excluded from all 9 of the EEGs. The “Occipital” analysis was able to be performed in all EEGs. This was due to the fact that we proactively attempted to record sleep EEGs in the babies of the Validation cohort (whereas we had attempted to record waking EEGs in the babies of the Initial cohort).
3.2.3 Standard Visual EEG Interpretation Results
Standard clinical interpretation had a sensitivity of 80% (4/5) and a specificity of 100% (4/4) in the Validation cohort. The SWS EEGs which were identified as asymmetric showed voltage asymmetry of the posterior basic rhythm, an asymmetry of normal background theta activity and asymmetric sleep spindles. No epileptiform activity was seen in any EEG in this cohort as well. The visual EEG interpretation was repeated by another fellowship-trained, board-certified electroencephalographer, with similar results.
4. Discussion
Our ultimate goal is to develop a clinically useful method for assigning risk of developing SWS brain involvement to babies born with facial PW birthmark, in order to target prophylactic interventions to babies at highest risk and to provide education for their parents, while providing reassurance to the parents of babies at lowest risk. At this time, the diagnosis of SWS is often made only when a child has already become symptomatic, however. MRI is the gold-standard neuroimaging technique to look for brain involvement, however the risks associated with repeated administration of sedation and contrast make serial MRI of the asymptomatic baby with PW birthmark impractical.
We hypothesize that a qEEG method may be a non-invasive, quick and relatively inexpensive method that can be repeated in order to permit optimal timing of MRI. This study builds on previous research by demonstrating that qEEG can discriminate between infants with SWS brain involvement and those who have a neurologically asymptomatic facial PW birthmark. Although this study does not (nor was designed to) demonstrate that qEEG is statistically better than a fellowship-trained pediatric electroencephalographer experienced in reading EEGs from individuals with SWS, we do note that the diagnostic accuracy of the clinical reads increased significantly from the Initial cohort to the Validation cohort, perhaps suggesting that accuracy in assessing these sorts of asymmetries by visual inspection comes with experience. An electroencephalographer with such qualifications and experience would not be available to most pediatricians who are seeing an infant with a PW birthmark, while this qEEG technique could be made widely available. In this technique, the only operator-dependent steps were the selection of epochs and the decision whether to exclude a channel with persistent artifact. This was accomplished by research assistants who were trained in recognition of artifact but who had no other formal EEG training. The use of research assistants helps demonstrate that this technique is not reliant on the same level of expertise that produced the standard clinical visual EEG interpretations in this study.
This study demonstrates the feasibility of a qEEG technique for discriminating reliably between infants with SWS vs. those with neurologically asymptomatic PW birthmarks. Our ultimate goal is to determine whether this technique can detect asymmetries before a patient becomes symptomatic, but the current study is limited by a dearth of infant subjects who were asymptomatic at the time of EEG but who later went on to develop either clinical or radiological signs of SWS. It remains to be seen whether qEEG changes can be detected before a patient becomes symptomatic and can be used to guide whom to image and when. The fact that 3 subjects had a positive qEEG prior to the development of symptoms provides at least a proof of principle that this may be the case.
Given that only a portion of at-risk babies with a PW birthmark develops the neurological involvement characteristic of SWS, it will require significantly larger numbers of subjects to power a study to look at the ability of qEEG to screen presymptomatically for brain involvement. To that end, our center has begun to collect EEGs performed at outside locations for analysis. In this way, we aim to have a sufficient sample to determine whether or not this technique will be helpful as a screening tool.
Although we currently conceive of qEEG’s potential as a “gatekeeper” to MRI, it is difficult to study these modalities together, e.g., with MRIs performed at predetermined ages. However, the performance of sedated MRIs with contrast is widely considered to be an unacceptable research risk in infants, and all MRIs in this study were preformed in the line of standard clinical care.
Given larger numbers of participants, we expect to be able to determine positive and negative predictive values for qEEG, as well as perhaps a graduated scale of risk (rather than a dichotomous scale). In order to make this technique widely available, we aim to automate the process as much as possible. We expect that the approach will be modified as we extend its utility to different age groups and clinical situations. We aim to modify the qEEG threshold to be used for following cerebral function for research and clinical purposes (e.g., to predict and evaluate response to neuroprotective interventions). Future work will address not only these issues, but will also look at serial data collected to chart the natural history of qEEG in SWS, to determine whether serial qEEG can predict clinical deterioration and to determine whether qEEG can be used as a marker of “brain health,” in situations such as the use of prophylactic interventions. This work may make use of statistical methods that can find efficient thresholds to differentiate between groups, such as Linear Discriminant Analysis, Bayesian Classifiers or Support Vector Machine.
In summary, quantitative EEG shows promise as an objective and measurable biomarker of neurological involvement in children with potential SWS brain involvement. A threshold that was developed in one cohort of infants with PW birthmark was validated on a second cohort of 9 infants. Further research involving infants who are negative at the time of qEEG but who later go on to develop signs SWS brain involvement will be needed to demonstrate qEEG as a valid predictor.
Acknowledgements/Financial Interests
This study was supported by grants from Hunter’s Dream for a Cure and by supplementary funds on NIH/NINDS Grant R01NS40596. We would also like to thank the Sturge-Weber Foundation for their support. We recognize, with great appreciation, the participation of our patients and their families. Finally, we would like to thank Laura Hatfield and Ronnie Abi-Raad for their contributions to data analysis. The authors have no additional financial interests to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Brenner RP, Sharbrough FW. Electroencephalographic evaluation in Sturge-Weber syndrome. Neurology. 1976;26:629–32. doi: 10.1212/wnl.26.7.629. [DOI] [PubMed] [Google Scholar]
- Comi AM. Advances in Sturge-Weber syndrome. Curr Opin Neurol. 2006;19:124–8. doi: 10.1097/01.wco.0000218226.27937.57. [DOI] [PubMed] [Google Scholar]
- Comi AM. Sturge-Weber syndrome and epilepsy: an argument for aggressive seizure management in these patients. Expert Rev Neurother. 2007;7:951–6. doi: 10.1586/14737175.7.8.951. [DOI] [PubMed] [Google Scholar]
- Hatfield LA, Crone NE, Kossoff EH, Ewen JB, Pyzik PL, Lin DD, et al. Quantitative EEG asymmetry correlates with clinical severity in unilateral Sturge-Weber syndrome. Epilepsia. 2007;48:191–5. doi: 10.1111/j.1528-1167.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218–22. [PubMed] [Google Scholar]
- Jansen FE, van Huffelen AC, Witkamp T, Couperus A, Teunissen N, Wieneke GH, et al. Diazepam-enhanced beta activity in Sturge Weber syndrome: its diagnostic significance in comparison with MRI. Clin Neurophysiol. 2002;113:1025–9. doi: 10.1016/s1388-2457(02)00105-0. [DOI] [PubMed] [Google Scholar]
- Jordan LC, Wityk RJ, Dowling MM, DeJong MR, Comi AM. Transcranial Doppler ultrasound in children with Sturge-Weber syndrome. J Child Neurol. 2008;23:137–43. doi: 10.1177/0883073807307079. [DOI] [PubMed] [Google Scholar]
- Mazereeuw-Hautier J, Syed S, Harper JI. Bilateral facial capillary malformation associated with eye and brain abnormalities. Arch Dermatol. 2006;142:994–8. doi: 10.1001/archderm.142.8.994. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E. Historical Aspects. In: Niedermeyer E, Silva FLd, editors. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 1–15. 2005. [Google Scholar]
- Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10:49–58. doi: 10.1177/088307389501000113. [DOI] [PubMed] [Google Scholar]
- Tallman B, Tan OT, Morelli JG, Piepenbrink J, Stafford TJ, Trainor S, et al. Location of port-wine stains and the likelihood of ophthalmic and/or central nervous system complications. Pediatrics. 1991;87:323–7. [PubMed] [Google Scholar]
- van Putten MJ. Extended BSI for continuous EEG monitoring in carotid endarterectomy. Clin Neurophysiol. 2006;117:2661–6. doi: 10.1016/j.clinph.2006.08.007. [DOI] [PubMed] [Google Scholar]
- van Putten MJ. The revised brain symmetry index. Clin Neurophysiol. 2007;118:2362–7. doi: 10.1016/j.clinph.2007.07.019. [DOI] [PubMed] [Google Scholar]
- van Putten MJ, Peters JM, Mulder SM, de Haas JA, Bruijninckx CM, Tavy DL. A brain symmetry index (BSI) for online EEG monitoring in carotid endarterectomy. Clin Neurophysiol. 2004;115:1189–94. doi: 10.1016/j.clinph.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Ville D, Enjolras O, Chiron C, Dulac O. Prophylactic antiepileptic treatment in Sturge-Weber disease. Seizure. 2002;11:145–50. doi: 10.1053/seiz.2001.0629. [DOI] [PubMed] [Google Scholar]