Abstract
Growth plate chondrocytes produce proteoglycan-rich type II collagen extracellular matrix (ECM). During cell maturation and hypertrophy, ECM is reorganized via a process regulated by 1α,25(OH)2D3 and involving matrix metalloproteinases (MMPs), including MMP-3and MMP-2. 1α,25(OH)2D3 regulates MMP incorporation into matrix vesicles (MVs), where they are stored until released. Like plasma membranes (PM), MVs contain the 1α,25(OH)2D3-binding protein ERp60, phospholipase A2 (PLA2), and caveolin-1, but lack nuclear vitamin D receptors (VDRs). Chondrocytes produce 1α,25(OH)2D3 (10−8M), which binds ERp60, activating PLA2, and resulting lysophospholipids lead MV membrane disorganization, releasing active MMPs. MV MMP-3 activates TGF-β1 stored in the ECM as large latent TGF-β1 complexes, consisting of latent TGF-β1 binding protein, latency associated peptide, and latent TGF-β1. Others have shown that MMP-2 specifically activates TGF-β2. TGF-β1 regulates 1α,25(OH)2D3-production, providing a mechanism for local control of growth factor activation. 1α,25(OH)2D3 activates PKCα in the PM via ERp60-signaling through PLA2, lysophospholipid production, and PLCβ. It also regulates distribution of phospholipids and PKC isoforms between MVs and PMs, enriching the MVs in PKCζ. Direct activation of MMP-3 in MVs requires ERp60. However, when MVs are treated with 1α,25(OH)2D3, PKCζ activity is decreased and PKCα is unaffected, suggesting a more complex feedback mechanism, potentially involving MV lipid signaling.
Keywords: 1α,25(OH)2D3; Matrix vesicles; Growth plate chondrocytes; Rapid actions; Matrix metalloproteinases; TGF-β1 activation; TGF-β2
INTRODUCTION
The post-fetal growth plate is a specialized cartilagenous structure that permits growth of bones by interstitial expansion of the extracellular matrix, even against gravity. The classic version of the growth plate is found in long bones. Chondrocytes at the top of the growth plate are surrounded by a proteoglycan-rich type II collagen extracellular matrix. This region is termed a resting zone (RC) or reserve zone. At the base of this zone, the chondrocytes undergo a set number of divisions (proliferating cell zone) and then enter a state of maturation (prehypertrophic cell zone) prior to becoming hypertrophic. In the upper hypertrophic cell zone, the chondrocytes begin to reorganize their extracellular matrix to facilitate their rapid increase in cell size. The large sulfated proteoglycan aggregates are degraded by specific enzymes, including matrix metalloproteinases (MMPs). Not only is matrix degraded, but a new matrix is produced that contains type X collagen. At the base of the prehypertrophic/hypertrophic cell zones (GC, growth zone), the extracellular matrix is calcified. Once this occurs, osteoclasts can resorb the calcified cartilage, permitting vascular ingrowth and bone formation.
These changes in matrix composition and accompanying changes in cell shape and function require complex regulation in time and space. Resting zone cells and growth zone cells not only produce different extracellular matrices, but they also control the synthesis, maturation and turnover of the matrix in distinctly different ways. In both zones, the chondrocytes must manage events in the matrix at sites that are distant from the cell itself. One mechanism that they use is to produce matrix processing enzymes as zymogens to be activated at the appropriate time by mechanisms that are not well understood [1]. A subset of these MMPs, including stromelysin-1 (MMP-3) and 72kD gelatinase (MMP-2), are packaged in extracellular matrix vesicles (MVs) [2].
MVs produced by resting zone cells contain neutral MMPs but the levels of these enzymes are lower than are found in MVs produced by growth zone cells [2]. In addition, the lipid composition of the MV membrane in cultures of resting zone cells differs from that of MVs in growth zone cells [3] and the activity of MV membrane enzymes like alkaline phosphatase and phospholipase A2 is lower [4], suggesting that the mechanisms that determine MV composition during the formation of the organelles differs between the two cells and that the mechanisms by which the cells control their behavior out in the extracellular matrix may differ as well.
ROLE OF VITAMIN D IN MATRIX SYNTHESIS AND GROWTH FACTOR ACTIVATION
Metabolites of vitamin D, 24,25(OH)2D3 and 1,25(OH)2D3, are important to the regulation of events in the extracellular matrix of the growth plate. 24R,25(OH)2D3 regulates matrix synthesis in the resting zone, including MV composition and production, whereas 1α,25(OH)2D3 regulates matrix synthesis and MV composition in growth zone cells [5–8]. These effects on matrix vesicle content are genomic. However, both vitamin D metabolites also elicit non genomic regulation of the organelles once they are in the matrix. For example, MVs treated directly with 1α,25(OH)2D3 and incubated in gelatin gels containing proteoglycan aggregate, are able to reduce proteoglycan-dependent inhibition of calcium phosphate deposition [9]. Because MVs do not contain DNA or RNA, there is no potential for new protein synthesis. Thus, any effect of 1α,25(OH)2D3 must occur via a non genomic pathway. This may explain the association of MVs with new mineral deposition in the lower hypertrophic zone of the growth plate. As will be shown below, both genomic and nongenomic mechanisms are involved in the role of the vitamin D metabolites in growth factor activation.
1α,25(OH)2D3 and 24R,25(OH)2D3 also differentially regulate transforming growth factor beta-1 (TGF-β1) availability in the extracellular matrix of their respective target cells. Growth plate chondrocytes synthesize TGF-β1 in latent form consisting of the heterodimeric protein linked to a latency associated peptide (LAP) [10]. This small latent complex is stored in the matrix as a large latent complex due to non-covalent interactions with latent TGF-β binding protein-1 (LTPB1). When rat costochondral growth plate cells are treated with 1α,25(OH)2D3, but not 24R,25(OH)2D3, there is a decrease in the amount of latent TGF-β in the conditioned media [10]. This is not due to an increase in active growth factor, however, suggesting that 1α,25(OH)2D3 is affecting storage of the latent form in the matrix. This has proved to be the case. 24R,25(OH)2D3 increases LTBP1 mRNA and protein levels in cultures of resting zone chondrocytes and 1α,25(OH)2D3 increases LTBP1 mRNA and protein levels in cultures of growth zone cells [10]. By controlling the amount of LTBP1, 1α,25(OH)2D3 and 24R,25(OH)2D3 control the amount of TGF-β1 present in the extracellular matrix for later activation and paracrine/autocrine regulation of the cells.
The ability to carefully modulate TGF-β availability is important for growth plate physiology. Chondrocytes in the post-fetal rat costochondral growth plate are particularly sensitive to TGF-β1, responding to concentrations that are one tenth those active in osteoblast cultures [11; 12]. Moreover, the effects of the growth factor vary over very small concentration gradients. At concentrations activity. At concentrations of 0.4 ng/ml, however, TGF-β1 increases chondrocyte proliferation. Interestingly, both TGF-β1 and 24R,25(OH)2D3 cause resting zone cells to acquire a growth zone phenotype with respect to 1α,25(OH)2D3 responsiveness [13], suggesting that they promote differentiation of of 0.1 to 0.2 ng/ml, TGF-β1 causes an increase in matrix synthesis and an increase in alkaline phosphatase these cells, although by different mechanisms. When used together, they cause a synergistic increase in alkaline phosphatase activity in resting zone cells [14], supporting this hypothesis. In contrast, when growth zone cells are treated with 1α,25(OH)2D3 and TGF-β1 together the effect of TGF-β1 predominates. Because the effect of TGF-β1 on alkaline phosphatase is less stimulatory than the effect of 1α,25(OH)2D3, these observations suggest that the growth factor promotes early differentiation events but retards the rate at which hypertrophic maturation of the growth zone can occur.
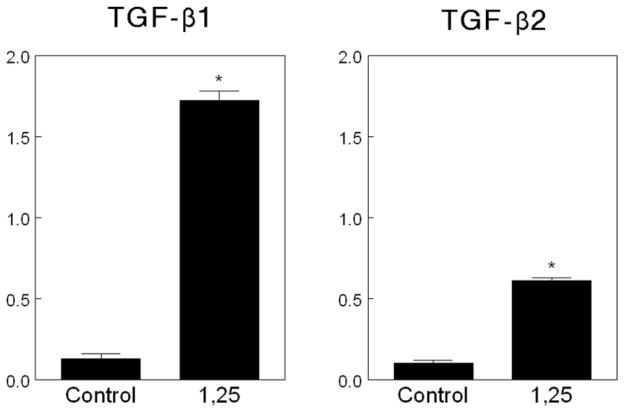
1α,25(OH)2D3 also plays a critical role in regulating the availability of active growth factor. Matrix vesicles are the key to this process through the action of two MV MMPs: MMP-3 and MMP-2. We have shown that when recombinant latent simian TGF-β1 or recombinant latent simian TGF-β2 are incubated directly with MVs in the presence of 1α,25(OH)2D3, active TGF-β1 and TGF-β2 are released [15] (Figure 1), indicating that MVs contain factors capable of removing LAP. These early studies indicated that decreased pH was not responsible since the experiments were conducted at neutral pH. Subsequently we showed that antibodies to MMP-3 blocked the activation of TGF-β1, but antibodies to MMP-2 had no effect on this isoform of the growth factor [16]. Others have now shown that MMP-2 specifically activates TGF-β2. We have subsequently confirmed that MMP-3 can release active TGF-β1 from the extracellular matrix produced by growth plate chondrocytes and that MVs do so via MMP-3 action [16]. Moreover, these studies show that a two step process is involved. Based on time course experiments, large latent complex is first released from the matrix making the small latent complex accessible to the enzyme, which can then act on the complex to release active growth factor [17].
Figure 1.
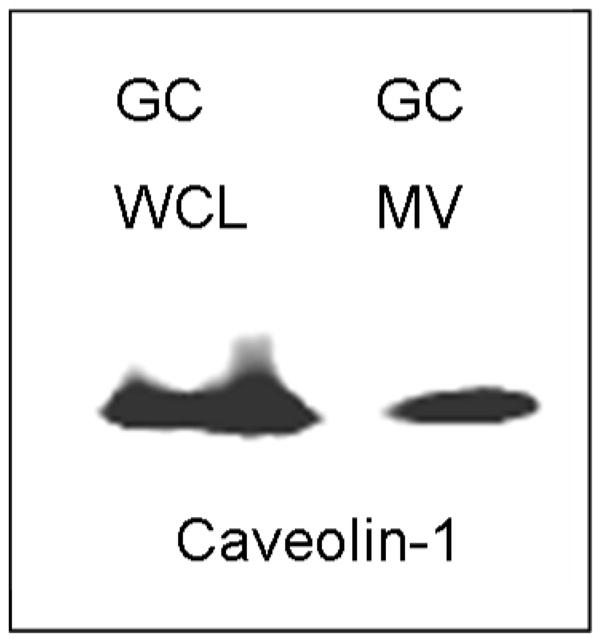
Matrix vesicles produced by rat costochondral growth zone chondrocyte cultures contain caveolin-1. Western blot showing immunoreactive caveolin-1 in whole cell lysates (WCL) and isolated matrix vesicles (MV).
Interestingly, 24R,25(OH)2D3 has no effect on the ability of MVs to activate either form of TGF-β, but when MVs produced by resting zone cells are treated directly with 1α,25(OH)2D3, they are able to release active TGF-β [15]. This is one of the few demonstrations of an effect of 1α,25(OH)2D3 on resting zone cells and suggests that the role of this metabolite in TGF-β activation is specific and receptor mediated. The fact that resting zone chondrocyte MVs are less potent activators is likely due to the lower levels of MMPs as well as to differences in the membrane composition of the extracellular organelles.
MECHANISM OF 1α,25(OH)2D3 ACTION IN THE EXTRACELLULAR MATRIX
We have used the rat costochondral cartilage as a model to investigate the mechanisms by which vitamin D metabolites regulate events in the growth plate. Our studies have shown that 1α,25(OH)2D3 and 24R,25(OH)2D3 elicit their effects on their target cells through traditional nuclear receptor pathways as well as through rapid signaling pathways involving protein kinase C (PKC) resulting in increased mitogen activated protein kinase (MAPK) activity, but different mechanisms are involved. These pathways have been reviewed in the literature [18–20] and are briefly outlined here.
1α,25(OH)2D3 increases PKCα activity via activation of PLA2. This is dependent on the presence of a membrane associated 1α,25(OH)2D3-binding protein ERp60 based on antibody blocking studies [21] and on the presence of phospholipase A2 activating protein [22]. Arachidonic acid released by the action of PLA2 serves as a substrate for cyclooxygenase-1, producing prostaglandin the acts via its EP1 receptor to increase PKC. The arachidonic acid also acts as a co-factor for PKCα directly, and it can contribute to the 1α,25(OH)2D3-dependent changes in the cells by binding its own nuclear receptors. In addition, the lysophospholipid that is produced also participates in the mechanism by increasing PLC activity, resulting in production of diacylglycerol (DAG) and inositol-1,4,5 trisphosphate (IP3). DAG bind PKCα and translocates it to the plasma membrane while IP3 binds to its receptors on the endoplasmic reticulum increasing intracellular Ca++ that is a necessary co-factor for PKCα.
24R,25(OH)2D3 stimulates PKCα in resting zone chondrocytes via a phospholipase D (PLD) dependent pathway which does not involve ERp60 [23]. This also results in production of DAG, but via a two-step process, and peak activation occurs later than is seen in 1α,25(OH)2D3 treated growth zone cells. PLA2 and PLC are not involved either in the activation of PKC or in the activation of PKC-dependent MAPK.
1α,25(OH)2D3 and 24R,25(OH)2D3 also regulate PKC activity in matrix vesicles, both during MV biogenesis and through direct action on the MV membrane. When growth zone cells are treated with 1α,25(OH)2D3 or resting zone cells are treated with 24R,25(OH)2D3 for 24 hours, PKC activity in the extracellular organelles is increased due to the specific incorporation of PKC-zeta during production [24]. PKCζ is an atypical form of PKC, requiring neither lipid not Ca++ as co-factors. Although MV PKC activity is increased as a result of the presence of the enzyme, direct action of the metabolites on matrix vesicles produced by their target cells causes an inhibition of PKCζ activity specifically. Why this is the case is not clear; however, it suggested that the mechanisms by which these metabolites exert their direct effects on MVs may differ considerably from their mechanism of action on the cells, thereby providing a differential process for controlling events in the matrix at distant sites.
To test this hypothesis we have focused on the regulation of PKC in MVs produced by growth zone cells. These MVs are enriched in alkaline phosphatase and phospholipase A2 activities [4] and they contain ERp60 [25], as well as caveolin-1 (Figure 2), which has been shown to co-localize with ERp60 in growth zone cell membranes [19]. 1α,25(OH)2D3 directly causes an increase in MV PLA2 activity [5], resulting in production of arachidonic acid and lysophospholipids. These studies have shown that MV PKC activity can be increased by treatment with arachidonic acid [26], which is a cofactor for PKCα [27]. Low levels of PKCα are present in MV and may participate in the overall response through this mechanism. Recently, we showed that lysophospholipids produced as a result of the activation of PLA2 also participate in the release and activation of latent TGF-β1 [28]. This effect is most likely due to their detergent properties, either by causing loss of MV membrane integrity and release of MMP-3 or by disrupting the three dimensional structure of the extracellular matrix, making the small latent complex more accessible.
Figure 2.
Effect of 1α,25(OH)2D3 on matrix vesicle dependent activation of TGF-β1 and TGF-β2 from recombinant simian TGF-β1 and recombinant simian TGF-β2, respectively. Matrix vesicles were isolated from cultures of rat costochondral growth zone chondrocytes and incubated directly with latent growth factor in the presence of 10−9 M 1α,25(OH)2D3. Active TGF-β in the samples was measured by ELISA specific for the active form of the growth factor.
The pivotal role of phospholipase A2 in 1α,25(OH)2D3-dependent activation of TGF-β1 was shown in a series of experiments using inhibitors of this enzyme [16]. When phospholipase A2 activity was inhibited, MVs were not able to activate latent TGF-β1, even when treated with 1α,25(OH)2D3. This effect of 1α,25(OH)2D3 also depended on the presence of the membrane associated receptor ERp60. Antibodies to ERp60 blocked the activation of the growth factor by MVs from growth zone chondrocytes. This supports the hypothesis that phospholipase A2 activation is mediated by ERp60, as it is in the cell membranes. However, in the cell the downstream consequence of this activation is an increase in PKCα. Thus, it is not likely that PKCζ is playing a role in the activation of TGF-β1 by MVs.
Although 24R,25(OH)2D3 does not participate directly in the activation of TGF-β1, it does have an indirect effect on the process by regulating local production of 1,25(OH)2D3. Both resting zone and growth zone cells possess enzymes involved in metabolism of 25(OH)D3 and produce and secrete 24R,25(OH)2D3 and 1α,25(OH)2D3 into their extracellular environment at levels as high as 10−7 and 10−8 M, respectively [29]. 24R,25(OH)2D3 regulates production of 1,25(OH)2D3 via activation of the 1-hydroxylase [30] and 1α,25(OH)2D3 regulates production of 24,25(OH)2D3 via transcriptional regulation of the 24-hydroxylase [30]. Interestingly, TGF-β1 also regulates activity of these enzymes [29], providing a mechanism for feed-back regulation of MV-dependent TGF-β1 activation.
SUMMARY
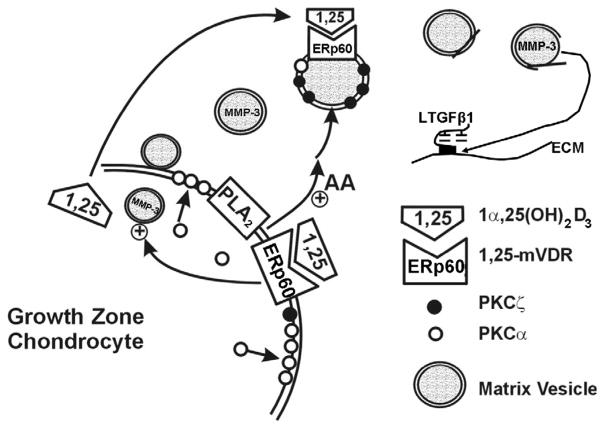
The work described here shows that growth plate chondrocytes are able to control events in their extracellular matrix through multiple pathways involving the vitamin D metabolites 1α,25(OH)2D3 and 24R,25(OH)2D3. These pathways, shown in Figure 3, include cell maturation specific actions of the metabolites on extracellular matrix synthesis and turnover, including production of matrix vesicles. Regulated secretion of 1α,25(OH)2D3 and 24R,25(OH)2D3 provides a mechanism for nongenomically controlling MV function. This will have consequences for matrix composition, including release and activation of latent growth factors like TGF-β1 and TGF-β2. Thus, by their inter-related actions as paracrine and autocrine regulators of growth plate chondrocyte function, 1α,25(OH)2D3 and 24R,25(OH)2D3 can fine tune the rate and extent of chondrocyte proliferation and hypertrophy.
Figure 3.
Schematic showing the formation of MMP-3 and PKCζ-enriched matrix vesicles via genomic regulation by 1α,25(OH)2D3. Once in the matrix, 1α,25(OH)2D3 (1,25) acts directly on matrix vesicles through an ERp60 mediated pathway resulting in increased phospholipase A2 (PLA2) activity and subsequent hydrolysis of matrix vesicle phospholipids, production of lysophospholipids and arachidonic acid, and loss of matrix vesicle membrane integrity. Hydroxyapatite crystals form within the matrix vesicle during this time. Breakdown of the vesicle membrane results in release of matrix vesicle MMP-3, which then acts on the matrix to release large latent TGF-β1 from the extracellular matrix and ultimately to release active TGF-β1 from the small latent growth factor. TGF-β1 can then act back on the cell to regulate formation and release of 1α,25(OH)2D3. 1α,25(OH)2D3 can also activate plasma membrane PLA2 via ERp60, and released arachidonic acid may also participate in the activation of matrix vesicle PKC.
Acknowledgments
This work has been funded by the National Institutes of Health, the National Science Foundation, the Georgia Research Alliance and the Price Gilbert, Jr. Foundation. The authors thank the students and staff that have contributed to these studies over the past 20 years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sylvia VL, Schwartz Z, Ellis EB, Helm SH, Gomez R, Dean DD, Boyan BD. Nongenomic regulation of protein kinase C isoforms by the vitamin D metabolites 1α,25-(OH)2D3 and 24R,25-(OH)2D3. J Cell Physiol. 1996;167:380–393. doi: 10.1002/(SICI)1097-4652(199606)167:3<380::AID-JCP2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Dean DD, Schwartz Z, Muniz OE, Gomez R, Swain LD, Howell DS, Boyan BD. Matrix vesicles are enriched in metalloproteinases that degrade proteoglycans. Calcif Tissue Int. 1992;50:342–349. doi: 10.1007/BF00301632. [DOI] [PubMed] [Google Scholar]
- 3.Boyan BD, Schwartz Z, Swain LD, Carnes DL, Jr, Zislis T. Differential expression of phenotype by resting zone and growth region costochondral chondrocytes in vitro. Bone. 1988;9:185–194. doi: 10.1016/8756-3282(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz Z, Boyan BD. The effects of vitamin D metabolites on phospholipase A2 activity of growth zone and resting zone cartilage cells in vitro. Endocrinology. 1988;122:2191–2198. doi: 10.1210/endo-122-5-2191. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz Z, Schlader DL, Swain LD, Boyan BD. Direct effects of 1,25-dihydroxyvitamin D3 and 24,25- dihydroxyvitamin D3 on growth zone and resting zone chondrocyte membrane alkaline phosphatase and phospholipase-A2 specific activities. Endocrinology. 1988;123:2878–2884. doi: 10.1210/endo-123-6-2878. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz Z, Schlader DL, Ramirez V, Kennedy MB, Boyan BD. Effects of vitamin D metabolites on collagen production and cell proliferation of growth zone and resting zone cartilage cells in vitro. J Bone Miner Res. 1989;4:199–207. doi: 10.1002/jbmr.5650040211. [DOI] [PubMed] [Google Scholar]
- 7.Swain LD, Schwartz Z, Boyan BD. Regulation of matrix vesicle phospholipid metabolism is cell maturation-dependent. Bone Miner. 1992;17:192–196. doi: 10.1016/0169-6009(92)90735-v. [DOI] [PubMed] [Google Scholar]
- 8.Sylvia VL, Schwartz Z, Holmes SC, Dean DD, Boyan BD. 24,25-(OH)2D3 regulation of matrix vesicle protein kinase C occurs both during biosynthesis and in the extracellular matrix. Calcif Tissue Int. 1997;61:313–321. doi: 10.1007/s002239900341. [DOI] [PubMed] [Google Scholar]
- 9.Boskey AL, Boyan BD, Doty SB, Feliciano A, Greer K, Weiland D, Swain LD, Schwartz Z. Studies of matrix vesicle-induced mineralization in a gelatin gel. Bone Miner. 1992;17:257–262. doi: 10.1016/0169-6009(92)90747-2. [DOI] [PubMed] [Google Scholar]
- 10.Pedrozo HA, Schwartz Z, Mokeyev T, Ornoy A, Xin-Sheng W, Bonewald LF, Dean DD, Boyan BD. Vitamin D3 metabolites regulate LTBP1 and latent TGF-β1 expression and latent TGF-β1 incorporation in the extracellular matrix of chondrocytes. J Cell Biochem. 1999;72:151–165. [PubMed] [Google Scholar]
- 11.Sylvia VL, Mackey S, Schwartz Z, Shuman L, Gomez R, Boyan BD. Regulation of protein kinase C by transforming growth factor-beta-1 in rat costochondral chondrocyte cultures. J Bone Miner Res. 1994;9:1477–1487. doi: 10.1002/jbmr.5650090921. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz Z, Bonewald LF, Caulfield K, Brooks B, Boyan BD. Direct effects of transforming growth factor-beta on chondrocytes are modulated by vitamin D metabolites in a cell maturation-specific manner. Endocrinology. 1993;132:1544–1552. doi: 10.1210/endo.132.4.8462452. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz Z, Dean DD, Walton JK, Brooks BP, Boyan BD. Treatment of resting zone chondrocytes with 24,25-dihydroxyvitamin D3 [24,25-(OH)2D3] induces differentiation into a 1,25-(OH)2D3-responsive phenotype characteristic of growth zone chondrocytes. Endocrinology. 1995;136:402–411. doi: 10.1210/endo.136.2.7530645. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz Z, Sylvia VL, Dean DD, Boyan BD. The synergistic effect of TGFβ and 24,25-(OH)2D3 on resting zone chondrocytes is metabolite-specific and mediated by protein kinase C. Connect Tissue Res. 1996;35:101–106. doi: 10.3109/03008209609029180. [DOI] [PubMed] [Google Scholar]
- 15.Boyan BD, Schwartz Z, Park-Snyder S, Dean DD, Yang F, Twardzik D, Bonewald LF. Latent transforming growth factor-β is produced by chondrocytes and activated by extracellular matrix vesicles upon exposure to 1,25-(OH)2D3. J Biol Chem. 1994;269:28374–28381. [PubMed] [Google Scholar]
- 16.Maeda S, Dean DD, Gay I, Schwartz Z, Boyan BD. Activation of latent transforming growth factor beta1 by stromelysin 1 in extracts of growth plate chondrocyte-derived matrix vesicles. J Bone Miner Res. 2001;16:1281–1290. doi: 10.1359/jbmr.2001.16.7.1281. [DOI] [PubMed] [Google Scholar]
- 17.Maeda S, Dean DD, Gomez R, Schwartz Z, Boyan BD. The first stage of transforming growth factor β1 activation is release of the large latent complex from the extracellular matrix of growth plate chondrocytes by matrix vesicle stromelysin-1 (MMP-3) Calcif Tissue Int. 2002;1:54–65. doi: 10.1007/s002230010032. [DOI] [PubMed] [Google Scholar]
- 18.Boyan BD, Jennings EG, Wang L, Schwartz Z. Mechanisms Regulating Differential Activation of Membrane-mediated Signaling by 1α,25(OH)2D3 and 24R,25(OH)2D3. In: Feldman D, Glorieux FH, Pike JW, editors. Vitamin D. Academica Press; San Diego, CA: 2004. [DOI] [PubMed] [Google Scholar]
- 19.Boyan BD, Jennings EG, Wang L, Schwartz Z. Mechanisms regulating differential activation of membrane-mediated signaling by 1alpha, 25(OH)2D3 and 24R,25(OH)2D3. J Steroid Biochem Mol Biol. 2004;89–90:309–315. doi: 10.1016/j.jsbmb.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Boyan BD, Schwartz Z. Rapid vitamin D-dependent PKC signaling shares features with estrogen-dependent PKC signaling in cartilage and bone. Steroids. 2004;69:591–597. doi: 10.1016/j.steroids.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Nemere I, Farach-Carson MC, Rohe B, Sterling TM, Norman AW, Boyan BD, Safford SE. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc Natl Acad Sci USA. 2004;101:7392–7397. doi: 10.1073/pnas.0402207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz Z, Graham EJ, Wang L, Lossdorfer S, Gay I, Johnson-Pais TL, Carnes DL, Sylvia VL, Boyan BD. Phospholipase A2 activating protein (PLAA) is required for 1alpha,25(OH)2D3 signaling in growth plate chondrocytes. J Cell Physiol. 2005;203:54–70. doi: 10.1002/jcp.20212. [DOI] [PubMed] [Google Scholar]
- 23.Sylvia VL, Schwartz Z, Del Toro F, DeVeau P, Whetstone R, Hardin RR, Dean DD, Boyan BD. Regulation of phospholipase D (PLD) in growth plate chondrocytes by 24R,25(OH)2D3 is dependent on cell maturation state (resting zone cells) and is specific to the PLD2 isoform. Biochim Biophys Acta. 2001;1499:209–221. doi: 10.1016/s0167-4889(00)00120-8. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz Z, Sylvia VL, Larsson D, Nemere I, Casasola D, Dean DD, Boyan BD. 1α,25(OH)2D3 regulates chondrocyte matrix vesicle protein kinase C directly via G-protein dependent mechanisms and indirectly via incorporation of PKC during matrix vesicle biogenesis. J Biol Chem. 2003;5:11828–11837. doi: 10.1074/jbc.M110398200. [DOI] [PubMed] [Google Scholar]
- 25.Nemere I, Schwartz Z, Pedrozo H, Sylvia VL, Dean DD, Boyan BD. Identification of a membrane receptor for 1,25-dihydroxy vitamin D3 which mediates rapid activation of protein kinase C. J Bone Miner Res. 1998;13:1353–1359. doi: 10.1359/jbmr.1998.13.9.1353. [DOI] [PubMed] [Google Scholar]
- 26.Boyan BD, Sylvia VL, Curry D, Chang Z, Dean DD, Schwartz Z. Arachidonic acid is an autocoid mediator of the differential action of 1,25-(OH)2D3 and 24,25-(OH)2D3 on growth plate chondrocytes. J Cell Physiol. 1998;176:516–524. doi: 10.1002/(SICI)1097-4652(199809)176:3<516::AID-JCP8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Nicolas R, Lopez-Andreo MJ, Marin-Vicente C, Gomez-Fernandez JC, Corbalan-Garcia S. Molecular mechanisms of PKCalpha localization and activation by arachidonic acid. The C2 domain also plays a role. J Mol Biol. 2006;357:1105–1120. doi: 10.1016/j.jmb.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Gay I, Schwartz Z, Sylvia VL, Boyan BD. Lysophospholipid regulates release and activation of latent TGF-B1 from chondrocyte extracellular matrix. Biochem Biophys Acta. 2004 doi: 10.1016/j.bbalip.2004.04.006. In Press. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz Z, Brooks BP, Swain LD, Del Toro F, Norman AW, Boyan BD. Production of 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 by growth zone and resting zone chondrocytes is dependent on cell maturation and is regulated by hormones and growth factors. Endocrinology. 1992;130:2495–2504. doi: 10.1210/endo.130.5.1572278. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz Z, Pedrozo HA, Sylvia VL, Gomez R, Dean DD, Boyan BD. 1α,25-(OH)2D3 regulates 25-hydroxyvitamin D3 24R-hydroxylase activity in growth zone costochondral growth plate chondrocytes via protein kinase C. Calcif Tissue Int. 2001;69:365–372. doi: 10.1007/s00223-001-1009-y. [DOI] [PubMed] [Google Scholar]