Abstract
There is a substantial unmet need for new classes of drugs that block TNF-α-mediated inflammation, and particularly for small molecule agents that can be taken orally. We have screened a library of natural products against an assay measuring TNF-α secretion in lipopolysaccharide (LPS)-stimulated THP-1 cells, seeking compounds capable of interfering with the TNF-α inducing transcription factor Lipopolysaccharide Induced TNF Alpha Factor (LITAF). Among the active compounds were several produced by the kava plant (Piper mysticum), extracts of which have previously been linked to a range of therapeutic effects. When tested in vivo, a representative of these compounds, kavain, was found to render mice immune to lethal doses of LPS. Kavain displays promising pharmaceutical properties, including good solubility and high cell permeability, but pharmacokinetic experiments in mice showed relatively rapid clearance. A small set of kavain analogs was synthesized, resulting in compounds of similar or greater potency in vitro compared to kavain. Interestingly, a ring-opened analog of kavain inhibited TNF-α secretion in the cell based assay and suppressed LITAF expression in the same cells, whereas the other compounds inhibited TNF-α secretion without affecting LITAF levels, indicating a potential divergence in mechanism of action.
Keywords: TNF-α, inflammation, kava root extract, kavain, medicinal chemistry, optimization, LITAF
Introduction
In recent years, a range of new drugs that block the inflammatory cytokine TNF-α have led to significant advances in the treatment of serious inflammatory diseases such as RA and Crohn's Disease (1–3). However, these TNF-α blockers show full efficacy only in a minority of patients, and can display serious side effects.(4, 5) Therefore, there remains an urgent need to develop new strategies to regulate TNF-α levels in inflammatory diseases. Moreover, because all current TNF-α blockers are protein therapeutics (a.k.a. `biologics') and therefore must be administered parenterally, there is a particular need for TNF-α antagonists that can be taken orally, both as a means to increase patient compliance and also to allow anti-TNF-α therapy to be extended to other inflammatory conditions for which an injected drug is not appropriate or desirable. In addition, the cost of biological therapeutics tends to be significantly higher than small molecule drug formulations, limiting their accessibility for some patients.
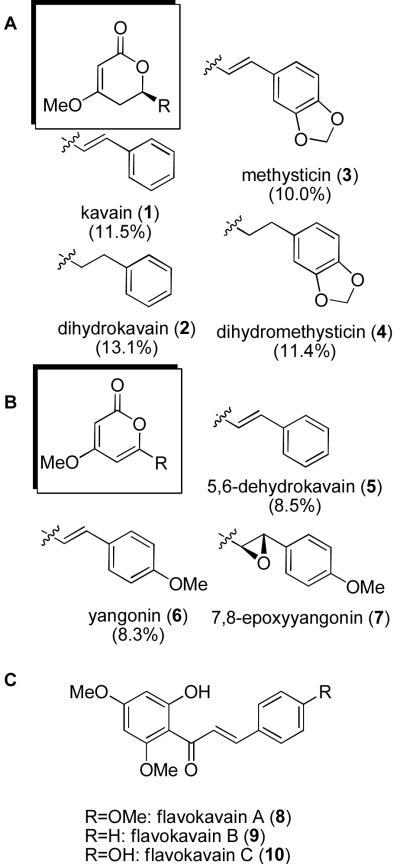
Kawa is a local beverage on the island of Fiji that is prepared from the roots of Piper methysticum, and has been suggested to be responsible for the low incidence of cancer in natives of this island (6). The beverage has also been utilized for its analgesic (7), relaxant (8), and anticonvulsant effects (9). Kava extract itself is marketed as a `nutraceutical', but has been reported to cause adverse biological effects, including hepatotoxicitiy (10), dermatological side-effects after oral ingestion (11) and neurological effects (12). In vitro identification of electrophilic, quinoid-based metabolic products of specific components of kava have been hypothesized as a potential source of toxicity (13). In light of these reports (14, 15), there has been increased interest in fully characterizing the identities and quantities of active components in kava-based nutraceuticals, with the goal of identifying the specific components that are associated with both the proposed beneficial and the toxic properties of the mixture. Seven known kavalactones (1–7, Figure 1) have been isolated from kava extract using a variety of techniques, including preparative thin-layer chromatography of ether extracts (16) and HPLC analysis of methanol extracts (17). A number of studies have quantified the components of the kava extract nutraceutical products (18–22). The levels of the natural products vary considerably depending on the particular preparation of the product. The most abundant components of kava, and their relative abundance as measured from acetone extracts of the kava rhizome of Hawaiian cv. mahakea, are shown in Figure 1 (23).
Figure 1.
Representatives of the kavalactone family. (A) Kavalactones, (B) kavapyrones, and (C) flavokavain compounds. Relative abundance of the major constituents as measured in extracts from Hawaiian cv mahakea are shown in parentheses.
We have recently cloned a transcription factor, Lipoplysaccharide Induced TNF-α Activating Factor (LITAF), and have shown it to control TNF-α gene expression (24–27). We subsequently reported that LITAF associates with STAT6B, and that the resulting complex plays a major role in transcription of several inflammatory cytokines including TNF-α (25, 26). Although the mechanism by which it regulates LITAF has not been fully investigated, activation of p38 MAPK is required for LITAF-dependent gene expression in response to LPS stimulation (27). In a quest to identify compounds capable of inhibiting LITAF signaling as a possible route to a novel class of oral TNF-α modulators, we developed an assay for inhibitors of TNF-α secretion in lipopolysaccharide (LPS)-stimulated THP-1 cells and used it to screen a library of natural products. Several kava derivatives were among the active compounds that were identified in that screen. Here, we report this result and describe the additional characterization of these compounds, their ADME and pharmacokinetic properties, their activity in vivo in a TNF-α–driven model of inflammatory disease, and the synthesis and characterization of a first set of analogs. The compelling biological activities of these compounds together with their promising pharmaceutical properties and ease of synthesis suggest that they might have potential as starting points for the development of a new class of orally available inhibitors of TNF-α mediated inflammation.
Experimental Section
Compound collection
A 800-member screening set that included “Genuine Natural Compound” (GNC) compounds was purchased from Biomodel, Inc (Wellesley, MA) and was used as received. Samples were prepared by dissolving the drug substance in DMSO at a concentration of 100 μg/mL, and were stored in 96-well plates at −80°C until used. The GNC library was assembled at Biomodel from nutritional supplements purchased from GNC Corporation.
Compound screening
The cell-line used to evaluate the impact of compounds on TNF-α production was THP-1, a human acute monocytic leukemia derived cell line. These cells were grown in RPMI-1640 medium supplemented with 2-mercaptoethanol (0.05 mM), 2 mM L-glutamine, 10% fetal bovine serum and antibiotics (all tissue culture reagents were from ATCC). These conditions were maintained throughout the assay process.
LPS-stimulated TNF-α production was evaluated by plating THP-1 cells in log phase onto standard 96-well tissue culture plates at 4 × 105 cells per well. Phorbol myristyl acetate (PMA, Sigma) was added at a concentration of 50 nM 24 hours after plating, along with test compound at 5 μg/ml. The media was replaced with media containing only test sample at 5 μg/ml 24 hours after the addition of PMA. After an additional 24 hours, lipopolysaccharide (LPS) was added at 10 μg/ml, and supernatants were collected 2 hours after the LPS addition. Each plate contained wells that received no test sample, wells that received no PMA, wells that received no LPS, and wells that received neither test sample, PMA or LPS. Each test was performed in triplicate. Once harvested, supernatants were stored at −80°C until they were tested for TNF-α expression. TNF-α ELISAs were performed using a huTNF-α ELISA kit (R&D systems) in accordance with the manufacturer's instructions.
LITAF-stimulated TNF-α production was evaluated by plating THP-1 cells in log phase onto standard 96-well tissue culture plates at 4 × 105 cells per well. PMA was added to 50 nM concentration 24 hours after plating, along with test compound at 5 μg/ml. The media was replaced with media containing LITAF peptide B at 100 μg/ml and test compound at 5 μg/ml, 24 hours after the addition of PMA. As we reported previously, LITAF peptide B was found to the peptide stretch isolated from LITAF that has kept LITAF activity (25). Supernatants were collected 24 hours after LITAF addition. Each plate contained wells that received no test sample, wells that received no PMA, wells that received no LITAF peptide B, and wells that received neither test sample, PMA or inhibitor. Each test was performed in triplicate.
Data analysis
Levels of TNF-α expression were calculated from the standard curve supplied with the ELISA kit. The data from any given plate was discarded if any of the following criteria were met: (1) The background (untreated cells only) exceeded an absorbance of 0.1; (2) The highest calibration standard was less than 1.0; (3) The cells treated with PMA and LPS/inhibitor but no test sample was lower than the highest calibration standard; or (4) The cells treated with either PMA, LPS or inhibitor was more than 2× background. For those experiments that did not meet the above criteria for discarding, the percent inhibition for each test sample on the plate was calculated. When testing was complete, test samples were ranked on their percent inhibition of TNF-α expression.
The impact of the compounds on TNF-α production in these cells was evaluated in response to both LITAF stimulation and LPS stimulation. Those pharmaceuticals or nutritional supplements that reduced LITAF-stimulated, but not LPS-stimulated TNF-α production were assumed to be specific for the LITAF pathway. In this way, pharmaceuticals or nutritional supplements that are toxic to the THP-1 cells are eliminated, since they will also be toxic under conditions of LPS stimulation. Using this analysis, three lists were generated: (1) those compounds that reduce LITAF-induced TNF-α expression by ≥ 80%, but LPS-stimulated TNF-α expression by ≤ 20%; (2) those compounds that reduce LPS-stimulated TNF-α expression by ≥ 80%, but LITAF-stimulated TNF-α expression by ≤ 20% and (3) those that inhibit both LPS- and LITAF-stimulated TNF-α expression and ≥ 80%. All extracts that exhibited more than 80% inhibition were retested to confirm their activity and to test for cytotoxicity by trypan blue exclusion , wherein apoptosis was assessed by TUNEL (Roche Diagnostics) (28). The effects of the compounds on LITAF expression was tested by western blot as we described earlier (27).
Compounds procurement and synthesis
Samples of kavain 1, dihydrokavain 2, dihydromethysticin 3, and methysticin 4 were purchased from Avachem, Ltd., (San Antonio, TX). All reagents for chemical synthesis and d-galactosamine were purchased from Aldrich Chemical Company (St. Louis, MO) and were used as received. Chemical reactions were carried out under a nitrogen atmosphere and monitored by thin layer chromatography using Merck 60 F254 pre-coated silica gel plates (0.25 mm thickness). Analytical LC was performed on a Waters Acquity UPLC with PDA, ELS and SQ detectors. An Acquity UPLC BEH 2.1 × 50 mm 1.7 μM C18 column was used for analytical LC. Analytical LC was performed on a Waters Acquity UPLC with PDA, ELS and SQ detectors. An Acquity UPLC BEH 2.1 × 50 mm 1.7 μM C18 column was used for analytical LC. The following compounds were prepared using the General Method, and analytical characterization was identical to that previously reported: 11 (29), 12 (30), 13 (31), 14 (32).
General synthetic method (32)
To a rapidly stirred solution of MeOH (2.0 equiv) in CH2Cl2 was added n-BuLi (2.5 M in hexanes, 1.0 equiv) followed by the desired aldehyde (5.0 equiv). PMe3 (1.0 M in THF, 0.25 equiv) and methyl allenoate (1.0 equiv) were added successively and stirred at ambient temperature for 0.5 to 5 hours. After stirring for the specified time, saturated NaHCO3/brine (1:1, 10 mL) was added to the reaction mixture and the organic phase was isolated. The aqueous phase was extracted using CH2Cl2 (10 mL) and the combined organic phase was dried (Na2SO4), filtered and reduced in vacuo. The resultant residues were purified by flash chromatography using the conditions stated.
6-(Biphenyl-4-yl)-4-methoxy-5,6-dihydro-2H-pyran-2-one (15) and (E)-Methyl 5-(biphenyl-4-yl)-5-hydroxy-3-methoxypent-2-enoate (16). Using the General Method, the reaction of MeOH (0.040 mL), n-BuLi (0.200 mL), methyl allenoate (0.049 mg), PMe3 (0.130 mL, 1.0 M), and 4-biphenylcarboxaldehyde (0.474 mg) for 40 min gave the product was an off-white solid (41 mg, 30%) after column chromatography (20 – 50% EtOAc in hexanes). 1H NMR (400 MHz) δ 7.56 – 7.71 (m, 4H), 7.42 – 7.56 (m, 4H), 7.34 – 7.42 (m, 1H), 5.49 (dd, J=12.0, 3.3, 1H), 5.28 (s, 1H), 3.80 (s, 3H), 2.87 (dd, J=16.7, 12.0, 1H), 2.65 (dd, J=16.7, 3.3, 1H). 13C NMR δ (100 MHz) 172.5, 166.8, 141.5, 140.4, 137.1, 128.8, 127.5, 127.4, 127.1, 126.4, 90.6, 76.9, 56.2, 35.0. LCMS (C18): tR (min) = 1.70, (ESI) found 281.2 [M + H]+. The ring-opened compound (16) was also isolated (15 mg, 10%) as a white solid. 1H NMR (400 MHz) δ 7.57 – 7.67 (m, 4H), 7.49 – 7.56 (m, 2H), 7.45 (t, J=7.4, 2H), 7.31 – 7.40 (m, 1H), 5.23 (s, 1H), 5.05 (d, J=9.4 Hz, 1H), 3.84 (br. s., 1H), 3.74 (s, 3H), 3.69 (s, 3H), 3.38 (dd, J=13.7, 9.8, 1H), 2.96 (dd, J=13.7, 3.1, 1H). 13C NMR δ (100 MHz) 173.2, 169.7, 143.6, 141.0, 140.3, 128.7, 127.2, 127.12, 127.09, 126.0, 92.7, 72.7, 55.8, 51.4, 42.4. LCMS (C18): tR (min) = 1.84, (ESI) found 625.2 [2M + H]+.
In vitro ADME and pharmacokinetic experiments
These experiments were performed at an external vendor. The experimental protocols are included in the Supporting Information.
In vivo efficacy experiments
The importance of the compound in inhibiting response to LPS in vivo was determined by comparing LPS-induced lethality in mice as we published recently (27). Drug compound was given i.p. to age-matched C57 mice (8–12 weeks, n = 8) weighing 20–25 g, immediately followed by a lethal dose of LPS (0.25 μg), also administered i.p..The animals were closely monitored for mortality and morbidity every hour for 24 hours following i.p. injection with d-galactosamine (d-GalN, dose = 40 μg/g, as 4 μg/μL solution in DMSO), followed by 0.25 μg of E. coli LPS per mouse (total volume of 0.1mL of PBS buffer containing 1% bovine serum albumin). Control animals were given an equivalent dose of DMSO, followed by LPS. In murine models, it is well accepted that d-GalN dramatically sensitizes mice to the lethal effects of LPS via its toxic effects on hepatocytes (33). There is agreement that death in LPS/d-GalN-challenged animals is due to TNF-α toxicity (34) such that d-GalN-sensitized mice are sensitive to the lethal effect of LPS at a 100-fold lower dose than are unsensitized littermates (35).
Results and Discussion
Compound Screening
We screened an 800-membered compound library that included representatives of the “Genuine Natural Compound” (GNC) collection, comprised of natural product extracts derived from commercial nutraceutical products (some representatives are listed in the Supporting Information). Extracts were tested at a single concentration of 50 μg/ml for their ability to inhibit TNF-α secretion by LPS-stimulated THP-1 cells, and for their ability to reduce levels of the p65 subunit of NFκB and of the TNF-α-inducing transcription factor LITAF in cell lysate, as described in the Experimental Section. All extracts that exhibited more than 80% inhibition of TNF-α secretion and/or NFκB levels were retested to confirm their activity and to test for cytotoxicity, wherein apoptosis was assessed by the TUNEL method. Among the confirmed active extracts were kava root extract, barberry extract and quercetin. Kava root extract was considered to be of particular interest due to its reported medicinal properties in inflammatory diseases (7).
A variety of effects of kava derivatives on TNF-α levels have been described previously. For example, 5,6-dehydrokavain 5 and yangonin 6 were found to significantly inhibit TNF-α release from NIH3T3 cells treated with okadaic acid, with moderate potency (IC50 values of 17 and 40 μM, respectively) (17). While dihydrokavain 2 was demonstrated to be the most potent inhibitor of TNF-α release in mice, it showed lower activity in NIH3T3 cells. Inhibition of both NF-κB-driven reporter gene expression and TNFα-induced binding of NF-κB to a consensus response element was achieved at concentrations of 320 μM (8), 175 μM (9) and 870 μM (1 and 2) (36). Interestingly, treatment with kavain 1 and flavokavains A and B (8 and 9) led to Inhibitor of κB (IκB) degradation and subsequent translocation of p50 and p65 NF-κB subunits from the cytoplasm to the nucleus, as shown by Western blot analysis. Kinase selectivity screening demonstrated that 8, but not 1 or 9, inhibits the IκB kinase (IKK) as well as PRAK (p38-regulated/activated kinase), MAPKAP-K3 (MAPK-activated protein kinase 3), DYRK1A (dual-specificity tyrosine-phosphorylated and regulated kinase 1A), and Aurora B. Thus, the various derivatives of kava extract collectively display an array of inhibitory and activating effects on NFkB signaling pathway that provide one potential starting point for elucidating the molecular mechanism(s) underlying the TNF-α suppression effects of specific family members. Although there are numerous synthetic approaches to kavalactones (37–42), despite the compelling biological activities reported for this class of compounds no systematic medicinal chemistry optimization program has been described.
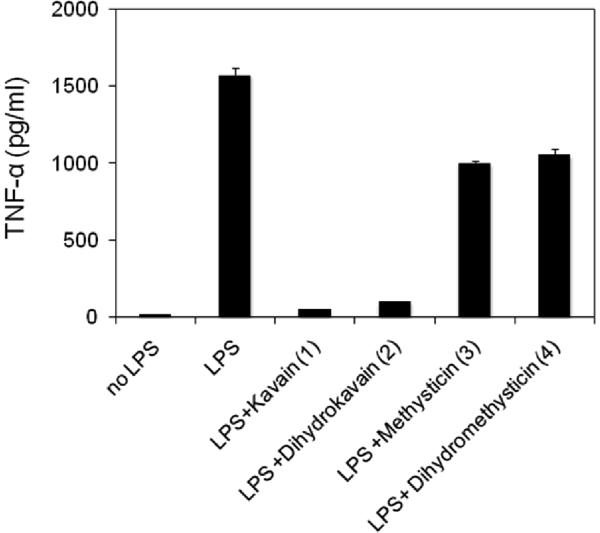
The most abundant discrete components of kava root extract are shown in Figure 1. For further characterization, we purchased the four most abundant components, kavain 1 and dihydrokavain 2, dihydromethysticin 3 and methysticin 4, all of which are commercially available. These four compounds were tested individually in the TNF-α secretion assay. Figure 2 shows that compounds 1 and 2 were reproducibly active at concentrations that did not show any cytotoxicity. Compounds 3 and 4 were found to be essentially inactive in the TNF-α secretion assay.
Figure 2.
Suppression of TNF-α secretion by compounds 1–4 at 50 μg/ml. Bars show the mean of three measurements; error bars represent the Standard Deviation.
In vivo efficacy of kavain and dihydrokavain
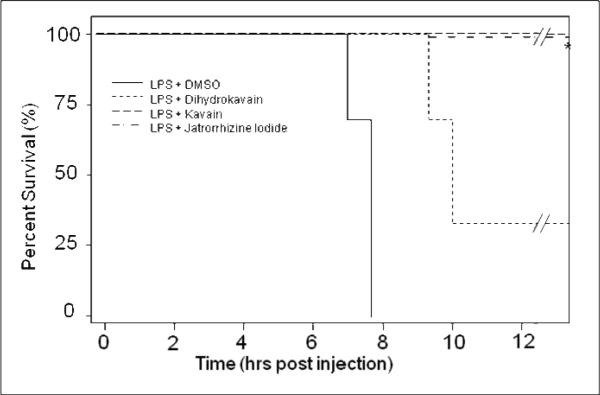
Compounds 1 and 2 were assessed in vivo in an LPS-induced endotoxic shock model of inflammation. In this model, mortality and morbidity are known to be driven by TNF-α {Mαρυψαμα, 2000#17}. The efficacy of the compounds was determined by comparing LPS-induced lethality in mice, as we have published recently (27). Figure 3 shows that animals that were stimulated with LPS and were treated with vehicle only all died within 6– 8 h. In striking contrast, mice pretreated with 40 mg/kg kavain, given intraperitoneally, were rendered immune to lethal doses of LPS, with no mortality or overt morbidity observed for at least 24 hours after receiving LPS. Mice pretreated with 40 mg/Kg dihydrokavain were only partially protected from the effects of LPS, with a 30% survival rate at 24 h, and with the animals that died showing about a two hour delay in time of death compared to the vehicle treated animals. Jatrorrhizine iodide, a compound unrelated to kavalactones that emerged from the HTS and displayed TNF-α suppression (unpublished results) was also shown to render mice immune to endotoxic shock-mediated death. However, these animals were in poor health and were euthanized after 24h.
Figure 3.
Compound mediated survival of mice in an LPS-induced endotoxic shock model of inflammation. Compounds were dosed ip at 40 mg/kg.
Assessment of Pharmaceutical Properties and Pharmacokinetics
Pharmacologic activity is only one of many properties that are required for a compound to be useful as a drug. Also important are a compound's “pharmaceutical properties”, and particularly its absorption, distribution, metabolism, and excretion (ADME) behavior in vivo. While it is the profile of properties of the final clinical candidate that is obviously most critical, ADME properties tend to track to a significant degree with chemotype. The best current practice in the pharmaceutical industry is therefore to assess the ADME properties of evolving drug candidates from an early stage, and to weigh these properties quite heavily when deciding which compound series to select for continued medicinal chemistry optimization. We therefore set out to assess the pharmaceutical properties of kavain, using a combination of predictive in vitro and in vivo assays, as part of a broad evaluation of the potential of this class of compounds as starting points for drug discovery. This work was performed through an outside vendor (Apredica, Inc.). The results are summarized in Table 1.
Table 1.
ADME and PK assessment of kavain.
| Property | Result |
|---|---|
| Solubility | >500 μM, PBS Buffer pH=7 |
| Chemical Stability | <10% degradation at 1 hr, pH=7 |
| Mouse plasma stability | 99% remaining at 2h (5 μM dose) |
| Caco-2 permeability | Papp(B→A)/Papp(A→B)=0.7 |
| Pharmacokinetics | t1/2<15 min (1 mg/kg iv) |
| t1/2<40 min (10 mg/kg po) |
Among the properties that can be measured in vitro that are considered most predictive of in vivo ADME are solubility and cell permeability. Kavain is highly soluble at pH 7, and displayed a high level of cell permeability in a Caco-2 assay. This combination of properties suggests that this class of compounds has excellent prospects for oral absorption, low serum protein binding, and straightforward formulation into preclinical and clinical dosage forms. In addition, kavain displayed good chemical stability both in aqueous buffer and in mouse plasma. A preliminary pharmacokinetic evaluation was also performed on kavain. The results show that kavain is rapidly cleared following dosing, displaying t1/2 <15 minutes when dosed iv, and t1/2 <40 minutes when dosed orally. The latter number presumably reflects a balance between distribution and elimination, rather than elimination alone. Because of its rapid clearance, the compound levels were not detectable at enough time-points to allow the compound's oral bioavailability to be determined. These results nevertheless confirm the impressive bioavailability of kavalactones such as kavain for in vivo studies, while identifying improvement of elimination half-life as a key goal for optimization in future medicinal chemistry work. Interestingly, although the pharmacokinetic profile of kavain is characterized by very rapid clearance, when tested in vivo these compounds protected against LPS-induced death beyond 24 hours. This observation suggests that the TNF-α suppression activity of kavain is mediated by longer-lasting factors or effects that are initiated by dosing with this compound.
Overall, the profile of pharmaceutical properties displayed by kavain is quite promising for a compound at this relatively early stage in its development. As this compound series is further advanced through medicinal chemistry the measurement of additional properties will become important. These will include assessment of the compounds as inhibitors of cytochrome P450 (CYP) enzymes, measurement of serum protein binding, inhibition of hERG channel activity, and activity against the P-glycoprotein (P-gp) transporter. Kavalactones including kavain have been reported to have weak (17-90 μ-M) activity as P-gp inhibitors (43), and so it will be important to ensure that as more potent and specific analogs are developed the potency against P-gp is decreased or eliminated. It will also be important to identify the major metabolites of active kavalactones to rule out the possibility that the biological activity of the compounds in vivo is due to a metabolite rather than to the parent compound (44).
Initial Analog Panel
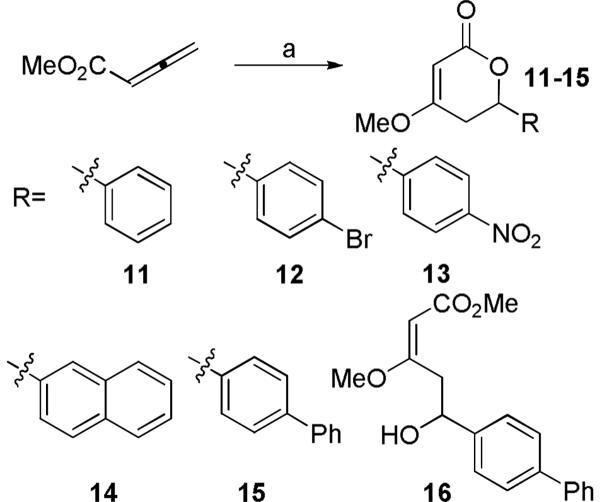
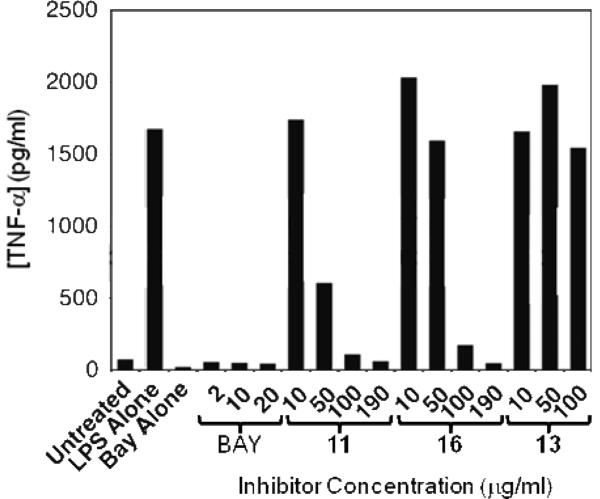
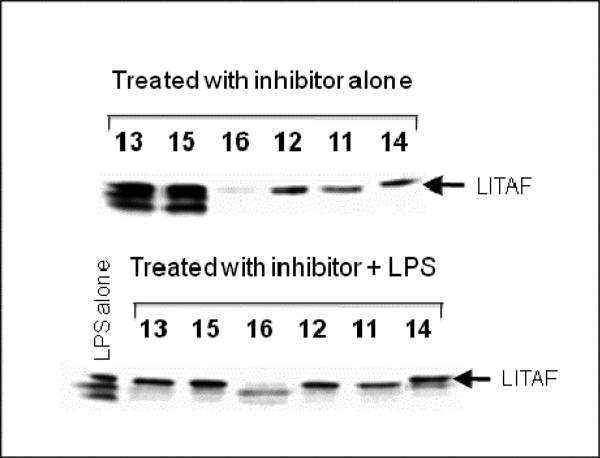
A key predictor of the potential for an early stage discovery compound to be optimized through medicinal chemistry to achieve drug-like properties is the observation of a promising structure-activity relationship (SAR). An ideal profile would establish that relatively small modifications to the structure of the compound result in analogues that display a range of potencies in a relevant biochemical assay, indicative of a specific interaction between the compound and its target. In addition, it is useful to identify sites on the compound that can tolerate modification without losing significant activity, which would indicate that there is scope to modify or grow the molecule to improve potency and selectivity and to optimize its pharmaceutical properties. We designed an initial set of analogues to probe the C6 region of kavain, shown in Figure 4. The primary strategy was to explore the effects of varying the length of the side-chain and the functionality of the aromatic group. Application of a phosphine-catalyzed preparation of pyrones, starting from methyl allenoate and various aryl aldehydes (Figure 4), was used to make the first few key analogs of kavain with a view to accomplishing a broad initial survey of the steric and electronic requirements for activity in the portion of the molecule comprising the C6 R-group. A ring-opened analog (16) was also isolated during the preparation of 15, and tested to ascertain whether the opened ring affects the biological activity of kavalactones. The analogs in Figure 4 were screened in the TNF-α secretion assay at a single concentration of 50 μg/ml. The effect of each compound on the level of LITAF protein in the cells was also measured, as a first step in elucidating the mechanism of action of the compounds, and their activities are tabulated in Table 2. Figure 5 shows that compounds 11 and 16 suppressed TNF-α secretion in the cellular assay in a dose-responsive manner.
Figure 4.
Preparation of an initial set of kavalactone analogs. Reagents and conditions: PMe3 (25 mol%), MeOH, n-BuLi, RCHO, CH2Cl2, rt
Table 2.
Percent inhibition of TNF-α secretion from LPS-treated THP-1 cells by kavain and related compounds at 100 μg/ml.
| Compound | % Inhibition (replicates) |
|---|---|
| 1 | 98 (3) |
| 2 | 95 (3) |
| 3 | 37 (3) |
| 4 | 43 (3) |
| 11 | 81 (3) |
| 12 | −2 (2) |
| 13 | −3 (3) |
| 14 | −11 (2) |
| 15 | −9 (2) |
| 16 | 58 (3) |
Figure 5.
Dose response data for compounds 11, 13 and 16 in the TNF-α secretion assay (data are representative of three independent experiments). BAY 11–7085, a selective inhibitor of NFKB activity interfering with IKB phosphorylation (45), was used as positive control of TNF-α inhibition.
These results show that (a) kavain tolerates substitutions on the linker carbons, and (b) that the presence of a hydrophobic substituent in this position that is large enough to reach at least as far as the styryl side chain present on kavain is important for activity. However, the inactivity of methysticin and dihydromethysticin would suggest that the region into which these compounds bind at its molecular target does have a maximum size allowable, and/or that it does not tolerate more electron-rich aromatic substituents. The fact that some of these compounds were inactive is also important, as it provides preliminary evidence of the sort of well-defined SAR that one expects for a specific and optimizable drug-target interaction.
Interestingly, the ring-opened analog 16 also gave ≥50% suppression of TNF-α secretion, indicating that an intact lactone ring is not essential for this activity. Moreover, unlike the other active compounds, this compound nearly ablated LITAF expression (Figure 6). This striking result suggests that, while both intact and ring-opened analogs of 15 can suppress TNF-α secretion in mouse macrophages, they might do so by different mechanisms. The results for 16 are consistent with the possibility that this compound suppresses TNF-α by suppressing LITAF levels, perhaps by inhibiting LITAF transcription or, by analogy with NFκB, by accelerating the proteolytic degradation of this protein. The other active compounds in Figure 4 cannot act by this mechanism, but must inhibit some other step in the LITAF pathway or another pathway. The generality of this difference in activity clearly warrants further exploration.
Figure 6.
Western blot analysis of compound suppression of LITAF.
Conclusions and Future Directions
We have demonstrated that kavain and related kavalactones suppress TNF-α secretion in cells, and provide a striking degree of protection in vivo in a TNF-α driven model of inflammation. Moreover, kavain displays highly favorable pharmaceutical properties including oral bioavailability, although with a relatively short elimination half-life. Taken together, these results indicate that kavalactones potentially represent a promising starting point for the development of a new class of anti-TNF-α agents. Clearly, there is much to be done to establish the compounds' molecular mechanism of action. First steps towards identifying the molecular target responsible for the observed biological effects showed that an open-ring kavalactone derivative may work in part by suppressing LITAF expression, whereas kavain itself and other closed ring analogs evidently do not. Importantly, the initial panel of compound analogs identified regions of the molecule that are tolerant of structural modification, suggesting that kavain and related kava derivatives represent a good starting point for the medicinal chemistry optimization of compound potency, elimination half-life, and such other properties as are required to generate a bona fide candidate for preclinical evaluation. We will report the results of such optimization experiments in due course.
Supplementary Material
Acknowledgments
The work was supported by a grant NIH/NIDCR R01 DE014079 (SA). We gratefully acknowledge the Center for Chemical Methodology & Library Development at Boston University (CMLD-BU, P50 GM067041) for support of this work (MP). Molecular modeling performed during the course of the compound designs was performed using software provided by OpenEye under a cost-free academic license agreement.
Footnotes
Appendices Supporting information includes general synthetic details and the experimental details for the ADME/PK experiments performed at Apredica, Inc. Also included is a list of representatives of the members of the GNC collection.
References
- 1.Pirzada S, Tomi Z, Gulliver W. A review of biologic treatments for psoriasis with emphasis on infliximab. Skin Therapy Lett. 2007 Apr;12(3):1–4. [PubMed] [Google Scholar]
- 2.Majithia V, Geraci SA. Rheumatoid arthritis: diagnosis and management. Am J Med. 2007 Nov;120(11):936–9. doi: 10.1016/j.amjmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Panes J, Gomollon F, Taxonera C, Hinojosa J, Clofent J, Nos P. Crohn's disease: a review of current treatment with a focus on biologics. Drugs. 2007;67(17):2511–37. doi: 10.2165/00003495-200767170-00005. [DOI] [PubMed] [Google Scholar]
- 4.Caviglia R, Boskoski I, Cicala M. Long-term treatment with infliximab in inflammatory bowel disease: safety and tolerability issues. Expert Opin Drug Saf. 2008 Sep;7(5):617–32. doi: 10.1517/14740338.7.5.617. [DOI] [PubMed] [Google Scholar]
- 5.Ravindran V, Scott DL, Choy EH. A systematic review and meta-analysis of efficacy and toxicity of disease modifying anti-rheumatic drugs and biological agents for psoriatic arthritis. Ann Rheum Dis. 2008 Jun;67(6):855–9. doi: 10.1136/ard.2007.072652. [DOI] [PubMed] [Google Scholar]
- 6.Singh YN. Kava: an overview. Journal of Ethnopharmacology. 1992;37(1):13–45. doi: 10.1016/0378-8741(92)90003-a. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson DD, Duffield PH. The antinociceptive actions of kava components in mice. Clinical and Experimental Pharmacology and Physiology. 1990 Jul;17(7):495–508. doi: 10.1111/j.1440-1681.1990.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 8.Meyer HJ, Kretzschmar R. Kava pyrones: a new group of components in central muscle relaxing agents of the mephenesin type. Klin Wochschr. 1966;44:902–3. doi: 10.1007/BF01711971. 1966. [DOI] [PubMed] [Google Scholar]
- 9.Tonks A. Treating generalised anxiety disorder. BMJ. 2003 Mar 29;326(7391):700–2. doi: 10.1136/bmj.326.7391.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGhee HW. Hepatic toxicity possibly associated with kava-containing products - United States, Germany, and Switzerland, 1999-2002. Journal of the American Medical Association. 2003 Jan;289(1):36–7. [PubMed] [Google Scholar]
- 11.Guro-Razuman S, Anand P, Hu QL, Mir R. Dermatomyositis-like illness following kava-kava ingestion. Jcr-Journal of Clinical Rheumatology. 1999 Dec;5(6):342–5. doi: 10.1097/00124743-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Stevinson C, Huntley A, Ernst E. A systematic review of the safety of kava extract in the treatment of anxiety. Drug Safety. 2002;25(4):251–61. doi: 10.2165/00002018-200225040-00003. [DOI] [PubMed] [Google Scholar]
- 13.Johnson BM, Qiu SX, Zhang SD, Zhang FG, Burdette JE, Yu LN, et al. Identification of novel electrophilic metabolites of Piper methysticum Forst. (Kava) Chemical Research in Toxicology. 2003 Jun;16(6):733–40. doi: 10.1021/tx020113r. [DOI] [PubMed] [Google Scholar]
- 14.Anke J, Ramzan I. Kava Hepatotoxicity: Are we any Closer to the Truth? Planta Medica. 2004;(3):193–6. doi: 10.1055/s-2004-815533. [DOI] [PubMed] [Google Scholar]
- 15.Lechtenberg M, Quandt B, Schmidt M, Nahrstedt A. Is the alkaloid pipermethystine connected with the claimed liver toxicity of Kava products? Pharmazie. 2008 Jan;63(1):71–4. [PubMed] [Google Scholar]
- 16.Young RL, Hylin JW, Plucknett DL, Kawano Y, Nakayama RT. Analysis for kawa pyrones in extracts of Piper methysticum. Phytochemistry. 1966;5:795–8. [Google Scholar]
- 17.Hashimoto T, Suganuma M, Fujiki H, Yamada M, Kohno T, Asakawa Y. Isolation and synthesis of TNF-alpha release inhibitors from Fijian kawa (Piper methysticum) Phytomedicine. 2003 May;10(4):309–17. doi: 10.1078/094471103322004802. [DOI] [PubMed] [Google Scholar]
- 18.Bobeldijk I, Boonzaaijer G, Spies-Faber EJ, Vaes WHJ. Determination of kava lactones in food supplements by liquid chromatography-atmospheric pressure chemical ionisation tandem mass spectrometry. Journal of Chromatography A. 2005 Mar;1067(1–2):107–14. doi: 10.1016/j.chroma.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Bilia AR, Scalise L, Bergonzi MC, Vincieri FF. Analysis of kavalactones from Piper methysticum (kava-kava) Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2004 Dec;812(1–2):203–14. doi: 10.1016/j.jchromb.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 20.Lasme P, Davrieux F, Montet D, Lebot V. Quantification of kavalactones and determination of kava (Piper methysticum) chemotypes using near-infrared reflectance spectroscopy for quality control in Vanuatu. Journal of Agricultural and Food Chemistry. 2008 Jul;56(13):4976–81. doi: 10.1021/jf800439g. [DOI] [PubMed] [Google Scholar]
- 21.Ganzera M, Khan IA. Analytical techniques for the determination of lactones in Piper methysticum forst. Chromatographia. 1999 Dec;50(11–12):649–53. [Google Scholar]
- 22.Hu LH, Jhoo JW, Ang CYW, Dinovi M, Mattia A. Determination of six kavalactones in dietary supplements and selected functional foods containing Piper methysticum by isocratic liquid chromatography with internal standard. J AOAC Int. 2005 Jan-Feb;88(1):16–25. [PubMed] [Google Scholar]
- 23.Lim STS, Dragull K, Tang CS, Bittenbender HC, Efird JT, Nerurkar PV. Effects of kava alkaloid, pipermethystine, and kavalactones on oxidative stress and cytochrome P450 in F-344 rats. Toxicological Sciences. 2007 May;97(1):214–21. doi: 10.1093/toxsci/kfm035. [DOI] [PubMed] [Google Scholar]
- 24.Myokai F, Takashiba S, Lebo R, Amar S. A novel lipopolysaccharide-induced transcription factor regulating tumor necrosis factor alpha gene expression: molecular cloning, sequencing, characterization, and chromosomal assignment. Proc Natl Acad Sci U S A. 1999 Apr 13;96(8):4518–23. doi: 10.1073/pnas.96.8.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang X, Fenton MJ, Amar S. Identification and functional characterization of a novel binding site on TNF-alpha promoter. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4096–101. doi: 10.1073/pnas.0630562100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang X, Marciano DL, Leeman SE, Amar S. LPS induces the interaction of a transcription factor, LPS-induced TNF-alpha factor, and STAT6(B) with effects on multiple cytokines. Proc Natl Acad Sci U S A. 2005 Apr 5;102(14):5132–7. doi: 10.1073/pnas.0501159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang X, Metzger D, Leeman S, Amar S. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: Evidence for LITAF-dependent LPS signaling pathways. Proc Natl Acad Sci U S A. 2006 Sep 12;103(37):13777–82. doi: 10.1073/pnas.0605988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X, Amar S. Secreted frizzled-related protein 1 (SFRP1) protects fibroblasts from ceramide-induced apoptosis. J Biol Chem. 2004 Jan 23;279(4):2832–40. doi: 10.1074/jbc.M308102200. [DOI] [PubMed] [Google Scholar]
- 29.Dugger RW, Heathcock CH. A general synthesis of 5,6-dihydro-.alpha.-pyrones. The Journal of Organic Chemistry. 1980;45(7):1181–5. [Google Scholar]
- 30.Haifeng Du DZKD. Enantioselective Catalysis of the Hetero-Diels-Alder Reaction between Brassard's Diene and Aldehydes by Hydrogen-Bonding Activation: A One-Step Synthesis of (S)-(+)- Dihydrokawain. Chemistry - A European Journal. 2004;10(23):5964–70. doi: 10.1002/chem.200400515. [DOI] [PubMed] [Google Scholar]
- 31.Creech GS, Kwon O. Alcohol-Assisted Phosphine Catalysis: One-Step Syntheses of Dihydropyrones from Aldehydes and Allenoates. Organic Letters. 2008;10(3):429–32. doi: 10.1021/ol702462w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mowafak Y, Shandala MTAMJM. Reaction of ethyl gamma-bromo-beta-methoxycrotonate with carbonyl compounds. Synthesis of 4-methoxy-6-substituted-5,6-dihydro-2-H-pyran-2-ones and 3-substituted 3-pyrazolin-5-ones. Journal of Heterocyclic Chemistry. 1984;21(6):1755–6. [Google Scholar]
- 33.Silverstein R. D-galactosamine lethality model: scope and limitations. J Endotoxin Res. 2004;10(3):147–62. doi: 10.1179/096805104225004879. [DOI] [PubMed] [Google Scholar]
- 34.Maruyama H, Kikuchi S, Kawaguchi K, Hasunuma R, Ono M, Ohbu M, et al. Suppression of lethal toxicity of endotoxin by biscoclaurine alkaloid cepharanthin. Shock. 2000 Feb;13(2):160–5. doi: 10.1097/00024382-200013020-00011. [DOI] [PubMed] [Google Scholar]
- 35.Osakabe N, Yasuda A, Natsume M, Takizawa T, Terao J, Kondo K. Catechins and their oligomers linked by C4 --> C8 bonds are major cacao polyphenols and protect low-density lipoprotein from oxidation in vitro. Exp Biol Med. 2002 Jan;227(1):51–6. doi: 10.1177/153537020222700109. [DOI] [PubMed] [Google Scholar]
- 36.Folmer F, Blasius R, Morceau F, Tabudravu J, Dicato M, Jaspars M, et al. Inhibition of TNFalpha-induced activation of nuclear factor kappaB by kava (Piper methysticum) derivatives. Biochem Pharmacol. 2006 Apr 14;71(8):1206–18. doi: 10.1016/j.bcp.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 37.Israili ZH, Smissman EE. Synthesis of kavain, dihydrokavain, and analogs. J Org Chem. 1976;41(26):4070–4. doi: 10.1021/jo00888a004. [DOI] [PubMed] [Google Scholar]
- 38.Spino C, Mayes N, Desfossés H, Sotheeswaran S. Enantioselective synthesis of (+)- and (−)- dihydrokawain. Tetrahedron Letters. 1996;37(36):6503–6. [Google Scholar]
- 39.Lin L, Chen Z, Yang X, Liu X, Feng X. Efficient Enantioselective Hetero-Diels-Alder Reaction of Brassard's Diene with Aliphatic Aldehydes: A One-Step Synthesis of (R)-(+)-Kavain and (S)-(+)- Dihydrokavain. Org Lett. 2008;10(6):1311–4. doi: 10.1021/ol8002282. [DOI] [PubMed] [Google Scholar]
- 40.Kostermans D. Synthesis of Kawain. Nature. 1950;166(4227):788–9. doi: 10.1038/166788c0. [DOI] [PubMed] [Google Scholar]
- 41.Klohs M, Keller F, Williams R. Notes: Piper Methysticum Forst. II. The Synthesis of dl-Methysticin and dl-Dihydromethysticin. J Org Chem. 1959;24(11):1829–30. [Google Scholar]
- 42.Castellino S, Sims JJ. The total synthesis of (±) kawain via a hetero-diels-alder cycloaddition. Tetrahedron Letters. 1984;25(37):4059–62. [Google Scholar]
- 43.Weiss J, Sauer A, Frank A, Unger M. Extracts and kavalactones of Piper methysticum G. Forst (kava-kava) inhibit P-glycoprotein in vitro. Drug Metab Dispos. 2005 Nov;33(11):1580–3. doi: 10.1124/dmd.105.005892. [DOI] [PubMed] [Google Scholar]
- 44.Tarbah F, Mahler H, Kardel B, Weinmann W, Hafner D, Daldrup T. Kinetics of kavain and its metabolites after oral application. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jun 5;789(1):115–30. doi: 10.1016/s1570-0232(03)00046-1. [DOI] [PubMed] [Google Scholar]
- 45.Hu X, Janssen WE, Moscinski LC, Bryington M, Dangsupa A, Rezai-Zadeh N, et al. An IkappaBalpha inhibitor causes leukemia cell death through a p38 MAP kinase-dependent, NF-kappaB-independent mechanism. Cancer Res. 2001 Aug 15;61(16):6290–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.