Abstract
We previously showed that estrogen regulates baboon fetal ovarian follicle development and oocyte integrity. Because iron incorporated into cells by the transferrin receptor is essential for cell/nuclear function, we determined whether fetal oocyte expression of transferrin receptor and the nuclear protein Ki67 were developmentally regulated by estrogen and associated with DNA integrity/fragmentation. Transferrin-receptor expression was minimal at midgestation and abundant in late gestation and localized to the cytoplasm and surface of oocytes of primordial follicles. Expression of transferrin receptor, however was negligible in oocytes in fetuses in which serum estradiol-17β levels were suppressed (>95%) by daily maternal treatment between mid and late gestation with the aromatase inhibitor letrozole and partially restored by treatment with letrozole and estradiol benzoate. Ki67 was localized to pregranulosa and germ cells at midgestation and throughout the oocyte nucleus in late gestation in estrogen-replete fetuses. In contrast, in estrogen-suppressed fetuses, Ki67 was localized to a limited number of foci around the oocyte nucleus. Apoptosis detected in pregranulosa and germ cells at midgestation was not observed in late gestation in estrogen-replete/-suppressed fetuses. We conclude that estrogen regulates fetal oocyte transferrin receptor expression and that inhibition of receptor development is associated with alterations in Ki67 expression by the oocyte but not apoptosis. Collectively these results and our previous studies further define the essential role of estrogen in regulating development of follicles comprised of healthy oocytes by the baboon fetal ovary.
Keywords: estrogen, transferrin receptor, fetal oocytes, baboon, ovary
INTRODUCTION
Using the baboon as an experimental model for studies of human reproductive biology, our laboratories have shown that fetal ovarian folliculogenesis is regulated by estrogen (1), the levels of which increase between mid and late gestation in human and nonhuman primates (2). Thus, in baboons in which estrogen was suppressed by treatment with an aromatase inhibitor during the second half of gestation, the number of follicles formed was reduced by more than 50% (3) and the majority of follicles that did develop contained oocytes which were unhealthy and characterized by cytoplasmic vacuolization and a marked reduction in microvilli (4), structures essential for uptake of nutrients from surrounding granulosa cells (5, 6). Importantly, formation of follicles and oocyte microvilli were normal in fetal baboons treated with aromatase inhibitor and estradiol, supporting our hypothesis that estrogen regulates development of follicles comprised of healthy oocytes with microvilli critical for long-term survival (1, 4).
It is well established that iron typically bound to transferrin is essential for several aspects of cell function including proliferation and DNA synthesis (7, 8). Availability of iron to cells is facilitated by binding of the iron-transferrin complex to the transferrin receptor typically expressed on the surface of cells, including microvilli, and which is internalized by invagination of clathrin-coated pits and formation of endocytotic vesicles (9). Although the transferrin receptor is expressed in oocytes of adult ovaries (10, 11), it is not known whether the receptor protein is developed in utero and associated with the formation of oocyte microvilli.
Numerous studies have confirmed that oocytes of primordial follicles formed in utero initiate meiosis and exhibit extensive DNA replication (12, 13). Although oocytes arrest in prophase I of the first meiotic division, which is considered equivalent to the G2 phase of a mitotic division (14), they continue to exhibit intense biosynthetic activities including DNA replication and RNA synthesis (13, 15). Studies have shown that Ki67, which is abundantly expressed during all active phases of the mitotic cell cycle (16, 17) is also expressed in primordial germ cells/oogonia and pregranulosa during early gestation (18, 19). Therefore, because oocytes of estrogen suppressed fetuses appeared unhealthy and devoid of microvilli, it is possible that nuclear function/activity (e.g. DNA replication; RNA synthesis) has been compromised and that cells may be susceptible to demise and/or programmed to undergo apoptosis.
Because estrogen regulates fetal oocyte microvilli development/oocyte integrity, and iron incorporated into cells by the transferrin receptor is thought to be essential for aspects of nuclear function, the current study utilized our nonhuman primate baboon model to determine whether transferrin receptor and Ki67 proteins were expressed in fetal oocytes and/or granulosa cells and test the hypothesis that expression is developmentally regulated by estradiol and associated with DNA fragmentation/apoptosis.
MATERIALS AND METHODS
Animals
Fetal ovaries were obtained on days 98–105 (n = 5) and days 162–175 (n = 5) of gestation (term = day 184) from untreated baboons and on days 160–178 of gestation from animals treated with the highly specific aromatase inhibitor, letrozole (CGS 20267; 4,4-[1,2,3-triazol-1yl-methylene] bis-benzonitrite, Novartis Pharma AG, Basel, Switzerland; 115 µg/kg body weight/day; n = 5) or with letrozole plus estradiol benzoate (each at 115 µg/kg; n = 4) administered sc to the mother beginning on day 100 of gestation as described previously (20). Blood samples were obtained from the maternal saphenous vein at 1–4 day intervals during the study period and from the maternal saphenous vein and from the umbilical vein at the time of placental-fetal delivery, and serum stored at −20°C until assayed for estradiol levels using an automated chemiluminescent immunoassay (Immulite; Diagnostic Products Corp., Los Angeles, CA) as described previously (20). Animals were cared for and used strictly in accordance with USDA regulations and the NIH Guide for the Care and Use of Laboratory Animals (86-23, 1985). The Animal Care and Use Committee of Eastern Virginia Medical School approved the experimental protocol used in this study.
Immunocytochemistry
The immunocytochemical detection of transferrin-receptor, Ki67 and DNA fragmentation was determined essentially as described previously (21, 22). Briefly, paraffin-embedded sections (4 µm) of formalin-fixed fetal ovaries were mounted onto Superfrost microscope slides (Fisher Scientific Co., Arlington, VA), heat fixed and endogenous peroxidase blocked with 0.3% H2O2 in methanol. Sections (n = 5–10 per ovary/animal) were incubated for 24 h (4°C) with mouse monoclonal antibodies to transferrin-receptor (Calbiochem, Los Angeles, CA) or Ki67 (Vector Laboratories, Burlingame, CA) and diluted 1:500 or 1:100, respectively, in 5% normal goat serum (NGS) in phosphate buffered saline (PBS). Ovarian sections were then washed in PBS and incubated with biotinylated goat anti-mouse IgG (Dako Corp., Carpinteria, CA) and proteins localized using diaminobenzidine (DAB)-imidazole-H2O2 (brown precipitant) or by immunofluorescence using streptavidin conjugated with Alexa Fluor 488 Green (Molecular Probes, Inc., Eugene, OR). For detection with DAB, sections were treated with VectaStain Elite ABC Kit (Vector), rinsed, stained with DAB-imidazole-H2O2, counterstained with hematoxylin, mounted in Bio mount (Fisher) and examined by light microscopy (21). For immunofluoresence, sections were incubated with streptavidin conjugated with Alexa Fluor Green (Molecular Probes) diluted 1:500 with 5% NGS, and then treated with Sudan Black (Sigma Chemical Corp., St. Louis, MO) to quench autofluorescence, rinsed and treated with propidium iodide (0.1 µg/ml PBS) to stain nuclei red. Following application of mounting media, slides were sealed with nail polish and stored in the dark at 4°C until examined using an Olympus BX41 microscope (Optical Elements Corp., Melville, NY) equipped with an Olympus DP70 digital camera with FITC and/or TRITC filter sets. DNA fragmentation was determined using the ApopTag plus peroxidase In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA) and methods/reagents supplied by the manufacturer.
Statistical analyses
Estradiol levels in maternal saphenous serum samples were analyzed using linear regression; values in umbilical venous samples were examined using ANOVA and the Student Newman-Keuls multiple comparison statistic.
RESULTS
Serum estradiol concentrations
As shown previously (3, 20), maternal serum estradiol levels in untreated baboons gradually increased (P<0.05) from approximately 1 ng/ml on days 85–120 of gestation to approximately 3.0 ng/ml by days 160–175. Approximately 72–96 h after onset of treatment with letrozole, maternal serum estradiol decreased to and was maintained at 0.10 – 0.15 ng/ml. Estradiol levels (mean ± SE) in umbilical venous serum on the day of delivery in untreated baboons at midgestation (0.21 ± 0.14 ng/ml) was increased (P<0.05) approximately 5-fold by late gestation (1.25 ± 0.55 ng/ml) and decreased (P<0.05) at term by >95% in letrozole-treated baboons (0.04 ± 0.01 ng/ml). In animals treated with letrozole and estrogen, maternal and umbilical venous serum estradiol levels were restored to 90–120% (P<0.05) and 10–20% of normal, respectively.
Histology of the baboon fetal ovary
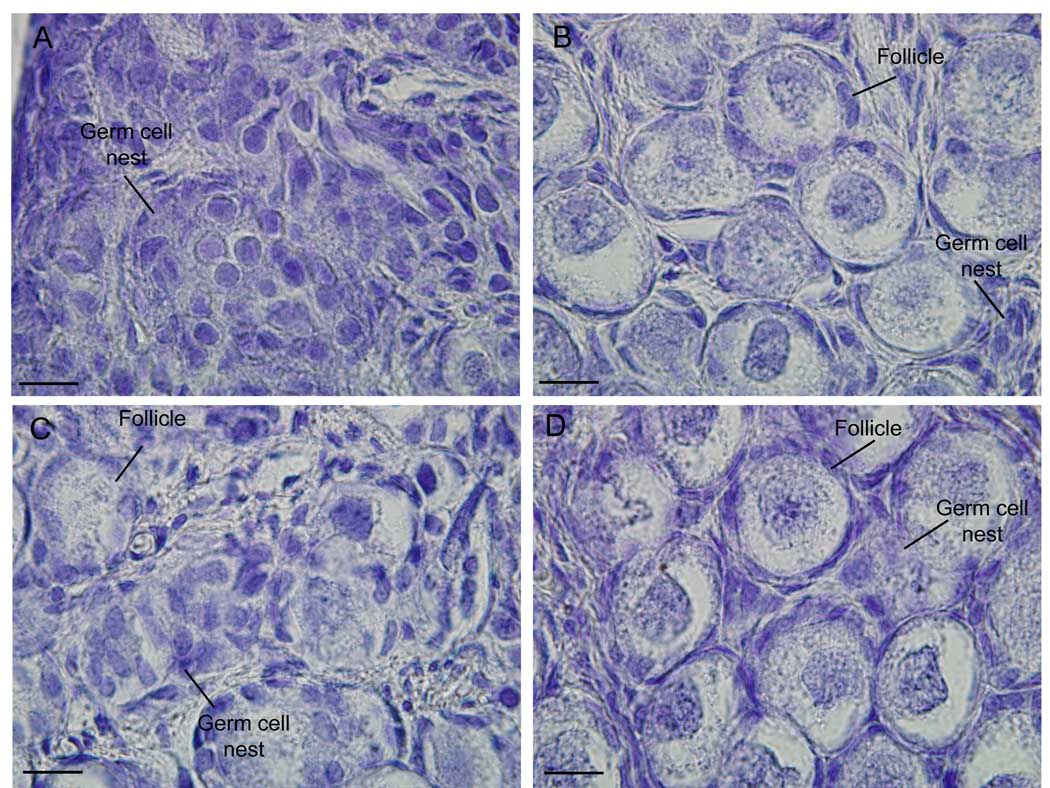
As previously demonstrated (21), the baboon fetal ovary at midgestation was comprised primarily of germ cell nests containing numerous oogonia/oocytes and pregranulosa cells (Fig 1A). By late gestation, the fetal ovary was comprised almost exclusively of primordial follicles with only a few germ cell nests from which follicles emerged still evident (Fig 1B). In contrast, in the fetal ovary of estrogen-suppressed baboons, germ cell nests were still present and primordial follicles in the ovarian cortex less abundant (Fig 1C). In baboons in which estrogen was restored, fetal ovarian follicle formation (Fig 1D) was similar to that of untreated (i.e. estrogen-replete) animals.
Fig 1.
Representative photomicrographs of hematoxylin histology of the baboon fetal ovary at day 100 (A) and day 165 (B) of gestation in untreated animals and at day 165 in baboons treated with letrozole (115 µg/kg BW/day; C) or letrozole and estradiol benzoate (115 µg/kg BW/day each; D) administered sc to the mother beginning on day 100 (term = day 184). Original magnification =x 1000. —— 10 µm magnification bar.
Expression of transferrin-receptor
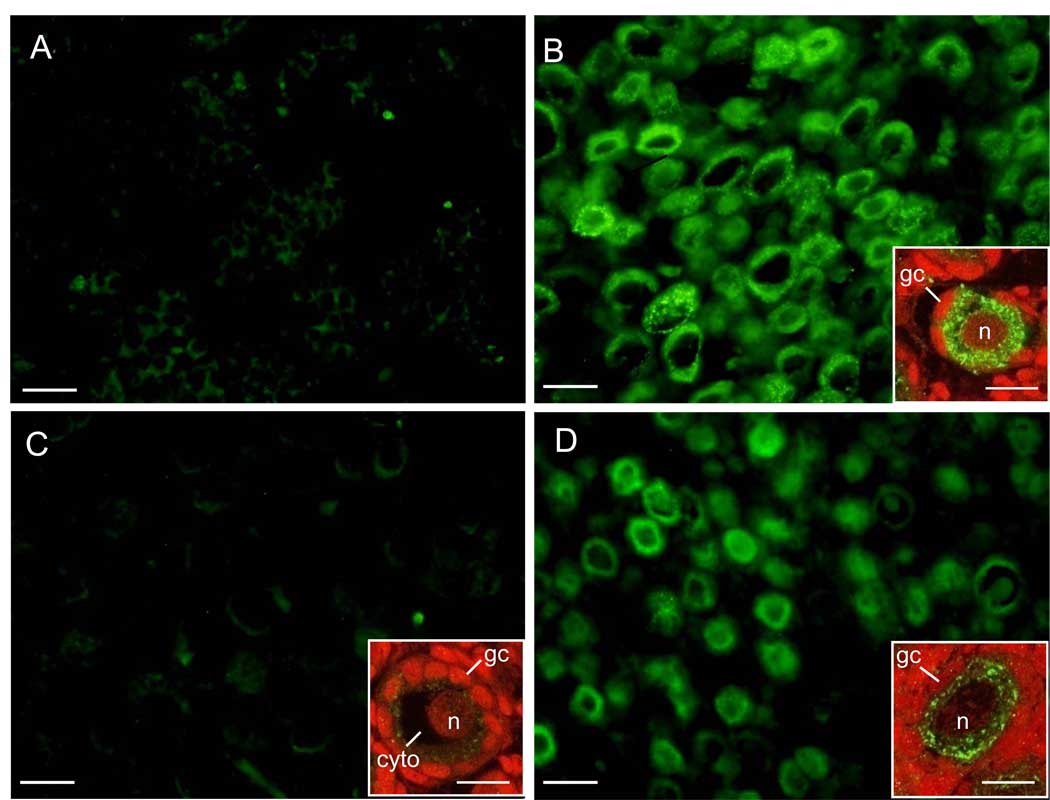
Immunocytochemistry confirmed that the apparent intensity of transferrin-receptor protein expression in the fetal ovary was very low at midgestation (Fig 2A) and moderate to extensive in virtually all sections of fetal ovary examined in late gestation and localized to oocytes of primordial follicles (Fig 2B and insert). Thus, in late gestation, punctuate expression of the transferrin receptor was abundant and detected throughout the oocyte cytoplasm. Moreover, expression extended to the surface of the oocyte and in close proximity to the cytoplasm of the granulosa cell, presumably reflecting expression of the receptor in the microvilli of the oocyte which we previously showed extend to the cytoplasm of the granulosa cells (4). Transferrin receptor protein was not detected on the surface of the granulosa cells adjacent to the oocyte (Fig 2B insert) and was minimal in the oocyte nucleus as evidenced by absence of yellow dots and thus absence of colocalization of protein (green) with propidium iodide stained (red) nuclei (Fig 2B). In fetal ovaries of animals in which estradiol levels were suppressed and folliculogenesis and the number/height of oocyte microvilli reduced (4) by in vivo treatment with letrozole, the apparent intensity of transferrin receptor expression by oocytes of primordial follicles was minimal (Fig 2C and insert) and in several sections not detectable. Significantly, in late gestation fetal ovary of baboons treated with letrozole and estradiol which restored oocyte microvilli (4) intensity of transferrin receptor expression was moderate in oocytes of virtually all primordial follicles examined (Fig 2D and insert). Moreover, as in ovaries of untreated baboons, punctuate expression of the transferrin receptor was abundant throughout the cytoplasm, extended to the surface of the oocyte but was absent in granulosa cells and the oocyte nucleus. Specificity of the primary antibody was confirmed by absence of signal in sections of fetal term ovary incubated without primary antibody (not shown).
Fig 2.
Representative photomicrographs of transferrin receptor expression in fetal ovary on day 100 (A) and 165 (B and insert) of gestation in baboons untreated and on day 165 in animals treated with letrozole (C) or letrozole and estradiol benzoate (D) as described in legend to Fig 1. Paraffin-embedded sections were incubated with antibody to transferrin receptor, stained green with streptavidin conjugated with AlexaFluor 488 and treated with propidium iodide to stain nuclei red. Original magnification = x400 A-D; inserts = x1000. gc = granulosa cell; n = oocyte nucleus; cyto = oocyte cytoplasm. —— 25 µm magnification bar; magnification bar for inserts to B, C, and D = 10 µm.
Expression of Ki67
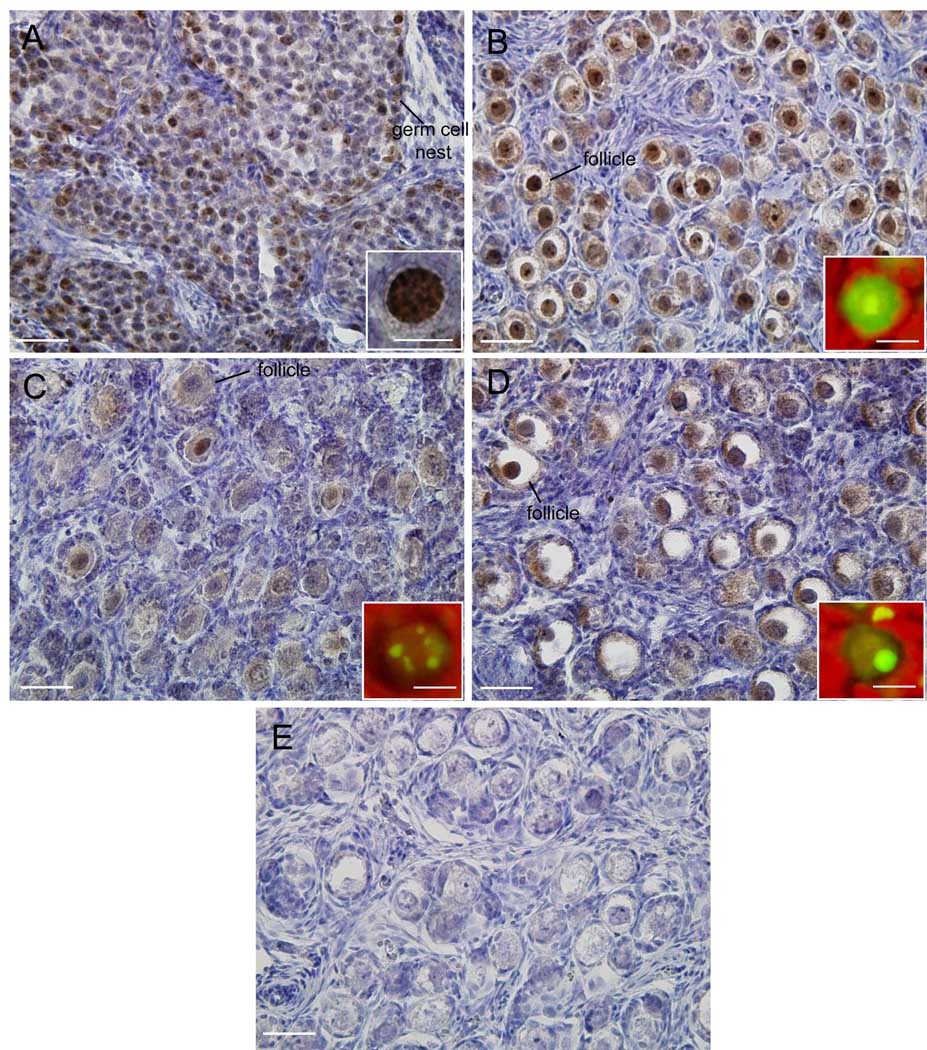
At midgestation, Ki67 was abundantly expressed and localized to the nuclei of several, but not all pregranulosa cells, and oogonia/oocytes but not oocytes that had become encapsulated by pregranulosa to form a primordial follicle (Fig 3A and insert). By late gestation, Ki67 was primarily expressed throughout the oocyte nucleus and either dispersed in numerous nucleolar foci or a single nucleolus in oocytes of primordial follicles (Fig 3B and insert). In contrast, in ovaries of estrogen-suppressed fetuses, Ki67 expression was not only reduced in intensity, but appeared to be localized to limited number of distinct nucleolar foci throughout the oocyte nucleus (Fig 3C and insert). Significantly, in baboons treated with letrozole and estradiol, Ki67 expression was partially restored to that in untreated animals and thus abundant in nuclei of oocytes of primordial follicles (Fig 3D) and either dispersed throughout numerous nucleolar foci or a single nucleolus (Fig 3D insert). Specificity of primary antibody was confirmed by absence of signal in sections of fetal term ovary incubated without primary antibody (Fig 3E).
Fig 3.
Representative photomicrographs of Ki67 expression in fetal ovary on day 100 (A and insert) and 165 (B and insert) of gestation in baboons untreated and on day 165 in animals treated with letrozole (C and insert) or letrozole and estradiol benzoate (D and insert) as described in legend to Fig 1. Paraffin-embedded sections were incubated with primary antibody stained with diaminobenzidine and Ki67 detected (brown precipitate) by light microscopy or stained with streptavidin conjugated with AlexaFluor 488 Green and treated with propidium iodide to stain nuclei red (inserts). E. Section of late gestation fetal ovary incubated without primary antibody. Original magnification = x400; inserts x1000 and enlarged approximately 2-fold. —— 25 µm magnification bar; magnification bar for inserts = 5 µm (A); 10 µm (B-D).
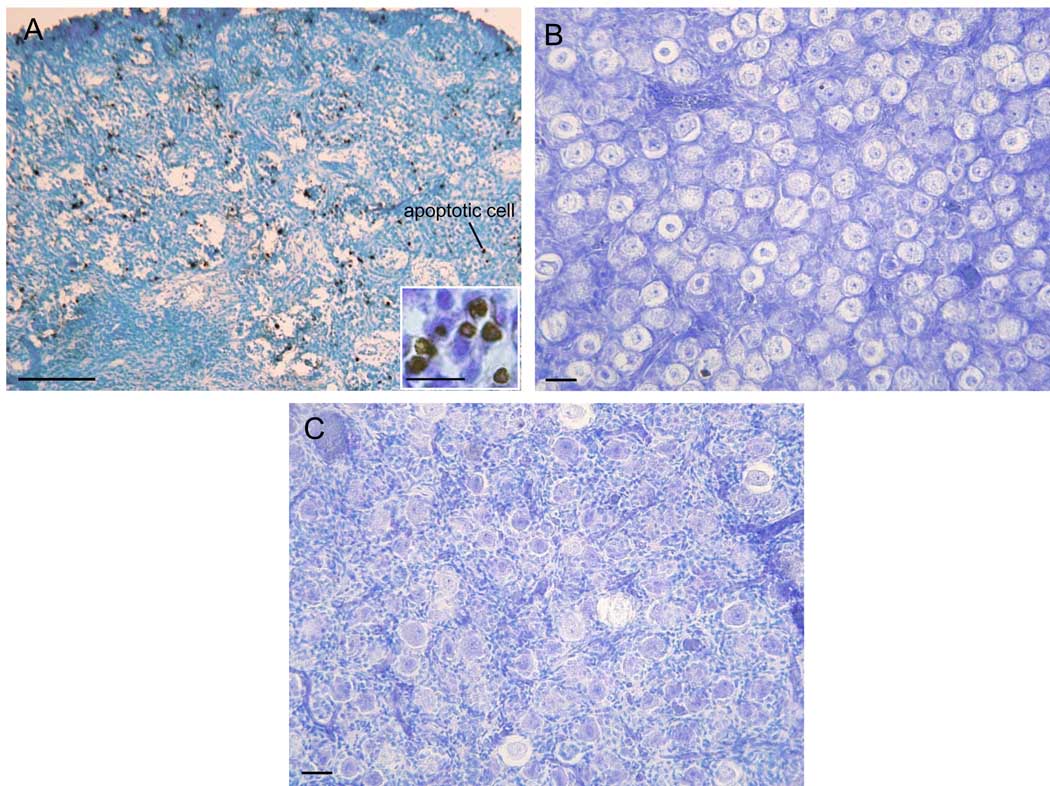
As seen in Fig 4, at midgestation, DNA fragmentation was detected in several oogonia/oocytes present in germ cells nests (Fig 4A). However, by late gestation, DNA fragmentation was negligible in ovaries of baboons untreated (Fig 4B) or treated with letrozole (Fig 4C).
Fig 4.
Representative photomicrographs of DNA fragmentation in the baboon fetal ovary on day 100 (A) and day 165 (B) of gestation in baboons untreated and on day 165 in animals treated with letrozole (C) as described in legend to Fig 1. Paraffin-embedded sections incubated with ApopTag In Situ Apoptosis kit and sections examined by light microscopy. Original magnification = x100 A; x200 B-C. —— 100 µm (A) and 25 µm magnification bar (B,C); magnification bar for insert = 10 µm.
DISCUSSION
The results of the current study demonstrate that oocytes of primordial follicles in the late gestation baboon fetal ovary expressed the transferrin receptor on the surface and throughout the cytoplasm of the oocyte. Moreover, although transferrin receptor expression appeared to extend into the cytoplasm of surrounding granulosa cells, protein was not detected on the opposite surface (i.e. away from the oocyte) of these same granulosa cells. We previously showed that the surface of the fetal oocytes of primordial follicles in late, but not midgestation, is comprised of numerous microvilli which extend into the cytoplasm of surrounding granulosa cells (4). Therefore, because the transferrin receptor typically resides in and/or is a component of microvilli in other organ systems (9), we suggest that the transferrin receptor is expressed by the fetal oocyte but not the granulosa cells of primordial follicles and becomes localized to the oocyte microvilli. Although additional studies are required to examine whether trafficking of the receptor occurs in the fetal oocyte, the abundant punctuate expression of the receptor detected throughout the oocyte cytoplasm is consistent with this suggestion. It is also possible, but remains to be determined, that the transferrin receptor is internalized by the fetal oocyte of primordial follicles, as shown to occur in several other cells when the receptor is bound by iron-transferrin complex (23, 24).
Our study also showed that the ontogenesis between mid and late gestation of oocyte transferrin receptor expression and localization to the microvilli were prevented in estrogen-suppressed fetuses and restored to normal in baboons treated with letrozole and estrogen. Therefore, we propose that the development of transferrin receptor protein expression by the fetal oocyte is regulated by estrogen.
As previously demonstrated in the human fetal ovary (18), pregranulosa cells and oogonia/oocytes in germ cell nests that comprise the baboon fetal ovary at midgestation expressed the nuclear protein Ki67. By late gestation, although Ki67 was not detected in granulosa cells of primordial follicles, the protein continued to be abundantly expressed by the oocytes and localized to the nucleus and either dispersed throughout numerous nucleolar foci or a distinct nucleolus. Ki67 has also been detected in oocytes of primordial follicles in the human fetal ovary at weeks 28–32 and although expression was highly variable (18) when detected was also localized to the nucleolus (18). Most interestingly, although Ki67 was detected in oocytes (but not granulosa cells) of primordial follicles in estrogen-suppressed fetuses of the current study, oocyte expression was localized to only a few discrete nucleolar foci and not throughout the nucleus or to a concentric nucleolus as in estrogen-replete animals. In mitotic somatic cells, Ki67 expression during early G1 is restricted to numerous foci (considered sites of nucleoli reformation) throughout the nucleus and only becomes localized to the nucleolus in late G1, i.e. after the nucleoli are reassembled (17). Therefore, we suggest that oocytes of primordial follicles exhibit a similar pattern of nucleolus dispersion/formation during follicle formation and that the latter is apparently an estrogen-dependent process.
It has been amply demonstrated that iron is required for aspects of somatic cell function, most notably DNA synthesis/cell proliferation (7), and that maturation of mammalian cell systems in vitro will not occur if transferrin and iron are excluded (25). In the adult human and rodent ovary, the concentrations of iron and transferrin in follicular fluid increase with advancing follicular maturation and steroidogenesis (26, 27) and transferrin is often added to culture media for studies of follicle or granulosa-oocyte complexes (28). Moreover, transferrin receptor is expressed in oocytes of the human adult ovary (10) and oocyte uptake of transferrin increased with follicle development (11). Therefore, it is possible that the inability of oocytes of estrogen-suppressed baboons to express transferrin receptor and presumably to accumulate iron underpins the changes noted in Ki67 expression.
The mechanism(s) by which estrogen acts to regulate transferrin receptor and Ki67 expression by the fetal oocyte remains to be determined. We recently demonstrated that baboon fetal oocytes isolated by laser capture microdissection from ovaries in late gestation express mRNA and protein for estrogen receptor (ER)β but not ERα (29). Therefore, estrogen may regulate transferrin receptor and Ki67 expression by exerting a direct effect on the oocyte although the latter remains to be determined.
Devine and colleagues (30) have previously shown in the adult rat ovary that oocytes undergoing demise but not programmed cell death exhibited unique morphological changes, including cytoplasmic vacuolization, retraction of their microvilli as well as condensation of and loss of cristae from mitochondria (30). In estrogen-suppressed baboons of the current study, however, alterations in oocyte expression of the transferrin receptor and Ki67, as well as failure to develop microvilli (4), oocytes did not exhibit DNA fragmentation and apparently may not be apoptotic. Therefore, it is possible that the cytoplasmic vacuolization and changes in mitochondria previously observed in oocytes of estrogen-suppressed baboon fetuses (4) may be a sign of another form of cell demise although the latter remains to be determined.
In summary, the results of the current study showed that there was a developmental increase in expression of the transferrin receptor by the oocyte of primordial follicles of the baboon fetal ovary in late gestation which was prevented in fetuses in which estrogen production was suppressed and restored to normal by concomitant treatment with estrogen. Therefore, we propose that the development of transferrin receptor expression by the fetal oocyte is regulated by estrogen. The current study also showed that oocytes of primordial follicles developed in estrogen-replete animals expressed Ki67 which was localized to nucleolar foci throughout the nucleus or to a distinct nucleolus. In contrast, in estrogen-suppressed fetuses, Ki67 expression by oocytes in late gestation was reduced and localized to a few foci around the nucleus suggesting that nuclear maturation may be compromised. We suggest that the concomitant failure of oocytes of estrogen-suppressed baboons to express transferrin receptor and presumably to accumulate iron which is required for DNA synthesis/cell proliferation (7) are interrelated and perhaps underpin the changes noted in Ki67 expression. Collectively, the results of the current study and our previous studies further define the critical role of estrogen in regulating fetal ovarian development in non-human primates.
ACKNOWLEDGMENTS
The authors sincerely appreciate the secretarial assistance of Ms. Sandra Huband with the manuscript and preparation of the photomicrographs. The authors greatly appreciate the generous provision of letrozole by Novartis Pharma AG, Basel, Switzerland.
*This research was supported by NICHD/NIH through cooperative agreement U54 HD 36207 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
REFERENCES
- 1.Pepe GJ, Billiar RB, Albrecht ED. Mol. Cell. Endocrinol. 2006;247:41–46. doi: 10.1016/j.mce.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht ED, Pepe GJ. Endocr. Rev. 1990;11:124–150. doi: 10.1210/edrv-11-1-124. [DOI] [PubMed] [Google Scholar]
- 3.Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Biol. Reprod. 2002;67:1148–1156. doi: 10.1095/biolreprod67.4.1148. [DOI] [PubMed] [Google Scholar]
- 4.Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Endocrinology. 2004;145:959–966. doi: 10.1210/en.2003-1078. [DOI] [PubMed] [Google Scholar]
- 5.Eppig JJ. J. Exp. Zool. 1979;209:345–353. doi: 10.1002/jez.1402090216. [DOI] [PubMed] [Google Scholar]
- 6.Heller DT, Cahill DM, Schultz RM. Dev. Biol. 1981;84:455–464. doi: 10.1016/0012-1606(81)90415-2. [DOI] [PubMed] [Google Scholar]
- 7.Robbins E, Pederson T. Proc. Natl. Acad. Sci. U.S.A. 1970;66:1244–1251. doi: 10.1073/pnas.66.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogt V. Nature. 1969;223:854–855. doi: 10.1038/223854a0. [DOI] [PubMed] [Google Scholar]
- 9.Rothenberger S, Iacopetta BJ, Kuhn LC. Cell. 1987;49:423–431. doi: 10.1016/0092-8674(87)90295-9. [DOI] [PubMed] [Google Scholar]
- 10.Balboni GC, Vannelli GB, Barni T, Orlando C, Serio M. Fertil Steril. 1987;48:796–801. doi: 10.1016/s0015-0282(16)59533-8. [DOI] [PubMed] [Google Scholar]
- 11.Briggs DA, Sharp DJ, Miller D, Gosden RG. Mol. Hum. Reprod. 1999;5:1107–1114. doi: 10.1093/molehr/5.12.1107. [DOI] [PubMed] [Google Scholar]
- 12.Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. Nat Genet. 2006;38:1430–1434. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- 13.Cohen PE, Pollack SE, Pollard JW. Endocr. Rev. 2006;27:398–426. doi: 10.1210/er.2005-0017. [DOI] [PubMed] [Google Scholar]
- 14.Nebreda AR, Ferby I. Curr. Opin. Cell Biol. 2000;12:666–675. doi: 10.1016/s0955-0674(00)00150-2. [DOI] [PubMed] [Google Scholar]
- 15.Strich R. Curr. Top. Dev. Biol. 2004;61:29–60. doi: 10.1016/S0070-2153(04)61002-7. [DOI] [PubMed] [Google Scholar]
- 16.Endl E, Gerdes J. Exp. Cell Res. 2000;257:231–237. doi: 10.1006/excr.2000.4888. [DOI] [PubMed] [Google Scholar]
- 17.Scholzen T, Gerdes J. J. Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Stoop H, Honecker F, Cools M, de Krijger R, Bokemeyer C, Looijenga LH. Hum. Reprod. 2005;20:1466–1476. doi: 10.1093/humrep/deh800. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL, Liu L. Dev. Biol. 2007;306:112–120. doi: 10.1016/j.ydbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Albrecht ED, Aberdeen GW, Pepe GJ. Am. J. Obstet. Gynecol. 2000;182:432–438. doi: 10.1016/s0002-9378(00)70235-3. [DOI] [PubMed] [Google Scholar]
- 21.Pepe GJ, Billiar RB, Leavitt MG, Zachos NC, Gustafsson JA, Albrecht ED. Biol. Reprod. 2002;66:1054–1060. doi: 10.1095/biolreprod66.4.1054. [DOI] [PubMed] [Google Scholar]
- 22.Pepe GJ, Burch MG, Albrecht ED. Endocrionology. 2006;147:2986–2996. doi: 10.1210/en.2005-0887. [DOI] [PubMed] [Google Scholar]
- 23.Ciechanover A, Schwartz AL, utry-Varsat A, Lodish HF. J. Biol. Chem. 1983;258:9681–9689. [PubMed] [Google Scholar]
- 24.Klausner RD, van Renswoude J, Ashwell G, Kempf C, Schechter AN, Dean A, Bridges KR. J. Biol. Chem. 1983;258:4715–4724. [PubMed] [Google Scholar]
- 25.Gatter KC, Brown G, Trowbridge IS, Woolston RE, Mason DY. J. Clin. Pathol. 1983;36:539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Entman SS, Maxson WS, Bradley CA, Osteen K, Webster BW, Vaughn WK, Wentz AC. J. In Vitro Fert. Embryo. Transf. 1987;4:98–102. doi: 10.1007/BF01555447. [DOI] [PubMed] [Google Scholar]
- 27.Mantzavinos T, Dalamanga N, Hassiakos D, Dimitriadou F, Gregoriou O, Zourlas PA. Clin. Exp. Obstet. Gynecol. 1993;20:32–36. [PubMed] [Google Scholar]
- 28.Eppig JJ, Schroeder AC. Biol. Reprod. 1989;41:268–276. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- 29.Bocca SM, Billiar RB, Albrecht ED, Pepe GJ. Endocrine. 2008 doi: 10.1007/s12020-008-9081-y. published May 17, 2008; PMID: 18484193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devine PJ, Payne CM, McCuskey MK, Hoyer PB. Biol. Reprod. 2000;63:1245–1252. doi: 10.1095/biolreprod63.5.1245. [DOI] [PubMed] [Google Scholar]