Abstract
The dopamine transporter (DAT) is a crucial regulator of dopaminergic neurotransmission which undergoes constitutive and substrate-mediated trafficking to and from the membrane. Although, considerable research has been done to elucidate the regulation of substrate-stimulated DAT trafficking, less is known about which trafficking proteins are involved in constitutive DAT trafficking. Rab proteins are GTPases known to regulate the trafficking of proteins to and from specific endocytic compartments. Rabs 8 and 11, in particular, are involved in trafficking proteins from intracellular compartments to the plasma membrane. In this study, we sought to determine whether Rabs 8 and 11 would modulate DAT activity and trafficking in N2A neuroblastoma cells. We used Rab mutations known to confer constitutively active or dominant negative activity of these proteins to investigate the role of Rab activity in constitutive DAT trafficking and function. We found that constitutively active Rab 11 upregulates DAT function and surface expression while neither the constitutively active nor the dominant negative mutant of Rab 8 had any effect on DA uptake. Furthermore, immunofluorescence experiments revealed that dominant negative Rab 11 overexpression results in decreased surface DAT indicating a necessary function of Rab 11 in DAT trafficking to the plasma membrane. These data show for the first time a functional role of Rab proteins in the constitutive recycling of DAT to the plasma membrane.
Keywords: Rab, DAT: Dopamine transporter, trafficking
The neurotransmitter dopamine (DA) can efficiently be removed from the synaptic cleft by reuptake into the nerve terminal by the dopamine transporter (DAT). DAT is an important regulator of dopaminergic neurotransmission as it is the key mechanism by which dopamine signaling is terminated [1]. The DAT is a presynaptic plasma membrane protein with a predicted topology of twelve transmembrane domains with intracellular N and C tails [9]. Substantive evidence demonstrates dynamic regulation of DAT trafficking to and from the membrane which occurs constitutively and in the presence of DAT substrates (For review, see [15, 19]). Constitutive DAT internalization was found to be clathrin-mediated in various heterologous cell lines including madin-darby canine kidney (MDCK), human embryonic kidney (HEK) and porcine aortic endothelial (PAE) cells [6, 20, 23]. DAT undergoes constitutive internalization into early and recycling endosomes as indicated by its co-localization with endosomal markers [16, 22, 23]. Rab proteins regulate trafficking of proteins to specific endosomal compartments and are thought to mediate tethering or delivery to the plasma membrane [29]. Rab 8 localizes to the trans golgi network (TGN) and regulates the trafficking of proteins from the TGN to the plasma membrane [2]. Rab 11 predominantly localizes to the pericentriolar recycling endosomes and is thought to regulate trafficking of proteins from the recycling endosomes to the plasma membrane [21, 25, 29]. The DAT recycles into the endocytic recycling compartment [16]. Loder and Melikian [13] demonstrated that DAT proteins recycle into Rab 5 containing endosomes in PC12 cells. Treatment with the PKC activator phorbol myristate acetate or the DAT substrate amphetamine (AMPH) for 1-2 hours induced DAT internalization into Rab 11- and Rab 5-containing compartments [22]. Notably, the authors in that study found a small amount of DAT co-localized in Rab 11-containing endosomes in the absence of treatment indicating constitutive trafficking to these endosomes. Furthermore Rab proteins have been implicated in trafficking of other transporters such as the insulin-sensitive glucose transporter GLUT4 [28]. The role of Rab protein function in DAT trafficking and activity has not been investigated. In the present study, we utilized constitutively active (CA, GTP-bound) or dominant negative (DN, GDP-bound) mutants of Rab to investigate their role in DAT function. Here we show in N2A neuroblastoma cells that a constitutively active form of Rab 11 upregulates DAT activity and trafficking to the surface while the dominant negative decreases DAT surface expression. These data indicate a sufficient and necessary role of Rab 11 in constitutive DAT trafficking.
Rab GTPase DNA constructs with single point mutations known to confer either a GDP bound/dominant negative state or a GTP-bound/constitutively active state were generated. N2A neuroblastoma cells stably expressing human DAT were transiently transfected with the constitutively active mutants of Rab 8 (Q67L) and 11 (Q70L) or the dominant negative mutant of Rab 8 (T22N) and Rab 11 (S25N) using Lipofectamine-PLUS reagent kit. These mutants have previously been described [8]. All Rab proteins contain an N-terminal Green Fluorescent Protein (GFP) tag for labeling. GFP-Rab fusion proteins correctly localize to membranes [2, 21]. As a transfection control, cells were transiently transfected with the GFP-vector alone. [3H]dopamine uptake assay: 24 hours after transfection, cells were plated in quadruplicate (4 wells/transfection) onto coated 24-well plates and incubated for another 24 hours. Cells were washed twice with Krebs Ringers Hepes (KRH) buffer composed of (in mM): 25 HEPES, 125 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.3 CaCl2, 1.2 MgSO4, and 5.6 glucose. KRH was removed and uptake was initiated with the addition of [3H]dopamine (10 nM, Perkin Elmer) + unlabeled dopamine in KRH at 25°C in the presence or absence of the DAT blocker 10 μM GBR12935 to determine non-specific binding. Five minutes after treatment, cells were rapidly washed twice with KRH and 1% sodium dodecyl sulfate was added to solubilize cells. Lysed cells were collected and scintillation fluid was added to the vials before radioactive counting on a Beckman Liquid Scintillation counter. One well per transfection was assayed for protein content (Bio-Rad DC protein assay kit) and data were plotted as pmol DA uptake/mg protein. Specific uptake was determined in the presence and absence of the DAT inhibitor GBR12935 (10 μM). [3H]WIN35428 binding assay: Cells were plated as described for the [3H]dopamine uptake assay. Cells were washed twice with 4°C KRH to stop constitutive DAT trafficking. [3H]WIN35428 binding was initiated by the addition of 4 nM [3H]WIN35428 + 70 nM unlabeled 2 beta-carbomethoxy-3-beta-(4-fluorophenyl)-N-methyltropane in 4°C KRH buffer in the presence or absence of 10 μM GBR12935 to determine non-specific binding. Thirty minutes after [3H]WIN35428 treatment, cells were rapidly washed two times with cold KRH. Cells were counted for radioactivity as in the [3H]dopamine uptake assay. One well per transfection was assayed for protein content and data were plotted as pmol [3H]WIN35428 bound/mg protein. Immunofluorescence experiments: N2A cells were transiently co-transfected with DAT containing a hemagglutinin (HA)-epitope in the extracellular loop (generously supplied by Dr. Jonathan Javitch, Columbia University) and GFP vector, GFP-Rab 11CA, or GFP-Rab 11DN. Twenty-four hours after transfection cells were replated onto coated glass coverslips and incubated at 37°C for 24 hours. Immunofluorescence assays were carried out at ∼10-15°C. Cells were washed twice with phosphate buffered saline supplemented with calcium and magnesium (PBS/Ca/Mg) and blocked with 2% normal goat serum. Surface labeling of DAT was achieved by incubating cells with 1:250 anti-HA (Covance) followed by 1:250 of secondary goat α-mouse AF594 (Invitrogen). In some cases, cells were permeabilized to determine total DAT content using 0.1% triton X-100 in PBS/Ca/Mg followed by anti-DAT (MAB369: Millipore Biomedical Research) or anti-HA and corresponding secondary antibody conjugated to AF594 (Goat anti-mouse) or AF647 (Goat anti-rat, Invitrogen). Cells were mounted onto glass slides using ProLong Gold anti-fade reagent (Invitrogen). Cells were imaged on an Olympus FluoView 500 confocal microscope. Sequential scans were taken to prevent overlap of laser signal. Z-slices of cells were compressed and quantified using Image J software (NIH). Background was subtracted from each image. A total of 28 cells (28 for surface, 23 for total) were analyzed from three separate cultures.
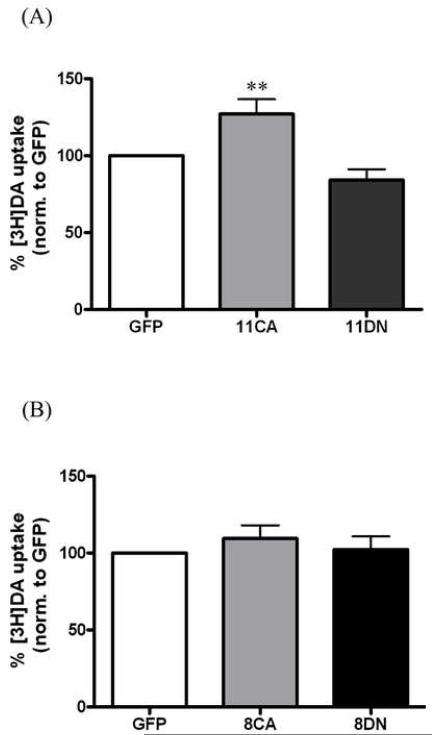
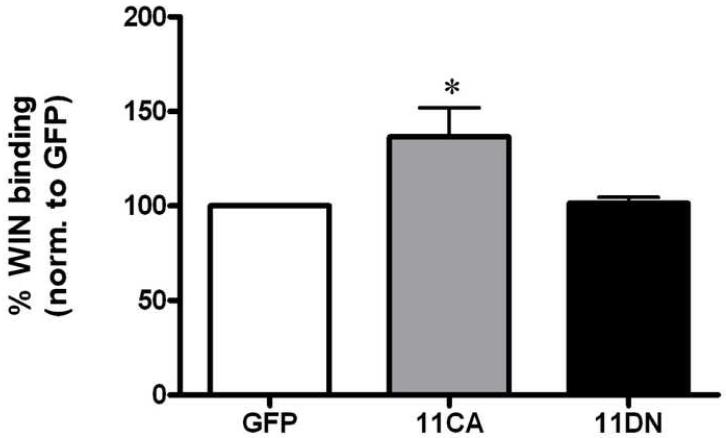
Both Rab 11 and Rab 8 have been shown to facilitate trafficking of proteins from intracellular compartments to the plasma membrane. If these proteins affect constitutive DAT trafficking and thus surface DAT, we would expect a change in DAT function reflected in the amount of [3H]dopamine uptake. In order to determine whether Rab 11 or Rab 8 regulates DAT function, N2A-human DAT cells were overexpressed with the CA or DN forms of GFP tagged Rab 11 and 8. As shown in Figure 1A, overexpression of GFP-Rab 11CA increased [3H]dopamine uptake to 127% of GFP vector control (p<0.01 by one-way ANOVA). Although GFP-Rab 11DN slightly decreased DA uptake to 84 ± 7% of control, there was no statistical significance when compared to GFP vector alone. Transfection of either the constitutively active or dominant negative form of GFP-Rab 8 had no effect on [3H]dopamine uptake as shown in Figure 1B. To determine whether this increase in function with GFP-Rab11CA was due to more DAT on the surface, [3H]WIN35428 binding experiments were performed. WIN 35428 has been shown to measure DAT surface expression in numerous experiments and is presumed to specifically label plasma membrane DAT [5, 12]. Figure 2 demonstrates an increase in [3H]WIN35428 binding in cells transiently expressing GFP-Rab 11CA to 137% of control (GFP vector alone), p<0.05 by one-way ANOVA. No significant difference was found between GFP vector alone- and GFP-Rab 11DN-transfected cells. Changes in [3H]dopamine uptake and [3H]WIN35428 binding will only be reflected when there is a large effect because cells which do not contain overexpressed Rab 11 (i.e. cells which did not take up DNA) will also be measured in these assays. This may result in an under-representation of the effect that occurs in transfected cells.
Figure 1.
Rab 11CA increases [3H]DA uptake. A, DAT-N2A cells were transiently transfected with GFP-vector (GFP, n = 17), GFP-Rab 11CA (11CA, n = 9) or GFP-Rab 11DN (11DN, n = 14) and assayed for [3H]DA uptake (specific activity = 10 nM). p<0.0001 by one-way ANOVA. Post hoc Bonferroni analysis shows a significant difference between GFP and 11CA (**p<0.01) and between 11CA and 11DN (***p<0.0001, stars not shown) B, Cells were transiently transfected with GFP-vector (GFP, n = 10), GFP-Rab 8CA (8CA, n = 5) or GFP-Rab 8DN (8DN, n = 6). No significant difference by one-way ANOVA between any groups was seen (p = 0.49). Data are plotted as pmol DA uptake per mg protein as a percentage of GFP vector. Error bars represent SEM.
Figure 2.
Rab 11CA increases [3H]WIN35428 binding. A, DAT-N2A cells were transiently transfected with GFP-vector (GFP, n = 5), GFP-Rab 11CA (11CA, n = 5) or GFP-Rab 11DN (11DN, n = 5) and assayed for [3H]WIN35428 binding (specific activity = 4 nM). p <0.05 by one-way ANOVA. Post hoc Bonferroni analysis shows a significant difference between GFP and 11CA (*p <0.05). Data are plotted as pmol [3H]WIN35428 bound per mg protein as a percentage of GFP vector. Error bars represent SEM.
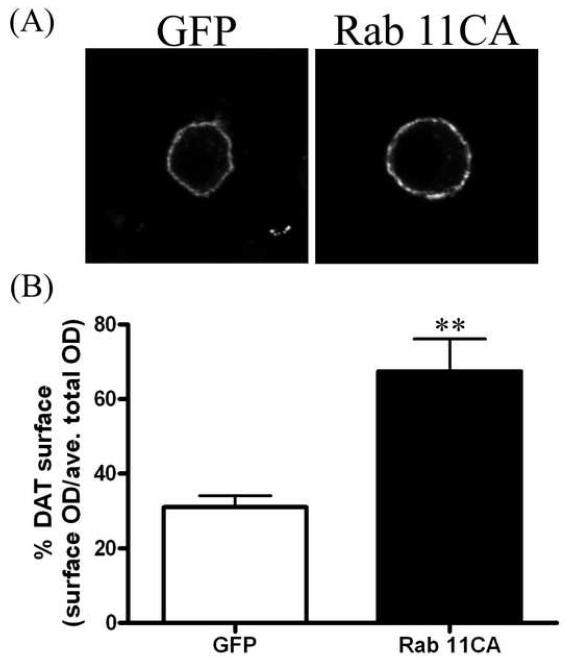
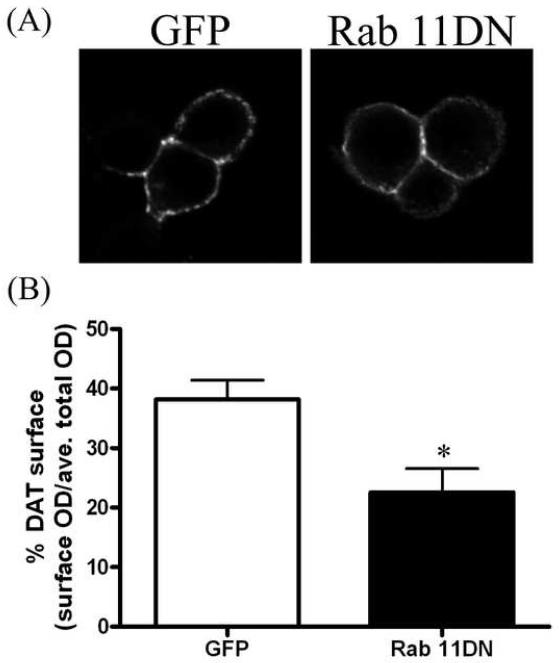
In order to measure effects of Rab 11 on DAT surface expression in individual cells, we performed immunofluorescence experiments. These experiments enabled us to exclude cells which did not contain a sufficient (visible) amount of GFP, GFP-Rab 11CA or GFP-Rab 11DN. In order to visualize and quantify surface DAT, a human DAT construct containing an HA tag in the extracellular loop was used. The HA-DAT construct used in these experiments has previously been described and has comparable function to wild-type DAT [24]. Non-permeabilized cells were reacted with anti-HA to label surface DAT followed by a secondary antibody conjugated to a fluorescent (AF594, red) tag. To ensure that changes in surface DAT did not reflect total amounts of DAT within the cell, some cells were permeabilized and reacted with either an anti-DAT antibody that binds to the N-terminal tail of DAT or anti-HA. Cells that were permeabilized and reacted with anti-HA for total DAT were not previously exposed to antibody. The cells were then reacted with a secondary antibody conjugated to a fluorescent tag. Figure 3A shows the surface labeling (anti-HA) of representative cells transfected with GFP-vector (left panel) or GFP-Rab 11CA (right panel). Cells transfected with GFP-Rab 11CA show increased surface DAT fluorescence intensity as compared to those transfected with GFP vector alone. GFP-Rab 11CA transfected cells had a 2-fold increase in DAT surface expression as compared to GFP vector alone (GFP = 31 ± 3.1%, 11CA = 67 ± 8.7 %, Figure 3B). In contrast, GFP-Rab 11DN transfected cells demonstrated significantly decreased surface staining as compared to GFP-vector alone as shown in figure 4. Figure 4A shows representative cells co-transfected with HA-DAT and GFP vector (left) or GFP-Rab 11DN (right). Quantification of surface DAT images demonstrates a significant decrease in surface labeling in GFP-Rab11DN transfected cells compared to GFP vector transfected cells (GFP = 38 ± 3.3%, 11DN = 23 ± 4.5%, Figure 4B). Analysis of cells permeabilized and stained for total DAT revealed lower but statistically insignificant total DAT levels (optical density measurement) for 11CA and 11DN as compared to GFP; p = 0.14, by unpaired t-test for 11CA (303 ± 24, n = 4) v. GFP (419 ± 59, n = 5) and p = 0.15 for 11DN (363 ± 77, n = 8) v. GFP (612 ± 160, n = 6).
Figure 3.
Rab 11CA increases DAT surface expression. A, Representative images of N2A cells co-transfected with HA-DAT and GFP-vector (left) or GFP-Rab 11CA (right). Surface labeling of DAT is shown (average of 10 z-slices in the middle of the cell). B, Quantitation of surface labeling of GFP vector (n = 7) or GFP-Rab 11CA (n = 9) using Image J software. Data are plotted as percent DAT surface expression: optical density (OD) of surface (HA) staining divided by the average total DAT staining. Error bars represent SEM. **p<0.01 by unpaired two-tailed t-test.
Figure 4.
Rab 11DN reduces DAT surface expression. A, Representative images of N2A cells co-transfected with HA-DAT and GFP-vector (left) or GFP-Rab 11DN (right). Surface labeling of DAT is shown (average of 10 z-slices in the middle of the cell). B, Quantitation of surface labeling of GFP vector (n = 5) or Rab 11DN (n = 7) using Image J software. Data are plotted as percent DAT surface expression: optical density (OD) of surface (HA) staining divided by the average total DAT staining. Error bars represent SEM. *p<0.05 by unpaired two-tailed t-test.
This paper describes the novel finding that Rab 11 activity regulates DAT function and trafficking. By utilizing mutants of Rab GTPase proteins, we demonstrated that DAT trafficking and function was increased in cells expressing a constitutively active form of Rab 11. For Rab 11CA transfected cells, [3H]WIN35428 binding and [3H]dopamine uptake both increased to a similar degree (127 ± 9.7% of GFP vector for [3H]dopamine uptake and 136.6 ± 15% of GFP vector for [3H]WIN35428 binding). When looking at individual cells to ensure that all cells analyzed contained GFP-Rab 11 DNA, we found even greater effects with GFP-Rab 11CA as compared to GFP vector alone (Figure 3). Although we did not see an effect of GFP-Rab 11DN in the [3H]dopamine uptake or [3H]WIN35428 binding assays, when measuring individual cell effects, we found decreased DAT surface expression in GFP-Rab 11DN-transfected cells as compared to GFP vector-transfected cells (Figure 4). While Rab 8 had no effect on [3H]dopamine uptake, it should be noted that we can’t rule out a role of Rab 8 in DAT trafficking as immunofluorescence experiments with Rab 8 were not performed.
These data demonstrate a specific role for Rab 11 to regulate trafficking of DAT to the plasma membrane. Based on co-fractionation studies in DAT-PC12 cells, the predominant location of intracellular DAT at steady state is in the pericentriolar recycling endosome [16], which is also the main location of Rab 11 [29]. The most parsimonious explanation of our data, therefore, is that upregulation of Rab11 increased trafficking to the membrane of DAT which originated from these recycling endosomes. Our data support the finding that intracellular DAT is localized to Rab 11-containing vesicles constitutively and in response to DAT substrates [22].
Historically, proteins known to be sorted by Rabs have been receptors such as transferrin receptor, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor and epidermal growth factor receptor [4, 8, 17]. However, increasing evidence has showed that channels and transporters are also trafficked through Rab dependent pathways [10, 14]. Since endosomal compartments can be somewhat fluid, it can be difficult to distinguish in which compartments a protein resides. Both Rabs 11 and 8 have been localized to the TGN as well as the pericentriolar recycling endosome [2, 21, 29], however Rab 8 is thought to be the predominant mediator of protein trafficking from the TGN to the plasma membrane [29]. Differentiating the role of Rab 11 versus Rab 8 in affecting DAT function helps determine the specific compartment to which DAT is localized.
Numerous studies have focused on DAT internalization, usually following PKC activation or substrate treatment, while fewer studies demonstrate trafficking proteins necessary for constitutive DAT recycling. The mechanism of neurotransmitter transporter fusion to the plasma membrane has been a strong research area of interest. It is now evident that soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins are important regulators of monoamine transporter trafficking [18]. The SNARE protein syntaxin 1A has been shown to interact with the N-terminus of DAT and regulate its activity [3, 11]. Furthermore, in the norepinephrine transporter (NET), syntaxin has been demonstrated to regulate clearance capacity of norepinephrine in a calcium and PKC-dependent manner [26, 27]. We found that syntaxin 1A is important for the substrate-stimulated translocation of DAT to the plasma membrane [7]. It is generally believed that SNARE proteins are necessary for the fusion of vesicles to the plasma membrane but that other proteins such as rabs may be necessary to mediate the delivery and tethering of vesicles to allow for fusion. A potential role for Rab 11 could be to assist in fusion of DAT-containing vesicles to the plasma membrane by delivery or tethering. Since Rab 11CA mimics the rapid trafficking of DAT to the surface upon amphetamine (AMPH) treatment [7], it is tempting to speculate that AMPH may act through a Rab-11 dependent pathway to traffic DAT from the recycling endosome to the plasma membrane. A similar mechanism has been demonstrated with another transporter GLUT4 which requires Rab 11 for transport from the endocytic recycling compartment to the GLUT4 storage vesicles which are primed for GLUT4 translocation to the plasma membrane [10].
However, it remains to be determined whether Rab 11 functions to increase the rate of DAT exocytosis or slow down the rate of endocytosis. Also, further studies are needed to determine whether Rab 11 directly interacts with DAT or whether there is an indirect interaction via a Rab 11 binding protein.
Overall, these data demonstrate that Rab 11 is an integral part of constitutive DAT trafficking.
Acknowledgments
This work was supported by National Institutes of Health Grants: DA 011697 (MEG), and T32-GM07767 (CAF). We would like to thank Dr. Jonathan A. Javitch (Columbia University, NY) for the HA-EL2-DAT construct and Dr. Karley Little (Baylor College of Medicine, TX) for the DAT-N2A cell line. Finally, we would like to acknowledge the pharmacology confocal microscope facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cheryse A. Furman, University of Michigan Medical School, Department of Pharmacology, Ann Arbor, MI 48109
Charles B. Lo, University of Cincinnati College of Medicine, Department of Pharmacology and Cell Biophysics, Cincinnati, OH 45267-0575
Stephanie Stokes, University of Michigan Medical School, Department of Pharmacology, Ann Arbor, MI 48109.
Jose A. Esteban, Centro de Biología Molecular “Severo Ochoa” (CSIC/Universidad Autónoma de Madrid)
Margaret E. Gnegy, University of Michigan Medical School, Department of Pharmacology, Ann Arbor, MI 48109
References
- [1].Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- [2].Ang AL, Folsch H, Koivisto UM, Pypaert M, Mellman I. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J Cell Biol. 2003;163:339–350. doi: 10.1083/jcb.200307046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, Zhang M, Sen N, Colbran RJ, Gnegy ME, Gether U, Javitch JA, Erreger K, Galli A. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol Pharmacol. 2008;74:1101–1108. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ceresa BP. Regulation of EGFR endocytic trafficking by rab proteins. Histol Histopathol. 2006;21:987–993. doi: 10.14670/HH-21.987. [DOI] [PubMed] [Google Scholar]
- [5].Chen N, Zhen J, Reith ME. Mutation of Trp84 and Asp313 of the dopamine transporter reveals similar mode of binding interaction for GBR12909 and benztropine as opposed to cocaine. J Neurochem. 2004;89:853–864. doi: 10.1111/j.1471-4159.2004.02386.x. [DOI] [PubMed] [Google Scholar]
- [6].Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- [7].Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW, Gnegy M. Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J Neurosci. 2009;29:3328–3336. doi: 10.1523/JNEUROSCI.5386-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gerges NZ, Backos DS, Esteban JA. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J Biol Chem. 2004;279:43870–43878. doi: 10.1074/jbc.M404982200. [DOI] [PubMed] [Google Scholar]
- [9].Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- [10].Ishikura S, Koshkina A, Klip A. Small G proteins in insulin action: Rab and Rho families at the crossroads of signal transduction and GLUT4 vesicle traffic. Acta Physiol (Oxf) 2008;192:61–74. doi: 10.1111/j.1748-1716.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- [11].Lee KH, Kim MY, Kim DH, Lee YS. Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem Res. 2004;29:1405–1409. doi: 10.1023/b:nere.0000026404.08779.43. [DOI] [PubMed] [Google Scholar]
- [12].Li LB, Reith ME. Modeling of the interaction of Na+ and K+ with the binding of dopamine and [3H]WIN 35,428 to the human dopamine transporter. J Neurochem. 1999;72:1095–1109. doi: 10.1046/j.1471-4159.1999.0721095.x. [DOI] [PubMed] [Google Scholar]
- [13].Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez-Lluhi JA, Van Genderen KM, Martens JR. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem. 2007;282:29612–29620. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- [15].Melikian HE. Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol Ther. 2004;104:17–27. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- [16].Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mohrmann K, van der Sluijs P. Regulation of membrane transport through the endocytic pathway by rabGTPases. Mol Membr Biol. 1999;16:81–87. doi: 10.1080/096876899294797. [DOI] [PubMed] [Google Scholar]
- [18].Quick MW. The role of SNARE proteins in trafficking and function of neurotransmitter transporters. Handb Exp Pharmacol. 2006:181–196. doi: 10.1007/3-540-29784-7_9. [DOI] [PubMed] [Google Scholar]
- [19].Robertson SD, Matthies HJ, Galli A. A Closer Look at Amphetamine-Induced Reverse Transport and Trafficking of the Dopamine and Norepinephrine Transporters. Mol Neurobiol. 2009 doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sorkina T, Doolen S, Galperin E, Zahniser NR, Sorkin A. Oligomerization of dopamine transporters visualized in living cells by fluorescence resonance energy transfer microscopy. J Biol Chem. 2003;278:28274–28283. doi: 10.1074/jbc.M210652200. [DOI] [PubMed] [Google Scholar]
- [23].Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6:157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- [24].Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis. J Neurosci. 2006;26:8195–8205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-5-reviews3007. REVIEWS3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sung U, Apparsundaram S, Galli A, Kahlig KM, Savchenko V, Schroeter S, Quick MW, Blakely RD. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J Neurosci. 2003;23:1697–1709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sung U, Blakely RD. Calcium-dependent interactions of the human norepinephrine transporter with syntaxin 1A. Mol Cell Neurosci. 2007;34:251–260. doi: 10.1016/j.mcn.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J. 2008;413:201–215. doi: 10.1042/BJ20080723. [DOI] [PubMed] [Google Scholar]
- [29].Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]