Abstract
Little is known of combined utility of magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) biomarkers for prediction of Alzheimer’s disease (AD) and longitudinal data is scarce. We examined these biomarkers at baseline and longitudinally in incipient AD. Forty-five subjects [21 controls (NL-NL), 16 stable MCI (MCI-MCI), 8 MCI who declined to AD (MCI-AD)] received MRI and lumbar puncture at baseline and after 2 years. CSF measures included total and phosphorylated tau (T-tau, P-tau231), amyloid-β (Aβ42/Aβ40) and isoprostane. Voxel-based morphometry identified gray matter concentration (GMC) differences best distinguishing study groups and individual GMC values were calculated. Rate of medial temporal lobe (MTL) atrophy was examined using regional boundary shift (rBS) method. At baseline, for MRI, MCI-AD showed reduced GMC-MTL, and for CSF higher CSF T-tau, P-tau231, IP and lower Aβ42/Aβ40 as compared with MCI-MCI or NL-NL. Longitudinally, rBS-MTL atrophy was higher in MCI-AD than in either MCI-MCI or NL-NL, particularly in the left hemisphere. CSF data showed longitudinally greater increases of isoprostane in MCI-AD as compared with NL-NL. Combining baseline CSF-P-tau231 and GMC-MTL significantly increased overall prediction from 74% to 84% (pstep <0.05). These results provide support for including multiple modalities of biomarkers in the identification of memory clinic patients at increased risk for dementia.
Keywords: Alzheimer’s disease, brain atrophy, cerebrospinal fluid biomarkers, early diagnosis
INTRODUCTION
As new Alzheimer’s disease (AD) treatments become available, the diagnosis of AD at the Mild Cognitive Impairment (MCI) stage becomes particularly important. Identification of suitable patients for early and high-risk treatments requires both high sensitivity and specificity. Recent data suggests that the combination of magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) modalities can improve preclinical diagnostic accuracy of AD [1–3].
MRI estimates of medial temporal lobe (MTL) atrophy are among the most sensitive predictors of decline from MCI to AD although they are not specific to AD (for a review, see [4]). CSF biomarkers are both promising for early diagnosis and, in the case of hyperphosphorylated tau (P-tau) and amyloid-β (Aβ), measurements afford AD pathological specificity [5,6]. The most promising CSF biomarkers include: P-tau total tau (T-tau), Aβ1–42 and Aβ1–40, and isoprostanes (IP) (for a review, see [7]). However, little is known of the combined utility of imaging and CSF biomarkers in the prediction and longitudinal course of the transitions between MCI and AD.
This longitudinal MRI and CSF study investigated the relationships of five CSF biomarkers for AD in combination with two automated MRI methods for assessing brain atrophy in three clinical groups: MCI who declined to AD (MCI-AD), stable MCI subjects (MCI-MCI), and clinically stable normal elderly controls (NL-NL). This study tested the hypothesis that the MRI and CSF biomarkers are complementary predictors of the decline from MCI to AD.
MATERIAL AND METHODS
Subjects
From a pool of 56 consecutive AD research center patients and controls, who completed a 2-year, 2-timepoint examination with CSF and MRI, 45 were selected (see below). The standardized longitudinal protocol included: history, physical, neurological, neuropsychological, and psychiatric evaluations, and clinical laboratory analysis including apolipoprotein E (APOE) genotyping. Subjects with at least one APOE ε4 allele were classified as “APOE4 carriers”. All subjects signed IRB approved informed consent.
NL subjects were highly functioning individuals, defined as having a Clinical Dementia Rating (CDR [8]) score of 0, a Global Deterioration Scale (GDS [9]) score of 1 or 2 and a Mini-Mental State Examination (MMSE) score ≥ 28. The diagnosis of MCI was based on: progressive cognitive (typically memory) complaints corroborated by an informant, a CDR = 0.5, GDS score = 3 and clinically recognizable cognitive impairment without fulfilling either the DSM-IV [10] or NINCDS-ADRDA [11] criteria for dementia or AD. AD patients fulfilled the DSM-IV criteria for dementia and the NINCDS-ADRDA criteria for probable AD, and had GDS scores ≥4 and CDR ≥ 1.0.
Individuals with medical conditions or a history of significant conditions that affect brain structure or function (e.g; other neurodegenerative or metabolic diseases) were not enrolled in the study. Additionally, all subjects with MRI-based evidence of cortical infarctions or mass lesions as assessed with T1- and T2-weighted images at baseline were excluded. 11 enrolled subjects from the total pool (n = 56) were excluded from the study: 9 subjects due to significant motion artifact on MRI preventing accurate brain measurements (6 NL and 3 MCI-MCI) and 2 MCI patients due to a change in clinical diagnosis at follow-up (1 Normal Pressure Hydrocephalus, 1 Frontotemporal Dementia).
Three study groups were created: a normal control group that remained normal (NL-NL: n = 21), an MCI patient group that did not deteriorate (MCI-MCI: n = 16); and an MCI patient group that declined to AD (MCI-AD: n = 8). The creation of the study groups was based solely on the clinical diagnosis and was blinded to all CSF, quantitative MRI, and APOE data.
MRI acquisitions
Images were acquired on a 1.5T GE Signa Imager scanner (General Electric, Milwaukee, USA). All subjects received a diagnostic and a research MRI study. The diagnostic study was used to satisfy the exclusion criteria and included 2mm coronal T2 -weighted and contiguous 3mm Fluid-attenuated Inversion-Recovery fast spin echo axial images. The research study was used for quantitative longitudinal measurements of brain atrophy and included a high-resolution T1-weighted 3D fast gradient-echo acquired in a coronal orientation which encompassed the entire brain without gap or wrap artifact (TR:35 ms, TE:9 ms, FA:60°, FOV:18 × 18 cm, matrix:256 × 192), obtaining 124 coronal slices spanning the entire brain with a 1.7mm section thickness.
The MR images were stripped of demographic information and were transferred to our central image data bank and satellite workstations for further processing. Image analysis was done using proprietary MIDAS software running on a UNIX operating system [12].
Image preprocessing
Voxel Based Morphometry (VBM) analysis
All T1-weighted MRI images were analyzed using Statistical Parametric Mapping (SPM’2, Wellcome Department of Cognitive Neurology, London, UK). Briefly, the method allows assessment of differences in gray matter concentrations (GMC) on a voxel-wise basis [13]. After correcting all T1 images for signal intensity distortions the MRI scans were realigned and spatially normalized to a reference MRI image in the standardized anatomical space and resliced with sinc interpolation to a final voxel size of 1.5 × 1.5 × 1.5 mm. The normalized MRI scans were then segmented into gray matter (GM), white matter and CSF images, after removal of all non-brain voxels and after applying an image intensity non-uniformity correction [13]. The GM images were retained for analyses and re-normalized to a customized GM template by using the same normalization parameters described above. To preserve the volume of a particular tissue compartment within each voxel, the images were modulated by the Jacobian determinants derived from the spatial normalization, and smoothed using an 8-mm FWHM isotropic Gaussian kernel. Normalization for global differences in voxel intensity across scans was effected by inclusion of the global mean GM voxel value as a covariate, while preserving regional differences in GM intensity. Additionally, individual differential GMC images (follow-up GMC minus baseline GMC) were calculated for each study participant. In order to simplify the description of the VBM results, all local maxima within the medial temporal lobe (including hippocampus, parahippocampal gyrus, entorhinal cortex and amygdala) are collectively described as the “MTL”.
Medial temporal lobe atrophy rate assessment
The longitudinal MTL atrophy rate, using the regional boundary shift (rBS) method [14] as described by Rusinek et al. was computed from the coregistered and intensity normalized MR images. The method extends the original whole-brain protocol of Fox et al [15]. This protocol assesses the proportion of CSF and brain tissue per voxel over time and thus provides an atrophy measure. The atrophy rates were defined as the follow-up minus the baseline brain tissue volume, divided by the baseline volume and by the time interval between the two MRI scans (%/year) [16].
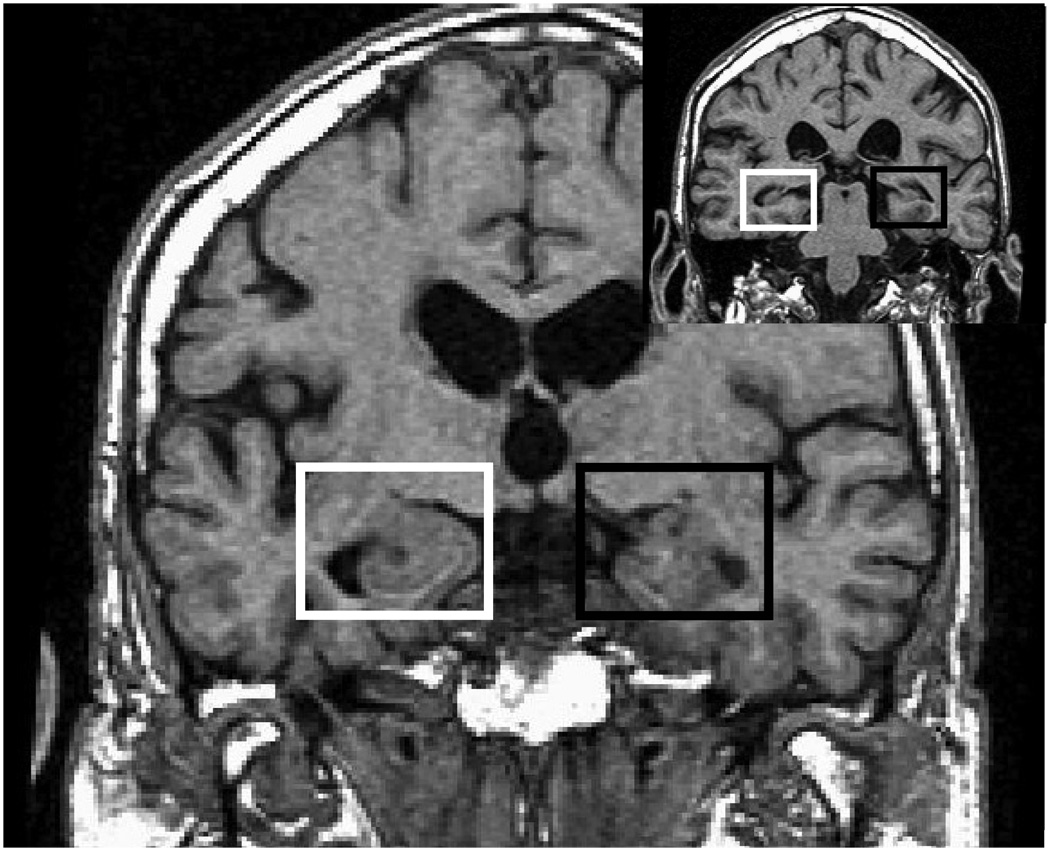
The rBS-MTL was assessed for the right and left MTL using standardized semi-automated regions of interest (ROIs) using published protocols with intra-rater reliability >90% [14]. The rectangular solid-shaped bilateral MTL ROI was generated using the following criteria [14]. Horizontal and vertical box sizes were defined as 0.25 times the left-to-right and craniocaudal dimensions of the cranial cavity. The anterior plane was defined as 4.5 mm posterior to the frontal-temporal junction and the posterior plane was defined by the anterior crux of the fornix. The operator placed the MTL box on all coronal images in the anterior to posterior MTL range by manually selecting within each scan section centers of both hippocampi with a mouse click. These centers became the centers of the left and the right MTL boxes (see Fig. 1). The contents of the rBS-MTL ROI included nearly the entire hippocampus, and parts of the piriform lobe, entorhinal cortex, amygdala and parahippocampal gyrus. ROIs were drawn by single operator (M.B.) and independently verified by a co-author.
Fig. 1.
Placement of right (white box) and left (black box) MTL ROIs on sinc interpolated, coronal T1 MRI image cut to “pathological” angle (offset shows location of the ROI on one of the posterior slices).
CSF collection and analysis
After an overnight fast, at 11:00 A.M. and on the same day as the MRI scan, CSF was acquired using a 25-gauge Sprott lumbar puncture needle. Samples were centrifuged, aliquoted to polypropylene tubes and stored at −80°C. Assays were blinded to clinical data. Analysis of both baseline and follow-up samples was performed in the same batch assay without refreezing.
A sandwich ELISA assay was used to detect tau phosphorylated at threonine 231 (P-tau231) using tau-1 and CP-27 antibodies in the first step and P-tau231-specific CP9 antibody in the second step [17]. CSF T-tau levels were determined using a commercially available INNOTEST hTAU antigen kit (Innogenetics®, Gent, Belgium). The CSF Aβ levels (Aβ40 and Aβ42) were measured using a monoclonal antibody 6E10 (specific to an epitope present on Aβ-16) and to rabbit antisera to Aβ40 and Aβ42 respectively, in a double antibody sandwich ELISA [18]. As some prior studies demonstrated that the Aβ42/Aβ40 ratio is superior to either the Aβ42 or the Aβ40 level in discriminating AD from NL or from other dementias [6], for predicting future MCI [19] or MCI transition to AD [20], the ratio was used in this study. CSF levels of IP (8,12-iso-iPF2α-VI) were assayed by negative ion chemical ionization gas chromatography/mass spectrometry, after CSF samples were spiked with a fixed amount of internal standard (d4-8,12-iso-iPF2α-VI), extracted on a C18 cartridge column and purified by thin-layer chromatography [21].
Statistical analysis
All results for the SPM analyses are presented in terms of clusters of significant differences in gray matter concentration (GMC) between study groups. The General Linear Model/univariate analysis with pairwise post-hoc t-tests was used to test for GMC cross-sectional and longitudinal differences across study groups. Results were assessed over the whole-brain at a conservative p < 0.001 level, and uncorrected for multiple comparisons. Anatomical location of areas showing GMC effects was described using the Talairach and Tournoux coordinates [22].
Analysis of covariance (ANCOVA) with subsequent post-hoc Tukey tests was used to examine between-group differences in demographic data, rBS-MTL atrophy and CSF biomarker levels. Significant baseline effects were tested in binary logistic regression models as predictors of decline among MCI patients. Logistic regression prediction models were also used to characterize the incremental diagnostic classification properties of the best univariate CSF and MRI measures. p values <0.05 were considered significant. All analyses were performed with SPSS 12.0 (SPSS, Chicago, IL 2004).
RESULTS
The demographic data of the study participants are presented in Table 1. During the 2 year observation, 8 (33%) out of the 24 evaluable MCI subjects seen at baseline declined to AD and 16 retained the diagnosis of MCI. None of the 21 normal controls changed diagnostic group. There were no significant age, education, or length of observation period differences between the study groups (p > 0.05; see Table 1). MCI-AD patients had lower MMSE scores than MCI-MCI patients at follow-up (p < 0 05) and against controls at both baseline and follow-up (p < 0.01). MCI-AD patients had a higher prevalence of APOE4 carriers than either stable MCI or control (p < 0.05). Both MCI-AD and control groups demonstrated a higher prevalence of females than MCI-MCI (χ2 = 9.17,p < 0.05). As previous brain studies show age effects [23], age (in addition to APOE genotype and gender) was controlled for in the subsequent analyses.
Table 1.
Demographics of study participants
| NL-NL (n = 21) | MCI-MCI (n = 16) | MCI-AD (n = 8) | |
|---|---|---|---|
| age at baseline | 65.0 ± 10.0 | 71.1 ± 6.9 | 70.3 ± 8.3 |
| ApoE4 (carriers/non-carriers) | 6/15 | 6/10 | 7/1a |
| Gender (males/females) | 6/15 | 11/5b | 1/7 |
| education [years] | 15.0 ± 3.8 | 14.4 ± 3.6 | 12.3 ± 3.2 |
| observation period [years] | 2.1 ± 0.7 | 1.7 ± 0.5 | 2.0 ± 0.5 |
| MMSE score at baseline [points] | 29.7 ± 0.5 | 28.4 ± 1.7 | 27.6 ± 1.9c |
| MMSE score at follow-up [points] | 29.3 ± 1.1 | 28.9 ± 1.3 | 24.9 ± 2.7c |
Values for continuous variables are Mean ± SD.
indicates value significantly different than in NL-NL and MCI-MCI groups.
indicates value significantly different than NL-NL and MCI-AD groups.
indicates value significantly different than in NL-NL group and MCI-MCI.
Voxel-based gray matter concentration results
Baseline effects
Univariate ANOVA over the 3 study groups at baseline showed several GMC clusters in the right hemisphere (including MTL, superior, middle and inferior temporal gyri) and in the left (including MTL and posterior cingulate gyrus). Similarly, at follow-up the analysis detected clusters in the right (including MTL, superior and inferior temporal gyri) and in the left hemisphere (including MTL and posterior cingulate gyrus).
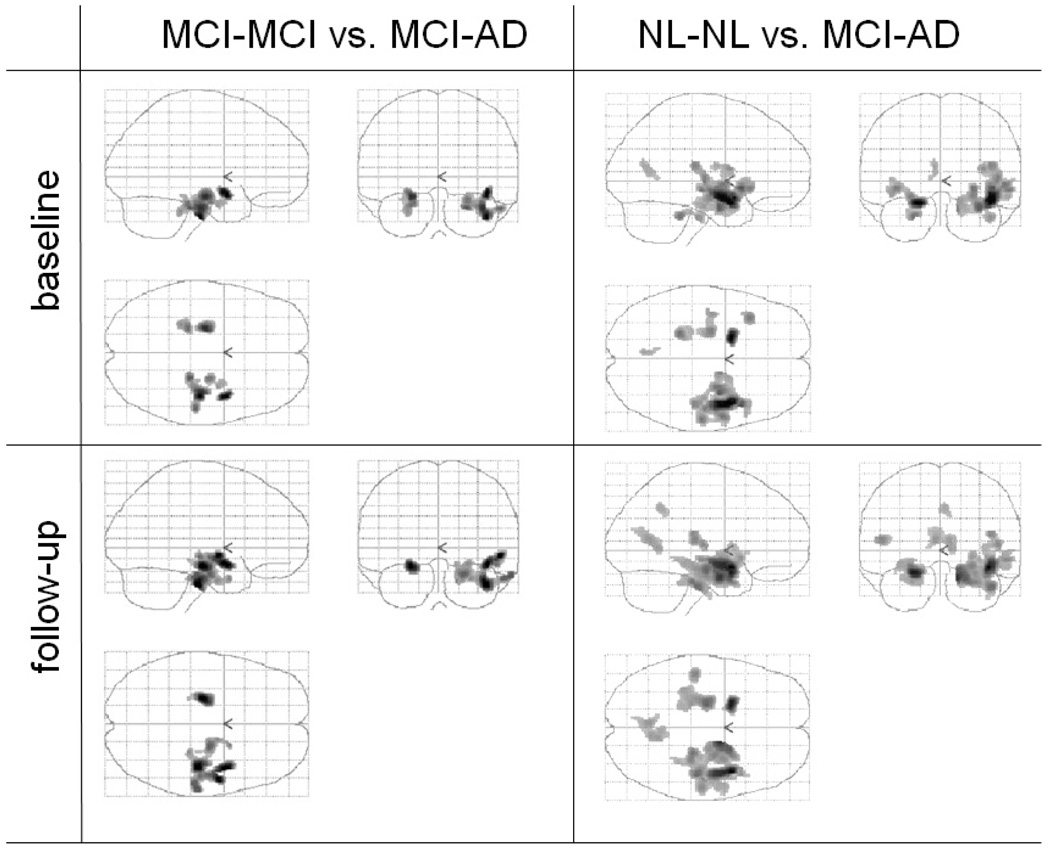
Post-hoc analysis showed that MCI-AD subjects as compared with MCI-MCI, at baseline demonstrated reduced GMC bilaterally in MTL and in right inferior temporal gyrus (for local maxima and Brodman areas see Table 2, Fig. 2). Similar effects were found at the follow-up. Comparison of NL-NL with MCI-AD subjects at baseline showed bilateral MTL effects with superior and middle temporal gyri in the right hemisphere and inferior frontal and posterior cingulate gyri in the left hemisphere. Similar effects were found at follow-up (Table 2, Fig. 2). No significant differences in GMC between NL-NL and MCI-MCI groups were detected at either baseline or follow-up.
Table 2.
Voxel-based morphometry: local maxima and Brodman areas (BA) of clusters of significant baseline and follow-up differences in gray matter concentration between MCI-MCI and MCI-AD and between NL-NL and MCI-AD
| Cluster extent (Ke) | Brain Region | coordinates (x, y, z) | z value | p | |
|---|---|---|---|---|---|
| MCI-MCI vs. MCI-AD | BASELINE | ||||
| 1108 | Right inferior temporal gyrus, BA 20 | (+35, −24, −33) | 4.05 | p <0.001 | |
| Right parahippocampal gyrus, hippocampus | (+25, −15, −21) | 3.73 | p <0.001 | ||
| 359 | Left parahippocampal gyrus, BA 28 | (−21, −20, −17) | 4.36 | p <0.001 | |
| Left Parahippocampal gyrus, hippocampus | (−26, −15, −20) | 3.88 | p <0.001 | ||
| FOLLOW-UP | |||||
| 1152 | Right inferior temporal gyrus, BA 20 | (+41, −22, −28) | 4.22 | p <0.001 | |
| Right parahippocampal gyrus, hippocampus | (+26, −13, −19) | 4.00 | p <0.001 | ||
| 364 | Left parahippocampal gyrus, BA 28 | (−24, −18, −13) | 4.18 | p <0.001 | |
| Left parahippocampal gyrus, hippocampus | (−24, −13, −16) | 4.15 | p <0.001 | ||
| NL-NL vs. MCI-AD | BASELINE | ||||
| 2978 | Right superior temporal gyrus, BA 38 | (+42, +4, −17) | 4.89 | p <0.001 | |
| Right middle temporal gyrus, BA 21 | (+56 −20 −6) | 3.89 | p <0.001 | ||
| Right parahippocampal gyrus, BA 28 | (+17, −7, −14) | 3.57 | p <0.001 | ||
| Right parahippocampal gyrus, hippocampus | (+30, −12, +21) | 3.36 | p <0.001 | ||
| 218 | Left parahippocampal gyrus, BA 34 | (−18, +4, −17) | 4.87 | p <0.001 | |
| 189 | Left inferior frontal gyrus, BA 47 | (−38, +20, −6) | 3.84 | p <0.001 | |
| 357 | Left amygdala | (−24, −12, −12) | 3.80 | p <0.001 | |
| 109 | Left posterior cingulate gyrus, BA 31 | (−5, −65, 16) | 3.47 | p <0.001 | |
| 2978 | Right superior temporal gyrus, BA 38 | (+42, +4, −17) | 4.89 | p <0.001 | |
| FOLLOW-UP | |||||
| 3612 | Right superior temporal gyrus, BA 38 | (+41, +5, −13) | 5.06 | p <0.001 | |
| 138 | Right limbic lobe, uncus, BA 34 | (+20, +2, −20) | 4.26 | p <0.001 | |
| Right superior temporal gyrus, BA 22 | (+48, −17, +6) | 3.80 | p <0.001 | ||
| 280 | Left parahippocampal gyrus, BA 34 | (−23, +5, −17) | 4.86 | p <0.001 | |
| 794 | Left parahippocampal gyrus BA 27 | (−24, −30, −8) | 3.51 | p <0.001 | |
| 552 | Left posterior cingulate gyrus BA 30 | (−5, −61, +13) | 3.64 | p <0.001 | |
Fig. 2.
Voxel-based morphometry results: clusters with significantly different GMC in MCI-MCI subjects vs. MCI-AD subjects (A) and NL-NL subjects vs. MCI-AD subjects (B) at baseline and at follow-up.
Longitudinal
The between-group analysis of the differential GMC images (follow-up minus baseline) did not detect differences in GMC. Further, the unprotected paired-t-tests analyses within each study group also failed to reach statistical significance.
In summary, at baseline and follow-up for the VBM analyses, only the MTL showed consistent bilateral effects separating decliners from the other groups.
Longitudinal MRI rBS-determined atrophy rates
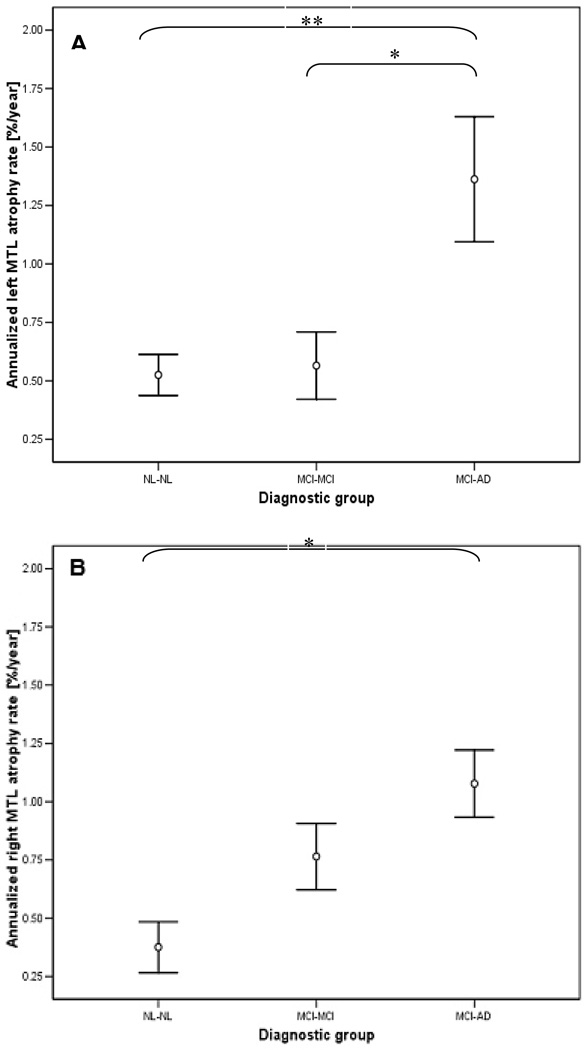
Given our hypothesized longitudinal MTL effects, the MTL region was specifically examined with the rBS method. After controlling for confounds, annualized atrophy rates for both the left (F(2,36) = 9.5, p < 0.001) and right rBS-MTL (F(2,36) = 4 4 p < 0.05) differed among three study groups (see Table 3 and Fig. 3). Post-hoc analysis showed that atrophy rates in the left MTL were higher in MCI-AD patients than in either NL-NL or MCI-MCI (p < 0.01). Atrophy rates in the right MTL were only higher in MCI-AD patients as compared with NL-NL (p < 0 01) For neither hemisphere were the annual MTL atrophy rates different between MCI-MCI patients and NL-NL (p > 0.05)
Table 3.
Baseline and follow-up measures of CSF biomarkers and annualized rates of change for CSF and MRI measures by diagnostic group
| NL-NL (n = 21) | MCI-MCI (n = 16) | MCI-AD (n = 8) | overall group difference (ANCOVA) | |
|---|---|---|---|---|
| Baseline Measures | ||||
| T-tau level [pg/ml] | 312 ± 126 | 398 ± 202 | 643 ± 147a,b | p < 0.01 |
| P-tau231 level [pg/ml] | 15.1 ± 15.0 | 21.8 ± 26.5 | 46.1 ± 20.6a,b | p < 0.01 |
| IP level [pg/ml] | 31.5 ± 12.3 | 30.7 ± 12.2 | 48.3 ± 12.9a,b | p < 0.01 |
| Aβ42 / Aβ40 ratio* | 12.1 ± 4.8 | 10.3 ± 3.4 | 6.8 ± 1.6a,b | p < 0.01 |
| Follow-up Measures | ||||
| T-tau level [pg/ml] | 328 ± 156 | 429 ± 237 | 659 ± 184a,b | p < 0.01 |
| P-tau231 level [pg/ml] | 14.6 ± 15.2 | 24.0 ± 27.5 | 52.8 ± 23.0a,b | p < 0.01 |
| IP level [pg/ml] | 34.6 ± 12.5 | 40.4 ± 26.3 | 60.1 ± 13.9a,b | p < 0.05 |
| Aβ42/Aβ40 ratio* | 11.3 ± 3.2 | 11.3 ± 3.1 | 8.3 ± 5.8 | n.s. |
| Annualized Measures | ||||
| rBS-MTL left [%] | 0.53 ± 0.09 | 0.57 ± 0.14 | 1.36 ± 0.27a,b | p < 0.01 |
| rBS-MTL right [%] | 0.38 ± 0.11 | 0.76 ± 0.14 | 1.08 ± 0.14a | p < 0.05 |
| rBS-MTL left + right [%] | 0.45 ± 0.08 | 0.67 ± 0.14 | 1.22 ± 0.19a,b | p < 0.01 |
| T-tau rate of change [pg/ml/year] | +16.29 ± 16.3 | +31.50 ± 29.0 | +16.13 ± 62.1 | n.s. |
| P-tau231 rate of change [pg/ml/year] | −0.54 ± 1.7 | +2.27 ± 0.8 | +6.67 ± 2.2 | n.s. |
| IP rate of change [pg/ml/year] | +3.10 ± 1.7 | +9.69 ± 4.1 | +11.88 ± 2.0a | p= 0.07 |
| Aβ42/Aβ40 ratio* rate of change | −0.81 ± 1.0 | +1.03 ± 0.5 | +1.57 ± 1.7 | n.s. |
| MMSE rate of change [pts/year] | +0.33 ± 0.2 | +0.50 ± 0.4 | −2.75 ± 1.2a,b | p < 0.01 |
Values are Mean ± SD.
value significantly different than in NL-NL group.
value significantly different than in MCI-MCI group*the values of the Aβ42/Aβ40 ratio are multiplied by 100.
Fig. 3.
rBS atrophy rates in the left (A) and right (B) MTL region for the three diagnostic outcome groups. The bars represent mean value ± SEM. One star indicates p < 0.05, two stars: p < 0.001.
The rBS showed consistent longitudinal MTL effects distinguishing the decliners from the other groups.
CSF biomarkers analysis: baseline, follow-up and longitudinal results
Baseline data
Across all groups, there were significant baseline effects for all CSF analytes (see Table 3). The MCI-AD patients showed significantly higher values of T-tau, P-tau231 and IP levels, and lower values of Aβ42/Aβ40 ratio when compared with either MCI-MCI patients or with NL-NL (p < 0.05; see Table 3). Similar significant effects were found at the follow-up with exception the Aβ42/Aβ40 ratio (see Table 3). No differences were found between MCI-MCI and NL-NL subjects at baseline, or follow-up.
Longitudinal data
Analysis of longitudinal changes in CSF biomarker levels showed a trend for overall between-group differences for IP (p = 0.07; see Table 3). Exploratory post-hoc analysis showed that a significantly greater increase in the IP level was found in MCI-AD patients as compared with NL-NL (p < 0.01). No other CSF biomarker showed longitudinal diagnostic group effects.
In summary, consistent baseline and follow-up effects were found in MCI-AD as compared with the other groups for T-tau, P-tau231 and IP levels. Only IP showed a trend towards a longitudinal effect.
The prediction of decline to AD
Univariate predictors
Logistic regression outcome models classifying MCI-MCI and MCI-AD subjects showed that at baseline, both right and left GMC-MTL values predicted MCI to AD decline with ≥74% accuracy (p < 0.01, see Table 4). All baseline CSF measures discriminated the groups in the logistic regression models (≥70%, p < 0.05, see Table 4).
Table 4.
Significant predictors of MCI to AD decline corrected for confounding measures
| AUC | sensitivity [%] | specificity [%] | overall accuracy [%] | p for the step increase | |
|---|---|---|---|---|---|
| Univariate predictors – MR measures: | |||||
| GMC-MTL (right) at baseline: | 0.83 | 100% | 67% | 78% | p <0.01 |
| GMC-MTL (left) at baseline: | 0.78 | 100% | 60% | 74% | p <0.01 |
| rBS-MTL (left) | 0.71 | 50% | 87% | 74% | p <0.01 |
| rBS-MTL (right) | n/a | n/a | n/a | n/a | p >0.05 |
| Univariate predictors – CSF measures: | |||||
| IP at baseline | 0.75 | 63% | 80% | 74% | p <0.01 |
| P-tau231at baseline | 0.72 | 75% | 73% | 74% | p <0.01 |
| Aβ42/Aβ40 ratio at baseline | 0.73 | 100% | 53% | 70% | p = 0.05 |
| T-tau at baseline | 0.76 | 63% | 73% | 70% | p <0.05 |
| Combined measures (CSF + MR): | |||||
| baseline P-tau231 + GMC-MTL (right) | 0.85 | 100% | 73% | 84% | p <0.05 |
| baseline P-tau231 + rBS-MTL (left) | 0.78 | 50% | 100% | 84% | p <0.05 |
| baseline IP + GMC-MTL (right) | 0.85 | 100% | 67% | 78% | p <0.05 |
| baseline IP + rBS-MTL (left) | 0.85 | 75% | 87% | 78% | p <0.05 |
Multivariate predictors
Multivariate CSF and MRI models were used to test for incremental prediction effects among the significant baseline measures (see Table 4). The results showed that adding the right GMC-MTL to baseline CSF P-tau231 raised the overall prediction accuracy to 84% (pstep < 0.05). Addition of right GMC-MTL to baseline CSF IP levels increased the prediction accuracies to 78% (pstep < 0.05).
Longitudinal correlates of decline
Univariate correlates
Longitudinally, atrophy of the left MTL using the rBS method discriminated MCI-MCI from MCI-AD with 74% overall accuracy (p < 0.01). The right rBS-MTL atrophy and longitudinal changes in CSF biomarkers levels were not significant group discriminators (p > 0.05). No longitudinal correlations were observed between the MRI and CSF biomarkers.
Multivariate correlates
Combining the rBS-MTL atrophy rate with the baseline IP level resulted in a significant increment in the correlation with decline (p < 0.05). Combining the rBS-MTL atrophy rate with the IP change did not result in a significant increment in the correlation with decline (p > 0.05).
Follow-up diagnostic classifications
All follow-up CSF measures with the exception of the Aβ42/40 ratio discriminated the groups in the logistic regression models (≥70%,p < 0.05). Similarly, both right and left GMC-MTL at follow-up discriminated the groups in the logistic regression models (≥78%, p < 0.05).
DISCUSSION
This longitudinal study examined both CSF biomarkers and MRI measures of brain atrophy in the prediction and course of the MCI to AD conversion. We applied an automated regional boundary shift algorithm and voxel-based morphometry.
An important finding from our study was the observation that individually, GMC-MTL atrophy measure and CSF biomarkers for tauopathy are both useful in the separation of MCI-AD from both normal and non-declining MCI. Further, the combination of the baseline, AD-specific CSF P-tau231 [5] with the baseline MRI medial temporal lobe gray matter atrophy measure improved the prediction accuracy for the MCI to AD conversion from 74% to 84%. Although previous studies of from our lab [24,25] and of Bouwman et al. [2] demonstrated the added value of combining the CSF and MRI biomarkers in the diagnostic workup of MCI subjects, to our knowledge the present longitudinal study is the first to evaluate the conversion of MCI to AD using 2-timepoints to estimate CSF biomarker levels and quantitative measures of brain atrophy.
We previously showed that both longitudinal CSF and MRI measures in MCI-MCI correlate with psychometric progression and with each other [3,26]. In the present study, we observed the conversion of MCI to AD and demonstrated that MRI-MTL and CSF measures – while accurate and additive predictors of decline from MCI to AD – are not related to each other either at cross-section or longitudinally.
MRI effects
Prior computed tomography (CT) and MRI studies demonstrated that hippocampal atrophy (assessed either qualitatively or volumetrically) is one of the earliest features predicting among MCI patients future AD [26–28]. Similarly, our VBM analysis identified clusters of decreased MTL gray matter density in MCI patients who progressed to AD both at both timepoints. These results largely confirm previous findings of reduced MTL GMC in subjects with AD [29] and MCI [30]. One of the major findings of the study is that at an individual level, the GMC-MTL can serve as a valuable predictor of MCI to AD decline. Other studies suggest that MTL atrophy is likely due to neuronal loss [31] secondary to neurofibrillary tangle pathology which is toxic to neurons and known to affect the entorhinal cortex and hippocampus in pre-dementia [32] and even presymptomatic stages [33]. Another imaging study showed that other cortical regions (including lateral temporal and parietal lobes) were predictive of MCI to AD decline [34]. However, in that study the observation period was longer than ours and different image analysis procedures were used. In our study, when NL-NL were compared with MCI-AD, temporal and posterior cingulate and frontal cortical regions were also identified.
In our study, no longitudinal differences in GMC were detected, but we did detect differences in rBS-MTL atrophy rates between the groups. In our opinion, the most likely explanation of this finding refers to the pre-processing of the MRI images: the spatial normalization technique used with VBM renders the technique less sensitive to detect subtle atrophy changes within subjects. In contrast, the rBS method works on individual, non-spatially-normalized scans and may be more sensitive to small atrophic changes. Another factor contributing to the different results yielded with the two techniques is that boundary shift method detects changes in CSF volume, while VBM detects changes in gray matter concentration.
Although both left and right rBS-MTL atrophy was greater in the MCI-AD than in controls, only left rBS-MTL differentiated MCI decliners from MCI non-decliners. Our right and left rBS-MTL atrophy rates are within the range reported by others (e.g., approximately 2–3%/year for the MTL structures) [35–37]. The high MTL atrophy rates among MCI-AD suggest that loss of brain tissue in this region is an important feature of AD distinguishing it from controls and stable MCI. The lack of difference in atrophy rates between controls and stable MCI subjects may be explained in several ways. First, MCI is a clinically defined concept and not a biologically defined early AD stage. Thus, the inhomogeneity of the diagnosis of MCI may be expected to differentially affect their atrophy rates. While it is likely that many of the MCI subjects have AD, an unknown fraction of this group is expected to progress to other types of dementia or even revert back to normal. It is also possible that heterogeneity in the NL-NL group accounts for some of the variance. The fact that the MCI-MCI group was also not different from controls with the CSF measures suggests that the subjects in fact did not have early AD or that it was below delectability thresholds.
Interestingly, left rBS-MTL atrophy was better correlated with the transition to AD than right rBS-MTL atrophy, which is consistent with our previous atrophy rate study [16] as well as with the studies of other authors [38,39]. Similarly, left GMC-MTL (but not right) predicted AD in this study.
CSF effects
At baseline, all the CSF measures provided good prediction of incipient AD. The results are consistent with an extensive literature (see [40] for a review), including our previously published study [20]. The best predictions found were P-tau231 and IP. The presence of pathological levels of P-tau231, T-tau, IP and Aβ42/40 ratio in MCI decliners points to the fact that changes in the CSF biochemical composition appear before the onset of clinically overt AD. Prior validation studies show that pathological CSF levels of biomarkers reflect AD pathology: amyloid plaques [41] neurofibrillary tangles [42] and oxidative damage to neuronal cell membranes [43].
We previously reported that only IP showed longitudinal effects [3,20]. In this report, only IP levels showed a trend towards increasing over 2 years in association with the MCI-AD conversion as compared with NL subjects. Since IP is a marker of membrane lipid peroxidation and inflammation, these data continue to suggest that the increase of IP levels in cognitively deteriorating patients reflects progressive neuronal damage [21].
Additive effects
The observed additive effect of MRI and CSF biomarkers offers a potential diagnostic advance. We demonstrated that the combination of baseline levels of either P-tau231 or IP with the right MTL GMC raised the prediction accuracy levels to ≥78%.
There are few published reports where both CSF biomarkers and MRI measures of brain atrophy were applied in diagnosis of AD. In a cross-sectional study by Schoonenboom et al., MTL atrophy was found to contribute to CSF measures of Aβ1–42 and P-Tau181 in incrementing the diagnostic separation of AD and control subjects [1]. Our previous work demonstrated that the longitudinal reductions in the hippocampal volume were associated with progressive elevations in P-Tau231 level and reductions in the Aβ42 [25]. Another study published by our group showed that in NL and MCI subjects, longitudinal increases in IP levels correlated with atrophy in both the left MTL and left inferior temporal gyrus regions [3].
There are several limitations of our study. First, the small sample size reduces statistical power and restricts detection of other effects. The results warrant a replication with a larger sample and perhaps testing in a community based random study predicting cognitive decline and AD. Second, in dementia centers the clinical diagnoses of AD correspond with the postmortem diagnoses about 85% of the time [44] and in our study the diagnosis of AD was not confirmed with pathology. Third, the observed CSF data combined with MRI needs to be evaluated for AD diagnostic specificity with diverse patient groups at the MCI stage, as prior diagnostic specificity studies have not included MCI patients. It will be important to learn if other forms of dementia will be correctly discriminated at their MCI stages. Fourth, the generalizability of the study results is restricted because of known selection bias of a memory clinic population. Nevertheless, our results probably will generalize to other memory clinics as our observed 30% decline rate over two years for the MCI subjects is consistent other AD center studies [45].
CONCLUSION
The high diagnostic accuracy of both MRI-MTL and AD CSF biomarkers to identify incident AD from MCI patients is improved when both measurements are combined in a prediction model. These results provide support for including CSF and MRI biomarkers in the evaluation of memory clinic patients, a position advocated in recent reviews [46]. Our data suggest that in the near future, both imaging and biomarker identification of preclinical AD subjects will be a part of pharmacologic and non-pharmacologic treatment and prevention strategies.
ACKNOWLEDGEMENTS
The authors thank Dr. Martin J. Sadowski for diagnostic evaluations and Drs. Elizabeth Javier and Yi Li for expert advice on image analysis. This study was supported by the following grants: NIH-NIA AG12101, AG08051, AG03051 and CRR MO1RR0096.
Dr. Zinkowski owns stock and stock options in Applied NeuroSolutions. Dr. de Leon serves as a consultant to and/or receives funding support from Baxter, Bayer, Elan, Forest labs, and Neuroptix. Dr. Pratico serves on the Advisory Board for Eisai. Dr. Blennow receives funding support from Innogenetics. Dr. Wallin serves on the advisory board of Lundbeck and has received lecture fees from GSK, Lundbeck, Pfizer and Novartis.
Part of this work was presented at the 60th Annual Meeting of the American Academy of Neurology, Chicago, IL, April 2008.
References
- 1.Schoonenboom NS, van der Flier WM, Blankenstein MA, Bouwman FH, Van Kamp GJ, Barkhof F, Scheltens P. CSF and MRI markers independently contribute to the diagnosis of Alzheimer’s disease. Neurobiol Aging. 2008;29:669–675. doi: 10.1016/j.neurobiolaging.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Bouwman FH, Schoonenboom SN, van der Flier WM, van Elk EJ, Kok A, Barkhof F, Blankenstein MA, Scheltens P. CSF biomarkers and medial temporal lobe atrophy predict dementia in mild cognitive impairment. Neurobiol Aging. 2007;28:1070–1074. doi: 10.1016/j.neurobiolaging.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 3.de Leon MJ, Mosconi L, Li J, De Santi S, Yao Y, Tsui WH, Pirraglia E, Rich K, Javier E, Brys M, Glodzik L, Switalski R, Saint Louis LA, Pratico D. Longitudinal CSF isoprostane and MRI atrophy in the progression to AD. J Neurol. 2007;254:1666–1675. doi: 10.1007/s00415-007-0610-z. [DOI] [PubMed] [Google Scholar]
- 4.de Leon MJ, Golomb J, Convit A, DeSanti S, McRae TD, George AE. Measurement of medial temporal lobe atrophy in diagnosis of Alzheimer’s disease. Lancet. 1993;341:125–126. doi: 10.1016/0140-6736(93)92610-6. [DOI] [PubMed] [Google Scholar]
- 5.Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, Andreasen N, Hofmann-Kiefer K, DeBernardis J, Kerkman D, McCulloch C, Kohnken R, Padberg F, Pirttila T, Schapiro MB, Rapoport SI, Moller HJ, Davies P, Hampel H. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002;59:1267–1272. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- 6.Lewczuk P, Esselmann H, Otto M, Maler JM, Henkel AW, Henkel MK, Eikenberg O, Antz C, Krause WR, Reulbach U, Kornhuber J, Wiltfang J. Neurochemical diagnosis of Alzheimer’s dementia by CSF Abeta42, Abeta42/Abeta40 ratio and total tau. Neurobiol Aging. 2004;25:273–281. doi: 10.1016/S0197-4580(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 7.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 9.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 12.Tsui WH, Rusinek H, Van Gelder P, Lebedev S. Analyzing multimodality tomographic images and associated regions of interest with MIDAS. In: Sonka M, Hanson KM, editors. SPIE Medical Imaging 2001: Image Processing. Bellingham, Washington: International Society for Optical Engineering; 2001. pp. 1725–1734. [Google Scholar]
- 13.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 14.Rusinek H, De Santi S, Frid D, Tsui WH, Tarshish CY, Convit A, de Leon MJ. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- 15.Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer’s disease. J Magn Reson Imaging. 1997;7:1069–1075. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- 16.Rusinek H, Endo Y, De Santi S, Frid D, Tsui WH, Segal S, Convit A, de Leon MJ. Atrophy rate in medial temporal lobe during progression of Alzheimer disease. Neurology. 2004;63:2354–2359. doi: 10.1212/01.wnl.0000148602.30175.ac. [DOI] [PubMed] [Google Scholar]
- 17.Kohnken R, Buerger K, Zinkowski R, Miller C, Kerkman D, DeBernardis J, Shen J, Moller HJ, Davies P, Hampel H. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett. 2000;287:187–190. doi: 10.1016/s0304-3940(00)01178-2. [DOI] [PubMed] [Google Scholar]
- 18.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 19.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 20.Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, Glodzik-Sobanska L, De Santi S, Zinkowski R, Mehta P, Pratico D, Saint Louis LA, Wallin A, Blennow K, de Leon MJ. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.08.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratico D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, FitzGerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000;48:809–812. [PubMed] [Google Scholar]
- 22.Talairach J, Tournoux P. Co-planar sterotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- 23.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 24.de Leon MJ, Segal S, Tarshish CY, DeSanti S, Zinkowski R, Mehta PD, Convit A, Caraos C, Rusinek H, Tsui W, Saint Louis LA, DeBernardis J, Kerkman D, Qadri F, Gary A, Lesbre P, Wisniewski T, Poirier J, Davies P. Longitudinal cerebrospinal fluid tau load increases in mild cognitive impairment. Neurosci Lett. 2002;333:183–186. doi: 10.1016/s0304-3940(02)01038-8. [DOI] [PubMed] [Google Scholar]
- 25.de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, Rusinek H, Li J, Tsui W, Saint Louis LA, Clark CM, Tarshish C, Li Y, Lair L, Javier E, Rich K, Lesbre P, Mosconi L, Reisberg B, Sadowski M, DeBernadis JF, Kerkman DJ, Hampel H, Wahlund LO, Davies P. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27:394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Convit A, de Asis J, de Leon MJ, Tarshish CY, De Santi S, Rusinek H. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer’s disease. Neurobiol Aging. 2000;21:19–26. doi: 10.1016/s0197-4580(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 27.de Leon MJ, George AE, Stylopoulos LA, Smith G, Miller DC. Early marker for Alzheimer’s disease: the atrophic hippocampus. Lancet. 1989;2:672–673. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- 28.Jack CR, Jr, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busatto GF, Garrido GE, Almeida OP, Castro CC, Camargo CH, Cid CG, Buchpiguel CA, Furuie S, Bottino CM. A voxel-based morphometry study of temporal lobe gray matter reductions in Alzheimer’s disease. Neurobiol Aging. 2003;24:221–231. doi: 10.1016/s0197-4580(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 30.Pennanen C, Testa C, Laakso MP, Hallikainen M, Helkala EL, Hanninen T, Kivipelto M, Kononen M, Nissinen A, Tervo S, Vanhanen M, Vanninen R, Frisoni GB, Soininen H. A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2005;76:11–14. doi: 10.1136/jnnp.2004.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobinski M, Wegiel J, Wisniewski HM, Tarnawski M, Reisberg B, De Leon MJ, Miller DC. Neurofibrillary pathology–correlation with hippocampal formation atrophy in Alzheimer disease. Neurobiol Aging. 1996;17:909–919. doi: 10.1016/s0197-4580(97)85095-6. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 33.Morris JC, Storandt M, McKeel DW, Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in "normal" aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 34.Karas G, Sluimer J, Goekoop R, van der Flier W, Rombouts SA, Vrenken H, Scheltens P, Fox N, Barkhof F. Amnestic mild cognitive impairment: structural MR imaging findings predictive of conversion to Alzheimer disease. AJNR Am J Neuroradiol. 2008;29:944–949. doi: 10.3174/ajnr.A0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, Rossor MN. Presymptomatic hippocampal atrophy in Alzheimer’s disease. A longitudinal MRI study. Brain. 1996;119(Pt 6):2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 36.Kaye JA, Swihart T, Howieson D, Dame A, Moore MM, Karnos T, Camicioli R, Ball M, Oken B, Sexton G. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 37.Visser PJ, Scheltens P, Verhey FR, Schmand B, Launer LJ, Jolles J, Jonker C. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 38.Thompson PM, Mega MS, Woods RP, Zoumalan CI, Lindshield CJ, Blanton RE, Moussai J, Holmes CJ, Cummings JL, Toga AW. Cortical change in Alzheimer’s disease detected with a disease-specific population-based brain atlas. Cereb Cortex. 2001;11:1–16. doi: 10.1093/cercor/11.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Thompson PM, Moussai J, Zohoori S, Goldkorn A, Khan AA, Mega MS, Small GW, Cummings JL, Toga AW. Cortical variability and asymmetry in normal aging and Alzheimer’s disease. Cereb Cortex. 1998;8:492–509. doi: 10.1093/cercor/8.6.492. [DOI] [PubMed] [Google Scholar]
- 40.Brys M, Mosconi L, de Santi S, Rich KE, de Leon MJ. CSF biomarkers for mild cognitive impairment. Aging Health. 2006;2:111–121. [Google Scholar]
- 41.DeMattos RB, Bales KR, Parsadanian M, O’Dell MA, Foss EM, Paul SM, Holtzman DM. Plaque-associated disruption of CSF and plasma amyloid-beta (Abeta) equilibrium in a mouse model of Alzheimer’s disease. J Neurochem. 2002;81:229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 42.Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- 43.Montine TJ, Markesbery WR, Morrow JD, Roberts LJ., 2nd Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Ann Neurol. 1998;44:410–413. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- 44.Becker JT, Boller F, Lopez OL, Saxton J, McGonigle KL. The natural history of Alzheimer’s disease. Description of study cohort and accuracy of diagnosis. Arch Neurol. 1994;51:585–594. doi: 10.1001/archneur.1994.00540180063015. [DOI] [PubMed] [Google Scholar]
- 45.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 46.Dubois B. Prodromal Alzheimer’s disease’: a more useful concept than mild cognitive impairment? Curr Opin Neurol. 2000;13:367–369. doi: 10.1097/00019052-200008000-00001. [DOI] [PubMed] [Google Scholar]