Abstract
Background and rationale
Hepatocellular carcinoma (HCC) remains a common cancer worldwide that lacks effective chemoprevention or treatment. Chronic liver disease often leads to impaired hepatic S-adenosylmethionine (SAMe) biosynthesis and mice with SAMe deficiency develop HCC spontaneously. SAMe is anti-apoptotic in normal but pro-apoptotic in cancerous hepatocytes. Our current study investigated SAMe’s effectiveness in prevention and treatment of HCC.
Results
Two weeks after injecting 2.5 million H4IIE cells into the liver parenchyma of ACI rats, they typically form a 1cm tumor. When SAMe (150mg/kg/day) was delivered by continuous intravenous (IV) infusion, hepatic SAMe levels reached 0.7mM (over 10-fold) 24 hours later. This regimen, started one day after injecting H4IIE cells and continued for 10 days, was able to reduce tumor establishment and growth. However, if IV SAMe was started after HCC had already developed, it was ineffective in reducing tumor growth for 24 days. Although plasma SAMe levels remained elevated, hepatic SAMe levels was minimally increased (30% higher). Chronic SAMe administration led to induction of hepatic methyltransferases, which prevented SAMe accumulation. To see if SAMe’s preventive effect on tumor establishment involves angiogenesis, effect of SAMe on angiogenesis genes was studied. SAMe treatment of H4IIE cells altered the expression of several genes with the net effect of inhibiting angiogenesis. These changes were confirmed at the protein level and functionally in human umbilical vein endothelial cells (HUVECs).
Conclusions
SAMe is effective in preventing HCC establishment but ineffective in treating established HCC because of induction of hepatic methyltransferases, which prevents SAMe level to reach high enough to kill liver cancer cells. SAMe’s chemopreventive effect may be related to its pro-apoptotic action and its ability to inhibit angiogenesis.
Keywords: Magnetic Resonance Imaging, Ultrasound, H4IIE cells, cell death, ACI rats
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most frequent cause of cancer death worldwide (1). The incidence of HCC is expected to rise due to the high prevalence of chronic hepatitis C in many parts of the world (2). Only 30% of HCC patients are considered candidates for surgical resection, and the overall 5-year survival for HCC patients is less than 10% (3). Treatments for advanced HCC patients have been largely ineffective although recently Sorafenib, a multikinase inhibitor, was shown in a randomized, placebo-controlled phase III trial (SHARP) to increase the median overall survival from 7.9 to 10.7 months without severe side effects (4). Effective chemoprevention against HCC would be highly desirable, given the grim outlook of HCC. A randomized controlled study showed that acyclic retinoid was able to significantly reduce the incidence of a second primary HCC in patients who had undergone curative removal of their original HCC (5). However, this remains to be confirmed by others.
S-adenosylmethionine (SAMe) is the principal biological methyl donor that is synthesized in all mammalian cells but the liver is the major site of its synthesis and degradation (6). In normal hepatocytes, SAMe synthesis is catalyzed by methionine adenosyltransferase 1A (MAT1A)-encoded isoenzymes MAT I/III (6). SAMe participates in three key biochemical pathways in hepatocytes – transmethylation, trans-sulfuration (which allows the conversion of methionine to cysteine), and polyamine biosynthesis (6). Importantly, most patients with cirrhosis have impaired SAMe biosynthesis due to reduced expression of MAT1A and inactivation of MAT I/III (6). Mice deficient in MAT1A exhibit chronic hepatic SAMe deficiency, are more susceptible to oxidative liver damage and develop spontaneous HCC (7). Interestingly, Pascale and colleagues demonstrated that SAMe administration prevented hepatocarcinogenesis in rodent HCC models (8,9). However, whether SAMe administration can be used to treat already established HCC is unknown.
An important property of SAMe that makes it an attractive agent for chemoprevention and treatment of HCC is its ability to selectively kill liver cancer cells (10,11). SAMe protects normal hepatocytes against apoptotic cell death while it induces apoptosis in liver cancer cells (10). One mechanism for this is SAMe’s ability to selectively induce the expression of Bcl-xs, a pro-apoptotic protein (11). Given that SAMe is now widely available and used as a nutritional supplement in the United States with little known side effects (12), whether SAMe can be an effective agent in preventing tumor development and suppress tumor growth is of interest and the topic of this investigation.
MATERIALS AND METHODS
Materials
Cell culture media and fetal bovine serum were obtained from Mediatech (Herndon, VA) and Omega Scientific (Tarzana, CA), respectively. Medium 200 for culturing HUVEC was obtained from Invitrogen (Carlsbad, CA). SAMe in the form of disulfate p-toluenesulfonate dried powder was generously provided by Gnosis SRL (Cairate, Italy). All other reagents were of analytical grade and were obtained from commercial sources.
Cell culture
H4IIE cells were obtained from the Cell Culture Core of the USC Research Center for Liver Diseases and cultured according to instruction provided from ATCC (Rockville, MD). The effect of SAMe on cell death was evaluated by treating H4IIE cells with varying concentrations of SAMe for 24 hours. In separate experiment, H4IIE cells were treated with SAMe (0.75mM) for 13 hours and the effect on expression was evaluated by real-time PCR, microarray or Western blot analysis as described below. HUVECs were obtained from Invitrogen and grown in Medium 200 containing low serum according to the manufacturers protocol.
Angiogenesis and proliferation assays
In vitro angiogenesis of HUVECs was performed using the Fibrin Gel Angiogenesis assay kit (Chemicon International Inc. Temecula, CA) according to the manufacturer’s protocol. After 24 or 72 hours of angiogenesis induction with 50ng/ml phorbol 12-Myristate 13-acetate (PMA) alone or in combination with SAMe (0.75mM), patterns of cell migration, capillary tube formation and lumina formation were observed under an inverted light microscope (Nikon Eclipse TE300) at 40X magnification. To assay for cell proliferation, HUVECs were plated in 96-well plates, treated with SAMe (0.75mM) or vehicle for 72 hours and incubated with bromodeoxyuridine (BrDU) during the last 16 hours. BrDU incorporation into DNA was measured according to manufacturer’s instructions (CalBiochem, San Diego, CA).
In vivo HCC model
The use and the care of the animals were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Southern California. The model used was previously described with slight modifications (13). Three-month old male ACI rats (Harlan, Indianapolis, Indiana) were anesthetized by intraperitoneal (ip) injection of pentobarbital (50mg/kg), ketamine (100mg/kg) and xylazine (1mg/kg) and after an adequate level of anesthesia has been achieved, a mini-laparatomy was performed under strictly sterile conditions. H4IIE cells (2.5×106 cells) were suspended in 0.1ml normal saline (0.9% wt/vol) and inoculated directly into the liver parenchyma with a 30-gauge needle. Following this, the rats were allowed to recover. Two weeks after injection, majority (80%) of the rats have 1cm tumor.
SAMe treatment
SAMe treatment was given either by ip injection or continuous intravenous (IV) infusion. In pilot experiment, SAMe 200mg/kg ip was given to rats and animals were sacrificed at various times afterwards for determination of liver and kidney SAMe and S-adenosylhomocysteine (SAH) levels. In the first experiment, SAMe was given at 200mg/kg ip once or twice/day for 11 days starting two weeks after H4IIE cells were injected into the liver parenchyma (n=6 to 7 per group, control rats received vehicle). Rats were sacrificed after 11 days of treatment and liver was harvested for measurement of tumor size and SAMe levels. In a second pilot experiment, SAMe (150mg/kg/day or 300mg/kg/day) or vehicle control was delivered by continuous IV infusion through a long-term central venous cannula. To do this, a long-term central venous cannula was placed under the same anesthesia for injection of the H4IIE cells. Placement of the central venous cannula and the anchoring button with swivel was described in detail previously (14). 24 hrs after cannula was placed, IV infusion with either SAMe (dissolved in 3ml phosphate-buffered saline or PBS, pH 7.0), or PBS (3ml, pH 7.0) was infused over 24 hrs using the PHD 22/2000 infusion pump from Harvard Apparatus (Holliston, MA). In the pilot experiment, infusion was stopped after 24 hours for determination of liver SAMe levels. In the next experiment, 24 hours after injecting ACI rats with H4IIE cells (2.5×106 cells) into the liver parenchyma and placement of the long-term central venous catheter, rats received either SAMe (150mg/kg in 3ml/day, prepared fresh daily) or PBS (3ml/day) by IV infusion for 11 days (n=6 per group). At the end of this treatment catheters were removed and rats were imaged using magnetic resonance imaging (MRI) as described below. Rats were sacrificed after imaging and serum and liver tissues were obtained for additional analyses as described below. In the final experiment, rats that had been previously injected with H4IIE cells as above underwent ultrasound imaging to document comparable tumor burden at the start of treatment. They were then divided into two groups (n=5 per group), one group received SAMe (150mg/kg/day as above) and the other group received PBS by IV infusion for 24 days. All were imaged on day 14 and day 24 of treatment. Rats were sacrificed after 24 days of treatment and plasma and liver tissues were obtained for additional analyses described below. SAMe treatment by ip or IV for up to 24 days was well tolerated, as no sign of toxicity was apparent. Body weight of the animals at the start and end of treatment for all SAMe groups were no different from controls.
SAMe and S-adenosylhomocysteine (SAH) determination
Plasma SAMe and liver SAMe and SAH levels were determined as previously described (15).
Gene and protein expression
Total RNA isolated by the TRIzol reagent (Invitrogen) was subjected to reverse transcription (RT) by using M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA). One µl of RT product was subjected to quantitative real-time PCR analysis. The primers and TaqMan probes for glycine N-methyltransferase (GNMT), phosphatidylethanolamine N-methyltransferase (PEMT) and Universal PCR Master Mix were purchased from ABI (Foster City, CA). 18SrRNA was used as housekeeping genes as described (16). The delta Ct obtained was used to find the relative expression of these genes according to the formula: relative expression=2−ΔΔCt, where ΔΔCt= ΔCt of those genes in experimental groups - ΔCt of the same genes in control group. RNA from H4IIE cells was analyzed using the OligoGEArray rat Angiogenesis microarray system (SABiosciences, Frederick, MD). Western blot analysis was done as described (16) using anti-GNMT antibodies kindly provided by Dr. Conrad Wagner (Department of Biochemistry, Vanderbilt University, Nashville, Tenn) (16), anti-Type XVIII collagen and anti- platelet-derived growth factor-α (PDGF-α) (Millipore, Temecula, CA), or anti-midkine antibodies (Novus Biologicals, Littleton, CA).
Liver histology
Replicate 4µm thick sections of formalin-fixed liver tissues containing tumor embedded in paraffin were cut and stained with hematoxylin and eosin (H&E) for the evaluation of HCC.
Serum alanine transaminase (ALT) levels
Serum ALT level was measured by a commercially available kit (Infinity ALT, Thermo Electron Corp., Waltham, MA) following manufacturer’s instruction.
MRI
Rats were initiated with 5% Isoflurane and maintained with 1-3% Isoflurane during the examination. Rats were scanned on a 7.05T Bruker Biospin Pharmascan MRI (Magnet type-B-C 70/16 US Proton NMR frequency (H1) 300,30MHz, 38mm RF coil, 2003, Billerica, Massachusetts) using Paravision 3.1 imaging software. Rare Mouse Body T2 imaging protocol was used (field of view = 5.0 – 8.0 cm, matrix = 256 × 128, scan average = 4, slice thickness = 0.67mm, number of slices = 48–64, TR = 4500–300ms). Following adequate sedation, ophthalmic ointment (Paralube) was placed on the surface of the eye to prevent corneal drying. The rats were placed in a tubular imaging restrain device designed to hold the rat's torso in a comfortable position. The rat's respiratory rate was monitored throughout the imaging period through use of noninvasive monitoring equipment. Following scanning, rats were removed from the restrain device and allowed to recover from anesthesia on a heating pad with continuous monitoring.
Ultrasonography
Rats were anesthetized with a mixture of pentobarbital, ketamine and xylazine ip. Hair was removed from the abdomen of the rats using a commercially available depilatory cream. Ultrasound scans were performed using a commercially available Toshiba Aplio unit. A near field focused linear array 7–14 MHz broadband transducer with a center frequency of 12 MHZ was used to assess the liver in all cases. Tumor volume was calculated in mm3 by multiplying the transverse, anteroposterior, and longitudinal dimensions of the masses.
Statistical Analysis
Data are given as mean ± SEM. Statistical analysis was performed using analysis of variance followed by Fisher's test for multiple comparisons. Significance was defined by p<0.05.
RESULTS
SAMe induces cell death in H4IIE cells
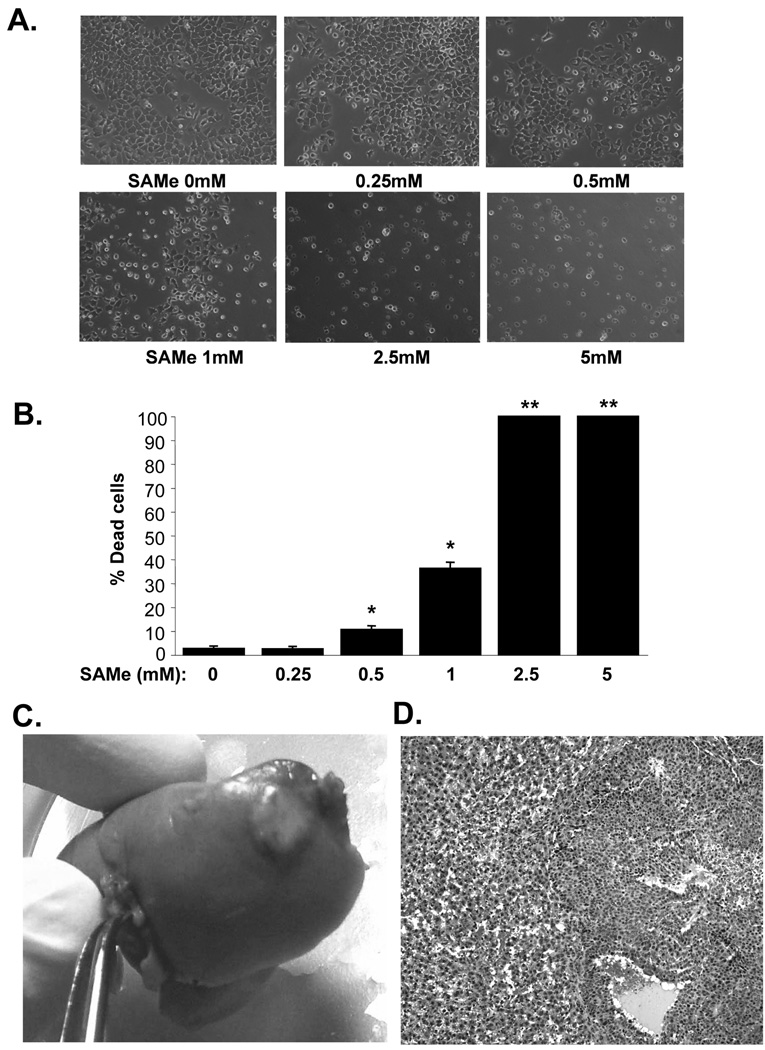
Consistent with our previous finding that SAMe is selectively pro-apoptotic to human liver cancer cells (10), SAMe also induced cell death in a dose-dependent manner in a rat hepatoma cell line, H4IIE (Fig. 1A and B). The lowest SAMe dose that induced cell death after 24 hours was 0.5mM. Two weeks after injecting 2.5×106 cells directly into the liver parenchyma, 80% of the rats develop liver tumor of about 1cm in size (Fig. 1C). Histologically the liver tumor is consistent with poorly differentiated HCC that can be seen in man (Fig. 1D).
Figure 1.
A and B) SAMe causes dose-dependent cell death in H4IIE cells. Cells were treated with varying doses of SAMe for 24 hours and phase contrast images are shown in A. while the graph in B. summarizes the results of 4 experiments shown as % of dead cells in mean±SEM. *p<0.001, **p<0.00001 versus (vs.) control (no SAMe). C) In vivo HCC model. Two weeks after injecting 2.5×106 H4IIE cells into the liver parenchyma, a clearly visible tumor can be seen. D) HCC histology on H&E (100X) shows the tumor is composed of cells having round to oval nuclei, small nucleoli, basophilic cytoplasm, and increase in the nuclear:cytoplasmic ratio. The cells predominantly form sheets as well as in areas thickened cords in part lined by flattened endothelial cells. Occasional variably-sized vascular spaces are also seen. The adjacent non-tumor liver shows variable sinusoidal dilatation and congestion.
Effect of SAMe ip treatment
Following SAMe 200mg/kg ip, tissue SAMe levels rose rapidly (Table 1). In liver, peak levels reached in 15 minutes to 434nmol/gm (or about 434µM, assuming 1gm liver is about 1ml) and fell back toward baseline by 4 hours after injection (Table 1). We next examined whether giving SAMe ip once or twice a day would have any effect on tumor growth. Rats that had been injected with H4IIE cells two weeks earlier were treated with SAMe 200mg/kg ip once or twice (every 12 hours) per day for 11 days. Unfortunately neither regimen had any effect on the size of the tumor or hepatic SAMe levels (measured either 12 or 24 hours after SAMe injection) (data not shown).
Table 1.
Tissue levels of SAMe and SAH after an acute ip SAMe injection
| Time | Liver SAMe | Liver SAH | Kidney SAMe | Kidney SAH |
|---|---|---|---|---|
| Min | nmol/gm | nmol/gm | nmol/gm | nmol/gm |
| Control | 71.4±6 | 26.8±4.8 | 26.0±8.1 | 20.9±9.2 |
| 15 | 434±162* | 69.6±30.3† | 3285±563* | 57.7±12.3† |
| 30 | 220±41* | 52.8±10.2* | 4398±726* | 98.0±30.2* |
| 60 | 219±48* | 51.8±9.5* | 5447±645* | 118.2±18.8* |
| 120 | 118±33† | 32.3±14.6 | 7272±1006* | 118.6±27.6* |
| 240 | 89.2±36 | 21.8±8.1 | 6856±545* | 103.1±17.1* |
Results are mean±SD from 4 rats for each group.
p<0.01,
p<0.05 vs. control.
Effect of SAMe IV treatment in preventing tumor development
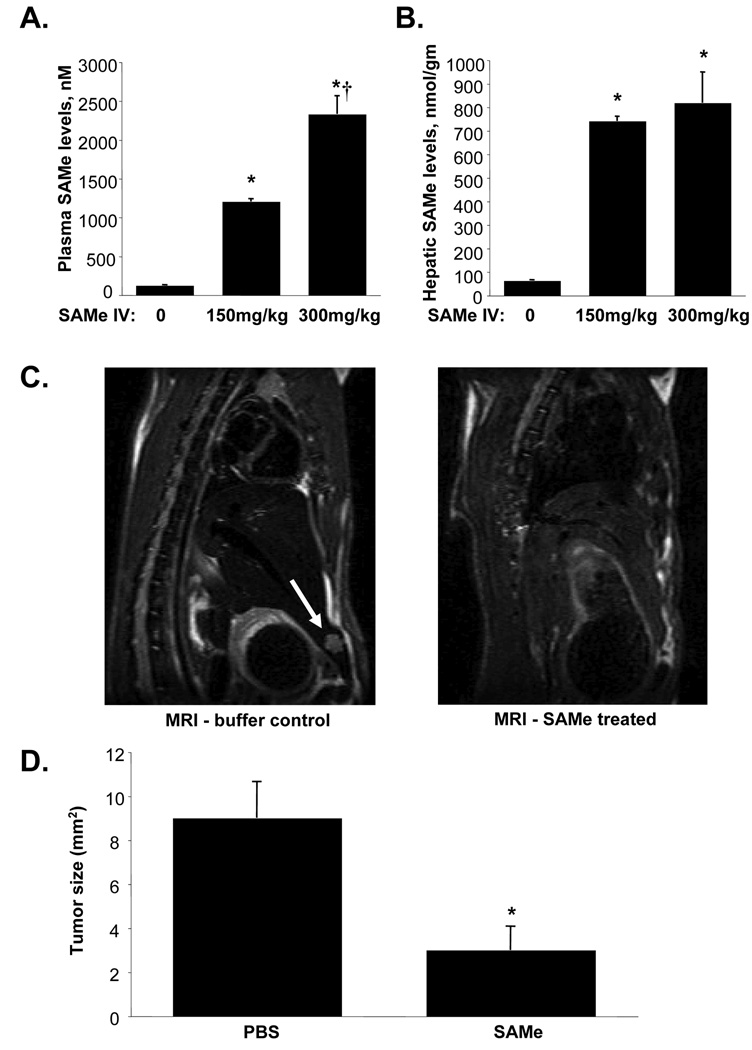
To have high sustained hepatic SAMe level, we switched to a continuous IV SAMe infusion. 24 hours after IV SAMe infusion plasma levels rose sharply (Fig. 2A) but more importantly, liver SAMe levels were over 10-fold higher or 700µM at the dose of 150mg/kg/day (Fig. 2B). Treatment with IV SAMe (150mg/kg/d) by continuous infusion for 11 days starting 24 hours after initial injection of H4IIE cells into the liver parenchyma significantly reduced the tumor size as documented on MRI and visual inspection after sacrifice (Fig. 2C and D). In SAMe treated group, 2 of 6 rats developed small tumors (6 and 3.9mm2) while the rest had no tumor evident. In contrast, 4 of 6 rats treated with PBS developed tumors (16, 14, 9.1 and 15 mm2), which were much larger than SAMe treated rats (Fig. 2D). Visual inspection of the tumors gave similar dimensions as MRI measurements. IV SAMe treatment did not result in any liver toxicity as measured by serum ALT levels (control = 50.2±11.9, SAMe = 45.6±6.3 IU, results represent mean±SEM from 6 animals per group).
Figure 2.
A) Plasma SAMe levels following 24 hours of continuous IV SAMe infusion. Results are mean±SEM from 3 animals for each group. *p<0.001 vs. PBS (0 SAMe), †p<0.01 vs. SAMe 150mg/kg. B) Hepatic SAMe levels following 24 hours of continuous IV SAMe infusion. Results are mean±SEM from 3 animals for each group. *p<0.0001 vs. PBS (0 SAMe). C) Representative MRIs in PBS and SAMe treated rats. Rats (n =6 per group) were injected with H4IIE cells as above and received continuous IV SAMe or PBS buffer control starting 24 hours later and continued for 11 days. Arrow points to liver tumor that developed in one of the buffer treated controls. This image is one representative slice through the tumor. D) Effect of IV SAMe treatment for 11 days on the development of HCC. Graph summarizes tumor sizes (mm2) as measured in two dimensions on MRI. *p<0.05 vs. PBS group.
Effect of SAMe IV treatment in halting progression and/or causing regression of already established HCC
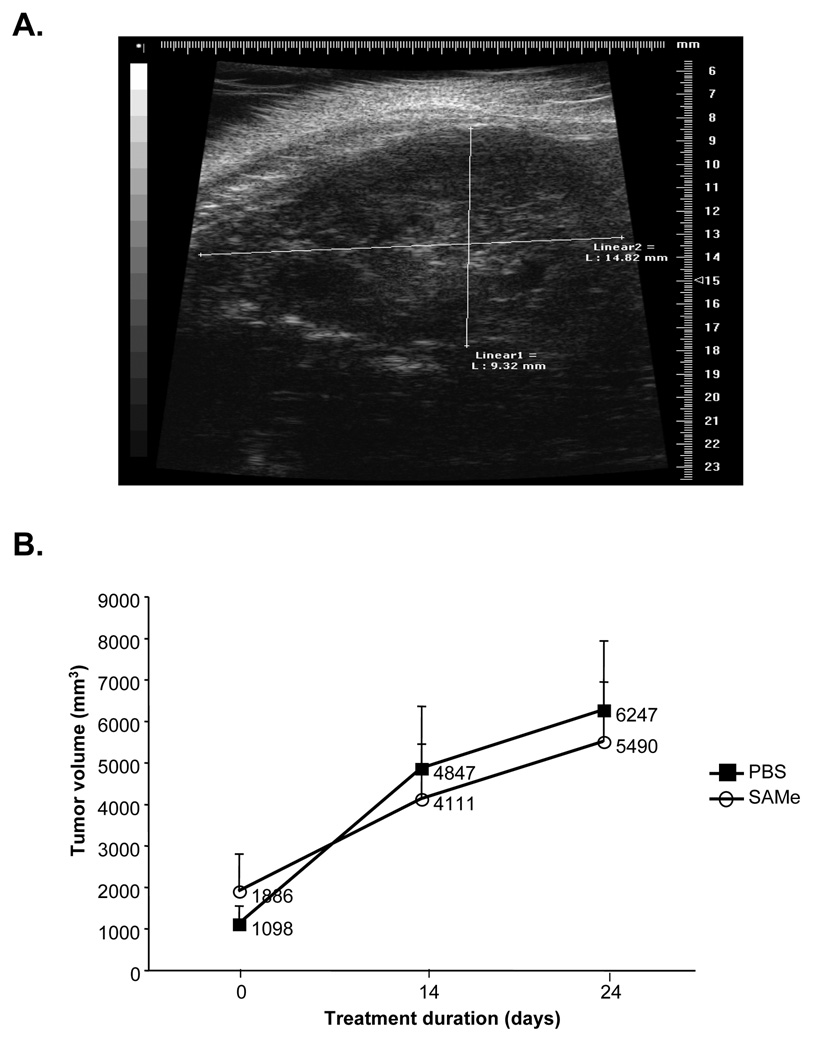
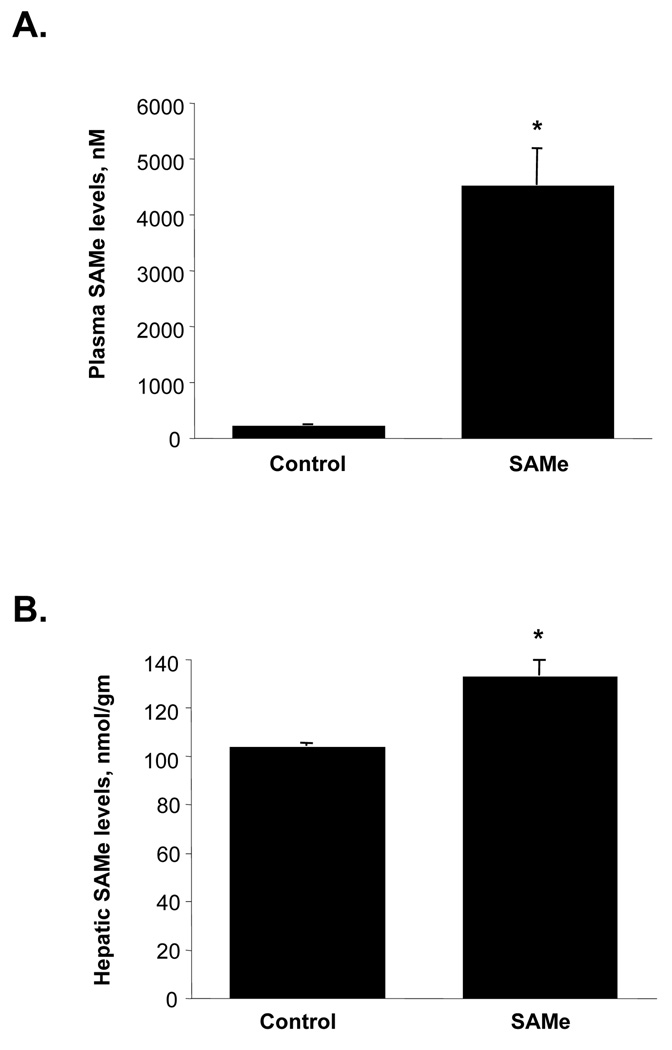
We next evaluated whether SAMe IV infusion can be used to treat already established HCC. For this purpose we switched to ultrasound for imaging because the metal catheter would interfere with MRI. Rats were treated with IV SAMe (150mg/kg/d) for 24 days and had serial ultrasound performed. Figure 3A shows an example of the ultrasound, which was able to easily measure the size of the tumor. Figure 3B shows that when HCC is already present (at least 1 cm in size), IV SAMe infusion failed to alter the rate of tumor growth. Figure 4A shows that plasma SAMe levels remained elevated at over 4µM in the treated rats. However, hepatic SAMe levels were only 30% above the control levels (Fig. 4B).
Figure 3.
A) Representative ultrasound image of a rat with HCC. B) Effect of IV SAMe treatment in already established HCC. Rats were previously injected with H4IIE cells (2.5×106 cells) and imaged by ultrasound (one representative slice through the tumor). They were then divided into two groups (n=5 per group) with comparable starting tumor volumes and treated with IV SAMe (150mg/kg/day) or PBS for 24 days. Ultrasound imaging was performed in all rats on day 14 of treatment and on day 24, at which time all rats were sacrificed. Graph summarizes change in tumor volume with time.
Figure 4.
A) Plasma SAMe levels following 24 days of IV SAMe infusion. *p<0.001 vs. PBS control (n=4 per group). B) Hepatic SAMe levels following 24 days of IV SAMe infusion. *p<0.001 vs. PBS control (n=4 to 5 per group).
Potential explanations for lack of SAMe effect in treating already established HCC
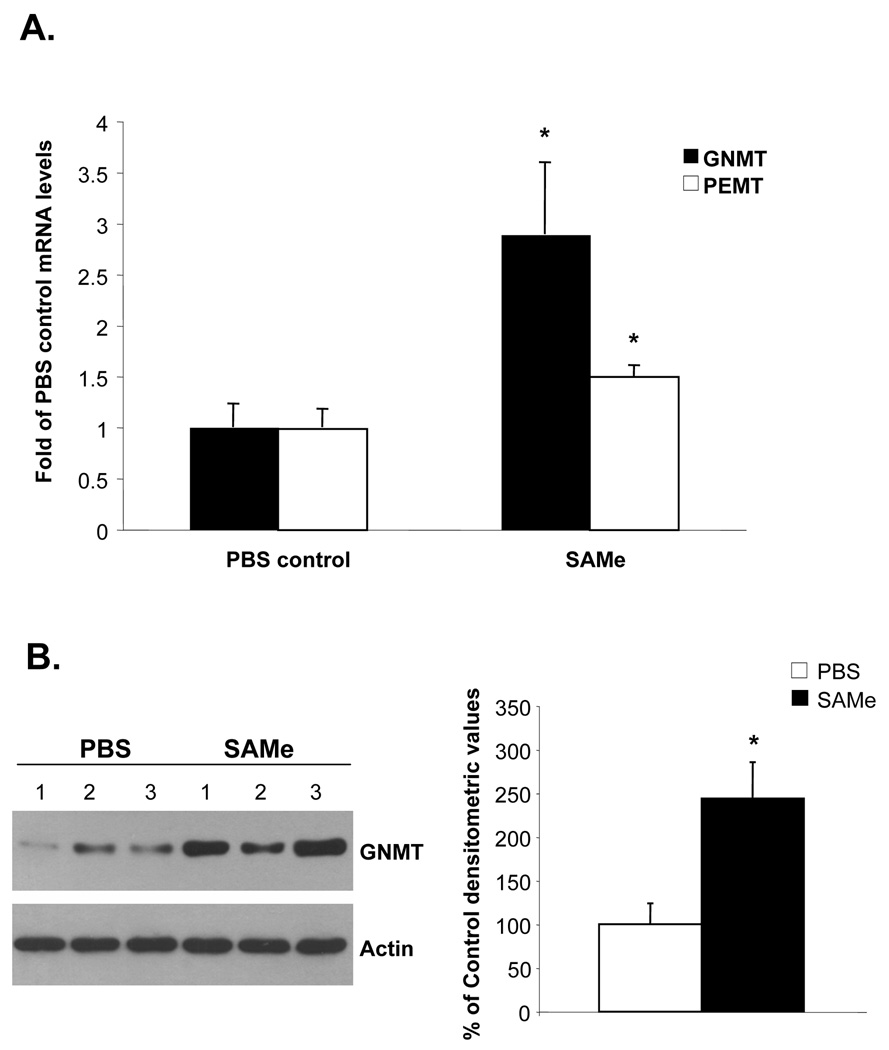
One possibility for the lack of sustained high hepatic SAMe level with chronic SAMe infusion is a compensatory response by the liver to metabolize SAMe. Consistent with this, Figure 5A shows that SAMe more than doubled the mRNA levels of GNMT in the liver and increased the PEMT mRNA levels by 50%. GNMT protein levels increased comparably (Fig. 5B).
Figure 5.
A) Effect of IV SAMe treatment for 24 days on hepatic GNMT and PEMT mRNA levels. GNMT and PEMT mRNA levels were measured by real-time PCR in 4 animals per group. *p<0.05 vs. control. B) Effect of IV SAMe treatment for 24 days on hepatic GNMT protein levels. Western blot analysis of GNMT was done as described in Methods. Graph on the right summarizes densitometric changes. *p<0.05 vs. PBS control (n=3 to 4 per group).
Potential mechanism(s) for SAMe’s inhibitory effect on tumor establishment
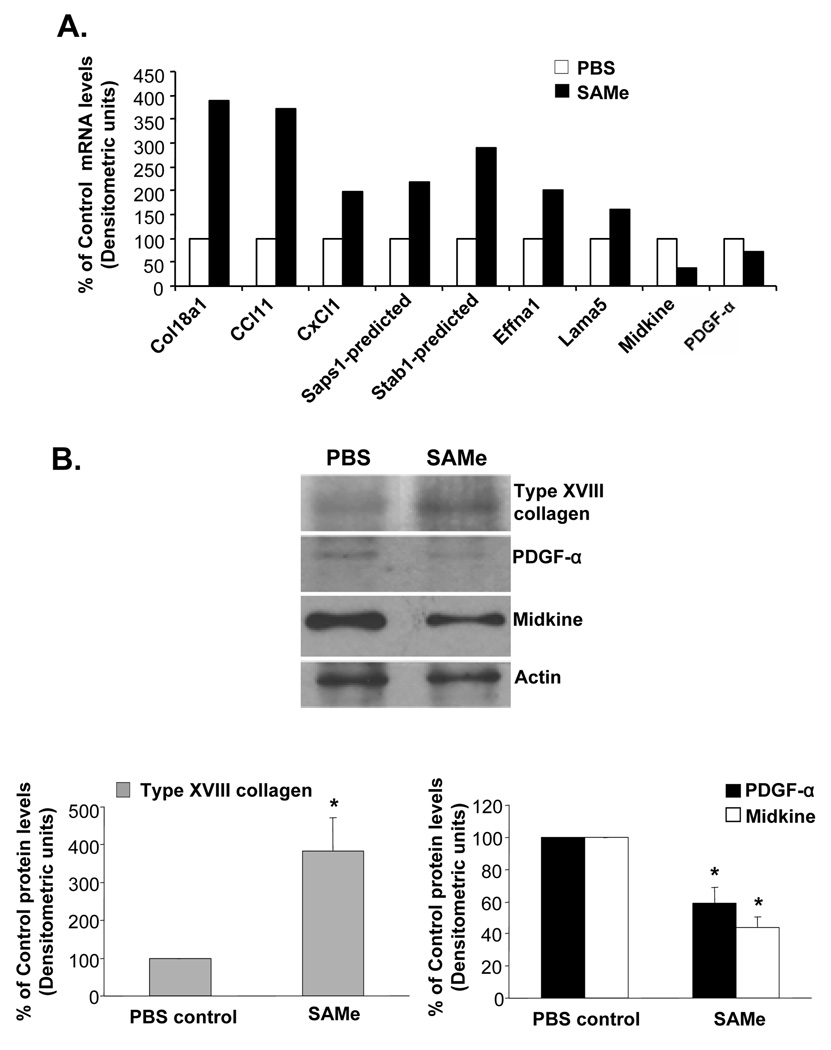
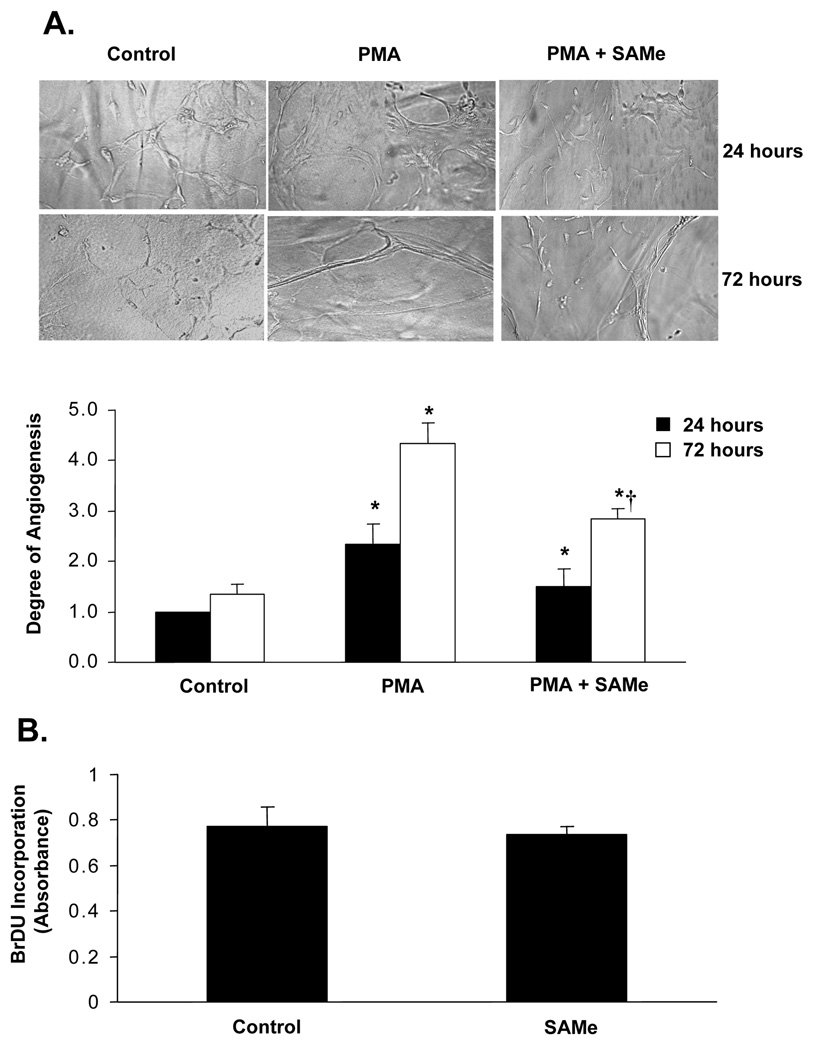
A plausible explanation for SAMe to inhibit the establishment of HCC is its pro-apoptotic effect during the acute setting, prior to the compensatory response of the methyltransferases. Other potential explanations include the possibility that SAMe may inhibit the expression of factors important for angiogenesis. Using a microarray-based approach we found that SAMe inhibited the expression of pro-angiogenic factors such as PDGF-α and midkine by 30% and 61% respectively (Fig. 6A). Interestingly, SAMe significantly induced the expression of Type XVIII collagen which is the precursor for an important anti-angiogenic peptide, endostatin (17) (Fig. 6A). These changes in expression were confirmed at the protein level (Fig. 6B). Furthermore, SAMe also inhibited in vitro PMA-stimulated angiogenesis in HUVECs by 35% at 72 hours (Fig. 7A). SAMe was not toxic to HUVECs as determined by the BrDU cell proliferation assay (Fig. 7B).
Figure 6.
A) Effect of SAMe on angiogenesis pathway genes in H4IIE cells. H4IIE cells were treated with 0.75mM SAMe for 13 hours and RNA was processed for microarray analysis as described in Methods. B) Effect of SAMe on the protein expression of three angiogenic markers strongly associated with expression in HCC. H4IIE cells were treated as in (A) and Western blot analysis of Type XVIII collagen, PDGF-α and midkine was done as described in Methods. Graphs below summarize the densitometric changes. *p<0.05 vs. PBS control (n=2 experiments).
Figure 7.
A) Effect of SAMe on HUVEC in vitro angiogenesis in fibrin gels. HUVECs were grown as described in Methods and plated on fibrin gels for 24 or 72 hours in the presence of PMA alone or in combination with 0.75mM SAMe. The degree of angiogenesis was quantitated by the pattern recognition method as suggested by the manufacturer. The graph summarizes the degree of angiogenesis from three experiments in quadruplets. *p<0.05 vs. control, †p<0.05 vs. PMA. B) Effect of SAMe on growth of HUVECs in culture. HUVECs were treated with 0.75mM SAMe for 72 hours and medium containing SAMe was replenished every 24 hours. BrDU was added to the cells during the last 16 hours and proliferation was measured as described in Methods. Results are expressed as mean±S.E from two experiments in duplicates.
DISCUSSION
HCC is one of the most difficult to treat cancers largely due to the advanced stage by the time it is diagnosed and poor response to treatment (2,3). Known risk factors for HCC include chronic infection with hepatitis B virus, hepatitis C virus (HCV), dietary aflatoxin, excessive alcohol intake, cigarette smoking, diabetes and obesity (18). The incidence of HCC is expected to rise as approximately 170 million individuals worldwide are estimated to be infected with HCV (2,19). In the United States the incidence of HCC doubled from 1979 to 1995 and projections for the future burden of HCC demonstrate a sizable increase in the number of HCC cases for the following 20 to 30 years (2). Given these projections, there has been tremendous interest in developing effective chemoprevention strategies. Although recent breakthrough in the treatment of advanced HCC with Sorafenib is encouraging (4), the increase in survival is only by a median of 3 months. Thus, improvement in both chemoprevention and treatment of HCC is sorely needed.
SAMe is a widely available nutritional supplement in the United States that is used therapeutically in European countries for the treatment of several cholestatic liver diseases (6,12). Although it is most well-known as a methyl donor and precursor for polyamines and glutathione (GSH), recent studies have shown that SAMe regulates diverse cellular processes such as growth and apoptosis independent of these roles (6,10,11). It is important to differentiate physiological SAMe level fluctuations from pharmacological SAMe administration. Thus, in normal hepatocytes, a fall in the SAMe level allows mitogens to activate the proliferative response necessary for liver regeneration (20,21). Indeed, if hepatic SAMe level is chronically depleted, as in the case of the MAT1A knockout mouse (7,22), there is at baseline increased hepatocyte proliferation (20,22) and the animals develop spontaneous HCC (7). These observations are important as most patients with cirrhosis have impaired SAMe biosynthesis due to low to absent expression of MAT1A and inactivation of its encoded isoenzymes (6). Taken together, these data would suggest preventing a fall in hepatic SAMe level may be a good strategy to prevent malignant degeneration as shown by Pascale and colleagues in rodent models of HCC (8,9,23).
When SAMe is administered at pharmacologic doses, many of its actions may actually be mediated by its by-product methylthioadenosine (MTA) (24). MTA is derived from SAMe spontaneously and in the polyamine biosynthesis pathway (24). In contrast to SAMe which is unstable, MTA is very stable and inhibits methylation and polyamine biosynthesis (24,25). MTA mimics SAMe in being selectively pro-apoptotic to liver cancer cells (10,11) and on the expression of pro-inflammatory cytokines (24) and MAT2A (25,26) at a lower dose than SAMe. MTA also prevented development of early neoplastic lesions in rats treated with diethylnitrosamine at a lower dose than SAMe (27). However, while SAMe administration increased DNA methylation, MTA did not (27). Thus, while an increase in methylation is due to SAMe, many of the other methylation-independent actions may be mediated by MTA.
Although MTA mimics much of SAMe’s actions, it is not available for human use and given the excellent safety profile of SAMe (12) and the strong animal data showing its chemopreventive capability (8,9,23,27), we investigated whether SAMe can prevent the establishment of HCC and cause regression of already established HCC. Our study differs from that of Pascale and colleagues in that no carcinogen was used so that there is no depletion of hepatic SAMe level. Thus, if SAMe is protective, it is not due to repletion of hepatic SAMe level. Whether SAMe can be used to treat already established HCC has not been examined.
In order to maintain a high hepatic SAMe level, SAMe was administered by continuous IV infusion. Twenty-four hours after infusion, hepatic SAMe levels reached over 700nmol/gm in rats that received 150mg/kg. This translates to about 0.7mM (one gm liver = 1ml), which is toxic to H4IIE cells in vitro. When IV SAMe infusion at 150mg/kg/day was started 24 hours after injecting H4IIE cells into the liver, it significantly prevented tumor development after 11 days. However, if SAMe infusion was started after sizable tumors had already formed, it failed to reduce the rate of tumor growth after 24 days of treatment. Surprisingly we found that while the plasma SAMe levels remained 20-fold elevated, hepatic SAMe levels were only 30% higher than controls. The explanation for this is a compensatory induction in hepatic methyltransferases, particularly GNMT, to prevent SAMe accumulation.
GNMT is the predominant methyltransferase in the liver that helps to maintain normal liver SAMe levels (28). Mice deficient in GNMT have markedly elevated hepatic SAMe levels and develop spontaneous liver injury and HCC (28). However, the mechanism for HCC formation differs from that of MAT1A knockout mice. In GNMT knockout mice, the supraphysiologic SAMe level (35-fold normal) led to aberrant DNA methylation, leading to hypermethylation and silencing of JAK/STAT inhibitors (28). In MAT1A knockout mice, chronic SAMe depletion facilitated expansion of liver progenitor cells, some of which have tumorigenic potential (29). In addition, we also found that SAMe is critical in stabilizing apurinic/apyrimidinic endonuclease redox effector (APE1/REF-1/APEX1), an enzyme that is essential for base excision repair of damaged DNA (30). MAT1A knockout mice have reduced APEX1 protein levels and increased genomic instability from one month of age and these changes may further contribute to hepatocarcinogenesis (30). SAMe’s chemopreventive effect may also be partly due to its ability to stabilize APEX1 and prevent genomic instability. Taken together, maintaining normal hepatic SAMe level is important as both depletion and excess are associated with bad outcomes.
In our model, the ability of SAMe to prevent HCC establishment cannot be explained by repleting SAMe levels. Potential mechanisms for SAMe’s effect against tumor establishment include 1) SAMe’s pro-apoptotic effect on H4IIE cells, at least in the acute setting prior to compensatory response of the methyltransferases, 2) SAMe’s inhibitory effect on MAT2A and MAT2β gene expression, both of which support liver cancer growth (26), and 3) SAMe’s inhibitory effect on factors important in tumor establishment, such as angiogenesis. Indeed our angiogenesis microarray and protein validation data support this hypothesis. Several angiogenic factors like chemokines (C×Cl1, CCl11) growth factors (PDGF-α and midkine), extracellular matrix proteins (Lama5) and transmembrane signaling proteins (Stab1, Saps1 and effna1) are affected by SAMe. In this study, we have validated only those angiogenic factors that were associated with HCC development. SAMe inhibited the expression of two pro-angiogenic growth factors, PDGF-α and midkine that are associated with HCC (31,32). We also found that SAMe induced the expression of Type XVIII collagen, an important hepatocyte-specific, anti-angiogenic protein (17). This could be an important mechanism of SAMe’s inhibition of tumor establishment in our model. This is further strengthened by our findings showing decreased functional angiogenesis by SAMe.
The observation that SAMe failed to exert any therapeutic effect in already established HCC is disappointing. However, the use of SAMe in chemoprevention is entirely a different story. SAMe has been previously shown to exert chemopreventive effects in several models of HCC in the rat, utilizing different carcinogenic protocols such as diethylnitrosamine, 1,2-dimethylhydrazine, orotic acid, and thiobenzamide in various combinations (8,9,27). Our findings further support this. As the number of individuals at risk of HCC increase, it is vitally important to address chemoprevention of HCC. The dose required for chemoprevention is most likely to be the dose necessary to normalize hepatic SAMe levels. Two earlier studies in man showed that oral SAMe at 1.2gm/day in three divided doses normalized hepatic GSH levels (33) and prolonged survival in patients with less advanced alcoholic liver disease (34). This can serve as a good starting point for a large double-blind, randomized, placebo-controlled trial to critically address this issue in man.
In summary, SAMe has a short half-life so that either frequent dosing or continuous IV infusion will be necessary to maintain a high hepatic SAMe level. SAMe is able to significantly reduce the development of HCC but is ineffective in treating established HCC. This is because chronic SAMe administration leads to a compensatory induction in hepatic methyltransferase expression, which prevents SAMe accumulation to a level necessary for it to exert a pro-apoptotic effect on liver cancer cells. Since MTA recapitulates many of SAMe’s actions, it will be worthwhile to examine whether MTA might be useful in treating HCC. However, whether SAMe can be effective in chemoprevention of HCC in man deserves study now.
Acknowledgments
The authors thank Gevorg Karapetyan for performing the MRIs and Qing-Gao Deng for placement of the IV catheters.
Financial support.
This work was supported by NIH grants DK51719 (SC Lu), AT1576 (SC Lu and JM Mato), and AT002311 (T Bottiglieri). Komal Ramani was also supported by the Institutional Training Program on Alcoholic Liver and Pancreatic Diseases from NIAAA/NIH (T32AA07578). H4IIE cells were provided by the Cell Culture Core of the USC Research Center for Liver Diseases (P30DK48522). Placement of IV catheters was done by the Animal Core and pathological sectioning & staining were done by the Morphology Core of the Southern California Research Center for Alcoholic Liver and Pancreatic Diseases and Cirrhosis (P50AA11999).
List of abbreviations (in alphabetical order)
- ALT
alanine transaminase
- APEX1
apurinic/apyrimidinic endonuclease redox effector
- BrDU
bromodeoxyuridine
- GNMT
glycine N-methyltransferase
- H&E
hematoxylin and eosin
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- ip
intraperitoneal
- IV
intravenous
- MAT
methionine adenosyltransferase
- MRI
magnetic resonance imaging
- MTA
methylthioadenosine
- PBS
phosphate-buffered saline
- PDGF-α
platelet-derived growth factor-α
- PEMT
phosphatidylethanolamine N-methyltransferase
- PMA
Phorbol 12-Myristate 13-acetate
- SAH
S-adenosylhomocysteine
- SAMe
S-adenosylmethionine
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000 Int. J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Sypsa V, Touloumi G, Papatheodoridis GV, Tassopoulos NC, Ketikoglou I, Vafiadis I, Hatzis G, et al. Future trends of HCV-related cirrhosis and hepatocellular carcinoma under the currently available treatments. J. Viral Hep. 2005;12:543–550. doi: 10.1111/j.1365-2893.2005.00588.x. [DOI] [PubMed] [Google Scholar]
- 3.Yeh FS, Yu MC, Mo CC, Luo S, Tong MJ, Henderson BE. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Res. 1989;49:2506–2509. [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, Cosme de Oliveira A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Takai K, Okuno M, Yasuda I, Matsushima-Nishiwaki R, Uematsu T, Tsurumi H, Shiratori Y, et al. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. updated analysis of the long-term follow-up data. Intervirology. 2005;48:39–45. doi: 10.1159/000082093. [DOI] [PubMed] [Google Scholar]
- 6.Mato JM, Lu SC. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Chantar ML, Corrales FJ, Martínez-Cruz LA, et al. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 8.Pascale RM, Marras V, Simile MM, Daino L, Pinna G, Bennati S, Carta M, et al. Chemoprevention of rat liver carcinogenesis by S-adenosyl-L-methionine: A long-term study. Cancer Res. 1992;52:4979–4986. [PubMed] [Google Scholar]
- 9.Pascale RM, Simile MM, De Miglio MR, Nufris A, Daino L, Seddaiu MA, Rao PM, et al. Chemoprevention by S-adenosyl-L-methionine of rat liver carcinogenesis initiated by 1,2-dimethylhydrazine and promoted by orotic acid. Carcinogenesis. 1995;16:427–430. doi: 10.1093/carcin/16.2.427. [DOI] [PubMed] [Google Scholar]
- 10.Ansorena E, García-Trevijano ER, Martínez-Chantar ML, Huang ZZ, Chen LX, Mato JM, Iraburu M, et al. S-adenosylmethionine and methylthioadenosine are anti-apoptotic in cultured rat hepatocytes but pro-apoptotic in human hepatoma cells. Hepatology. 2002;35:274–280. doi: 10.1053/jhep.2002.30419. [DOI] [PubMed] [Google Scholar]
- 11.Yang HP, Sadda MR, Li M, Zeng Y, Chen LX, Bae WJ, Ou XP, et al. S-Adenosylmethionine and its metabolite induce apoptosis in HepG2 cells: role of protein phosphatase 1 and Bcl-xS. Hepatology. 2004;40:221–231. doi: 10.1002/hep.20274. [DOI] [PubMed] [Google Scholar]
- 12.Mato JM, Lu SC. In: S-adenosylmethionine. In: Encyclopedia of Dietary Supplements. 1st edition. Coates PM, Blackman MR, Levine M, Moss J, White JD, editors. New York, NY: Marcel Dekker; 2005. http://www.dekker.com/sdek/abstract~db=enc~content=a713479736. [Google Scholar]
- 13.McKillop IH, Schmidt CM, Cahill PA, Sitzmann JV. Altered expression of mitogen-activated protein kinases in a rat model of experimental hepatocellular carcinoma. Hepatology. 1997;26:1484–1491. doi: 10.1002/hep.510260615. [DOI] [PubMed] [Google Scholar]
- 14.Tsukamoto H, Reidelberger RD, French SW, Largman C. Long-term cannulation model for blood sampling and intragastric infusion in the rat. Am. J. Physiol. 1984;247:R595–R599. doi: 10.1152/ajpregu.1984.247.3.R595. [DOI] [PubMed] [Google Scholar]
- 15.Lee TD, Sadda ME, Mendler MH, Bottiglieri T, Kanel G, Mato JM, Lu SC. Abnormal hepatic methionine and GSH metabolism in patients with alcoholic hepatitis. Alcoholism: Clin. and Exp. Res. 2004;28:173–181. doi: 10.1097/01.ALC.0000108654.77178.03. [DOI] [PubMed] [Google Scholar]
- 16.Ko KS, Yang HP, Noureddin M, Iglesia-Ara A, Xia M, Wagner C, Luka Z, et al. Changes in S-adenosylmethionine and glutathione homeostasis during endotoxemia in mice. Lab Invest. 2008;88:1121–1129. doi: 10.1038/labinvest.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: An Endogenous Inhibitor of Angiogenesis and Tumor Growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 18.Yuan JM, Lu SC, Van Den Berg D, Govindarajan S, Zhang ZQ, Mato JM, Yu MC. Genetic polymorphisms in the methylenetetrahydrofolate reductase and thymidylate synthase genes and risk of hepatocellular carcinoma. Hepatology. 2007;46:749–758. doi: 10.1002/hep.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okita K, Sakaida I, Hino K. Current strategies for chemoprevention of hepatocellular carcinoma. Oncology. 2002;62(suppl 1):24–28. doi: 10.1159/000048272. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Zeng Y, Yang HP, Lee TD, French SW, Corrales FJ, García-Trevijano ER, et al. Impaired liver regeneration in mice lacking methionine adenosyltransferase 1A. FASEB J. 2004 doi: 10.1096/fj.03-1204fje. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Chantar ML, Vázquez-Chantada M, Garnacho-Echevarria M, Latasa MU, Varela-Rey M, Dotor J, Santamaria M, et al. S-adenosylmethionine regulates cytoplasmic HuR via AMP-activated kinase. Gastroenterology. 2006;131:223–232. doi: 10.1053/j.gastro.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Lu SC, Alvarez L, Huang ZZ, Chen LX, An W, Corrales FJ, Avila MA, et al. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci USA. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcea R, Daino L, Pascale R, Simile MM, Puddu M, Ruggiu ME, Seddaiu MA, et al. Protooncogene methylation and expression in regenerating liver and preneoplastic liver nodules induced in the rat by diethylnitrosamine: effect of variations of S-adenosylmethionine:S-adenosylhomocysteine ratio. Carcinogenesis. 1989;10:1183–1192. doi: 10.1093/carcin/10.7.1183. [DOI] [PubMed] [Google Scholar]
- 24.Iglesias Ara A, Xia M, Ramani K, Mato JM, Lu SC. S-Adenosylmethionine inhibits lipopolysaccharide-induced gene expression via modulation of histone methylation. Hepatology. 2008;47:1655–1666. doi: 10.1002/hep.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Xia M, Lin M, Yang HP, Kuhlenkamp J, Li T, Sodir NM, et al. Role of methionine adenosyltransferase 2A and S-adenosylemthionine in mitogen-induced growth of human colon cancer cells. Gastroenterology. 2007;133:207–218. doi: 10.1053/j.gastro.2007.03.114. [DOI] [PubMed] [Google Scholar]
- 26.Ramani K, Yang HP, Xia M, Iglesias Ara A, Mato JM, Lu SC. Leptin’s mitogenic effect in human liver cancer cells requires induction of both methionine adenosyltransferase 2A and 2β. Hepatology. 2008;47:521–531. doi: 10.1002/hep.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascale RM, Simile MM, Satta G, Seddaiu MA, Daino L, Pinna G, Vinci MA, et al. Comparative effects of L-methionine, S-adenosylmethionine and 5’-methylthioadenosine on the growth of preneoplastic lesions and DNA methylation during the early stages of hepatocarcinogenesis. Anticancer Res. 1991;11 1617–19624. [PubMed] [Google Scholar]
- 28.Martinez-Chantar ML, Vázquez-Chantada M, Ariz U, Martínez N, Varela M, Luka Z, Capevila A, et al. Loss of the GNMT gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–1199. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rountree CB, Senadheera S, Mato JM, Crooks GM, Lu SC. Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A deficient mice. Hepatology. 2008;47:1288–1297. doi: 10.1002/hep.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasi ML, Iglesias Ara A, Yang H, Ramani K, Feo F, Pascale MR, Martínez-Chantar ML, et al. S-adenosylmethionine regulates Apurinic/Apyrimidinic Endonuclease 1 stability: implication in hepatocarcinogenesis. Gastroenterology. 2009;136:1025–1036. doi: 10.1053/j.gastro.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato M, Shinozawa T, Kato S, Awaya A, Terada T. Increased Midkine Expression in Hepatocellular Carcinoma: Immunohistochemical and In Situ Hybridization Analyses. Arch. Pathol. Lab Med. 2000;124:848–852. doi: 10.5858/2000-124-0848-IMEIHC. [DOI] [PubMed] [Google Scholar]
- 32.Kim SJ, Tae Kim KT, Jeong PDGFR-α Expression in Preneoplastic and Neoplastic Hepatocellular Lesions: A Rat Model N-nitrosomorpholine Stop Experiment. The Korean Journal of Pathology. 2006;40:354–360. [Google Scholar]
- 33.Vendemiale G, Altomare E, Trizio T, Le Grazie C, Di Padova C, Salerno MT, Carrieri V, et al. Effects of oral S-adenosyl-L-methionine on hepatic glutathione in patients with liver disease. Scand. J. Gastroenterol. 1989;24:407–415. doi: 10.3109/00365528909093067. [DOI] [PubMed] [Google Scholar]
- 34.Mato JM, Cámara J, Fernández de Paz J, Caballería L, Coll S, Caballero A, García-Buey L, et al. S-Adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081–1089. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]