Abstract
Defeat is a social stressor involving subordination by a threatening conspecific. Type 2 corticotropin-releasing factor receptors (CRF2) are abundant in brain regions implicated in defeat responses and are putative stress-related molecules. The present study sought to determine whether neuroactivation and CRF2 expression co-occurred at brain region or cellular levels following acute defeat. Male “intruder” Wistar rats were placed into the cage of an aggressive “resident” Long-Evans rat (n=6). Upon defeat, intruders (n=6) were placed in a wire-mesh chamber and were returned to the resident’s cage for an additional 75 min. Controls (n=6) were handled and returned to their home cage for the same duration. Coronal brain sections were stained for an immediate early gene product, Fos, as a neuronal activation marker. Combined immunohistochemistry with in situ hybridization was performed on a subset of brain sections from defeated intruders to visualize Fos immunoreactivity and CRF2 mRNA jointly. Defeated rats had fivefold, sevenfold, and 10-fold more Fos-positive cells than controls in the arcuate, ventromedial nucleus of the hypothalamus, and medial amygdala post-defeat. Significant colocalization of CRF2 mRNA and Fos-positive cells was observed in the posterior medial amygdala but not in the arcuate nucleus or ventromedial hypothalamus. The results indicate CRF2 receptor-positive neurons in the posterior medial amygdala are involved in the neural response to social defeat.
Keywords: corticotropin-releasing factor, CRF2 receptor, ventromedial or paraventricular hypothalamus, medial nucleus of the amygdala, lateral septum, social defeat stress or resident-intruder test
Defeat is a social stressor involving subordination by a threatening conspecific. In many species, a single defeat results in lasting autonomic, reproductive, and behavioral changes (Tornatzky and Miczek, 1993; Koolhaas et al., 1997; Blanchard et al., 2001; Korte and De Boer, 2003; Sapolsky, 2005). Victors develop increased offensive behaviors, whereas losers develop increased defensive behaviors and less territorial behavior (Blanchard et al., 1995; Tamashiro et al., 2004; Huhman, 2006; Shimozuru et al., 2006). Rodent subordinates show decreased testosterone production and decreased mounting behavior during chronic social conflict, whereas dominants exhibit normal or elevated testosterone levels (Blanchard et al., 1995; Tamashiro et al., 2004; Huhman, 2006). Even brief exposure to territorial, dominant males leads to weight loss, anorexia, and hyperthermia (Marini et al., 2006); reduced reproduction-relevant social behaviors such as territorial aggression, marking, and mating; and stress-like changes in hypothalamic–pituitary–adrenocortical and gonadal axis activity (Shively and Kaplan, 1984; Yoshimura and Kimura, 1991; Huhman, 2006).
Fos, the protein product of the immediate-early gene c-fos, has been used as a cellular marker of brain regions activated by defeat (Miczek et al., 2004). In rats, increased Fos protein or c-fos mRNA expression has been reported in the ventral lateral septum (LS), central and medial nuclei of the amygdala (CeA, MeA), bed nucleus of the stria terminalis (BNST), lateral hypothalamus, paraventricular nucleus of the hypothalamus (PVN), dorsal raphe, and hippocampus following acute defeat (Martinez et al., 1998; Gardner et al., 2005; Funk et al., 2006; Calfa et al., 2007). Defeated mice likewise showed higher c-fos mRNA in the septum, preoptic area, lateral hypothalamus, amygdala, and dorsal raphe (Stork et al., 1997). Hamsters showed high c-fos mRNA expression in the CeA, MeA, BNST, LS, and arcuate nucleus of the hypothalamus (ARH) following one defeat and also in the ventromedial hypothalamus (VMH) after repeated defeat (Kollack-Walker et al., 1997, 1999).
Corticotropin-releasing factor (CRF) family peptides are key mediators of behavioral, autonomic, and neuroendocrine responses to stress (Vale et al., 1981). CRF is distributed in the PVN, from which it elicits pituitary adrenocorticotropic hormone release following secretion from the median eminence (Sawchenko et al., 1993), and in extrahypothalamic brain regions where it subserves non-neuroendocrine, stress-related functions (Zorrilla and Koob, 2004). For example, acute defeat elevates CRF mRNA in the hippocampus (Marini et al., 2006), ventral BNST, and CeA (Funk et al., 2006).
Two genes encoding G-protein-coupled CRF receptors have been identified (CRF1 and CRF2) (Chen et al., 1993; Lovenberg et al., 1995). Localization studies show distinct distributions of these receptor subtypes, suggesting functional diversity (Fekete and Zorrilla, 2007). The CRF1 receptor, expressed throughout the brain including the cortex and cerebellum, mediates activational and anxiogenic-like components of stress-related behaviors (Zorrilla and Koob, 2004). CRF2 receptors are expressed in discrete brain areas, including the hypothalamus and limbic system, with CRF2 receptors in the hypothalamus and hindbrain regulating food intake and gastric motility (Fekete and Zorrilla, 2007). However, whether and which CRF2 receptors participate in endogenous responses to stress remain less clear, with data suggesting roles in the LS (Henry et al., 2006) and dorsal raphe (Hammack et al., 2003; Staub et al., 2005; Cooper and Huhman, 2007). Interestingly, CRF2 receptor mRNA is also present at medium-to-high levels in other brain regions where defeat induces c-fos gene expression, including the BNST, MeA, PVN, ARH, and VMH (Van Pett et al., 2000), raising the possibility that CRF2 neurons may mediate responses to social defeat.
Therefore, the present study tested the hypothesis that CRF2-containing neurons are activated by social defeat. Fos was used as a marker for neuronal activation to determine semiquantitatively whether social defeat activates neurons in areas that express CRF2. Combined immunohistochemistry and in situ hybridization was then performed to visualize Fos immunoreactivity and CRF2 mRNA simultaneously to identify neurons that express both Fos and CRF2.
EXPERIMENTAL PROCEDURES
Subjects
Adult male Wistar rats (n=12; 275–300 g on arrival; Charles River, Raleigh, NC, USA) were “intruders” in the resident–intruder defeat model of the present studies. Subjects were single-housed in wire-topped, plastic cages (48×27×20 cm) in a 12-h light/dark (08:00 h lights off), humidity- (60%) and temperature-controlled (22 °C) vivarium. Larger, adult male Long-Evans rats (n=6, 450–500 g on arrival) were housed in enclosures (48×69×50 cm) with sawdust-covered, stainless steel floors and were territorial “residents” in the defeat model. Each resident was stably housed with an adult female Wistar rat (n=6) that had received electrocauterization of the uterine coils under isoflurane anesthesia (1%–3% in oxygen) to prevent pregnancy. Food and water were available ad libitum unless stated otherwise. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication 85–23, revised 1996) and the “Principles of laboratory animal care” (http://www.nap.edu/readin-groom/bookslabrats) and were approved by the Institutional Animal Care and Use Committee of the Scripps Research Institute. All efforts were made to minimize the number of animals used and their suffering.
Resident-intruder social defeat procedure
Each resident (n=6) was housed with a sexually mature Wistar female for 1 month to promote territorial behavior. The female mate was removed from the cage before placing the intruder within the enclosure. Long-Evans rats were used as residents following the precedent of Miczek and colleagues (Miczek, 1979; Tornatzky and Miczek, 1993, 1994), who developed and characterized the resident–intruder model of social defeat. Long-Evans rats are used as residents in the model because of the strain’s propensity for territoriality and dominance behavior across the lifespan (Blanchard et al., 1984). Strains that show somewhat less social agonistic behavior are used as intruders in this model; here, intruders were from the less aggressive Wistar strain (Scholtens and Van de Poll, 1987; Schuster et al., 1993), also a well-studied model in neuroscience research. To potentiate dominance behavior, residents were exposed to “training” intruders—post-pubertal, smaller (200–225 g) male Wistar rats—for 16 days before experimental studies. Residents were exposed to different training intruders twice per day, every other day, with exposure lasting until the intruder submitted or, if submission did not occur, 5 min. Intruders were removed from the home cage immediately after defeat, and females were returned to the cage. The criterion for defeat was adoption of a submissive, supine posture by the intruder rat, as defined by Miczek and colleagues (Miczek, 1979; Tornatzky and Miczek, 1993). This training reduces the mean latency by which residents later achieve submissions over experimental intruders to <90 s and thereby reduces the duration of physical conflict that residents require to attain defeats. Potential residents that injured intruders during training, did not achieve 3 consecutive days of defeat, or had mean defeat latencies >120 s were excluded from the study. Training was conducted during the animals’ dark cycle under red lighting.
For testing, each experimental intruder (n=6) was placed inside the resident’s home cage until defeat. Upon submission, intruders were placed inside a protective wire-mesh enclosure (20×20×32 cm) that was then placed within the resident’s home cage. Intruders remained in the enclosure for 75 min to allow for Fos protein expression and to provide further psychosocial threat. The wire enclosure prevented injurious physical interactions but allowed auditory, olfactory, visual and limited physical contact (mouth/nose). Control rats (n=6) were picked up, briefly handled and returned to their home cage for 75 min.
Perfusion and tissue sectioning
After the 75-min post-defeat interval (within the protective wire-mesh enclosure for intruders or home cage for controls), subjects were anesthetized with an overdose of chloral hydrate (1 g/kg body weight, i.p.) and perfused transcardially with 150 ml of saline followed by 350 ml of 4% paraformaldehyde in borate buffer (pH 9.5). The whole brain was post-fixed in 25% sucrose in 4% paraformaldehyde at 4 °C for 24 h. Brains were stored at −80 °C until use. Coronal sections (25 μm) were cut on a sliding microtome and collected in a one-in-12 series. The tissue sections were stored until use at −20 °C in multiwell tissue culture plates containing cryoprotectant. The first two wells were used for immunocytochemistry. Sections from one defeated rat were not useable for counting Fos-labeled cells in the LS, MeA, ARC or BNST; sections from one control rats were not useable for the ARC or BNST.
Immunocytochemistry
Tissue sections were rinsed in 0.05 M potassium phosphate-buffered saline (KPBS) followed by treatment with 1% NaBH4–KPBS solution (Sigma, St. Louis, MO, USA). Sections were incubated with Fos protein antibodies raised in rabbit (1:60,000, EMD Biosciences, San Diego, CA, USA) in KPBS with 0.4% Triton X-100 at room temperature for 1 h, followed by 4 °C for 48 h. After incubation, sections were rinsed in KPBS and incubated in biotinylated donkey anti-rabbit IgG (1:600, Vector Laboratories, Burlin-game, CA, USA) in KPBS with 0.4% Triton X-100 for 1 h at room temperature, followed by a 1-h incubation at room temperature in avidin–biotin complex solution (4.5 μl of A and B each per ml of KPBS-0.4% Triton X-100; Vectastain ABC Elite Kit, Vector Laboratories). The antibody–peroxidase complex was visualized with a mixture of 3,3-diaminobenzidine (0.2 mg/ml) and 3% H2O2 (0.83 μl/ml) in 0.05 M Tris buffer–saline solution. Following the staining, sections were mounted on gelatin-coated slides, dehydrated, and coverslipped. For the Fos protein/CRF2 mRNA double-labeling study, brain sections were processed for Fos staining as described above, with the exception that all solutions were treated with diethylpyrocarbonate to prevent RNase contamination. Following Fos staining, brain sections were processed for in situ hybridization.
In situ hybridization
To determine whether some cells that expressed Fos protein following defeat were CRF2 receptor-expressing cells, immunocytochemistry and in situ hybridization were serially combined to visualize Fos protein and CRF2 mRNA simultaneously. All solutions were treated with diethylpyrocarbonate to protect brain sections from RNase contamination. For in situ hybridization, a [33P]uridine triphosphate-labeled (PerkinElmer, Boston, MA, USA) CRF2 complementary RNA (cRNA) probe was transcribed from a 460 base-pair 5′-region of the CRF2 cDNA linearized with XbaI (Li et al., 2002). The riboprobes were directed against the 5′ region of rat CRF2 receptors, covering the sequence up to the third presumed transmembrane region (Chalmers et al., 1995). The specific activity of the probe was ~5×105 cpm/ml of hybridization buffer. Following Fos immunostaining, brain sections were washed with KPBS, treated with acetic anhydride and exposed to the CRF2 cRNA probe overnight in moist chambers at 55 °C. Sections were then washed two times in 4× sodium chloride/sodium citrate (SSC) buffer, in RNase A (30 μg/ml final concentration, Sigma), one time in 2× SSC and finally in 0.1× SSC at 60 °C, and mounted on gelatin-coated slides that were then dipped in NTB-2 emulsion (Eastman Kodak, Rochester, NY, USA). The slides were exposed for 15 days at 4 °C and developed.
Imaging
Slides were examined using a Nikon (E600) light microscope (Lake Forest, CA, USA). The numerical apertures of the 4×, 10× and 40× lens were 0.2, 0.45 and 0.95, respectively. Four brain sections from each region of interest were analyzed, relative to bregma (anterior/posterior), as follows: from +1.2 to 0.6 for dorsal part of the lateral septum (dLS), from +0.48 to 1.3 for BNST, from −1.80 to −3.60 for MeA, from −1.0 to −2.12 for PVN, from −2.1 to −3.30 for VMH and from −1.80 to −3.60 mm for ARH per a rat brain atlas (Paxinos and Watson, 1998). Each coordinate range was further subdivided into 600 μm segments (two segments for PVN and VMH and three segments for dLS, BNST, MeA and ARH). One to two sections were analyzed from each 600 μm segment for data collection. Images were captured by a digital camera (Photometrics Cool-snap CF, Tucson, AZ, USA). Fos-positive cells in captured images were analyzed and counted using Image-Pro Plus (Media Cybernetics, Silver Spring, MD, USA). CRF2 positive cells were identified from the same regions that were analyzed for Fos immunoreactivity as clusters of silver grains, indicating [33P]uridine triphosphate-labeled CRF2 cRNA probe signals, in dark field photomicrographs. The images were cropped and adjusted to balance brightness and contrast in Adobe Photo-shop 8.0 (Adobe Systems, San Jose, CA, USA) before importing the images into Canvas 8.0 (Deneba Systems, Miami, FL, USA) for assembly into plates. The brain plates were then imported into Canvas for reordering.
Statistical analysis
For each rat, Fos-positive cells were counted bilaterally in four brain sections and averaged, with all sections from a given brain region evaluated by a single treatment-naive rater. Data were subjected to Student’s t-test for comparisons between the control and defeated groups or Welch’s t-test when variance significantly differed between groups. The software package used was InStat 3.0 (GraphPad, San Diego, CA, USA).
RESULTS
Distribution of Fos-positive cells in selected rat forebrain areas after social defeat
Intruders were rapidly defeated by residents (range: 31–126 s). An initial survey of the forebrain found that defeated rats showed more Fos-positive cells compared with control rats 75 min post-defeat in several regions in the limbic system and the hypothalamus (Table 1). In limbic structures, Fos-positive cells were observed in the dLS, MeA, and anterior cortical nuclei of the amygdala. A few Fos-positive cells were observed in the BNST of defeated subjects, but not more than in controls.
Table 1.
Semi-quantitative distribution of Fos-positive cells in selected areas in rat brain 75 min after acute defeat relative to control
| Forebrain region | Density of Fos-immunoreactive cells |
|---|---|
| Septum | |
| Dorsolateral nucleus | + |
| Intermediate nucleus medial nucleus | −/+ |
| −/+ | |
| Amygdala | |
| Anterior cortical nucleus | + |
| Posterior cortical nucleus | −/+ |
| Basolateral nucleus | −/+ |
| Basomedial nucleus | −/+ |
| Central nucleus | −/+ |
| Medial nucleus | +++ |
| BNST | |
| Rostral region | − |
| Posterior region | − |
| Hypothalamus | |
| Arcuate nucleus | ++/+++ |
| PVN | |
| Parvocellular | − |
| Magnocellular | −/+ |
| Supraoptic nucleus | + |
| VMH | ++/+++ |
| Lateral hypothalamus | + |
Semi-quantitative ratings reflect the density of Fos-positive cells relative to controls, with (−) representing control-like levels of Fos-labeled cells, and plus symbols indicating slightly (+), moderately (++), or highly elevated (+++) numbers of Fos-labeled cells compared with controls in a given cell group or field. The (−/+) symbol indicates control-like or slightly elevated numbers of Fos-labeled cells, varying across intruders.
In the hypothalamus, Fos-positive cells were consistently observed in the ARH and VMH (Table 1). The supraoptic nucleus and lateral hypothalamus showed few, but slightly increased numbers of Fos-positive cells after defeat. The PVN did not show Fos-positive cells 75 min post-defeat.
Quantification of Fos protein expression following acute social defeat
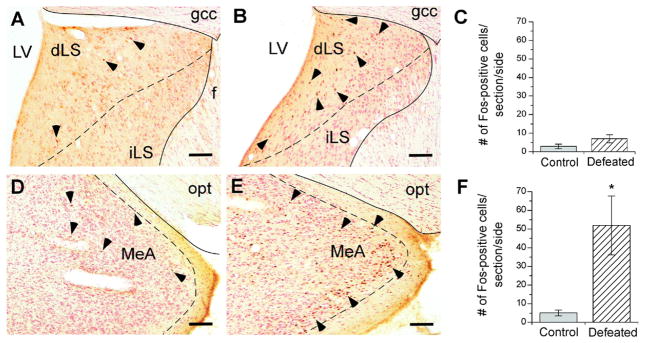
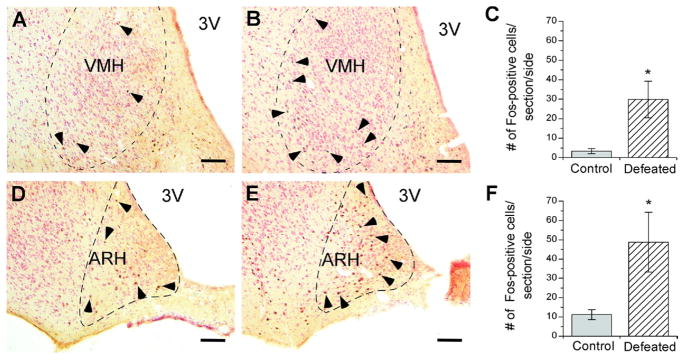
Fos-positive neurons were counted in selected brain regions 75 min after defeat. As shown in Figs. 1 and 2, compared with control rats, socially defeated rats showed significantly more Fos-positive cells in the MeA (Welch’s t4= 2.97, P≤0.05) (Fig. 1D, E, F), especially the posterior part of the MeA. In the hypothalamus, defeat induced significantly more Fos-positive cells in the VMH (Welch’s t5=2.80, P≤0.05) (Fig. 2A, B, C) and ARH (Student’s t8= 2.38, P≤0.05) (Fig. 2D, E, F). The majority of Fos immunostaining was evident in the ventrolateral part of the VMH and medial part of the ARH (Fig. 2B, E). No significant difference in Fos-positive cell counts was observed between control vs. defeated rats within the dLS (Fig. 1A, B, C), anterior BNST (defeated: 7.4±3.9, control: 7.2±2.8 cells), posterior BNST (defeated: 3.3±2.0, control: 9.5±4.8 cells), or PVN (defeated: 8.0±2.9, control: 11.4±5.5 cells).
Fig. 1.
Fos immunoreactivity in the dorsal lateral septum (A, B), and medial amygdala (D, E) 75 min after an acute defeat (B, n=5; E, n=5) or control (A, n=6; D, n=6) procedure. Dorsal LS and MeA panels are in the range of −0.26 to −0.30 and −2.80 to −3.14 mm from bregma, respectively. Bar graphs represent the mean (±SEM) number of Fos-positive cells per section per side in the dorsal lateral septum (C) and medial nucleus of the amygdala (F) after control (grey bars) or acute defeat (stripped bars) conditions. Selected Fos-positive cells are indicated with a black arrowhead. Symbols indicate significant difference between control and defeated rats: * P<0.05. Scale bar=100 μm. LV, lateral ventricle; gcc, genu of corpus callosum; iLS, intermediate part of the lateral septum; opt, optic tract.
Fig. 2.
Fos immunoreactivity in the ventromedial (A, B) and arcuate nuclei of the hypothalamus (D, E) 75 min after an acute defeat (B, n=6; E, n=5) or control (A, n=6; D, n=5) procedure. VMH and ARH panels are in the range of −2.30 to −3.14 and −2.30 to −2.56 mm (anterior/posterior) from bregma, respectively. Bar graphs represent the mean (±SEM) number of Fos-positive cells per section per side in the VMH (C) and the arcuate nucleus (F) after control (grey bars) or acute defeat (striped bars) conditions. Selected Fos-positive cells are indicated with a black arrowhead. Symbols indicate significant differences between control and defeated rats: * P<0.05. Scale bar=100 μm. 3V, Third ventricle.
Partial colocalization of Fos protein and CRF2 mRNA in the MeA
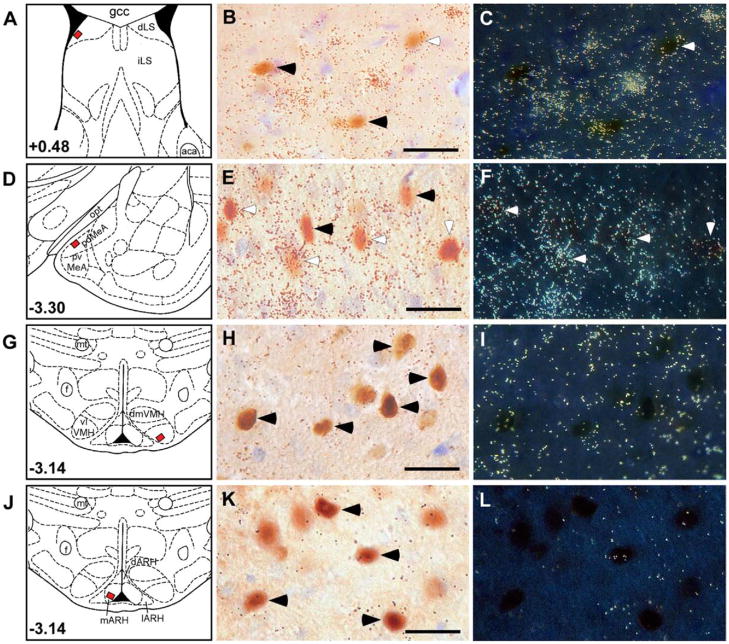
Similar to previous reports (Van Pett et al., 2000; Li et al., 2002), high levels of CRF2 receptor mRNA were observed in the intermediate part of the LS (Supplementary Fig. 1A) and dorsomedial VMH (Supplementary Fig. 1C). Moderate CRF2 mRNA density was observed in the dLS (Supplementary Fig. 1A), anterior and posterior MeA (Supplementary Fig. 1B), basomedial amygdala, and mainly in the posterior region of the BNST (not shown). Low levels of CRF2 mRNA were also evident in the hippocampal formation (not shown), ARH (Supplementary Fig. 1D), magno-cellular division of the PVN, and supraoptic nucleus (see Supplementary Table 1). The pattern of CRF2 expression significantly overlapped with that of defeat-induced Fos expression, suggesting that social defeat might activate CRF2-positive neurons in these areas. Therefore, dual-labeling of CRF2 mRNA and Fos immunoreactivity was performed to determine whether some of the Fos-positive cells in these areas were also CRF2-positive. As shown in Fig. 3D, E, and F, high degrees of colocalization of signals for Fos protein and CRF2 mRNA were found in the MeA, in which 49.7% of Fos-positive cells expressed CRF2 mRNA. Only moderate numbers (12.7%) of Fos-positive cells in the dLS were found to colocalize with CRF2 mRNA (Fig. 3A, B, C). Negligible or no colocalization of CRF2 mRNA with Fos protein was seen in the VMH (Fig. 3G, H, I; 14.2%) or ARH (Fig. 3J, K, L; 0.0%), respectively.
Fig. 3.
Acute defeat induces Fos protein expression at sites of CRF2 receptor mRNA expression in the rat brain. Schematic panels showing the bregma level (A, D, G, J) with the small black square filled with red within them indicating the region photographed at higher magnification and shown in B, C, E, F, H, I, K, and L. Brightfield photomicrographs of sections through the dorsal lateral septal (B), posterior medial amygdala (E), ventrolateral ventromedial hypothalamus (H), and medial arcuate nuclei (K) of the hypothalamus showing cells with acute defeat-induced nuclear Fos immunoreactivity and highlighted with silver grain clusters (dark dots), indicating a positive CRF2 receptor mRNA signal (white arrowheads). Darkfield photomicrographs of adjacent sections (C, F, I, L) compare the distribution of CRF2 mRNA (bright small dots, as clusters of silver grains, indicating [33P]uridine triphosphate-labeled CRF2 cRNA probe signals). Cells labeled singly for Fos (black arrowheads) or the CRF2 transcript colocalizing with Fos-labeled cells (white arrowhead) are shown for contrast. gcc, Genu of corpus callosum; iLS, intermediate lateral septum; aca, anterior part of anterior commissure; opt, optic tract; pd or pvMeA, posterodorsal or posteromedial medial amygdala; mt, mammillothalamic tract; f, fornix; vl or dmVMH, ventrolateral or dorsomedial ventromedial nuclei of hypothalamus; d or m or lARH, dorsal or medial or lateral arcuate nuclei of the hypothalamus. Scale bar=50 μm.
DISCUSSION
The present results indicate that CRF2 receptor-expressing neurons in the MeA are involved in the response to acute defeat, a model of antagonistic social stress relevant to several affective and reproductive disorders (Luiten et al., 1985; Kollack-Walker and Newman, 1995; Martinez et al., 1998; Blanchard et al., 2001; Dominguez et al., 2001). Confirming previous findings that used c-fos mRNA or Fos protein expression as markers of neuronal activation, several brain areas were activated following social defeat (Cullinan et al., 1995). Fos protein expression 75 min post-defeat was evident in the MeA, LS, ARH, and VMH, structures which all express high levels of urocortin-CRF2 system molecules (Van Pett et al., 2000; Li et al., 2002; reviewed in Fekete and Zorrilla, 2007). Combined in situ hybridization/immunocytochemistry demonstrated that a large proportion of Fos immunoreactive cells in the MeA, but not in the dLS, ARH, or VMH, were also positive for CRF2 mRNA.
The MeA, which receives input from the vomeronasal and olfactory systems via the cortical nucleus of the amygdala (Newman, 1999), projects to brain regions involved in stress- and sociosexual reproduction-related functions, including other amygdala divisions, extended amygdala (e.g. BNST), hippocampus (Canteras et al., 1995), and hypothalamic nuclei that modulate neuroendocrine systems and sexual and agonist behaviors (e.g. medial preoptic area, VMH and ventral premammillary nucleus; Paredes and Baum, 1997). Accordingly, the MeA has a role in several social behavioral responses, including behavioral arousal (Kollack-Walker and Newman, 1995), acquisition and expression of conditioned defeat (Markham and Huhman, 2008), social learning and memory processes (Luiten et al., 1985), and fear and anxiety-like behavior (Chen et al., 2006). Relevant to defeat, MeA lesions prevent males from avoiding conspecifics that recently defeated them (Luiten et al., 1985) and severely impair mating, parental behavior (Sheehan et al., 2001), and other reproductive functions (Dominguez et al., 2001). Lesions of the MeA also reduce defensive responses to predator odor and escape responses to noxious stimuli (Blanchard et al., 2005). Additionally, the MeA is a proposed regulator of hypothalamic–pituitary–adrenal axis activity (Dayas et al., 1999), with MeA stimulation increasing plasma glucocorticoid and adrenocorticotropic hormone levels (Herman et al., 1996). The results raise the hypothesis that CRF2-synthesizing neurons in the MeA may participate in consequences of, or counter-regulatory responses to, defeat.
As a structure subserving sociosexual and agonistic behavior, the MeA is structurally and functionally sexually dimorphic (Cooke and Woolley, 2005; Cooke, 2006), with the posterodorsal MeA ~50% larger in males than in females (Hines et al., 1992) in relation to circulating androgen levels. The MeA has many neurons that contain high levels of estrogen and/or testosterone and their receptors (Greco et al., 1998), a finding specifically true of CRF2-expressing neurons in the posterior MeA (Van Pett et al., 2000). Stressors, including defeat, suppress testosterone secretion in both rodents and primates, including man (Armario and Castellanos, 1984; Sapolsky, 1985; Schultheiss et al., 2005). Perhaps the defeat-induced changes in CRF2-expressing MeA circuitry are associated with the suppression of circulating testosterone.
A candidate natural ligand for MeA CRF2 receptors is urocortin 3. Many urocortin 3–positive neurons and fibers are present in the MeA, where its expression is increased by stress (Jamieson et al., 2006), and in the cortical nucleus of the amygdala, from which the MeA receives extensive projections (Li et al., 2002). Several studies suggest an anxiolytic-like role for CRF2 receptors. For example, administration of urocortin into the adjacent CeA reduced anxiety-like behavior during ethanol withdrawal (Valdez et al., 2004). I.c.v. urocortin 3 administration also reduced anxiety-like behavior in some, but not all, rodent models of anxiety-like behavior (Zhao et al., 2007). Some, but not all (Coste et al., 2000), studies of CRF2 knockout mice observed an anxiogenic-like phenotype of mice following stressor exposure (e.g. in the elevated plus maze [Bale et al., 2000; Kishimoto et al., 2000], emergence, open field [Bale et al., 2000], or light/dark box tests [Henry et al., 2006]). Conversely, some findings (Pelleymounter et al., 2004) support an alternate hypothesis that CRF2 receptor activation has anxiogenic-like effects, with lateral septal (Henry et al., 2006) or dorsal raphe (Hammack et al., 2003) CRF2 activation promoting defensive responses to uncontrollable stressors. However, to our knowledge, no studies have evaluated the behavioral effects of intra-MeA CRF2 agonist infusion. Thus, the specific anxiety-related or other behavioral role of CRF2 receptors in the MeA remains to be determined.
Defeat also increased the number of Fos-positive cells in the VMH, another androgen receptor–expressing nucleus that is larger in males than in females (Dugger et al., 2007). However, induction of Fos immunoreactivity was observed mostly in the ventrolateral VMH, whereas CRF2 mRNA predominated in the dorsomedial VMH, similar to previous studies (Lovenberg et al., 1995). Functional segregation of the dorsomedial vs. ventrolateral VMH has been proposed previously. For example, in males, the dorsomedial VMH is critical for ultrasonic vocalization and scent marking behaviors (Harding and McGinnis, 2005), whereas the ventrolateral VMH subserves mounting behavior (Pfaff and Sakuma, 1979). The dorsomedial VMH also putatively differentially subserves energy homeostasis (Flanagan-Cato, 2003), defensive aggression (Canteras et al., 1994; Canteras, 2002), and innate affective reactions to pain (Borszcz, 2006). The two VMH subregions receive innervation from topographically distinct aspects of the MeA (Canteras et al., 1995). The ventrolateral VMH also more closely innervates other hypothalamic regions that express gonadal steroid hormone receptors, including the medial preoptic, tuberal, and ventral premammillary nuclei, whereas the dorsomedial VMH differentially projects to anterior hypothalamic and dorsal premammillary nuclei (Canteras et al., 1994). Finally, the androgen-dependent sexual dimorphism of VMH volume is seen selectively in the ventrolateral, but not dorsomedial, subdivision (Dugger et al., 2007). The results suggest that defeat-induced Fos protein expression in the VMH may involve a subpopulation of cells distinct from those that express CRF2 receptors.
Defeat also induced Fos protein expression in a sub-population of cells in the medial ARH distinct from those that synthesize CRF2 receptors. Neurons in the medial ARH have a role in regulatory inhibition of the hypothalamic–pituitary–adrenal axis (Kollack-Walker et al., 1997) and of prolactin secretion via tuberoinfundibular dopamine projections to the median eminence (Freeman et al., 2000). Lesions of the ARH increased basal glucocorticoid levels and enhanced adrenocortical activity in response to stress (Cullinan et al., 1995). A separate population of medio-basal ARH neurons has recognized roles in energy homeostasis and reproductive functions via parallel ascending projections of orexigenic neuropeptide Y/agouti-related protein-expressing neurons and anorexigenic proopiomel-anocortin/cocaine and amphetamine-regulated transcript-expressing neurons to other hypothalamic nuclei (Hill et al., 2008). Arcuate neurons also are implicated in opioid-mediated stress-induced analgesia (Wang et al., 1990) following defeat (Miczek et al., 1985). Identification of the phenotypes of Fos-positive cells in the ARH may provide further insight into the physiological role and function of ARH neurons in social defeat stress.
The absence of PVN or BNST Fos protein expression following defeat stress was somewhat unexpected (Herman et al., 1996; Nail-Boucherie et al., 1998). The time course of analysis may be relevant, with PVN and BNST Fos expression perhaps occurring transiently and earlier in time. Indeed, in separate studies, increased Fos protein expression was observed in both the PVN and BNST 30 min post-defeat (ÉM Fekete, Y Zhao, C Li, V Sabino, WW Vale, EP Zorrilla, unpublished observations). Nonetheless, the present findings are consistent with reports that hypothalamic–pituitary–adrenal axis activation did not require PVN Fos expression (Brown and Sawchenko, 1997; Figueiredo et al., 2003) and that c-fos mRNA was not elevated in the LS, PVN, or BNST following defeat, while it was seen after foot shock or restraint (Funk et al., 2006). The functional significance of early BNST activation after defeat is similarly unclear. On the one hand, the BNST has been associated with fear-related behavior (Lee and Davis, 1997; Onaka and Yagi, 1998), and injection of antisauvagine-30 (a preferential CRF2 antagonist) into the BNST reduced conditioned defeat behavior (Cooper and Huhman, 2005). On the other hand, the number of threats received by an intruder correlated negatively with c-Fos expression in the BNST in a previous study (Martinez et al., 1998).
Importantly, c-fos activation does not provide a complete map of all neurons activated during a given stimulus (Robertson et al., 1989). Some activated neurons that express Fos protein may lead to downstream inhibition, and Fos protein is not known as a good marker of inhibition. Thus, induction of Fos immunoreactivity provides positive identification of brain areas activated during social conflict, but the absence of such labeling does not necessarily equal lack of involvement. Studies with other functional markers may help further delineate brain activation and inhibition patterns associated with social defeat. The present findings show, however, that CRF2-synthesizing neurons in the posterior MeA are part of the neural response to social defeat.
Supplementary Material
Acknowledgments
The authors thank Robert Lintz, Peilin Chen, Molly Brennan and Maegan Mattock for technical contributions and Mike Arends for editorial assistance. This is publication number 19600 from The Scripps Research Institute. Supported by grant 5P01 DK026741 from the National Institute of Diabetes and Digestive and Kidney Diseases and PT074076 from the Department of Defense Congressionally Directed Medical Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. É.M.F. was supported by a Hungarian Eötvös Fellowship.
Abbreviations
- ARH
arcuate nucleus of the hypothalamus
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- CRF
corticotropin-releasing factor
- CRF1
type 1 corticotropin-releasing factor receptor
- CRF2
type 2 corticotropin-releasing factor receptor
- cRNA
complementary RNA
- dLS
dorsal part of the lateral septum
- KPBS
potassium phosphate-buffered saline
- LS
lateral septum
- MeA
medial nucleus of the amygdala
- PVN
paraventricular nucleus of the hypothalamus
- SSC
sodium chloride/sodium citrate
- VMH
ventromedial hypothalamus
APPENDIX
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi: 10.1016/j.neuroscience.2009.03.078.
References
- Armario A, Castellanos JM. Effect of acute and chronic stress on testosterone secretion in male rats. J Endocrinol Invest. 1984;7:659–661. doi: 10.1007/BF03349502. [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a cat, cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Flannelly KJ, Layng M, Blanchard DC. The effects of age and strain on aggression in male rats. Physiol Behav. 1984;33:857–861. doi: 10.1016/0031-9384(84)90219-1. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko E, Dulloog L, Blanchard DC. Defense changes in stress nonresponsive subordinate males in a visible burrow system. Physiol Behav. 2001;72:635–642. doi: 10.1016/s0031-9384(00)00449-2. [DOI] [PubMed] [Google Scholar]
- Borszcz GS. Contribution of the ventromedial hypothalamus to generation of the affective dimension of pain. Pain. 2006;123:155–168. doi: 10.1016/j.pain.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ER, Sawchenko PE. Hypophysiotropic CRF neurons display a sustained immediate-early gene response to chronic stress but not to adrenalectomy. J Neuroendocrinol. 1997;9:307–316. doi: 10.1046/j.1365-2826.1997.00586.x. [DOI] [PubMed] [Google Scholar]
- Calfa G, Bussolino D, Molina VA. Involvement of the lateral septum and the ventral hippocampus in the emotional sequelae induced by social defeat: role of glucocorticoid receptors. Behav Brain Res. 2007;181:23–34. doi: 10.1016/j.bbr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Zorrilla E, Smith S, Rousso D, Levy C, Vaughan J, Donaldson C, Roberts A, Lee KF, Vale W. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J Neurosci. 2006;26:5500–5510. doi: 10.1523/JNEUROSCI.3955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Sexually dimorphic synaptic organization of the medial amygdala. J Neurosci. 2005;25:10759–10767. doi: 10.1523/JNEUROSCI.2919-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor type II (CRF2) receptors in the bed nucleus of the stria terminalis modulate conditioned defeat in Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2005;119:1042–1051. doi: 10.1037/0735-7044.119.4.1042. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology (Berl) 2007;194:297–307. doi: 10.1007/s00213-007-0849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Dominguez J, Riolo JV, Xu Z, Hull EM. Regulation by the medial amygdala of copulation and medial preoptic dopamine release. J Neurosci. 2001;21:349–355. doi: 10.1523/JNEUROSCI.21-01-00349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51:195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Flanagan-Cato L. Hypothalamic neural circuitry: a target for the behavioral effects of steroids. San Diego: Academic Press; 2003. [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Greco B, Edwards DA, Michael RP, Clancy AN. Androgen receptors and estrogen receptors are colocalized in male rat hypothalamic and limbic neurons that express Fos immunoreactivity induced by mating. Neuroendocrinology. 1998;67:18–28. doi: 10.1159/000054294. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Microlesions of the ventromedial nucleus of the hypothalamus: effects on sociosexual behaviors in male rats. Behav Neurosci. 2005;119:1227–1234. doi: 10.1037/0735-7044.119.5.1227. [DOI] [PubMed] [Google Scholar]
- Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–E832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Li C, Kukura C, Vaughan J, Vale W. Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology. 2006;147:4578–4588. doi: 10.1210/en.2006-0545. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinol. 1999;11:547–559. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Korte M, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiten PG, Koolhaas JM, de Boer S, Koopmans SJ. The cortico-medial amygdala in the central nervous system organization of agonistic behavior. Brain Res. 1985;332:283–297. doi: 10.1016/0006-8993(85)90597-9. [DOI] [PubMed] [Google Scholar]
- Marini F, Pozzato C, Andreetta V, Jansson B, Arban R, Domenici E, Carboni L. Single exposure to social defeat increases corticotropin-releasing factor and glucocorticoid receptor mRNA expression in rat hippocampus. Brain Res. 2006;1067:25–35. doi: 10.1016/j.brainres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Miczek K. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, 3rd, Nikulina EM, Jr, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic meso-corticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Naloxone injections into the periaqueductal grey area and arcuate nucleus block analgesia in defeated mice. Psychopharmacology (Berl) 1985;87:39–42. doi: 10.1007/BF00431775. [DOI] [PubMed] [Google Scholar]
- Nail-Boucherie K, Garcia R, Jaffard R. Influences of the bed nucleus of the stria terminalis and of the paraventricular nucleus of the hypothalamus on the excitability of hippocampal-lateral septal synapses in mice. Neurosci Lett. 1998;246:112–116. doi: 10.1016/s0304-3940(98)00230-4. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Onaka T, Yagi K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Res. 1998;788:287–293. doi: 10.1016/s0006-8993(98)00012-2. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Baum MJ. Role of the medial preoptic area/anterior hypothalamus in the control of masculine sexual behavior. Annu Rev Sex Res. 1997;8:68–101. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Robertson HA, Peterson MR, Murphy K, Robertson GS. D1-dopamine receptor agonists selectively activate striatal c-fos independent of rotational behaviour. Brain Res. 1989;503:346–349. doi: 10.1016/0006-8993(89)91689-2. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress-induced suppression of testicular function in the wild baboon: role of glucocorticoids. Endocrinology. 1985;116:2273–2278. doi: 10.1210/endo-116-6-2273. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. discussion: 21–29. [DOI] [PubMed] [Google Scholar]
- Scholtens J, Van de Poll NE. Behavioral consequences of agonistic experiences in the male S3 (Tryon maze dull) rat. Aggress Behav. 1987;13:213–226. [Google Scholar]
- Schultheiss OC, Wirth MM, Torges CM, Pang JS, Villacorta MA, Welsh KM. Effects of implicit power motivation on men’s and women’s implicit learning and testosterone changes after social victory or defeat. J Pers Soc Psychol. 2005;88:174–188. doi: 10.1037/0022-3514.88.1.174. [DOI] [PubMed] [Google Scholar]
- Schuster R, Berger BD, Swanson H. Cooperative social coordination and aggression. II. Effects of sex and housing among three strains of intact laboratory rats differing in aggressiveness. Q J Exp Psychol B. 1993;46:367–390. [PubMed] [Google Scholar]
- Sheehan T, Paul M, Amaral E, Numan MJ, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106:341–356. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- Shimozuru M, Kikusui T, Takeuchi Y, Mori Y. Social-defeat stress suppresses scent-marking and social-approach behaviors in male Mongolian gerbils (Meriones unguiculatus) Physiol Behav. 2006;88:620–627. doi: 10.1016/j.physbeh.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiol Behav. 1984;33:777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- Stork O, Welzl H, Cremer H, Schachner M. Increased intermale aggression and neuroendocrine response in mice deficient for the neural cell adhesion molecule NCAM. Eur J Neurosci. 1997;9:1117–1125. doi: 10.1111/j.1460-9568.1997.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80:683–693. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Behavioral and autonomic responses to intermittent social stress: differential protection by clonidine and metoprolol. Psychopharmacology (Berl) 1994;116:346–356. doi: 10.1007/BF02245339. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mao LM, Han JS. Naloxone-reversible analgesia produced by microstimulation of the arcuate nucleus of the hypothalamus in pentobarbital-anesthetized rats. Exp Brain Res. 1990;80:201–204. doi: 10.1007/BF00228862. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Kimura N. Ethopharmacology of copulatory disorder induced by chronic social conflict in male mice. Neurosci Biobehav Rev. 1991;15:497–500. doi: 10.1016/s0149-7634(05)80138-1. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther. 2007;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.