Abstract
Small membrane-bound extracellular organelles known as articular cartilage matrix vesicles (ACVs) participate in pathologic mineralization in osteoarthritic articular cartilage. ACVs are also present in normal cartilage, although they have no known functions other than mineralization. Recently, RNA was identified in extracellular vesicles derived from mast cells, suggesting that such vesicles might carry coding information from cell to cell. We found that ACVs from normal porcine and human articular cartilage and primary chondrocyte conditioned media contained 1 μg RNA/80μg ACV protein. No DNA could be detected. RT-PCR of ACV RNA demonstrated the presence of full length mRNAs for factor XIIIA, type II transglutaminase, collagen II, aggrecan, ANKH and GAPDH. RNA in intact ACVs was resistant to RNase, despite the fact that ACV preparations contained measurable levels of active RNases. Significantly, radiolabelled RNA in ACVs could be transferred to unlabelled chondrocytes by co-incubation and produced changes in levels of chondrocyte enzymes and proteins. The demonstration that ACVs contain mRNAs suggests that they may function to shuttle genetic information between articular cells and indicate novel functions for these structures in articular cartilage.
Keywords: articular cartilage vesicles, matrix vesicles, RNA, articular cartilage, chondrocytes
Articular cartilage matrix vesicles (ACVs) are small membrane-bound extracellular organelles characterized by their ability to form pathologic calcium-containing crystals [1]. When isolated from cartilage, ACVs generate calcium pyrophosphate dihydrate (CPPD) as well as hydroxyapatite-like basic calcium phosphate crystals (BCP) identical to those seen in human synovial fluids from arthritic joints [2]. These crystals are extremely common in joints affected by osteoarthritis, where their presence correlates with severity and progression of cartilage damage [3, 4].
ACVs, which can be isolated from whole articular cartilage or chondrocyte monolayers, are bound by a trilaminar membrane and are somewhat heterogeneous in size with a range from 60–120 nm [5]. ACVs belong to a family of mineralizing organelles known as matrix vesicles that bud from microvilli of hypertrophic chondrocytes, osteoblasts, odontoblasts and cells of calcifying neoplasms [6–8]. Matrix vesicles concentrate phosphate and pyrophosphate -regulating enzymes, growth factors and cytokines and serve as foci of calcium calcium crystal nucleation in the pericellular matrix [9].
Interestingly, ACVs are present in similar quantities in normal un-mineralized articular cartilage and in osteoarthritic cartilage, where matrix mineralization is common [10]. As few structures exist in nature to serve only a pathologic purpose, it is likely that ACVs have functions other than mineral formation. Sylvia et al. suggested that matrix vesicles in costochondral cartilage, for example, might repair the interterritorial matrix between the widely dispersed cells in this tissue [11].
Over the last ten years, there has been a burgeoning interest in extracellular organelles from non-mineralizing cells and tissues. These structures include ectosomes, exosomes, and microparticles [12, 13] that are released from a variety of cell types and serve multiple functions. In the immune system, these organelles may serve as antigen presenting devices [14]. During erythrocyte development, they remove unwanted cellular proteins and receptors [15]. Proteins that lack typical leader sequences, such as interleukin 1β, can also be exported to the extracellular space by vesicles [16], and vesicles can carry proteins and lipids from one cell to another [17]. Recently, Valadi et al. demonstrated the surprising presence of RNA in mast cell exosomes [18]. Exosomal mast cell RNA contained both mRNA for specific mast cell proteins as well as microRNAs with potential regulatory functions. Vesicle RNA could be transferred to other mast cells by co-incubation. Based on these observations, we asked whether ACVs also contain RNA, if this RNA included specific mRNAs, and if ACVs could transfer their RNA to chondrocytes.
MATERIALS AND METHODS
Materials
All reagents were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO) unless otherwise stated.
Cartilage and chondrocyte cultures
Healthy adult porcine hyaline cartilage was obtained from knee joints of freshly slaughtered 3–5 year old pigs. High density short term primary porcine chondrocytes were maintained in monolayer cultures in Dulbecco’s Modified Eagle’s medium (DMEM) as previously described [19], and placed in serum-free DMEM supplemented with 0.35 mg/ml bovine serum albumin 24 hours before an experiment was initiated. Snap frozen, de-identified, normal-appearing adult human knee cartilage was obtained from cadavers without known joint disease through the National Disease Research Interchange (NDRI) with permission from our local Institutional Review Board.
ACVs
ACVs were obtained from cartilage by sequential enzymatic digestion with hyaluronidase, trypsin and collagenase followed by differential centrifugation as previously described [20]. The ACV pellet was obtained after low speed centrifugation to clear cells and large particles (1100 × g), and then at 37,000 × g for 15 minutes to remove larger particles, organelles and cell fragments. The resultant supernatant was centrifuged at 140,000 × g for 60 minutes to obtain the ACV pellet. ACVs were also obtained with an identical centrifugation protocol from serum-free conditioned media of high density short term primary chondrocyte cultures as previously described [21]. ACVs were re-suspended in 500 μl DMEM and averaged 12–15 mg protein/ml[22].
RNA isolation and characterization
Total RNA was isolated from ACVs using TRizol (Invitrogen, Carlsbad, CA) followed by an RNeasy spin column (Qiagen, Valencia, CA). RNA was eluted with RNase-free water and quantified by spectrophotometry at 260 nm (Ratios of 260/280between 1.6 and 2.0 were obtained). For agarose gels, up to 5 ug total RNA was denatured at 65°C for 15 min and then run on a 1% formaldehyde agarose gel and visualized with ethidium bromide. Controls included total RNA from porcine chondrocytes that was isolated similarly. For conversion to cDNA, up to 3 μg RNA was reverse transcribed using random hexamers and a Superscript III Reverse Transcriptase kit (Invitrogen) according to manufacturer’s instructions. Three μl of the RT product were combined with Platinum Blue PCR Supermix (Invitrogen) containing 0.5 μM gene specific primers for specific chondrocytes proteins including aggrecan (ENSG00000157766), type II collagen (ENSG00000139219), type II transglutaminase ENSG00000198959, factor XIIIA (ENSG00000124491), and ANKH (ENSG00000154122), as well as generic genes such as GAPDH (ENSG00000111640). All primer sequences were chosen from published sequences to span a maximum number of exons. PCR conditions were as follows: 2 min at 94 C, followed by 40 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 30 s, followed by a final step at 72°C for 5min. Ten μl of sample were run on a 1.5% agarose TBE gel and stained with ethidium bromide. Band density was measured using a Kodak Image Station and Kodak Molecular Imaging Software. Bioanalyzer analysis was performed using 300 ng of chondrocyte or ACV RNA with an RNA 600 Nano Kit and an Agilent 2100 Bioanalyzer.
ACV protein synthesis
To determine whether ACVs were capable of protein synthesis, porcine ACVs were incubated with 1 μCi/ml 35S-methionine (American Radiolabeled Chemicals, St. Louis, MO) in methionine-free DMEM for 72 hours at 37°C. Protein was precipitated with trichloroacetic acid and 35S in the protein pellet was measured in a Packard TriCarb 1900 TR scintillation counter.
RNase-treated ACVs
The RNAseAlert Lab Kit (Ambion, Austin, TX) was used according to the manufacturer’s instructions to verify the presence of RNases in ACV preparations, and results were measured on a fluorescence plate reader. To assess the resistance of ACV-associated RNA to ribonucleases, either 5 U of RNaseONE (Promega, Madison, WI) or 5 ul of nuclease-free water was added to ACVs and mixed until a homogeneous suspension was obtained. Samples were incubated at 37°Cfor 30 min and TRIzol was added to inactivate RNases and homogenize the samples, which were stored for 24 hours at −70°C before RNA isolation. Additionally, ACVs with and without addition of 12 ug total RNA isolated from porcine chondrocytes were incubated with 250 ng RNase A at 37°C for 15 min. and processed similarly, except a final RNeasy column was used. RNAs were measured spectrophotometrically and analyzed on denaturing formaldehyde agarose gels.
Transfer of RNA from ACVs to chondrocytes
ACVs were isolated from the conditioned media of cells grown in the presence of 1 μCi/ml 3H-uracil (American Radiolabeled Chemicals,) for 72 hours. ACVs were then added to fresh chondrocyte monolayers or alcohol-fixed chondrocyte monolayers and incubated for 96hours. The cell layers were washed, exposed to scintillation fluid, and radioactivity incorporated into the cell layer was measured using a Packard TriCarb 1900 TR scintillation counter.
Enzyme and Protein levels in chondrocytes
To determine whether small quantities of ACVs affected chondrocytes in a measurable manner, we measured the activity of several key enzymes and proteins on chondrocytes after co-incubation with no additives, or with 1 or 10 μg/ml of ACVs for 72 hours. Cell layers were washed and activity levels of alkaline phosphatase and nucleoside triphosphate pyrophosphohydrolase (NTPPPH) were measured with standard colorimetric assays [3]. Osteopontin levels were measured by ELISA (Assay Designs, Ann Arbor, MI). While both alkaline phosphatase and NTPPPH are present on chondrocyte as well as on ACV membranes, osteopontin is not present in ACVs.
RESULTS
RNA is associated with ACVs
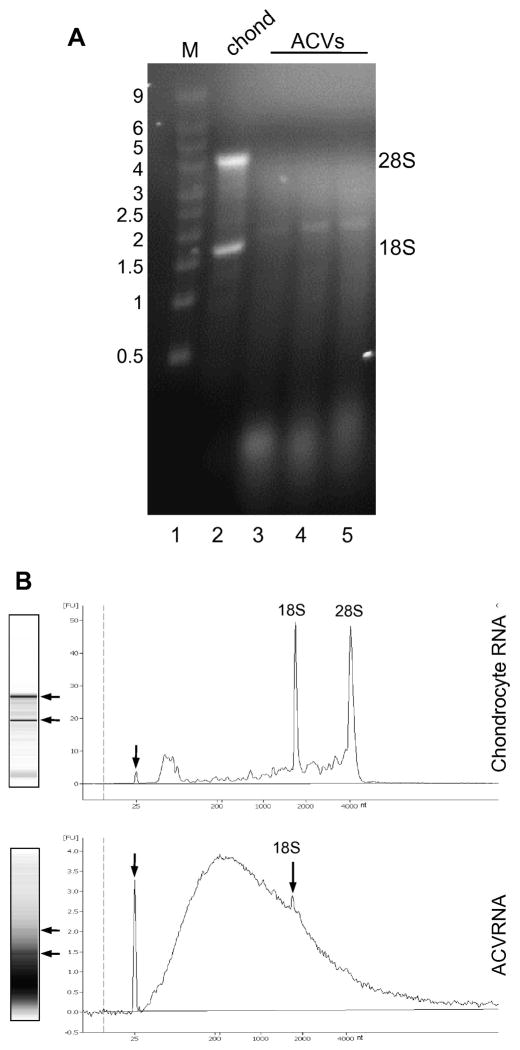
Because it was shown that exosomes from mouse and human mast cell lines contain RNA, we considered the possibility that this might also be true of ACVs. To test this proposal, we attempted to isolate RNA from porcine cartilage and chondrocyte-derived ACVs. Using sequential RNA purification protocols, one μg of RNA was obtained per 80 μg of vesicle protein (n=10 ACVs). It was also shown that ACVs derived from normal adult human cartilage had similar amounts of RNA to porcine ACVs (1 μg RNA/80 μg protein). No DNA was detectable in any ACVs fractions based on visual appearance on a DNA gel and after DNA isolation with TRizol according to manufacturer’s directions. RNA derived from primary chondrocytes showed sharp bands for the 28S and 18S ribosomal RNAs upon agarose gel electrophoresis (Fig. 1A, lane 2). In contrast, the majority of ACV RNA appeared to be small in size (Fig. 1A, lanes 3–5), although variable amounts of a band similar in size to 18S ribosomal RNA were consistently seen. Also evident in ACV RNA was a background smear of heterogeneously sized RNA consistent with mRNA.
Figure 1. RNA is associated with ACVs.
A. Total RNA was isolated from primary chondrocytes and three different preparations of ACVs, and RNA was run on a denaturing formaldehyde-agarose gel and stained with EtBr. Lane 1, size standards (Millenium Markers, 0.5 to 9 kb); lane 2, chondrocyte RNA (5 μg); lanes 3–5, RNA (5 μg) from 3 different porcine ACV preparations. The position of size standards are indicated on the left, and the 28S and 18S ribosomal RNA is indicated on the right. B. Total chondrocyte and ACV RNA was resolved on a Bioanalyzer. The data are presented as digitized pseudo-gels (left) and as an electropherograms (right). Arrows on the pseudo-gels indicate the positions of rRNA. The positions of an internal marker (small arrow) and rRNA are shown on the electropherograms. Size in nucleotides (nt) is shown along the x-axis.
Chondrocyte and ACVRNA was also analyzed on a Bioanalyzer (Fig 1B) This analysis confirmed the presence of a low but detectable amount of 18S rRNA in ACVs compared to cellular RNA, but little or no 28S rRNA. Furthermore, the bulk of the ACV RNA species were small, with a peak size of ~200nt, but RNAs of up to 4,000nt and larger were apparent. Thus, like mast cell exosomes, RNA is associated with ACVs.
RNA associated with ACVs is resistant to RNases
While RNA could be purified from ACVs, it was possible that the RNA was associated with the outside of the vesicles rather than inside. Arguing against this, RNase activity was easily detected in ACV preparations using a commercial assay. Levels of fluorescence were undetectable in the negative control, 229 ± 70 (arbitrary units) in the positive control, and 1055 ± 512 units in the ACV preparations (n=3). RNA exterior to the vesicles should be a substrate for the ACV-associated RNase, but RNA could be isolated even after several weeks of storage at 4°C, suggesting that ACV RNA is inside the vesicles. To explore this further, exogenous RNase was added to the intact ACVs and then RNA was isolated and quantified. As shown in Fig. 2, purified chondrocyte RNA and ACV RNA was destroyed by RNase within 15 minutes of exposure, demonstrating the effectiveness of the treatment, whereas RNA associated with intact ACVs was unaffected by similar treatment. When purified chondrocyte RNA was added to intact ACVs and exposed to RNase, the amount of RNA remaining corresponded to the amount of intact ACV RNA initially present. Taken together, these findings suggest that the RNA associated with ACVs is protected from degradation and is likely to be inside vesicles.
Figure 2. RNA associated with intact ACVs is resistant to RNase.
Total RNA (12 μg) from chondrocytes, or intact ACVs, were incubated for 30 min at 37°C in water, RNase buffer, or buffer with RNase, and RNA was purified. RNase treatment was also performed on total chondrocyte RNA that was mixed with intact ACVs, or on RNA purified from ACVs. RNA surviving the RNase treatment was then quantified spectroscopically.
ACVs contain mRNAs for several important chondrocyte proteins
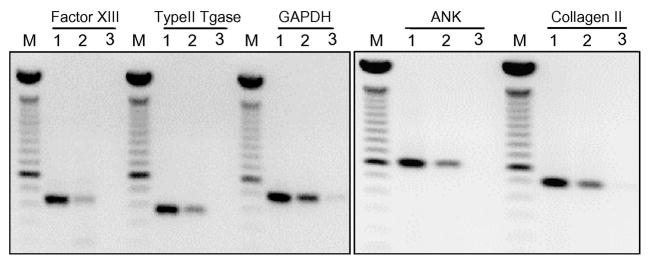
Given the Fig. 1 data that ACVs contain heterogeneously sized RNA, some of which is large, the presence of mRNA within the ACV RNA population was investigated by RT-PCR. In addition to the ubiquitously expressed GAPDH, mRNA for markers of chondrocytes, such as type II collagen and aggrecan, were present (Fig. 3). Also detected were mRNAs for other chondrocyte proteins including type II transglutaminase, factor XIIIA and ANKH. The PCR products were the same size as the corresponding products from healthy chondrocytes, indicating that the ACV mRNAs are intact. Incubation of ACVs with 35S-methionine revealed no evidence of new protein synthesis (data not shown), suggesting that ACVs do not possess the necessary components for translation of mRNA to protein.
Figure 3. mRNA is associated with ACVs.
Total RNA isolated from primary chondrocytes or ACVs was reverse transcribed using random hexamers for priming, and RT reactions were subjected to PCR with primers to the indicated genes. RT-PCR products were then resolved on an agarose gel. Water was used as a negative control. For each gene set, lane M contains size markers (100-, 600-, and 1500-bp bands are indicated), lane 1 contains RT-PCR products from chondrocyte RNA, lane 2 contains RT-PCR products from ACVs, and lane 3 shows the products of a PCR reaction using water.
Labeled ACV RNA is transferrable to living chondrocytes
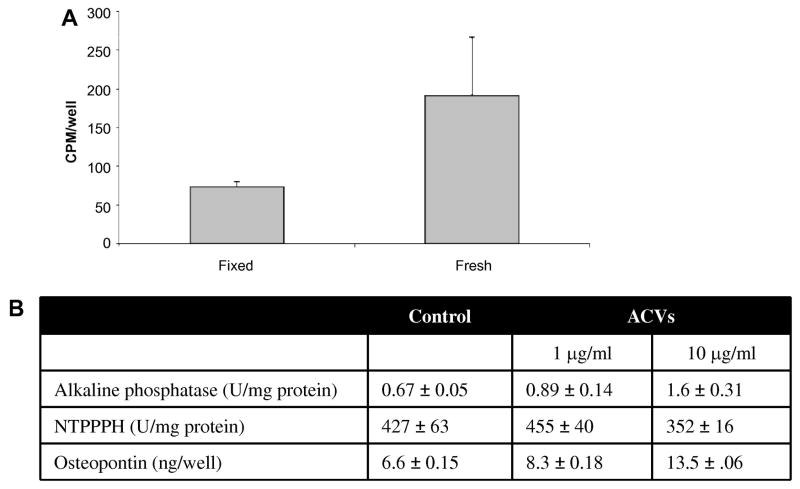
Because it was shown that RNA associated with mast cell exosomes could be transferred to other mast cells, we addressed the intriguing possibility that ACV RNA could be transferred to chondrocytes. To this end, chondrocytes were trace-labeled with 3H-uracil by incubating chondrocytes with radiolabled uracil, and ACVs were isolated from the conditioned media. Easily measurable quantities of 3H-uracil were detectable in the ACV fraction at 96 hours, with a specific activity of 600 CPM/μg protein, which is consistent with the above data that ACVs contain RNA. The radiolabeled vesicles (1 μg of protein) were then added to fresh unlabelled chondrocyte monolayers. Identical chondrocyte monolayers that were fixed with 70% alcohol before addition of labeled ACVs served as negative controls for non-specific binding. After a 96-hour incubation, significantly more 3H-uracil was present in fresh chondrocyte cell layers treated with trace-labelled ACVs compared to the fixed controls (p<0.01)(Fig. 4A). Unfixed controls maintained at 4°C, had identical background counts to the fixed controls. These data are consistent with the transfer of labeled RNA from ACVs to chondrocytes. To further address the possibility that ACVs were merely sticking to chondrocytes, the activity of two enzymes that are present in both ACVs and chondrocytes was measured, with the rationale that both activities should increase if ACVs were simply non-specifically adhered to the cell layer rather than specifically taken up by the cells. As shown in Figure 4B, co-incubation of chondrocytes with ACVs resulted in an increase in alkaline phosphatase activity, but there was no increase in NTPPPH activity. This suggested that ACVs were not just sticking to the cell layer as an increase in both enzymes would be expected. Moreover, there was a significant increase in osteopontin levels in chondrocytes co-cultured with ACVs. As there is no osteopontin in or on ACVs, this finding suggests that ACVs affect chondrocyte osteopontin production, and lend support to the hypothesis that ACVs alter chondrocyte behavior.
Figure 4. Co-incubation of ACVs with chondrocytes.
A. 1 μg (600 CPM) of ACVs labeled with 3H-uracil was added to each well of a 24 well plate containing fresh chondrocytes or alcohol-fixed chondrocytes, and cultures were incubated for 96 hours. Cell layers were washed thoroughly and 3H in cell lysates was quantified by liquid scintillation counting (n=6). More 3H accumulated in fresh chondrocytes than in the fixed cell layers (p<0.01). B. Chondrocytes were incubated with no additives, or 1 or 10 μg/ml of ACVs. Enzyme activity for alkaline phosphatase and NTPPPH, and protein levels for osteopontin were then measured in the cell layer after 72 hours.
DISCUSSION
Articular cartilage is a highly specialized tissue designed to protect joints by absorbing and distributing load and maintaining smooth surfaces for optimal joint motion. Ineffective repair of damaged articular cartilage results in osteoarthritis, which is frequently accompanied by pathologic mineral formation in what should be unmineralized cartilage matrix. While ACVs have been characterized by their ability to foster mineral formation, their consistent presence in normal cartilage remains unexplained. We show here that human and porcine ACVs contain significant quantities of stable RNA. The observation that extracellular vesicles from mineralizing and non-mineralizing tissues share this characteristic suggests that extracellular vesicles may also share other functions.
ACV RNA included mRNAs from a generic gene (GAPDH) and for chondrocyte-specific proteins, such as type II collagen and aggrecan. However, ACVs appear to be incapable of translating mRNA into protein. Abundant small RNAs were also observed in ACVs. This RNA may include micro-RNAs, which regulate gene expression at the level of mRNA stability and translation [23, 24]. In cartilage, alterations in microRNA may affect chondrocyte phenotype [25]and over-expression of some microRNAs may be seen in early osteoarthritis [26].
ACVs contained mRNA for specific cartilage matrix proteins as well as mRNA for repair factors such as the transglutaminase enzymes. The RNA in ACVs appears to be strongly protected from enzymatic degradation. ACVs also contain proteins, such as transglutaminase enzymes [27, 28]which withstand the rigorous enzymatic digestion process necessary for ACV isolation. We hypothesize that ACVs may provide a safe compartment in articular cartilage for storing important repair information during joint injury, stress, or disease.
The ability of ACVs to transfer RNA to naive chondrocytes taken together with the effects of co-culturing ACVs with chondrocytes suggests that ACVs may serve as communication shuttles between geographically distant cells in cartilage. ACVs are concentrated in the pericellular matrix of cartilage, a key region for biomechanical signal transduction [29].. Cartilage is often considered a solid matrix, however, there is a significant flux of water and small molecules into and out of cartilage [30]. This may be particularly true during early osteoarthritis [31], and maximized in the pericellular matrix with mechanical stress. This flux could conceivably facilitate exposure of cells to nearby ACVs and aid in disseminating coding information such as that necessary for repair or matrix production.
The finding of low levels of 18S ribosomal RNA in ACVs conflicts with the findings of Valadi et al. [18]that this species is lacking in mast cell exosomes. The occasional presence of ribosomes in matrix vesicles examined under electron microscopy is well documented [32], however, the relative paucity of the 28s ribosomal RNA is inconsistent with active ribosomes and our data indicate that ACVs are not translationally active. The ACV RNA is also distinguished from exosomal RNA in that it has a broader and larger size range, although the significance of this observation is not clear at present.
While RNA might contaminate ACVs after release from dying cells or damaged tissue, we do not believe that this is the origin of ACV RNA. ACV RNA is quite resistant to RNases, suggesting that it is not adhered to the outside of vesicles. Consistent quantities of RNA are present in ACVs derived from conditioned media of healthy chondrocytes and in enzymatically isolated ACVs from human and porcine cartilage. Matrix vesicles bear some resemblance to apoptotic bodies, which are similar sized RNA–containing structures that form during programmed cell death. However, apoptotic bodies also contain DNA, which was not detectable in our ACV preparations. There was no evidence of significant cell death in our healthy adult cartilage or in short-term monolayer cultures. Moreover, major functional and structural differences between apoptotic bodies and matrix vesicles have previously been elegantly delineated [33, 34].
The role of RNA in ACVs remains uncertain, and it is uncertain whether the transfer of protein, RNA, or other components mediates the effects of ACVs on chondrocytes. Indeed, in general, our knowledge of mechanisms of cell-vesicle interactions remains rudimentary. It is interesting that these structures were characterized in reference to their ability to form mineral, including calcium phosphate. Calcium phosphate is commonly used to promote cellular transfection in vitro. It is tempting to speculate that mineralization of ACVs may facilitate their interaction with cells and promote transfer of genetic information.
In summary, we show here that ACVs from normal adult porcine and human cartilage contain significant amounts of RNA that is protected from enzymatic degradation and transferrable to chondrocytes. Further studies of the contents and behavior of ACVs may increase our understanding of cartilage repair processes, and potentially lead to new interventions for degenerative cartilage diseases.
Acknowledgments
This work was supported by NIH grant AR- RO-1 AR 056215 (AKR).
REFERENCES CITED
- 1.Wortmann R, Chowdhury M, Rachow J. ATP-dependent mineralization of hyaline articular cartilage matrix vesicles. In: Mikanagi K, Nishioka K, Kelley W, editors. Purine and Pyrimidine Metabolism in Man. New York: Plenum; 1989. pp. 55–62. [Google Scholar]
- 2.Rosenthal A, Mattson E, Gohr C, Hirschmugl C. Characterization of articular calcium-containing crystals by synchrotron FTIR. Osteoarthritis Cartilage. 2008;16:1395–1402. doi: 10.1016/j.joca.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derfus B, Kurian J, Butler J, Daft L, Carrera G, Ryan L, Rosenthal A. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29:570–574. [PubMed] [Google Scholar]
- 4.Nalbant S, Martinez J, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher H., Jr Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthritis Cart. 2003;11:50–54. doi: 10.1053/joca.2002.0861. [DOI] [PubMed] [Google Scholar]
- 5.Derfus B, Steinberg M, Mandel N, Buday M, Daft L, Ryan L. Characterization of an additional articular cartilage vesicle fraction that generates calcium pyrophosphate dihydrate crystals in vitro. J Rheumatol. 1995;22:1514–1519. [PubMed] [Google Scholar]
- 6.Boyan B, Schwartz Z, Carnes DJ, V R. The effects of vitamin D metabolites on the plasma membrane and matrix vesicle membranes of growth and resting cartilage cells in vitro. Endocinol. 1988;122:2851–2860. doi: 10.1210/endo-122-6-2851. [DOI] [PubMed] [Google Scholar]
- 7.Bonewald L, Schwartz Z, Swain L, Ramirez V, Poser J, Boyan B. Stimulation of plasma membrane and matrix vesicle enzyme activity by transforming growth factor B in osteosarcoma cell cultures. J Cell Physiol. 1990;145:200–206. doi: 10.1002/jcp.1041450203. [DOI] [PubMed] [Google Scholar]
- 8.Slavkin H, Hu C, Sakakura Y, Diekwisch T, Chai Y, Mayo M, Braingas P, Simmer J, Mak G, Sasano Y. Gene expression, signal transduction and tissue specific biomineralizatoin during mammalian tooth development. Gene Expr. 1992;2:315–329. [PubMed] [Google Scholar]
- 9.Anderson H. Matrix vesicles and calcification. Curr Rheum Reports. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 10.Derfus BA, Kurtin SM, Camacho NP, Kurup I, Ryan LM. Comparison of matrix vesicles derived from normal and osteoarthritic human articular cartilage. Connective Tissue Research. 1996;35(1–4):391–396. doi: 10.3109/03008209609029209. [DOI] [PubMed] [Google Scholar]
- 11.Sylvia V, Schwartz Z, Holmes S, Dean D, Boyan B. 24,25-(OH)2D3 regulation of matrix vesicle protein kinase C occurs both during biosynthesis and in the extracellular matrix. Calcif Tissue Int. 1997;61:313–321. doi: 10.1007/s002239900341. [DOI] [PubMed] [Google Scholar]
- 12.Gasser O, Schifferli J. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Bood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 13.Messer L, Alsaleh G, GFreyssinet J-M, Zobairi F, Leray I, Gottenberg J-E, Sibilia J, Toti-Orfanoudakis F, Wachsman D. Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Arthritis Res &Therapy. 2009;11:R40. doi: 10.1186/ar2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raposo G, Nijman H, Stoorvogel W, Leijendekker R, Harding C, Meleif C, Geuze H. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle mediated secretion pathway during reticulocyte maturation. J Cell Sci. 1997;110:1867–1877. doi: 10.1242/jcs.110.16.1867. [DOI] [PubMed] [Google Scholar]
- 16.Verhoef P, Extacion M, Schilling W, Dubyak G. P2X7 receptor-depedent blebbing and the activation of Rho-effector kinases, caspases and IL-1beta release. J Immunol. 2003;170:5728–5738. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- 17.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cel-dervied exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 18.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee J, Lotvall J. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biol. 2007;9:654 –659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal A, Cheung H, Ryan L. Transforming growth factor beta 1 stimulates inorganic pyrophosphate elaboration by porcine cartilage. Arthritis Rheum. 1991;34:904–911. doi: 10.1002/art.1780340717. [DOI] [PubMed] [Google Scholar]
- 20.Derfus B, Rachow J, Mandel N, Boskey A, Buday M, Kushnaryov V, Ryan L. Articular cartilage vesicles generate calcium pyrophosphate dihydrate-like crystals in vitro. Arthritis Rheum. 1992;35:231–240. doi: 10.1002/art.1780350218. [DOI] [PubMed] [Google Scholar]
- 21.Derfus B, Camacho N, Olmez U, Kushnaryov V, Westfall P, Ryan L, Rosenthal A. Transforming growth factor beta-1 stimulates articular chondrocyte elaboration of matrix vesicles capable of greater calcium pyrophosphate precipitation. Osteoarthritis and Cartilage. 2001;9:189–194. doi: 10.1053/joca.2000.0375. [DOI] [PubMed] [Google Scholar]
- 22.Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Bartel D. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J. Control of protein synthesis and mRNA degradation by microRNAs. Curr Opin Cell Biol. 2008;20:214–221. doi: 10.1016/j.ceb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Lu J, Cobb B, Rodda S, McMahon A, Schipani E, Merkenschlager M, Kronenberg H. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. PNAS, USA. 2008;105:1949–1954. doi: 10.1073/pnas.0707900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamasaki K, Nakasa T, Miyaki S, Ishikawa M, Deie M, Adachi N, Yasunaga Y, Asahara H, Ochi M. Expression of micro-RNA 146a in osteoarthritis cartilage. Arthritis Rheum. 2009;60:1035–1041. doi: 10.1002/art.24404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenthal AK, Derfus BA, Henry LA. Transglutaminase activity in aging articular chondrocytes and articular cartilage vesicles. Arthritis Rheum. 1997;40(5):966–970. doi: 10.1002/art.1780400526. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal A, Masuda I, Gohr C, Derfus B, Le M. The transglutaminase, Factor XIIIA, is present in articular chondrocytes. Osteoarthritis and Cartilage. 2001;9:578–581. doi: 10.1053/joca.2000.0423. [DOI] [PubMed] [Google Scholar]
- 29.Guilak F, Alexopoulos L, Upton M, You I, Choi J, Cao L, Setton L, Haider M. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann NY Acad Sci. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- 30.Elfervig M, Graff R, Lee G, Kelley S, Sood A, Banes A. ATP induces Ca2+ signaling in human chondrons cultured in three-dimensional agarose films. Osteoarthritis Cart. 2001;9:518–526. doi: 10.1053/joca.2000.0435. [DOI] [PubMed] [Google Scholar]
- 31.Maroudas A, Venn M. Chemical composition and swelling of normal and osteoarthritic femoral head cartilage. II. Swelling. Ann Rheum Dis. 1977;36:399–406. doi: 10.1136/ard.36.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson H. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirsch T, Wang W, Pfander D. Functional differences bewteen growth plate apoptotic bodies and matrix vesicles. J Bone Mineral Res. 2003;18:1872–1881. doi: 10.1359/jbmr.2003.18.10.1872. [DOI] [PubMed] [Google Scholar]
- 34.Jaovisidha K, Hung J, Ning G, Ryan L, Derfus B. Comparative calcification of native articular cartilage vesicles and nitroprusside-generated vesicles. Osteoarthritis Cart. 2002;10:646–652. doi: 10.1053/joca.2002.0722. [DOI] [PubMed] [Google Scholar]