Abstract
Mammalian sterile 20—like kinase 1 (Mst1) is a ubiquitously expressed serine/threonine kinase and its activation in the heart causes cardiomyocyte apoptosis and dilated cardiomyopathy. Its myocardial substrates, however, remain unknown. In a yeast two-hybrid screen of human heart cDNA library with a dominant negative Mst1 (K59R) as bait, cardiac troponin I (cTnI) was identified as an Mst1-interacting protein. The interaction of cTnI with Mst1 was confirmed by co-immunoprecipitation in both co-transfected HEK293 cells and native cardiac myocytes, in which cTnI interacted with full length Mst1, but not with its N-terminal kinase fragment. In vitro phosphorylation assays demonstrated that cTnI is a sensitive substrate for Mst1 in its free form or in reconstituted troponin complex. In contrast, cardiac TnT was phosphorylated by Mst1 only when incorporated in the troponin complex. Mass spectrometric analysis indicated that Mst1 phosphorylates cTnI at Thr31, Thr51, Thr129, and Thr143. Substitution of Thr31 with Ala substantially reduced Mst1-mediated cTnI phosphorylation by approximately 90%, while replacement of either Thr51 orThr129, or Thr143 with Ala reduced Mst1-catalyzed cTnI phosphorylation by approximately 60%, suggesting that Thr31 is a preferential phosphorylation site for Mst1. Protein epitope analysis and binding assays showed that Mst1 mediated phosphorylation modulates the molecular conformation of cTnI and its binding affinity to TnT and TnC, thus indicating functional significances. Our results suggest that Mst1 is a novel mediator of cTnI and/or cTnT phosphorylation in the heart and may contribute to the modulation of myofilament function under a variety of physiological and pathophysiological conditions.
Keywords: Mst1, cardiac troponin I, substrate, myofilament, contractility, kinase
INTRODUCTION
Mammalian sterile 20—like kinase 1 (Mst1) is a ubiquously expressed serine/threonine kinase with a similarity to Ste20, an upstream activator of the mitogen-activated protein kinase pathway in budding yeast [1] [2]. Mst1 contains an N-terminal catalytic domain and an autoinhibitory segment followed by a dimerization domain and a nuclear localization motif in the non-catalytic C-terminal region [2]. In addition, Human Mst1 has two caspase cleavage sites suited between the catalytic and regulatory domains, which mediate the cleavage of the autoinhibitory domain [2] [3]. In response to a variety of apoptotic stimuli, Mst1 is cleaved by caspases to produce a 34-36-kDa N-terminal constitutively active fragment and this cleavage markedly increases Mst1 kinase activity and translocates the cleaved Mst1 to the nucleus where it phosphorylates histone H2B on Ser14, resulting in apoptotic cell death [3] [4] [5]. In addition to caspase cleavage, Mst1 phosphorylation has recently been proposed to contribute to the kinase activation [6]. Several phosphorylation sites have been identified in Mst1, namely Thr175, Thr177, Thr183, Thr187, Ser327 and Thr387 [6] [7], of which, Thr183 and Thr187 appear to be essential for kinase activation [7] [8]. The effect of phosphorylation at these sites on the activation of Mst1 may be further amplified by dimerization and eventually leads to the caspase cleavage, thereby constituting a powerful amplification loop of apoptotic responses [8].
Recently, the role of Mst1 in cardiac muscle function has become a novel focus of investigation. In cardiomyocytes, Mst1 is activated by pathological stimuli, such as hypoxia/reoxygenation in vitro and ischemia/reperfusion in vivo [9]. Cardiac-specific over-expression of Mst1 has been shown to cause dilated cardiomyopathy in mice [9]. Inhibition of endogenous Mst1 prevents apoptosis of cardiomyocytes and cardiac dysfunction after myocardial infarction without producing cardiac hypertrophy [9] [10]. However, little is known about the cellular function of Mst1 besides apoptosis. Investigating its pathophysiological substrates in the cardiac muscle will lead to a better understanding of myocardial adaptation to stress conditions. In the present study, we performed yeast two-hybrid screen of a human heart cDNA library using the dominant negative form of Mst1 (K59R) as bait to identify myocardial proteins that represent potential Mst1 substrates. Structural and functional characterization provided evidence that cardiac troponin I (cTnI) is a novel substrate of Mst1 in the heart. Mst1 directly interacts with and phosphorylates cTnI. The pertinent phosphorylation sites were identified by mass spectrometric analysis and confirmed by site-directed mutagenesis. Together with modulation of cTnI molecular conformation and function by Mst1 phosphorylation, our findings suggest that the Mst1 signaling pathway through cTnI phosphorylation may represent an important regulation of cardiac contractility under physiological and pathophysiological conditions.
MATERIALS AND METHODS
Screening of Yeast Two-Hybrid Library
MATCHMAKER GAL4 yeast 2-hybrid system 3 was purchased from Clontech Laboratories Inc. As previously described, the screening was performed on a human heart cDNA library using human dominant-negative Mst1(K59R) as bait [11]. Positive colonies were subjected to multiple rounds of additional selection in the appropriate media and ß-galactosidase filter assays to verify specificity. The confirmed cDNA clones were sequenced to reveal their identities in comparison with the GenBank databases.
Coimmunoprecipitation of cTnI and Mst1 in HEK293 Cells and Cardiomyocytes
HEK293 cells were transiently transfected with Flag-tagged cTnI and Myc-tagged Mst1 expression plasmids using FuGENE 6 as described by the manufacturer (Roche Applied Science). HEK293 Cells or cardiomyocytes were lysed with a buffer containing 1% Nonidet P-40, 150 mM NaCl, 50 mM Tris (pH 8), and protease inhibitors. Coimmunoprecipitation of cTnI and Mst1 was performed as described [11]. The following antibodies and beads were used for detection and immunoprecipitation: mouse monoclonal myc (Invitrogen), mouse monoclonal Flag M2 (Sigma), rabbit polyclonal Mst1 (Cell Signaling), mouse monoclonal TnI (Abcam), rabbit polyclonal TnI (Santa Cruz), anti-c-Myc-agarose affinity gel (Sigma), anti-Flag M2-agarose (Sigma), and Protein G Sepharose (Amersham).
Western blotting analysis to identify the immunoprecipitated proteins was performed using peroxidase-conjugated donkey anti-rabbit and sheep anti-mouse (GE Healthcare) second antibodies followed by detection using ECL reagents (Amersham).
In Vitro Phosphorylation Assays
To phosphorylate cTnI in vitro, recombinant cTnI, cTnC, cTnT (Lee Biosolutions, Inc), Myelin basic protein (MBP) (Upstate), and reconstituted troponin complexes were incubated with recombinant active Mst1 (Upstate) and [γ-32P]ATP (PerkinElmer) in 2× kinase assay buffer (40 mM HEPES-NaOH pH 7.4, 20 mM MgCl2, 1 mM DTT, 1 mM ATP, 1 mM Na3VO4, 50 mM NaF, and complete protease inhibitor [Roche]) at 30 °C for 60 min. The reaction was terminated by adding 1/2 volumes of 3×Laemmli sample buffer and incubated at 95 °C for 5min, then examined by 12% SDS-PAGE and autoradiography. For peptide mass spectrometric analysis, recombinant human cTnI was phosphorylated by active Mst1 in the presence of non-radio-labeled ATP.
Reconstitution of Troponin Complex
The troponin complex was reconstituted in vitro using recombinant wild-type or mutated cTnI together with recombinant wild-type cTnC and cTnT, as described [12]. The formation of the troponin complex was confirmed by non-denaturing SDS-PAGE.
Mass Spectrometric Analysis
Human cTnI was phosphorylated by Mst1 in vitro and then subjected to 10% SDS-PAGE, followed by Coomassie Blue R250 staining, tryptic in-gel digestion, and then analyzed by Matrix-Assisted Laser Desorption/Ionisation Mass Spectrometry (MALDI/MS). The phosphorylated residues were further identified by MS/MS analysis.
Expression and Purification of cTnI Mutants
In order to achieve high expression of cTnI in bacteria, two bases in the second and the fourth codons of cTnI cDNA (Ala2: GCG—GCC, and Gly4: GGG—GGT) were changed before cloning [13]. In vitro site-directed mutagenesis was performed to obtain the different mutated cTnI cDNA by using the QuikChange multi-site-directed mutagenesis Kit (Stratagene). The modified and mutated cTnI cDNA were cloned into the Nde I and BamH I sites of pET-21b vector and verified by DNA sequencing. BL21(DE3) competent cells (Stratagene) were transformed with the pET-21b constructs and induced with 1 mM IPTG at 37 °C for 3 hr. Recombinant cTnI was purified as previously described [14].
mAb Epitope Analysis
The binding affinity between an antibody and its antigenic epitope depends on the three dimensional structural fit. Enzyme-linked immunosorbant assay (ELISA) epitope analysis [15] was employed to examine conformational differences between the non-phosphorylated and Mst1 phosphorylated cTnI. An mAb TnI-1 against an epitope in the C-terminal domain of cTnI [16], an mAb 4H6 against the central region of TnI, and a polyclonal rabbit anti-TnI serum RATnI [17] were used to monitor conformational changes that alter the antibody binding affinity.
Protein Binding Assays
An ELISA-based solid phase protein binding assay [15] was used to investigate the interactions of the non-phosphorylated and Mst1 phosphorylated cTnI with TnT and TnC. All experiments were done in triplicate.
Statistical Analyses
The data for the epitope analysis and protein binding assays are presented as mean ± SD. Statistical analyses were performed using ANOVA or Student’s t test. Significant differences were determined at P<0.05.
RESULTS
Interaction of cTnI with Mst1
Since Mst1 plays an essential role in initiating apoptosis in cardiomyocytes and the development of dilated cardiomyopathy [9] [10], our study was aimed to identify the substrates of Mst1 in cardiomyocytes using yeast two-hybrid screening of a human heart cDNA library with the dominant negative Mst1 (K59R) as bait. After screening 2×106 clones, a total of 40 positive clones were identified, 9 of which bearing full-length cDNA encoding cardiac TnI (cTnI).
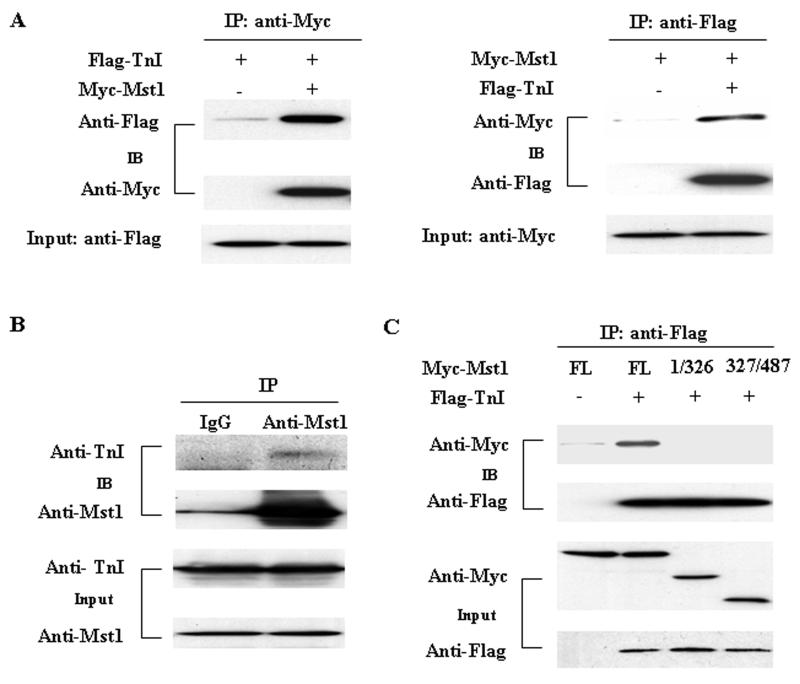
To confirm the interaction of Mst1 with cTnI does occur in mammalian cells, coimmunoprecipitation was performed in HEK293 cells cotransfectively expressing Myc-tagged Mst1 and Flag-tagged cTnI. Immunoprecipitation of Myc-tagged Mst1 led to coprecipitation of Flag-tagged cTnI. As a control, the anti-Myc antibody did not immunoprecipitate Flag-tagged cTnI in the absence of Myc-tagged Mst1. Similarly, immunoprecipitation of Flag-tagged cTnI resulted in coprecipitation of Myc-tagged Mst1, whereas the anti-Flag antibody did not immunoprecipitate Myc-tagged Mst1 in the absence of Flag-tagged cTnI (Figure 1A). These findings indicate that cTnI and Mst1 can form a tightly bound complex in mammalian cells.
Fig. 1.
Interaction of cTnI with Mst1. A. pCS2-Mst1, and pFLAG-cTnI expression vectors and empty vector control were cotransfected into HEK293 cells. Extracted proteins were precipitated by either anti-c-Myc-agarose (left) or anti-Flag M2-agarose (right) affinity beads and analyzed on 10% SDS-PAGE. The blotted membrane was examined with either horseradish peroxidase (HRP)-conjugated anti-Flag antibody or HRP-conjugated anti-Myc antibody. B. Cell lysates extracted from neonatal rat cardiomyocytes were immunoprecipitated with either anti-Mst1 antibody or control IgG and then analyzed on 10% SDS-PAGE. The protein bands resolved were transferred to nitrocellulose membrane and detected with either anti-TnI or anti-Mst1 antibody. C. FLAG-TnI expression vector or empty vector control together with expression vectors encoding Myc-Mst1 mutants were cotransfected into HEK293 cells. Extracted proteins were precipitated by anti-Flag M2-agarose and then analyzed on 15% SDS-PAGE. The protein bands were transferred to nitrocellulose membrane and examined with HRP-conjugated anti-Myc or HRP-conjugated anti-Flag antibody.
To determine whether there is an endogenous interaction of cTnI and Mst1 in cardiomyocytes, we performed immunoprecipitation with anti-Mst1 antibody using lysates obtained from neonatal rat cardiac myocytes. Indeed, cTnI coprecipitated with the anti-Mst1 antibody but not with the nonimmune IgG (Figure 1B). The result indicates that cTnI interacts with Mst1 in cardiomyocytes under physiological conditions.
Mst1 contains an N-terminal catalytic domain (aa 1-326) and a C-terminal regulatory domain (aa 327-487) [2]. To further map the cTnI interaction domain of Mst1, we constructed the kinase domain and regulatory domain of Mst1 in pCS26MT vector with a 6×Myc tag and transfected these constructs into HEK293 cells along with the Flag-cTnI expression plasmid. Lysates from transfected HEK293 cells were immunoprecipitated with anti-Flag antibody and analyzed by Western blotting analysis. We found that Flag-cTnI bound only to full length Mst1, but not to the N-terminal or C-terminal fragments (Figure 1C). As a negative control, the anti-Flag antibody did not immunoprecipitate Myc-Mst1 in cells cotransfected with Myc-Mst1 and empty Flag vector. These results suggest that full length Mst1 is required for the binding to cTnI.
Mst1-Mediated Phosphorylation of cTnI
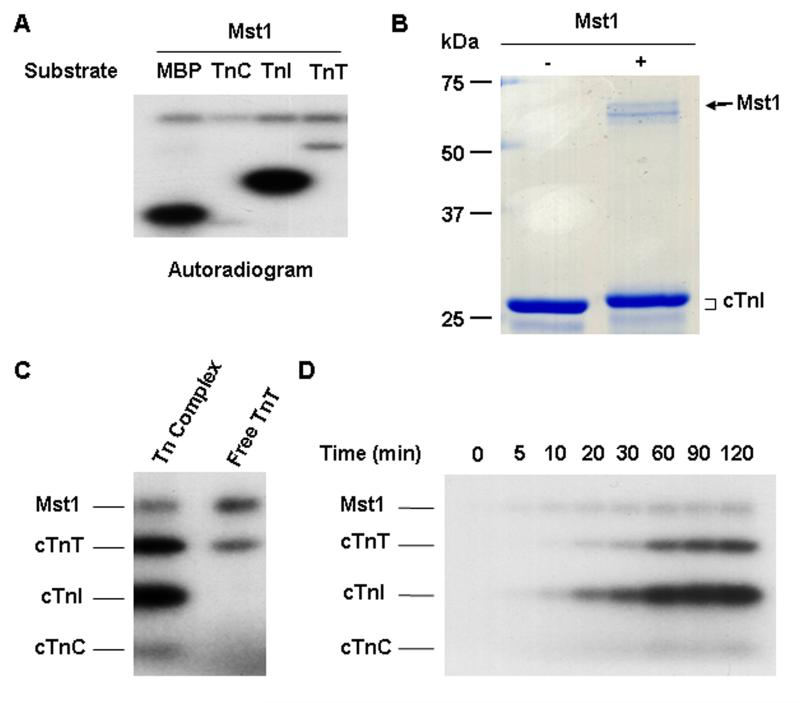
Since Mst1 is a serine/threonine kinase [1], the interaction of Mst1 with cTnI prompted us to investigate whether cTnI is a substrate of Mst1. Therefore, purified human cTnI was incubated with active Mst1 in the presence of 32P-ATP in an in vitro phosphorylation assay. Equal molar of MBP was included as a positive control. In order to study whether other troponin subunits were substrates of Mst1, human cTnC and cTnT were also tested in the in vitro kinase assay. As shown in Figure 2A, incubation of recombinant Mst1 with cTnI resulted in a robust phosphorylation of cTnI. The phosphorylation activity of Mst1 on cTnI was even higher than that on the known substrate MBP. In contrast, free cTnT and cTnC were both barely phosphorylated by Mst1. In addition, Mst1 mediated phosphorylation of cTnI produced a change in gel mobility as shown in the 12% SDS-PAGE (Figure 2B).
Fig. 2.
Mst1-mediated Phosphorylation of cTnI. A. Mst1 phsphorylates cTnI. Equal moles of MBP, cTnC, cTnI, and cTnT were incubated with 0.1 μg active Mst1 in the presence of 32P-ATP in an in vitro phosphorylation assay. Phosphorylation was detected by autoradiography. B. Mst1 mediated cTnI phosphorylation causes a change in mobility of the substrate by SDS-PAGE. 3 μg recombinant cTnI was incubated with 0.7 μg active Mst1 in an in vitro phosphorylation assay. After termination of the reaction, the samples were separated on 12% SDS-PAGE and stained with Coomassie Blue R250. C. Mst1 phosphorylates cTnT in reconstituted Tn complex. Equal moles of cTnT (1.3 μM) used in reconstituted Tn complex or in isolation were incubated with 0.1 μg active Mst1 in an in vitro phosphorylation assay. Phosphorylation was detected by autoradiography. D. The time course of Mst1 mediated phosphorylation of wild-type cTnI in reconstituted Tn complex.
To further investigate whether Mst1 phosphorylates cTnI in the Tn complex, Tn complex was reconstituted in vitro and then subjected to the in vitro phosphorylation assay in the presence of active Mst1. The results showed that cTnI in the Tn complex was also strongly phosphorylated by Mst1 (Figure 2C). Interestingly, although free cTnT was barely phosphosrylated by Mst1, it was markedly phosphorylated when incorporated in the Tn complex, albeit to a less extent, as compared with that of cTnI (Figure 2C). Together, these results indicate that cTnI is a good substrate for Mst1 not only in the free form but also when present in the Tn complex. Furthermore, cTnI may also serve as an Mst1 anchoring protein in the Tn complex and to facilitate the phosphorylation of cTnT by the kinase.
To determine the kinetics of Mst1-mediated phosphorylation of cTnI in the Tn complex, the time course of Mst1 phosphorylation of reconstituted Tn complex was determined in vitro. Mst1 phosphorylates cTnI in the Tn complex in a time-dependent manner and reached saturation at 90 minutes (Figure 2D).
Identification of the Phosphorylation Sites by MALDI-TOF Mass Spectrometry
To determine Mst1 phosphorylation sites on cTnI, we performed tryptic peptide mapping using MALDI-TOF mass spectrometric analysis after Mst1 phosphorylation treatment. As shown in Table 1, computer-assisted proteomic analysis revealed four phosphorylated tryptic ions with mass/charge ratios (m/z) of 1067.4532, 1589.9648, 1325.6299, and 997.527, corresponding to cTnI residues 28-36 + 1PO4, residues 45-57 + 1PO4, residues 121-131 + 1PO4, and residues 139-145 + 1PO4, respectively. The phosphorylated residues were further identified by MS/MS spectra (Supplementary material online, Figure S1). The results suggest that Mst1 phosphorylates cTnI at Thr31, Thr51, Thr129, and Thr143 (amino acids numbered as in human cTnI sequence) (Table 1). Alignments of cTnI sequences bearing Mst1 mediated phosphorylation sites indicated that they are conserved among multiple species (Figure 3).
Table 1. Mstl-mediated phosphorylation sites of human cTnl identified by MALDI-TOF MS.
Purified recombinant Tnl, after in vitro phosphorylation, were subjected to trypsin in-gel digestion and phosphorylation sites were analyzed by MALDI-TOF MS. The phosphorylated residues were further identified by MS/M Sspectra.
| Phosphopeptide | Mass calculated | Mass observed |
|---|---|---|
| 28AYATpEPHAK36 | 1067.4558 | 1067.4532 |
| 46KLQLKTpLLLQIAK58 | 1589.9753 | 1589.9648 |
| 121KITEIADLTpQK131 | 1325.6349 | 1325.6299 |
| 139FKRPTpLR145 | 997.5342 | 997.527 |
Fig. 3.
Sequence Alignments of cTnI from various species. Sequence alignments of cTnI indicate that Mst1 mediated phosphorylation sites in cTnI are mostly conserved among multiple species. Thr residues phosphorylated by Mst1 are shaded gray.
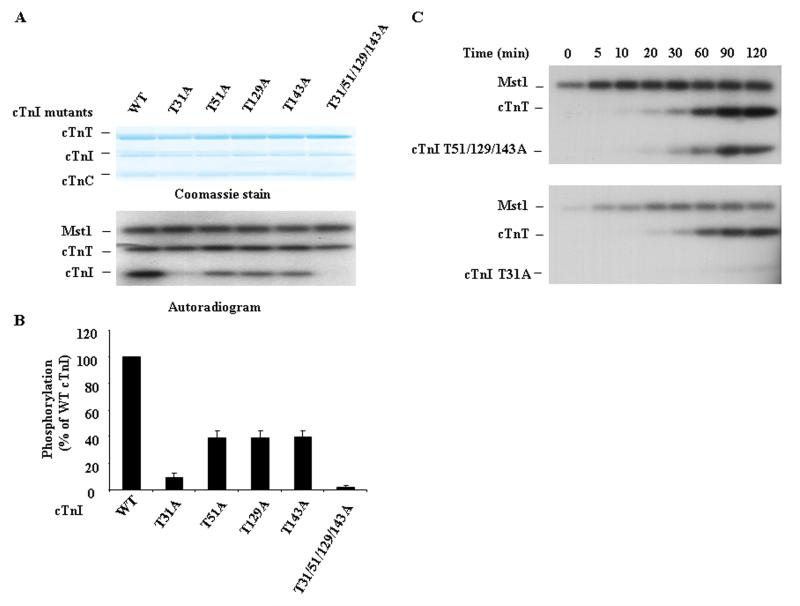
To identify the cTnI site(s) that are accessible for Mst1-mediated phosphorylation within the Tn complex, we further reconstituted the complex in vitro using recombinant wild-type or mutated (Thr31Ala, Thr51Ala, Thr129Ala, Thr143Ala, or Thr31/51/129/143Ala) cTnI and recombinant wild-type cTnC and TnT. Wild-type cTnI and each of the cTnI mutants were all incorporated into the troponin complex effectively (data not shown). Interestingly, the Thr31Ala mutation had a greater impact than the other single mutation on cTnI phosphorylation by Mst1. The Thr31/Ala mutation substantially attenuated Mst1-catalyzed cTnI phosphorylation by approximately 90%. In contrast, mutation of either Thr51 or Thr129 or Thr143 to Ala resulted in partial attenuation of Mst1 catalyzed cTnI phosphorylation by approximately 60% (Figure 4A and 4B). Furthermore, the combined replacement of all four Thr residues with Ala completely abolished Mst1-mediated phosphorylation of cTnI. These data further suggest that Mst1 phosphorylates Thr residues at positions 31, 51, 129, and 143 in cTnI and that Thr31 appears to be a preferential phosphorylation site.
Fig. 4.
The Contribution of Four Phosphorylation Sites to Mst1-Catalyzed cTnI Phosphorylation in Reconstituted Tn Complex. A. Tn complex consisting of wild-type or mutant cTnI was reconstituted in vitro and then incubated with 0.1 μg active Mst1 for 60 min in the presence of 32P-ATP in an in vitro phosphorylation assay. B. Phosphorylation levels of cTnI and its mutants by Mst1 were quantified by densitometry of autoradiograms. Results represent the mean ± SD of three separate experiments. C. The time course of Mst1 mediated phosphorylation of cTnI mutants (Thr51/129/143Ala or Thr31Ala) in reconstituted Tn complexes.
To further confirm that Mst1 preferentially phosphorylates cTnI at Thr31, the reconstituted troponin complexes containing cTnI mutants with or without Thr31Ala substitution were subjected to in vitro phosphorylation by Mst1 to determine the kinetics. In the Tn complex, wild type cTnI and mutations that retained Thr31 were effectively phosphorylated by Mst1 in a time-dependent manner, with maximal phosphorylation observed at 90 minutes. However, when Thr31 is mutated to Ala, the Mst1-mediated cTnI phosphorylation was markedly diminished (Figure 4C). These results further indicate that Mst1 preferentially phosphorylates cTnI at Thr31.
Mst1-Mediated Phosphorylation Induces Conformational Changes in cTnI
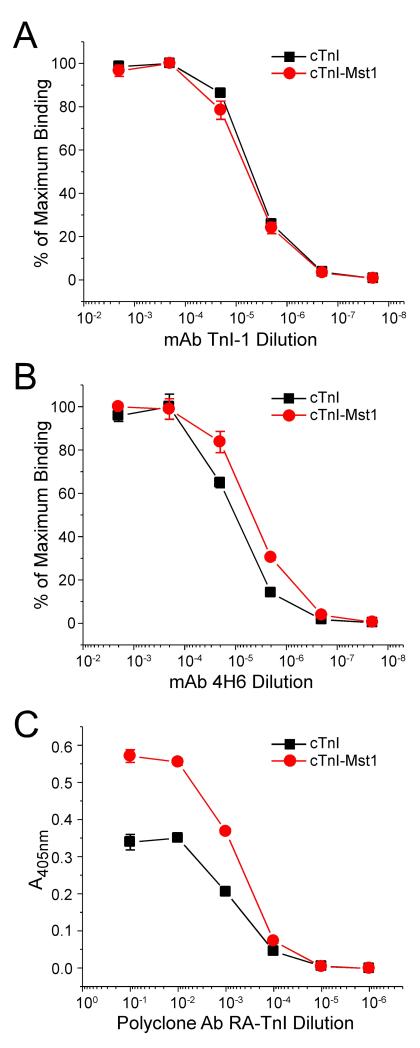
To investigate the structural and functional effects of Mst1 phosphorylation of cTnI, we investigated changes in cTnI molecular conformation upon Mst1 treatment. ELISA epitope analysis was performed to examine the binding affinity of monoclonal and polyclonal anti-TnI antibodies for non-phosphorylated and Mst1 phosphorylated cTnI. The titration curves of mAb TnI-1 that recognizes a COOH-terminal epitope [18], showed that Mst1-catalyzed phosphorylation of cTnI did not change molecular conformation in the COOH-terminal domain (Figure 5A). In contrast, the titration curves of mAb 4H6 that recognizes an epitope in the central region of TnI, demonstrated that Mst1-catalyzed phosphorylation of cTnI resulted in an increase in the binding affinity in comparison with that of the non-phosphorylated cTnI, as shown by the higher antibody dilution for 50% maximum binding (P < 0.05) (Figure 5B). This result indicates a change of molecular conformation in the central region of cTnI due to Mst1 catalyzed phosphorylation. This observation was further supported by epitope analysis using the polyclonal anti-TnI antibody RATnI. The RATnI titration curves demonstrated an increased maximum binding to Mst1- phosphorylated cTnI in comparison to the non-phosphorylated control (P < 0.05) (Figure 5C). The conformational analysis data suggest that Mst1-catalyzed phosphorylation resulted in a significant change in cTnI molecular conformation, laying a foundation for functional significance.
Fig. 5.
Mst1-Catalyzed Phosphorylation Induces Conformational changes in cTnI. ELISA epitope analysis was performed to measure the binding affinity of monoclonal and polyclonal anti-TnI antibodies against cTnI before and after Mst1 treatment for conformational changes due to Mst1 phosphorylation. A. The titration curves of mAb TnI-1 recognizing a COOH-terminal epitope showed that Mst1 phosphorylation of cTnI does not change molecular conformation of the COOH-terminal domain. B. The titration curves of mAb 4H6 that recognizes an epitope in the central region of cTnI demonstrated that Mst1 phosphorylated cardiac TnI had an increased affinity compared with that of the un-phosphorylated control, as shown by higher antibody dilution for the 50% maximum binding (P < 0.05). C. The titration curves of polyclonal antibody RATnI detected an increased maximum binding after Mst1 (P < 0.05), supporting an overall change in molecular conformation resulting from Mst1 phosphorylation. The data are shown as mean ± SD from triplicate assays wells.
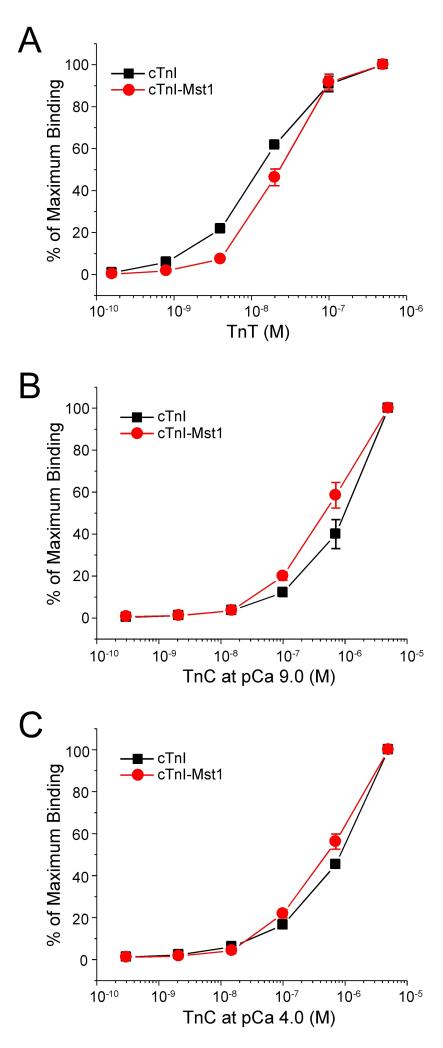
Altering Binding Affinity of cTnI to TnT and TnC by Mst1 Phosphorylation
To investigate the effects of Mst1-catalyzed cTnI phosphorylation on the interactions with cTnT and TnC, ELISA solid-phase protein binding experiments were performed [15]. The results showed that Mst1 phosphorylated cTnI had a lower binding affinity for TnT than that of unphosphorylated cTnI, as indicated by the rightward shift of the TnT concentration required for 50% maximum binding (P < 0.05) (Figure 6A). The binding curves for TnC demonstrated that Mst1 phosphorylated cTnI had an increased binding affinity for TnC as compared to that of unphosphorylated cTnI, as indicated by the significant right side shift of the TnT concentration required for 50% maximum binding, despite the presence or absence of Ca2+ (P < 0.01 and P < 0.05, respectively) (Figure 6B and 6C).
Fig. 6.
Mst1 Catalyzed Phosphorylation Affects the Binding Affinity of cTnI to TnT and TnC. ELISA solid-phase protein binding experiments were performed to investigate the effects of Mst1 phosphorylation of cTnI on interactions with TnT and TnC. The protein binding curves were normalized to the maximum binding. A. Mst1 phosphorylated cTnI had a lower affinity for TnT than that of the control, as shown by the rightward shift of the TnT concentration required for 50% maximum binding (P < 0.05). B & C. The binding curves for TnC demonstrated that Mst1 phosphorylated cTnI had an increased binding affinity for TnC compared to that of the control, as shown by the right side shift of the TnT concentration required for 50% maximum binding, despite the presence or absence of Ca2+ (P < 0.05 ). The data are shown as mean ± SD from triplicate assays wells.
DISCUSSION
Phosphorylation of cTnI is an important posttranslational mechanism in the regulation of thin filament function and thereby cardiac muscle contractility [19]. Altered phosphorylation of cTnI and other myofilament proteins may also contribute causally to cardiac dysfunction in the transition from compensated hypertrophy to heart failure [20] [21]. In the present study, we demonstrated that Mst1 interacts with and directly phosphorylates cTnI, resulting in conformational changes of cTnI and altered its interactions in the Tn complex.
The contraction of cardiac muscle is based on actin-myosin interactions regulated by intracellular Ca2+ via the thin filament-based troponin-tropomyosin system [22]. The troponin complex contains three subunits: the Ca2+-binding subunit TnC, the tropomyosin (Tm)-binding subunit TnT, and the inhibitory subunit TnI [23] [24]. During muscle contraction, the binding of Ca2+ to TnC releases the TnI inhibition of actomyosin ATPase through allosteric protein-protein interactions among the troponin complex, tropomyosin and actin, and leads to muscle contraction. A key step in this signaling mechanism is the release of inhibition of TnI on actin-myosin interaction [24]. Phosphorylation of specific serine and threonine residues in cTnI has been identified as a major physiological mechanism for alteration of myofilament properties [25] [21]. To date, much attention has been focused on the phosphorylation-mediated regulation of cTnI function by PKA and PKC. PKA has been demonstrated to phosphorylate cTnI at Ser22 and Ser23 (numbered as in the human cTnI sequence) in vitro [26] [27], thus leading to a reduction in myofilament Ca2+ sensitivity [28], an increase in cross-bridge cycling [29] [30], and increased the binding of cTnI to thin filament. PKC has been shown to phosphorylate cTnI mainly at Ser43/45 and Thr144 and reduce the maximal activity of actomyosin Mg2+-ATPase [31]. In addition, cTnI may also be specifically modified by protein kinase G and p21-activated kinases [21].
In the present study, our data demonstrated that Mst1 specifically targets cTnI and catalyzes the phosphorylation of cTnI at Thr31, Thr51, Thr129, and Thr143. These phosphorylation sites are mostly conserved in cTnI from multiple species. It is important to note that the decreases in phosphorylation of the mutant cTnI lacking one of the four Mst1 phosphorylation sites is non-proportional in comparison to that of the wild type cTnI, suggesting that the phosphorylation of cTnI at four different Thr residues by Mst1 may be dependent on each other. In reconstituted Tn complex, Thr31 that lies within the N-terminal extension of cTnI, appears to be a preferential phosphorylation site by Mst1, since replacement of Thr31 with Ala markedly reduced Mst1 catalyzed cTnI phosphorylation.
Another interesting finding is that when free cTnT was examined, it was not significantly phosphorylated by Mst1. However, when incorporated in the Tn complex, cTnT was markedly phosphosrylated by Mst1 (Figure 4). Moreover, our preliminary data suggest that cTnT did not directly interact with Mst1 both in vitro and in cotransfected HEK293 cells (data not shown). This implies that cTnI may serve as an anchoring protein to mediate the phosphorylation of cTnT by Mst1 in native cardiac muscle. The phosphorylation sites in cTnT by Mst1 remains to be identified in future studies.
In comparison with skeletal muscle TnI, cTnI has additional 27-33 amino acids at its N terminus, and phosphorylation of Ser22 and Ser23 by PKA in this cardiac specific segment has been shown to reduce myofilament Ca2+ sensitivity [21]. In the present study, we, for the first time, demonstrated that in this cardiac specific segment, Thr31 is a primary site phosphorylated by a novel kinase Mst1. Since Thr31 is close to the TnC binding domain in the N-terminal portion of cTnI, the phosphate introduced by Mst1 may possibly influence the interaction between the N terminus of cTnI and the C terminus of cTnC [32] [33]. This hypothesis is supported by the Mst1 phosphorylation-induced changes in the conformation of the 4H6 epitope (Figure 5) and binding affinity for TnC (Figure 6). In addition, Mst1 phosphorylation of Thr51 of cTnI in a site that binds to the C terminus of cTnT and the C-lobe of cTnC [32] [33] may also have an effect on the interaction of cTnI with cTnT and cTnC. Indeed, our results demonstrated that phosphorylation of cTnI by Mst1 significantly changed the global conformation of cTnI and its binding affinity to both cTnT and cTnC. The effect of Mst1 phosphorylatin of cTnI on interaction with TnT may also be attributed to the Thr129 site in the I-T arm of troponin complex.
The phosphorylation of cTnI by Mst1 at Thr143 located in the inhibitory region [34] is worth further investigating. Previously, Thr143 was identified to be one of the PKC phosphorylation sites in cTnI [35] and the PKC catalyzed Thr143 phosphorylation has been shown to result in a reduction in the Ca2+ sensitivity of filament sliding [36] and an increase in myofilament calcium sensitivity [37]. In this regard, Mst1 may represent another kinase that is able to phosphorylate cTnI at Thr143, for similar functional effects. Taken together, our results suggest that Mst1 is a novel kinase that phosphorylates cTnI at multiple Thr sites in several important structural and functional domains which are responsible for protein-protein interactions among the Tn complex, tropomyosin and actin. Thus, Mst1 may play important roles in modifying cardiac contractility through the phosphorylation of TnI and/or cTnT.
The functional significance of Mst1-mediated phosphorylation of cTnI in the heart remains elusive. In response to apoptotic stimuli, Mst1 is cleaved by caspases to 34-36-kDa N-terminal constitutive active fragment that subsequently translocates to the nucleus where it phosphorylates histone H2B on Ser14, resulting in apoptosis [3] [4] [5]. In the heart, Mst1 is activated by ischemia/reperfusion and targeted overexpression of Mst1 in the heart has been shown to cause dilated cardiomyopathy in mice [9]. In the Mst1 transgenic mice, increased Mst1 activity was found to be associated with the full-length form rather than its cleaved form [9]. Our results also demonstrated that cTnI interacted only with full length Mst1 but not the N-terminal fragment. This implies that cTnI may be a pathophysiologically relevant substrate of Mst1 in the cytoplasm in cardiac myocytes. Since the post-translational modification of cTnI, such as proteolysis and phosphorylation by kinase(s), is of particular importance in the modulation of cardiac myofilament function under stress conditions, the identification of cTnI as a novel substrate of Mst1 in the heart may open a new area of research toward a better understanding of cardiac contractile dysfunction associated with ischemic heart diseases and heart failure.
In summary, our study provides the first evidence to indicate that Mst1 is a novel kinase that interacts with and directly phosphorylates cTnI, resulting in conformational changes of cTnI and alterations of protein-protein interactions in the Tn complex. Therefore, Mst1 may play an important role in the regulation of cardiac muscle contractility under physiological and pathophysiological conditions, which deserves further investigation.
Supplementary Material
Acknowledgments
This work was supported by an American Heart Association Scientist Development Grant (0630047N to JS) and a grant from National Institutes of Health (HL078773 to J-PJ). ZZ was supported by a Postdoctoral Fellowship from American Heart Association, Great Midwest Affiliate.
The abbreviation used
- Mst1
mammalian sterile 20—like kinase 1
- Tn
troponin
- cTnI
cardiac Troponin I
- cTnT
cardiac troponin T
- cTnC
cardiac troponin C
- ELISA
enzyme-linked immunosorbant assay
REFERENCES
- 1.Creasy CL, Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995;270:21695–21700. doi: 10.1074/jbc.270.37.21695. [DOI] [PubMed] [Google Scholar]
- 2.Creasy CL, Ambrose DM, Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J Biol Chem. 1996;271:21049–21053. doi: 10.1074/jbc.271.35.21049. [DOI] [PubMed] [Google Scholar]
- 3.Ura S, Masuyama N, Graves JD, Gotoh Y. Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc Natl Acad Sci U S A. 2001;98:10148–10153. doi: 10.1073/pnas.181161698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Y, Khokhlatchev A, Figeys D, Avruch J. Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis. J Biol Chem. 2002;277:47991–48001. doi: 10.1074/jbc.M202630200. [DOI] [PubMed] [Google Scholar]
- 5.Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, Beeser A, Etkin LD, Chernoff J, Earnshaw WC, Allis CD. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell. 2003;113:507–517. doi: 10.1016/s0092-8674(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 6.Graves JD, Draves KE, Gotoh Y, Krebs EG, Clark EA. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J Biol Chem. 2001;276:14909–14915. doi: 10.1074/jbc.M010905200. [DOI] [PubMed] [Google Scholar]
- 7.Glantschnig H, Rodan GA, Reszka AA. Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J Biol Chem. 2002;277:42987–42996. doi: 10.1074/jbc.M208538200. [DOI] [PubMed] [Google Scholar]
- 8.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381:453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto S, Yang G, Zablocki D, Liu J, Hong C, Kim SJ, Soler S, Odashima M, Thaisz J, Yehia G, Molina CA, Yatani A, Vatner DE, Vatner SF, Sadoshima J. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest. 2003;111:1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odashima M, Usui S, Takagi H, Hong C, Liu J, Yokota M, Sadoshima J. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ Res. 2007;100:1344–1352. doi: 10.1161/01.RES.0000265846.23485.7a. [DOI] [PubMed] [Google Scholar]
- 11.Sun J, Yan G, Ren A, You B, Liao JK. FHL2/SLIM3 decreases cardiomyocyte survival by inhibitory interaction with sphingosine kinase-1. Circ Res. 2006;99:468–476. doi: 10.1161/01.RES.0000239410.65551.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward DG, Ashton PR, Trayer HR, Trayer IP. Additional PKA phosphorylation sites in human cardiac troponin I. Eur J Biochem. 2001;268:179–185. doi: 10.1046/j.1432-1327.2001.01871.x. [DOI] [PubMed] [Google Scholar]
- 13.al-Hillawi E, Minchin SD, Trayer IP. Overexpression of human cardiac troponin-I and troponin-C in Escherichia coli and their purification and characterisation. Two point mutations allow high-level expression of troponin-I. Eur J Biochem. 1994;225:1195–1201. doi: 10.1111/j.1432-1033.1994.1195b.x. [DOI] [PubMed] [Google Scholar]
- 14.Guo X, Wattanapermpool J, Palmiter KA, Murphy AM, Solaro RJ. Mutagenesis of cardiac troponin I. Role of the unique NH2-terminal peptide in myofilament activation. J Biol Chem. 1994;269:15210–15216. [PubMed] [Google Scholar]
- 15.Wang J, Jin JP. Conformational modulation of troponin T by configuration of the NH2-terminal variable region and functional effects. Biochemistry. 1998;37:14519–14528. doi: 10.1021/bi9812322. [DOI] [PubMed] [Google Scholar]
- 16.Jin JP, Chen A, Ogut O, Huang QQ. Conformational modulation of slow skeletal muscle troponin T by an NH(2)-terminal metal-binding extension. Am J Physiol Cell Physiol. 2000;279:C1067–1077. doi: 10.1152/ajpcell.2000.279.4.C1067. [DOI] [PubMed] [Google Scholar]
- 17.Jin JP. Alternative RNA splicing-generated cardiac troponin T isoform switching: a non-heart-restricted genetic programming synchronized in developing cardiac and skeletal muscles. Biochem Biophys Res Commun. 1996;225:883–889. doi: 10.1006/bbrc.1996.1267. [DOI] [PubMed] [Google Scholar]
- 18.Jin JP, Yang FW, Yu ZB, Ruse CI, Bond M, Chen A. The highly conserved COOH terminus of troponin I forms a Ca2+-modulated allosteric domain in the troponin complex. Biochemistry. 2001;40:2623–2631. doi: 10.1021/bi002423j. [DOI] [PubMed] [Google Scholar]
- 19.Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res. 2004;94:146–158. doi: 10.1161/01.RES.0000110083.17024.60. [DOI] [PubMed] [Google Scholar]
- 20.Murphy AM. Heart failure, myocardial stunning, and troponin: a key regulator of the cardiac myofilament. Congest Heart Fail. 2006;12:32–38. 39–40. doi: 10.1111/j.1527-5299.2006.04320.x. quiz. [DOI] [PubMed] [Google Scholar]
- 21.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 23.Leavis PC, Gergely J. Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. CRC Crit Rev Biochem. 1984;16:235–305. doi: 10.3109/10409238409108717. [DOI] [PubMed] [Google Scholar]
- 24.Perry SV. Troponin I: inhibitor or facilitator. Mol Cell Biochem. 1999;190:9–32. [PubMed] [Google Scholar]
- 25.Li MX, Wang X, Sykes BD. Structural based insights into the role of troponin in cardiac muscle pathophysiology. J Muscle Res Cell Motil. 2004;25:559–579. doi: 10.1007/s10974-004-5879-2. [DOI] [PubMed] [Google Scholar]
- 26.Mittmann K, Jaquet K, Heilmeyer LM., Jr. Ordered phosphorylation of a duplicated minimal recognition motif for cAMP-dependent protein kinase present in cardiac troponin I. FEBS Lett. 1992;302:133–137. doi: 10.1016/0014-5793(92)80423-e. [DOI] [PubMed] [Google Scholar]
- 27.Mittmann K, Jaquet K, Heilmeyer LM., Jr. A common motif of two adjacent phosphoserines in bovine, rabbit and human cardiac troponin I. FEBS Lett. 1990;273:41–45. doi: 10.1016/0014-5793(90)81046-q. [DOI] [PubMed] [Google Scholar]
- 28.Kranias EG, Solaro RJ. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature. 1982;298:182–184. doi: 10.1038/298182a0. [DOI] [PubMed] [Google Scholar]
- 29.Saeki Y, Takigiku K, Iwamoto H, Yasuda S, Yamashita H, Sugiura S, Sugi H. Protein kinase A increases the rate of relaxation but not the rate of tension development in skinned rat cardiac muscle. Jpn J Physiol. 2001;51:427–433. doi: 10.2170/jjphysiol.51.427. [DOI] [PubMed] [Google Scholar]
- 30.Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 31.Jideama NM, Noland TA, Jr., Raynor RL, Blobe GC, Fabbro D, Kazanietz MG, Blumberg PM, Hannun YA, Kuo JF. Phosphorylation specificities of protein kinase C isozymes for bovine cardiac troponin I and troponin T and sites within these proteins and regulation of myofilament properties. J Biol Chem. 1996;271:23277–23283. doi: 10.1074/jbc.271.38.23277. [DOI] [PubMed] [Google Scholar]
- 32.Krudy GA, Kleerekoper Q, Guo X, Howarth JW, Solaro RJ, Rosevear PR. NMR studies delineating spatial relationships within the cardiac troponin I-troponin C complex. J Biol Chem. 1994;269:23731–23735. [PubMed] [Google Scholar]
- 33.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 34.Szczesna D, Zhang R, Zhao J, Jones M, Potter JD. The role of the NH(2)- and COOH-terminal domains of the inhibitory region of troponin I in the regulation of skeletal muscle contraction. J Biol Chem. 1999;274:29536–29542. doi: 10.1074/jbc.274.41.29536. [DOI] [PubMed] [Google Scholar]
- 35.Noland TA, Jr., Kuo JF. Protein kinase C phosphorylation of cardiac troponin I or troponin T inhibits Ca2(+)-stimulated actomyosin MgATPase activity. J Biol Chem. 1991;266:4974–4978. [PubMed] [Google Scholar]
- 36.Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem. 2003;278:11265–11272. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Grant JE, Doede CM, Sadayappan S, Robbins J, Walker JW. PKC-betaII sensitizes cardiac myofilaments to Ca2+ by phosphorylating troponin I on threonine-144. J Mol Cell Cardiol. 2006;41:823–833. doi: 10.1016/j.yjmcc.2006.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.