Abstract
A new pentadentate ligand, α,α,α′,α′-tetra(pyrazolyl)lutidine, pz4lut, has been prepared by a CoCl2-catalyzed rearrangement reaction between 2,6-pyridinedicarboxaldehyde and dipyrazolylthione. The coordination chemistry with some divalent first-row transition metal (Mn, Fe, Co, Ni, Cu, and Zn) chlorides has been explored. The electronic properties indicate that the new κ5N ligand is a slightly stronger-field donor to Ni2+ and Co2+ than a related pentadentate ligand with five pyridyl donors presumably because of greater interaction between the metal and axial pyridyl.
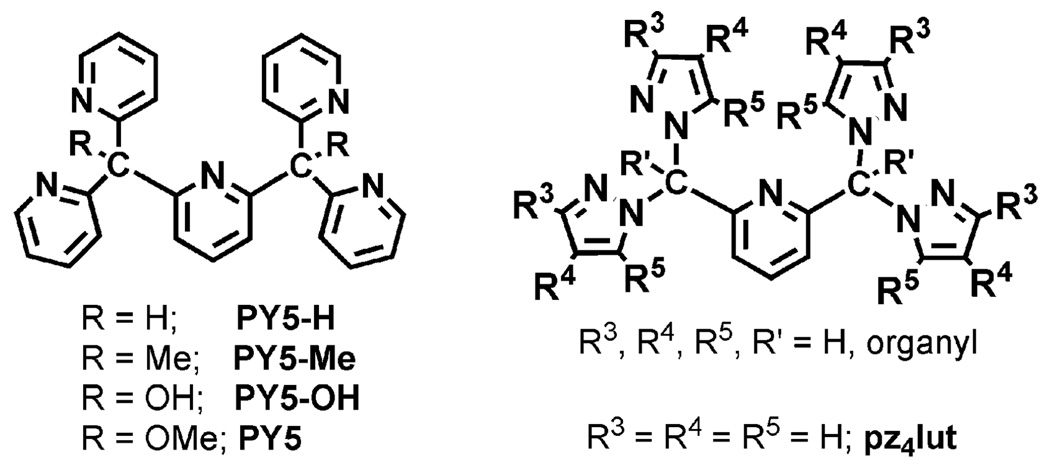
There has been recent interest in tetrapodal pentadentate ligands that bind metals in a MAE4 (M = metal; A = axial; E = equatorial) fashion for studies in fundamental coordination chemistry especially those involving modeling of the reactivity of biologically important species such as the hemes and cobalamins.1 The continued development of pentadentate ligands has led to the discovery of interesting new nonheme metal complexes capable of facilitating many spectacular organic transformations, highlighted by the oxidation of alkanes.2 Pentadentate ligands of particular relevance to the current study are those derived from tetrakis(2-pyridyl)-m-lutidine (Figure 1, left). Numerous coordination modes have been observed for each,3 with the pentadentate mode being more extensively structurally authenticated for PY54 than for other PY5-R derivatives.5 Transition-metal complexes of pentadentate PY5 have an unusual structural feature in that the dihedral angle between the axial pyridyl and the equatorial N4 plane significantly deviates from ideal orthogonality. Such a deformation likely reduces the efficiency of both σ- and π-orbital interactions between the axial hetero-cycle and the metal d orbitals, a possible factor influencing the reactivity of the complexes. Molecular models suggest that this unusual arrangement is enforced by steric interactions about the quaternary carbons specifically between the methoxy and pyridyl groups. While this deformation may not be as pronounced in solution,6 we conjectured that modification of the pentadentate ligand backbone might provide a key to tuning the properties and chemical reactivity of the resulting metal complexes because, in metal complexes, the cone angle for steric accessibility is larger for ligands with five-membered pyrazolyl rings compared to those with similarly substituted six-membered pyridyl rings. To this end, we have developed a new class of pentadentate ligands based on the α,α,α′,α′- tetra(pyrazolyl)lutidine, pz4lut, scaffold (Figure 1) and have initiated an investigation into their coordination chemistry. The new ligands offer some advantageous design features that can be exploited for further systematic studies, including (i) the ease of synthesis of pyrazoles with nearly limitless substitution patterns that allow for facile means to control steric and electronic properties of the resulting metal complexes and, by appropriate pyrazolyl substitution, allow for the deliberate construction of supramolecular assemblies7 and (ii) the flexible ligand synthesis permitting ready substitution of the fourth group (R′ in Figure 1, right) of the sp3 C atoms, which will allow further evaluation of the impact of this substituent on the properties of the resulting metal complex.
Figure 1.
Related pentadentate ligand scaffolds.
In this Communication, we report on the synthesis of the new ligand and its coordination complexes with a series divalent first-row transition-metal halides. A brief survey of the structural and electronic properties of the complexes is discussed. Future papers in this series will elaborate on the electronic properties and chemical reactivity of these and related complexes, where special attention will be paid to the effects of substitution along the organic skeleton and to the various ligand coordination modes.
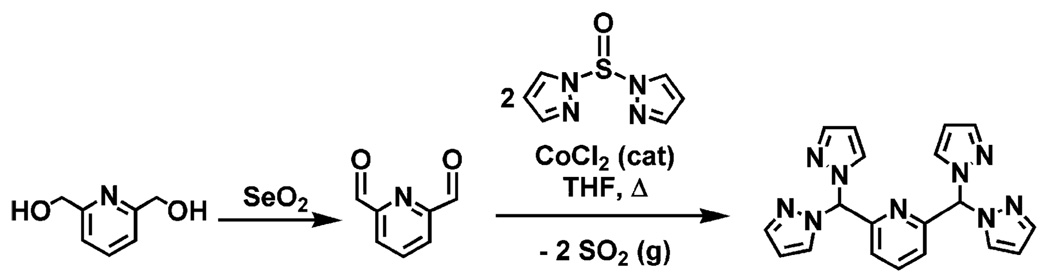
The synthesis of the new ligand, pz4lut (Scheme 1), exploits the CoCl2-catalyzed rearrangement reaction8 between 2,6-pyridinedicarboxaldehyde9 (prepared by SeO2 oxidation of 2,6-pyridinedimethanol) and the appropriate di(pyrazolyl)-sulfone (prepared in situ by the reaction between NaH, Hpz, and SOCl2)10 in THF. Typically, the CoCl2-catalyzed rearrangement reaction with dialdehydes affords high yields (>70%) of product. In the current case, the yield is typically only 40%. Presumably, the relatively higher stability and lower solubility of the cobalt complex of this ligand sequesters the catalyst, lowering its performance. We are currently working on methods to improve the catalyst performance or, otherwise, to optimize the synthesis of the ligand. Regardless, synthetically useful quantities of ligand are obtained.
Scheme 1.
Preparation of the pz4lut Ligand
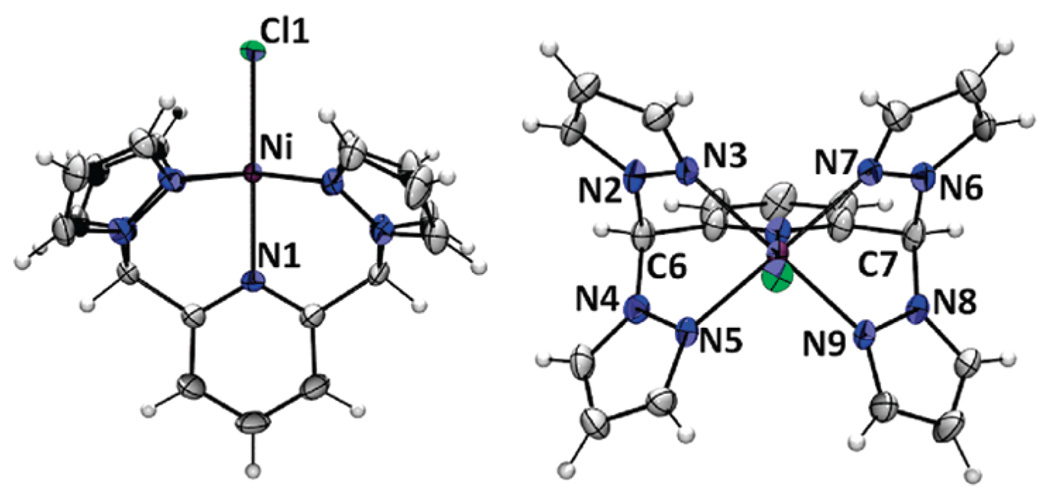
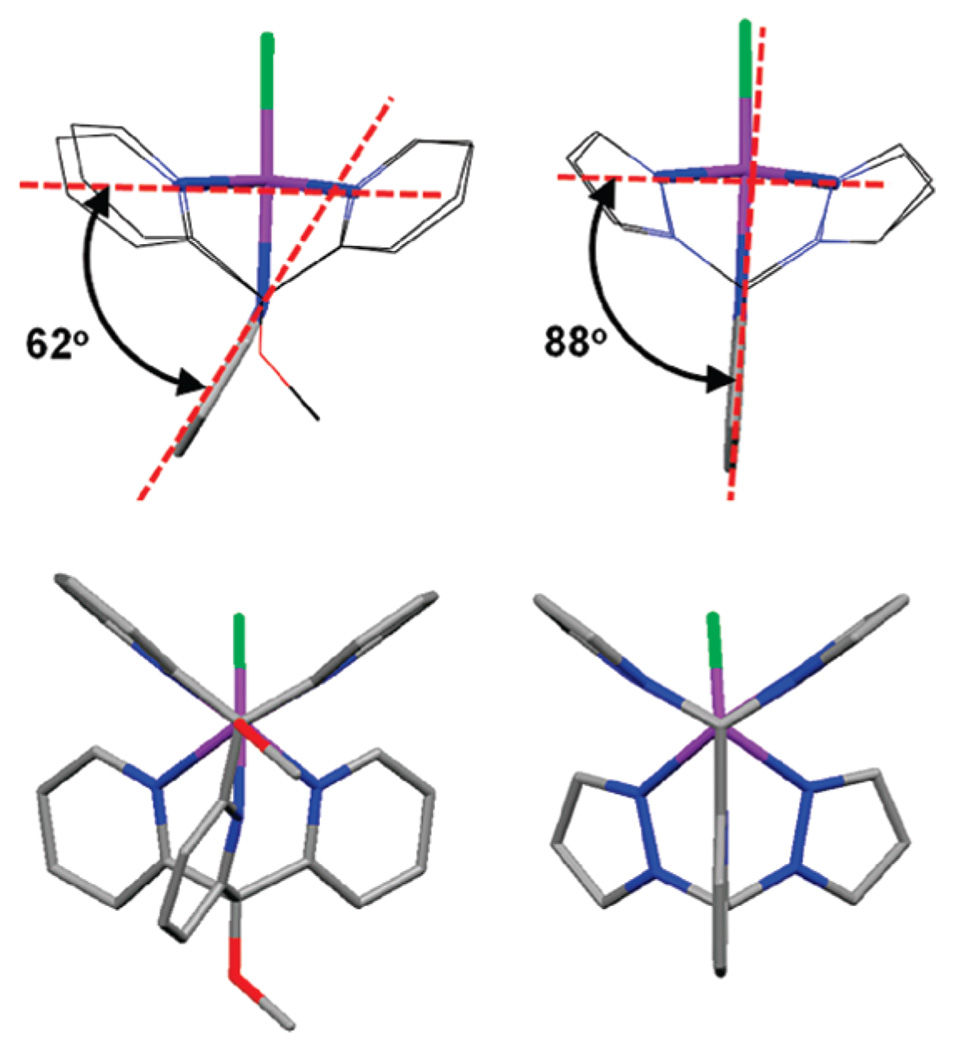
X-ray-quality crystals of divalent metal chloride complexes [MCl(κ5N-pz4lut)]+ were obtained by layering of methanol solutions of the transition-metal salts (except Ni, for which ethanol gave better quality crystals) onto CH2Cl2 or CHCl3 solutions of the ligands and allowing the solvents to slowly diffuse over several days. The complexes with M = Mn, Fe, Co, and Cu contain a chloride anion and a methylene chloride solvent molecule. The nickel derivative crystallizes with a chloride anion but as an ethanol solvate, while the zinc derivative contains the [ZnCl3(MeOH)]− anion. The structure and metrical parameters for each complex are given in the Supporting Information, while the structure of a representative cation, [NiCl(pz4lut)]+, is provided in Figure 2. The six metal cations prepared in this study share a number of common features. All have MN5Cl kernels as a result of κ5-ligand coordination with pyridyl and chloride axial groups and four pyrazolyl N atoms occupying the equatorial plane of the pseudooctahedral metal center. The average metal–nitrogen(pz) bond distances MnII–N 2.26 Å (d5), FeII–N 2.20 Å (d6), and CoII–N 2.15 Å (d7) are indicative of high-spin systems.11 The local MN4(pz)N(py) environment deviates from ideal C4υ symmetry because of various differences in the equatorial M–N bond distances and associated angles, as is more fully addressed in the Supporting Information. Also, with the exception of the copper derivative (see the Supporting Information), the bond distances and angles of the MN5Cl kernels are comparable to those for related high-spin [MCl(PY5)](Cl) complexes.4 One significant structural difference between the [MCl(κ5N-L)]+ cations (L = PY5 or pz4lut) is the angle between the mean plane of the axial pyridyl ring and that defined by the equatorial N atoms of each type of ligand; that for the PY5 complexes deviates from orthogonality to a greater extent than the corresponding angle in the pz4lut complexes, as is illustrated for the nickel cations in the top of Figure 3. This and other ligand distortions can also be quantified by examining the average heterocycle ring twisting,12 measured by the torsion angle MN-NC(sp3) for metal-bound pyrazolyls or MN-CC(sp3) for metal-bound pyridyls; ideally, this torsion angle should be 0°. In all cases, there is more ring twisting in the PY5 complexes than in the pz4lut cases, with the greatest contribution in the former cases from the axial pyridyl, illustrated in the bottom of Figure 3. For the two [NiCl(L)]+ cations, the average ring twisting for L = PY5 is 9.8°, whereas it is 1.8° for L = pz4lut.
Figure 2.
Two ORTEP (50% probability ellipsoids) views of the [NiCl(pz4lut)]+ cation.
Figure 3.
Comparison of the structures of related [NiCl(L)]+ cations (L = PY5, left; L = pz4lut, right). H atoms are omitted. Top: views orthogonal to the axial pyridyl. Bottom: views down C–O (PY5) or C–H (pz4lut) bonds.
Selected electronic properties of the complexes are summarized in Table 1. The magnetic and electronic properties, are consistent with the high-spin configurations of the MnII, FeII, and CoII derivatives (see the Supporting Information for the spectra). The solid-state magnetic moment of each [MIICl(pz4lut)]+ gives values close to the expected spin-only moments for MnII (high spin), NiII, and CuII, whereas those for FeII and CoII are a little higher because of the expected orbital angular momentum contribution. The various paramagnetic [MIICl(pz4lut)]+ derivatives as frozen (10 K) dispersions in silicon oil were also subject to X-band (9.6 GHz) electron paramagnetic resonance (EPR) spectroscopic studies to verify spin multiplicities. The data are collected in Table 1, while EPR spectra and detailed discussions are given in the Supporting Information. The complexes generally displayed axial signals, expected from the solid-state geometries. The MnII and CoII EPR spectra indicated the S = 5/2 and S = 3/2 high-spin configurations, respectively. The zero-field splitting, Δ, was much larger than the Zeeman interaction in both cases. In the case of MnII, it appears that excited-state doublets were populated at 10 K (i.e., 7 cm−1 ≤ Δ ≫ 0.3 cm−1), whereas for CoII, Δ ≫ 7 cm−1. No EPR signal was detected for the NiII complex, typical of a non-Kramers (S = 1) system with significant zero-field splitting. Interestingly, an EPR signal for [FeIICl(pz4lut)](Cl) was observed at geff ~ 9 at low temperatures despite a non-Kramers (S = 2) spin multiplicity owing to some proportion of the zero-field splitting envelope satisfying the condition Δ < 0.3 cm−1.13
Table 1.
Summary of the Electronic Properties of Transition-Metal Complexes of pz4lut
| complex | µeff,µB | λmax, nm (ε, M−1 cm−1), MeOH | 10 Dq, cm−1 | EPR X-band (9.6 GHz), geff |
|---|---|---|---|---|
| [MnCl(pz4lut)]+ | 5.9 | 215 (29 404), 264 (5614), 342 sh (53) | g⊥ 4.05, g‖ 0.99 | |
| [FeCl(pz4lut)]+ | 5.2 | 263 (8762), 300 sh (2677), 428 (282), 888 (19), 1050 sh (5) | 10 393 | geff ~9 |
| [CoCl(pz4lut)]+ | 3.6 | 264 (4936), 300 sh (608), 472 (20), 934 (4) | 11 582 | g⊥ 4.32, g‖ 2.30 (greal ~ 2.2) |
| [NiCl(pz4lut)]+ | 2.3 | 268 (3455), 300 sh (188), 536 (25), 764 sh (7), 874 (13) | 11 442 | |
| [CuCl(pz4lut)]+ | 1.8 | 270 (3611), 424 (278), 700 (43), 1150 sh (12) | ~9 820 | g⊥ 2.07, g‖ 2.28 (A‖ = 17 mT) |
The electronic spectra of the ligand and metal complexes are provided in the Supporting Information. For each, there are two rather intense bands near 200 nm (ε ~ 25 000 M−1 cm−1) and 260 nm (ε ~ 5000 M−1 cm−1), assigned to π-π* intraligand transitions on the basis of intensity. For all of the metal complexes, an additional shoulder near 300 nm (ε ~ 50–2700 M−1 cm−1) is observed that is absent in the free ligand and is tentatively assigned as metal-to-ligand charge transfer in nature. For FeII, CoII, NiII, and CuII, lower-energy low-intensity (ε < 50 M−1 cm−1) spin-allowed d–d bands are also observed; the spin-forbidden bands in MnII are not observed. The single d–d band in the spectra of each FeII and CuII shows a pronounced shoulder from the expected Jahn-Teller distortions. The crystal-field splitting parameters (Table 1) calculated from the observed d–d bands gave the metal-dependent spectrochemical series CoII > NiII > FeII > CuII for the pz4lut complexes.14 In the cases of CoII and NiII, where full data are readily available for comparison, the crystal-field splitting parameters for [MCl(L)]+ (L = pz4lut, PY5) indicate that the pz4lut ligand exerts a slightly stronger field (CoII, 10 Dq = 11 582 cm−1; NiII, 10 Dq = 11 442 cm−1) than the related PY5 ligand (CoII, 10 Dq = 10 915 cm−1; NiII, 10 Dq = 10 707 cm−1).4 Because pyridyls are generally stronger field donors than pyrazolyls, this rather surprising observation may be a result of the greater ligand distortions in the PY5 framework compared to the pz4lut cases. A greater degree of ring twisting in the former may potentially reduce the full σ-donor (and π-accepting) capabilities of the PY5 ligand.
In summary, a new pentadentate ligand, α,α,α′,α′-tetra(pyrazolyl) lutidine, pz4lut, and several coordination complexes with [MCl(pz4lut)]+ (M = Mn, Fe, Co, Ni, Cu, Zn) cations have been prepared. A survey of the structural features of the complexes shows that the ligand binds in the expected κ5N mode with the axial pyridyl group nearly orthogonal to the equatorial N4 plane. Analysis of ligand distortions in related [MCl(L)]+ (L = pz4lut, PY5) complexes indicated a lower degree of ring twisting in the current system relative to the PY5 complexes. This feature might be responsible for the rather surprising electronic properties because pz4lut is a slightly stronger field ligand to CoII and NiII compared to the all-pyridyl-based PY5 ligand, contrary to initial expectations. The simple synthesis of the new ligand architecture combined with ready modifications using numerous available substituted pyrazolyls permits easy access to derivatives with variable steric and electronic properties, chemical reactivity, and ligand denticity, as will be disseminated shortly.
Supplementary Material
Acknowledgment
J.R.G. thanks Marquette University and the donors of the Petroleum Research Fund for financial support. Support for EPR spectral studies (Grant NIH-RR001980) is also gratefully acknowledged.
Footnotes
Supporting Information Available:Full experimental details, ORTEP diagrams, a crystallographic information file (CIF), tables of bond distances and angles, and UV–vis/near-IR and EPR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Grohmann A. AdV. Inorg. Chem. 2004;56:179. [Google Scholar]
- 2.(a) Costas M, Mehn MP, Jensen MP, Que L., Jr Chem. ReV. 2004;104:939. doi: 10.1021/cr020628n. and references cited therein. [DOI] [PubMed] [Google Scholar]; (b) Kaizer J, Klinker EJ, Oh NY, Rohde J-U, Song WJ, Stubna A, Kim J, Münck E, Nam W, Que L., Jr J. Am. Chem. Soc. 2004;126:472. doi: 10.1021/ja037288n. [DOI] [PubMed] [Google Scholar]; (c) Jonas RT, Stack TDP. J. Am. Chem. Soc. 1997;119:8566. [Google Scholar]
- 3.(a) Kui SCF, Fang G-S, Zhu N, Che C-M. Inorg. Chem. 2008;47:5727. doi: 10.1021/ic8000043. [DOI] [PubMed] [Google Scholar]; (b) Canty AJ, Minchin NJ, Skelton BW, White AH. J. Chem. Soc., Dalton Trans. 1986:2205. [Google Scholar]
- 4.(a) Klein Gebbink RJM, Jonas RT, Goldsmith CR, Stack TDP. Inorg. Chem. 2002;41:4633. doi: 10.1021/ic025617r. [DOI] [PubMed] [Google Scholar]; (b) de Vries ME, La Crois RM, Roelfes G, Kooijman H, Spek AL, Hage R, Feringa BL. Chem. Commun. 1997:1549. [Google Scholar]
- 5.(a) Freedman DE, Jenkins DM, Iavarone AT, Long JR. J. Am. Chem. Soc. 2008;130:2884. doi: 10.1021/ja077527x. [DOI] [PubMed] [Google Scholar]; (b) Wong EL-M, Fang G-S, Che C-M, Zhu N. Chem. Commun. 2005:4578. doi: 10.1039/b507687k. [DOI] [PubMed] [Google Scholar]
- 6.López JP, Heinemann FW, Prakash R, Hess BA, Horner O, Jeandey C, Oddou J-L, Latour J-M, Grohmann A. Chem.–Eur. J. 2002;8:5709. doi: 10.1002/1521-3765(20021216)8:24<5709::AID-CHEM5709>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.For instance, see: Zhao N, Bullinger JC, Van Stipdonk MJ, Stern CL, Eichhorn DM. Inorg. Chem. 2008;47 doi: 10.1021/ic8003307. ASAP.Zhao N, Van Stipdonk MJ, Bauer C, Campana C, Eichhorn DM. Inorg. Chem. 2007;46:8662. doi: 10.1021/ic700628b..
- 8.(a) The KI, Peterson LK. Can. J. Chem. 1973;51:422. [Google Scholar]; (b) The KI, Peterson LK, Kiehlmann E. Can. J. Chem. 1973;51:2448. [Google Scholar]; (c) Peterson LK, Kiehlmann E, Sanger AR, The KI. Can. J. Chem. 1974;52:2367. [Google Scholar]
- 9.(a) Luening U, Baumstark R, Peters K, Von Schnering HG. Liebigs Ann. Chem. 1990;2:129. [Google Scholar]; (b) Steenland MWA, Lippens W, Herman GG, Goeminne AM. Bull. Soc. Chim. Belges. 1993;102:239. [Google Scholar]
- 10.(a) Reger DL, Grattan TC, Brown KJ, Little CA, Lamba JJS, Rheingold AL, Sommer RD. J. Organomet. Chem. 2000;607:120. [Google Scholar]; (b) Reger DL, Watson RP, Gardinier JR, Smith MD, Pellechia PJ. Inorg. Chem. 2006;45:10088. doi: 10.1021/ic0613154. [DOI] [PubMed] [Google Scholar]; (c) Reger DL, Watson RP, Smith MD. Inorg. Chem. 2006;45:10077. doi: 10.1021/ic0613053. [DOI] [PubMed] [Google Scholar]; (d) Reger DL, Watson RP, Smith MD, Pellechia PJ. Organometallics. 2006;25:743. [Google Scholar]; (e) Reger DL, Watson RP, Smith MD, Pellechia PJ. Organometallics. 2005;24:1544. [Google Scholar]; (f) Reger DL, Watson RP, Gardinier JR, Smith MD. Inorg. Chem. 2004;43:6609. doi: 10.1021/ic0491377. [DOI] [PubMed] [Google Scholar]
- 11.Chanaka D, De Alwis L, Schultz FA. Inorg. Chem. 2003;42:3616. doi: 10.1021/ic034077a. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 12.(a) Reger DL, Gardinier JR, Elgin JD, Smith MD, Hautot D, Long GJ, Grandjean F. Inorg. Chem. 2006;45:8862. doi: 10.1021/ic0607437. [DOI] [PubMed] [Google Scholar]; (b) De Bari H, Zimmer M. Inorg. Chem. 2004;43:3344. doi: 10.1021/ic0498992. [DOI] [PubMed] [Google Scholar]
- 13.Hendrich MP, Debrunner PG. Biophys. J. 1989;56:489. doi: 10.1016/S0006-3495(89)82696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Figgis BN, Hitchman MA. Ligand Field Theory and Its Applications. New York: Wiley-VCH; 2002. [Google Scholar]; (b) Lever ABP. Inorganic Electronic Spectroscopy. New York: Elsevier; 1968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.