Summary
Antibodies to HIV are potentially important reagents for basic and clinical studies. Historically, these reagents have been produced by random cloning of heavy and light chains in phage display libraries (Burton et al., 1991) and electrofusion techniques (Buchacher, 1992). Here we describe a method to identify and potentially enrich human memory B cells from HIV infected patients that show serum titers of neutralizing antibodies. When biotinylated gp140 is used to stain peripheral blood mononuclear cells it identifies a distinct population of gp140 binding B cells by flow cytometry.
Keywords: HIV, memory B cells
Introduction
The HIV surface protein is composed of a trimer of gp140, which is made up of two non-covalently associated polypeptides gp120 and gp41. Antibodies to either gp120 or gp41 can have neutralizing activity in vitro and in vivo. However, in order to neutralize, antibodies need to bind to their epitope in the context of the functional trimeric GP140 (Broder, 1994; Yang, 2000). In our search for a method to identify memory B cells in the blood that bind to the surface of HIV we made use of an artificially trimerized gp140 protein that has previously been shown to resemble the native envelope spike (Yang, 2000).
Materials and Methods
Participants
HIV-1 infected patient is part of the Elite Controller Study of the Partners Aids Research Center. The patient was identified as elite controller based on clinical data (Table 1), CD4+ T cell counts and plasma viral loads below 50 RNA copies/ml in the absence of retroviral therapy (Walker, 2007). The un-infected volunteer was a 31 year old male recruited at the Rockefeller University. Human samples were collected after signed informed consent in accordance with Institutional Review Board (IRB)-reviewed protocols of all participating institutions.
Table I. Clinical Data of CTR203, 1/10/06.
| GENDER | DATE OF BIRTH | DIAGNOSIS | ETHNICITY | VIRAL LOAD | CD4 + T Cells/μl | % CD4+ |
|---|---|---|---|---|---|---|
| MALE | 09-JAN-1974 | 17-JAN-2006 | CAUCASIAN | 49cop/ml | 408 | 34% |
Blood samples
Blood was obtained in syringes treated with acid citrate dextrose.
Neutralization screen
Neutralization screens were performed as previously described (Li et al., 2005). In brief, serum neutralization was detected as reduction in luciferase reporter gene expression after single round infection in Tzm-bl cells. In order to rule out unspecific antiviral activity in the plasma sample SIVmac251.WY5 was used as a negative control.
Biotinylated gp140
The Avitag biotinylation signal (LNDIFEAQKIEWHE) was added to the carboxylic terminus of Trimeric gp140 composed of the YU2 HIV-1 envelope amino acids 1 to 683 was fused to the T4 phage trimerization domain (Yang, 2000). The protein was produced by transient transfection of suspension cultured 293T cells with “293 fectin” according to the manufacturer's suggestion (Invitrogen). Supernatants from transfected cells were collected after 4 days of culture and recombinant protein concentrated by lentil lectin affinity chromatography before purification by affinity on a Ni column (GE Health care, Piscataway, NJ). The purified product was biotinylated using biotin ligase according to the manufacturer's suggestions (Avidity, Denver, Co) and checked for antigenic activity by ELISA assays with monoclonal antibodies to gp140.
Flow Cytometry
Mononuclear cells were purified from peripheral blood by Ficoll-Paque (GE Healthcare) density gradient centrifugation according to the manufacturer's instructions. B cells were enriched by depletion of non-B cells using a B Cell Isolation Kit (Miltenyi) Enriched cells were stained using anti-human CD19 PE, IgG APC (BD), biotinylated gp140. Biotinylated gp140 was used for staining at a concentration of 5 μg/ml and detected using Streptavidin-PE (Pharmingen) at a dilution of 1/1500. Samples were analyzed on FACSVantage (BD) using FACSDiva Software (BD).
Results
Antigen specific IgG+ B cells make up a small percentage of the circulating B cell pool. These cells can be identified by their expression of CD19 and IgG as well as a high affinity to their antigen and represent somatically hypermutated post germinal center IgG memory B cells (Klein, 1998). The presence of such cells in blood correlates in part with serum antibody titers (Crotty, 2003; Leyendeckers, 1999; Nanan, 2001, (Amanna et al., 2007).
To determine whether we could identify HIV-gp140 binding B cells in the circulation of individuals with serum titers of anti-HIV antibodies we studied one such individual and an uninfected control. Table 2 summarizes the neutralization breadth of the serum from the HIV-infected patient. Figure 1A shows that among CD19+IgG+ B cells in the infected individual we found a distinct population of cells that also bound biotinylated gp140. Further, we were unable to detect a significant number of gp140 binding CD19+IgG+ cells in the circulation of an uninfected control (Fig. 1B).
Table 2. Serum Neutralization of CTR203.
| TIER 1 | TIER 2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STRAIN | SF162.LS | BAL.26 | SS196.1 | 6535.3 | QH0692.4 2 |
SC422661 .8 |
PVO.4 | TRO.11 | AC10.0.2 9 |
RHPA425 9.7 |
THRO415 6.18 |
REJO454 1.67 |
TRJO455 1.58 |
WITO416 0.33 |
CAAN53 42.A2 |
SIVmac25 1.WY5 |
| ID50 | 9250 | 756 | 278 | 121 | 36 | 421 | 80 | 77 | <20 | 891 | 129 | 162 | 44 | 228 | 264 | <20 |
ID 50 values are the reciprocal dilutions required to achieve 50% inhibition of infectivity in a Tzm-bl assay (Li et al., 2005). The viral strains are divided into Tier 1 and Tier 2 viruses, the former being easier to neutralize (Li et al., 2005).
Figure 1. Gp140 staining of peripheral blood B cells.
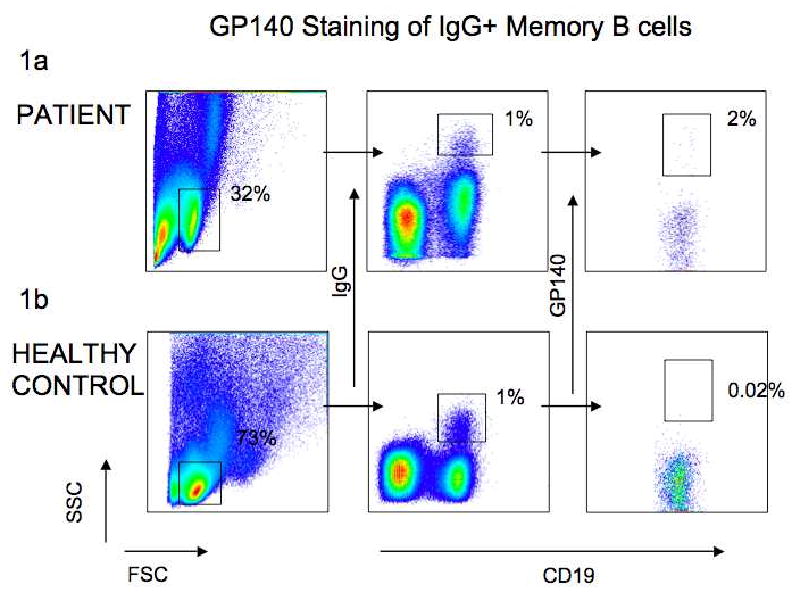
Gating strategy for GP140 binding IgG+ B cells from a HIV-1 infected patient and healthy control. 1A and 1b show the patient and control respectively. The plots on the left show the gating on lymphocytes according to their size and granularity, the plots in the middle indicate the gating for IgG+CD19+ B cells and the plots on the right show the gating of IgG+CD19+gp140+ cells. Numbers indicate the frequency of cells in the lymphocyte gate (left) and the frequency of CD19+IgG+ B cells (center) and gp140- binding CD19+IgG+ B cells (right) among all gated lymphocytes.
We conclude that biotin labeled gp140 trimer can be used to identify gp140 binding B cells in the circulation of HIV infected individuals with serum titers of neutralizing antibodies.
Discussion
Despite the importance of antibody responses to HIV there are no methods to identify, study and potentially purify circulating memory B cells that express such antibodies. We find only a low frequency of gp140 binding B cells in the IgG+ B cell compartment in the patient. However, the frequency of such cells was similar to the frequency of vaccinia specific circulating IgG memory B cells in vaccinia vaccinated individuals (Crotty, 2003). Furthermore these cells were not found in a control uninfected individual. In conclusion, the method we describe can potentially be used to identify and purify gp140 binding B cells by flow cytometry as a first step in the cloning and characterization of the antibodies they produce (Wardemann, 2003(Tiller et al., 2008).
Abbreviations
- Ig
immunoglobulin
- HIV
Human immunodeficiency virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- Buchacher A, Predl R, Tauer C, Purtscher M, Gruber G, Heider R, Steindl F, Trkola A, Jungbauer A, Katinger H. Human monoclonal antibodies against gp41 and gp120 as potential agent for passive immunization. Vaccines. 1992;92:191–195. [Google Scholar]
- Broder C, Earl P, Long D, Abedon S, Moss B, Doms R. Proc Natl Acad Sci USA. 1994;91:11699–11703. doi: 10.1073/pnas.91.24.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Barbas CF, III, Persson MAA, Koenig S, Chanock RM, Lerner RA. A large array of human monoclonal antibodies to type 1 immnodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Klein U, Rajewsky K, Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. 1998 doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyendeckers H, Odendahl M, Löhndorf A, Irsch J, Spangfort M, Miltenyi S, Hunzelmann N, Assenmacher M, Radbruch A, Schmitz J. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur J Immunol. 1999;29(4):1406–17. doi: 10.1002/(SICI)1521-4141(199904)29:04<1406::AID-IMMU1406>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–25. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine. 2001;20(34):498–504. doi: 10.1016/s0264-410x(01)00328-0. [DOI] [PubMed] [Google Scholar]
- Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–24. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BD. Elite control of HIV Infection: implications for vaccines and treatment. Top HIV Med. 2007;15:134–6. [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young J, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Yang X, Farzan M, Wyatt R, Sodroski J. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 2000;74:5716–5725. doi: 10.1128/jvi.74.12.5716-5725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


