Abstract
Methods enabling prion replication ex vivo are important for advancing prion studies. However, few such technologies exist, and many prion strains are not amenable to them. Here we describe a prion organotypic slice culture assay (POSCA) that allows prion amplification and titration ex vivo under conditions that closely resemble intracerebral infection. Thirty-five days after contact with prions, mouse cerebellar slices had amplified the abnormal isoform of prion protein, PrPSc, >105-fold. This is quantitatively similar to amplification in vivo, but fivefold faster. PrPSc accumulated predominantly in the molecular layer, as in infected mice. The POSCA detected replication of prion strains from disparate sources, including bovines and ovines, with variable detection efficiency. Pharmacogenetic ablation of microglia from POSCA slices led to a 15-fold increase in prion titers and PrPSc concentrations over those in microglia-containing slices, as well as an increase in susceptibility to infection. This suggests that the extensive microglial activation accompanying prion diseases represents an efficacious defensive reaction.
The defining trait of transmissible spongiform encephalopathies (TSEs) is transmissibility between individuals or across species1. The infectious agent causing TSEs is the prion, which may be identical to PrPSc, a partially protease-resistant isoform of a membrane glycoprotein termed PrPC. Mice devoid of the Prnp gene, which encodes PrPC, resist prion infections2.
To determine prion titers, endpoint dilutions of samples are inoculated into large numbers of indicator animals. The incubation time of prion infections is negatively correlated to the size of the inoculum, allowing a simplified incubation time bioassay that sacrifices precision, yet requires fewer animals3. Because small amounts of infectivity correlate with incubation times, the observation periods span the life of indicator animals. This is impractical and expensive. Minimizing the numbers of animals used for titration would also reduce the suffering wrought by prion infections.
The scrapie cell assay in endpoint format (SCEPA) eliminates some of the above concerns and allows dramatically faster assays4. Here, cells are exposed to endpoint titrations of prions and subsequently passaged several times. Prion titers are determined by counting PrPSc-positive tissue-culture wells. The SCEPA is equivalent to animal bioassays in that it detects actual transfer of prion infectivity from samples to susceptible cells. However, the reactions of the CNS to prion infection include highly diverse cell types such as neurons, astrocytes, oligodendrocytes and microglia acting in concert. This diversity cannot be reproduced in the SCEPA, which is based on monoclonal, homogeneous cells. Also, the range of cell lines that have been identified as susceptible to prion infection in vitro is limited5. At present this restricts the applicability of the SCEPA to a subset of mouse-adapted prion strains. These two limitations may be interdependent, as the machinery necessary for overcoming strain and species barriers may require cells distinct from those that replicate prions. Hence any infectivity titrations of prion variants that do not efficiently infect cultured cells still require animal bioassays.
Here we show that chronically cultured organotypic cerebellar slices rapidly amplify PrPSc after exposure to prions, thereby circumventing some of the above limitations. This protocol allows dissection of CNS pathologies in a complex cellular environment morphologically similar to the intact brain. The sensitivity of the prion organotypic slice culture assay (POSCA) was marginally lower than that of the SCEPA. Although the POSCA makes use of animals as the original tissue donors, many genetically identical slices can be produced from each individual mouse. Hence genetic background variation—which may account for the variability of mouse bioassays—can be controlled by comparing samples prepared from the same individual mouse.
Activation of microglia in prion infections is very extensive and precedes neurodegeneration6,7. Microglia are abundantly associated with prion plaques, and PrPSc can sometimes be found within microglia6,8. However, it is unclear whether microglial activation represents a precipitating or a defensive reaction. Using the POSCA we found that microglial ablation from slices from CD11b-HSVTK transgenic mice markedly increased prion titers and PrPSc deposition. Thus microglia may be important in containing prion loads during the course of prion infections.
RESULTS
Longevity of mouse cerebellar slices in long-term cultures
As prion infections progress extremely slowly in vivo, we reasoned that slice infection would require organotypic cultures of great longevity. We tested various parameters, including the method used for preparing slices, age of the mice, culture medium composition, and volume and frequency of media changes (data not shown). We achieved the best results when preparing slices by vibratome sectioning and culturing them on Millicell-CM polytetrafluoroethylene membranes, exchanging the full volume of medium thrice each week.
We assessed slice viability after 5 weeks of culture by morphometric quantitation of cells permeable to propidium iodide (PI) and by DEVDase activity assays, which measure the prevalence of dead cells and the substrate turnover rate of executioner caspases, respectively. Control slices were treated with staurosporine, which induces widespread apoptosis (Supplementary Fig. 1a–c online). Untreated slices showed 0.1% ± 0.02%(mean ± s.d.) of PI+ cells (or <0.1% ± 0.02% of the total tissue surface area) and 2.1% ± 0.6% of DEVDase levels seen in staurosporine-treated slices. Hence, slices were viable for up to 5 weeks. Morphological and immunohistochemical analyses with various markers of CNS constituents (Supplementary Fig. 1d–h) supported this conclusion.
The POSCA assay
Most TSE assays rely on the differential susceptibility of PrPC and PrPSc to digestion with proteinase K (PK)9. The relative resistance of PrPSc to PK depends on the prion strain and the composition of non-PrP contaminants within the samples. We therefore determined the minimal conditions under which all PrPC present in 5-week-old slices is fully degraded by PK (Supplementary Fig. 2a online). We found that digestion of 20 µg protein lysate with 25 µg ml−1 PK (in a reaction volume of 20 µl, corresponding to 1 µg ml−1 total protein) ensured complete, reproducible removal of PrPC. We used these conditions in all subsequent experiments.
In a first attempt, we prepared slices from prion-inoculated pups, but after 5 weeks ex vivo, we found no PrPSc in the slices (Supplementary Fig. 2b). Next, we prepared slices from 10-d-old PrPC-overexpressing tga20TK (offspring of tga20+/+ males crossed to CD11b-HSVTK females10) pups or from Prnpo/o (mice devoid of PrPC) pups11 and incubated groups of ten slices with 1 ml of buffer containing 20 mg ‘RML’ (Rocky Mountain Laboratory strain) inoculum, consisting of crude brain homogenate from pooled, terminally ill wild-type mice on a CD1 background infected with sixth-passage RML prions. To minimize any toxicity from cellular debris, slices were exposed to RML for 1 h at 4 °C in the presence of kynurenic acid.
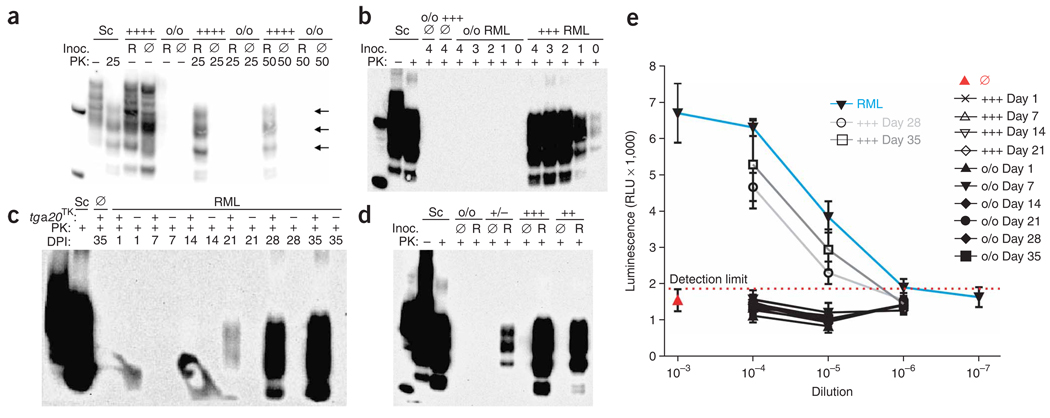
After 5 weeks, we analyzed slices by western blotting (Fig. 1a). In all experiments, we used monoclonal antibody POM1 (ref. 12) to detect PrP. To facilitate comparisons, we loaded 20 µg protein from the brain of a C57BL/6 mouse terminally ill with scrapie next to the marker on each blot. In lysates of RML-infected tga20TK cultures, we observed a characteristic triplet band pattern representing di-, mono- and unglycosylated forms of PrPSc after treatment with 25 or 50 µg ml−1 PK (Fig. 1a). RML-exposed Prnpo/o slices and slices exposed to uninfected brain homogenate never showed any PK-resistant material (Fig. 1a).
Figure 1.
Prions in slice cultures. (a) Immunoblot from tga20TK and Prnpo/o slices exposed to RML-infected (R) or uninfected (Ø) brain homogenate, cultured for 35 d, optionally digested with PK, and probed with antibody POM1 to PrP. Here and in all following figures, sample labels indicate the PrPC expression dictated by the slice genotype: o/o, Prnpo/o; +/−, hemizygous Prnp+/o; +/+, wild type; ++, hemizygous tga20 and Prnpo/o; +++, hemizygous for the tga20 allele and Prnp+/o; ++++, homozygous tga20 and Prnpo/o; Sc, positive-control homogenate from brain of a mouse with scrapie; inoc., inoculum. Left lane on all blots, molecular weight marker spiked with recombinant PrPC, yielding a PrP signal at 23 kDa with a cleavage product at 15 kDa. Arrows, PrPSc glycoforms. (b) Sensitivity of the POSCA. Immunoblot from tga20TK and Prnpo/o slices inoculated with decadal dilutions (104–100 µg; lane label, log(µg)) of prion-infected (RML) or uninfected (Ø) brain homogenate and cultured for 35 d before harvesting. (c) Time course of PrPSc accumulation in infected slices. Immunoblot from tga20TK (+) and Prnpo/o (−) slices inoculated with 100 µg prion-infected (RML) or uninfected (Ø) brain homogenate. (d) Immunoblot from Tga20TK, tga20+, Prnp+/o or Prnpo/o slices inoculated with 1 mg brain homogenate and harvested 35 dpi. (e) Time-dependent buildup of prion titers in slices. RML-infected or uninfected brain homogenate and slice homogenates from the experiment reported in panel c were serially tenfold diluted in uninfected brain homogenate, and infectivity was determined by transfer to PK1 cells. Means of three independent biological replicas ± s.d. RLU, relative light units.
Operating performance of the POSCA
The PrPSc signal observed in POSCA slices might partly represent residual inoculum adhering to slices. We therefore performed titration experiments using varying amounts of inoculum for the infection of slices. PrPSc was unambiguously detected in tga20TK slices exposed to as little as 1 µg RML homogenate (Fig. 1b). The amount of PrPSc recovered after 5 weeks was ≥ 40-fold higher than the total inoculum used to infect the cultures (Supplementary Fig. 3 online). Hence all PrP signals resulted from bona fide slice infection and de novo PrPSc amplification within slices. In RML-treated Prnpo/o cultures (n = 5) or in cultures treated with uninfected brain homogenates (n = 5), we never observed PK-resistant material, even in slices exposed to 10 mg of RML (Fig. 1b), indicating that PrPSc was amplified at least 104-fold.
Slices infected with small amounts of RML showed less PrPSc than those infected with larger amounts (Fig. 1b). This correlation was not explainable by variations in PrPC expression (Supplementary Fig. 4a online), and it suggests that PrPSc was accumulating exponentially at the time of analysis. We recorded PrPSc amplification within slices over time (Fig. 1c). One hour after inoculation we detected no residual inoculum (Fig. 1c), and 14 d after inoculation (dpi) we observed no indication of prion replication. After 21 d we observed a definite PrPSc signal (Fig. 1c). PrPC expression was similar in all cultures (Supplementary Fig. 4b).
We then examined the impact of host PrPC expression levels on prion replication. We infected cultures from tga20TK mice (heterozygous Prnp+/o mice carrying one copy of the tga20 transgene), Prnp+/o mice, or tga20+ mice (mice carrying one copy of the tga20 transgene), and compared PrPSc amounts. We observed a positive correlation between host expression of PrPC (tga20TK > tga20+ > Prnp+/o; Supplementary Fig. 4c) and the amount of PrPSc observed in the cultures 5 weeks after inoculation (tga20TK > tga20+ > Prnp+/o; Fig. 1d), as has been seen after intracerebral inoculation of mice13.
Infectivity amplification and relative sensitivity of POSCA
We then tested whether deposition of PrPSc accompanies prion replication, defined as increase in prion infectivity. Samples from the experiment in Figure 1c were used to infect the PK1 subclone4 of N2a neuroblastoma cells. Tga20TK culture homogenates showed no infectivity when assayed <28 d after exposure to RML. Slices assayed at 28 and 35 d contained >6.8 log TCI50 (tissue culture infectivity) units of prion infectivity per gram total protein (1 TCI50 unit being the dose required to infect 50% of PK1 culture wells) as determined by SCEPA4 (Fig. 1e). We detected no infectivity in RML-exposed Prnpo/o slice-culture lysates at any time. We compared the amount of infectivity in POSCA slices to a calibration curve of RML (Fig. 1e). RML contained >6.8 log TCI50 units g−1 protein (1 g RML homogenate contained 138 mg protein), similar to the amount of infectivity measured in RML-infected slices. Therefore, both PrPSc and prions were amplified in slices to amounts similar to those detected in terminally ill mouse brains.
To determine the dose required to infect 50% of the slice cultures (slice culture infectivity; SCI50), we performed an endpoint dilution experiment. We found that all slices infected with 1 µg RML, yet none of the cultures infected with 0.1 µg RML, accumulated PrPSc (Table 1, Supplementary Fig. 5a online and data not shown). The SCI50 was calculated according to standard methods14. Accordingly, RML homogenate contained >6.7 ± 0.1 log SCI50 g−1. To compare the sensitivity of the POSCA with that of other bioassays, we measured the titers of serially diluted RML homogenate by intracerebral inoculation into tga20 and CD1 mice as well as by SCEPA (Table 1). The tga20 bioassay measured 9.9 ± 0.2 log ID50 units (1 ID50 unit being the amount of infectivity that will cause infection in 50% of experimentally infected animals) and CD1 mice 9.3 ± 0.9 log ID50 units per gram of brain homogenate. In comparison, the SCEPA showed a sensitivity of 7.7 ± 0.5 log ID50 g−1 (Table 1).
Table 1.
Comparison of the sensitivity of four different prion infectivity assays
|
Tga20 inoculation |
CD1 inoculation |
|||||
|---|---|---|---|---|---|---|
| Dilution | Sick/total | Incubation time (d) ± s.d. | Sick/total | Incubation time (d) ± s.d. | SCEPA (positive/total) | POSCA (positive/total) |
| 10−1 | 4/4 | 54 ± 1 | 7/7 | 119 ± 1 | Not done | 5/5 |
| 10−2 | 4/4 | 59 ± 2 | 7/7 | 124 ± 1 | 6/6 | 5/5 |
| 10−3 | 3/3 | 69 ± 1 | 7/7 | 126 ± 1 | 6/6 | 15/15 |
| 10−4 | 4/4 | 77 ± 2 | 6/6 | 132 ± 4 | 12/12 | 5/5 |
| 10−5 | 6/6 | 86 ± 2 | 5/5 | 135 ± 3 | 12/12 | 9/9 |
| 10−6 | 6/6 | 93 ± 2 | 5/5 | 159 ± 4 | 12/24 | 0/9 (2/2a) |
| 10−7 | 6/6 | 119 ± 4 | 2/5 | 167 ± 16 | 5/24 | 0/3 |
| 10−8 | 1/6 | 121, >236 | 1/5 | 202, >239 | 0/24 | |
| 10−9 | 0/5 | >236 | 0/5 | >239 | ||
| log ID50 g−1 ± s.e.m. | 9.9 ± 0.2 | 9.3 ± 0.9 | 7.7 ± 0.5 | >6.7 ± 0.1 | ||
The assay sensitivity of the tga20 and CD1 bioassay, SCEPA and POSCA, compared by performing endpoint titrations of the same batch of RML brain homogenate in the various assays. The infectivity titers per gram of brain were determined according to ref. 14 and expressed as TCI50 units for the SCEPA, SCI50 for the POSCA, and LD50 for tga20 and CD1 mice upon intracerebral inoculation. Each data point represents the number of infected animals or cultures out by the total number of animals or cultures exposed to the inoculum. The sensitivity of POSCA lags behind that of SCEPA by 1 log, but is considerably enhanced by microglial depletion.
Microglia-depleted slices.
Infected slices appear healthy
We assessed the morphological integrity of slices by immunohistochemistry 1, 7, 21 and 35 dpi. Neurons (calbindin), astrocytes (glial fibrillary acidic protein (GFAP)) and myelin (myelin basic protein (MBP)) appeared intact. A progressive broadening of cell layers became evident at 7–35 d of culture (Supplementary Fig. 1d–f). Microglia and macrophages increased over time, confirming that slices underwent progressive microgliosis (Supplementary Fig. 1g,h). No difference was detected between tga20TK cultures treated with uninfected brain homogenates or RML-treated Prnpo/o and tga20TK cultures at any time point (Supplementary Fig. 1d–h).
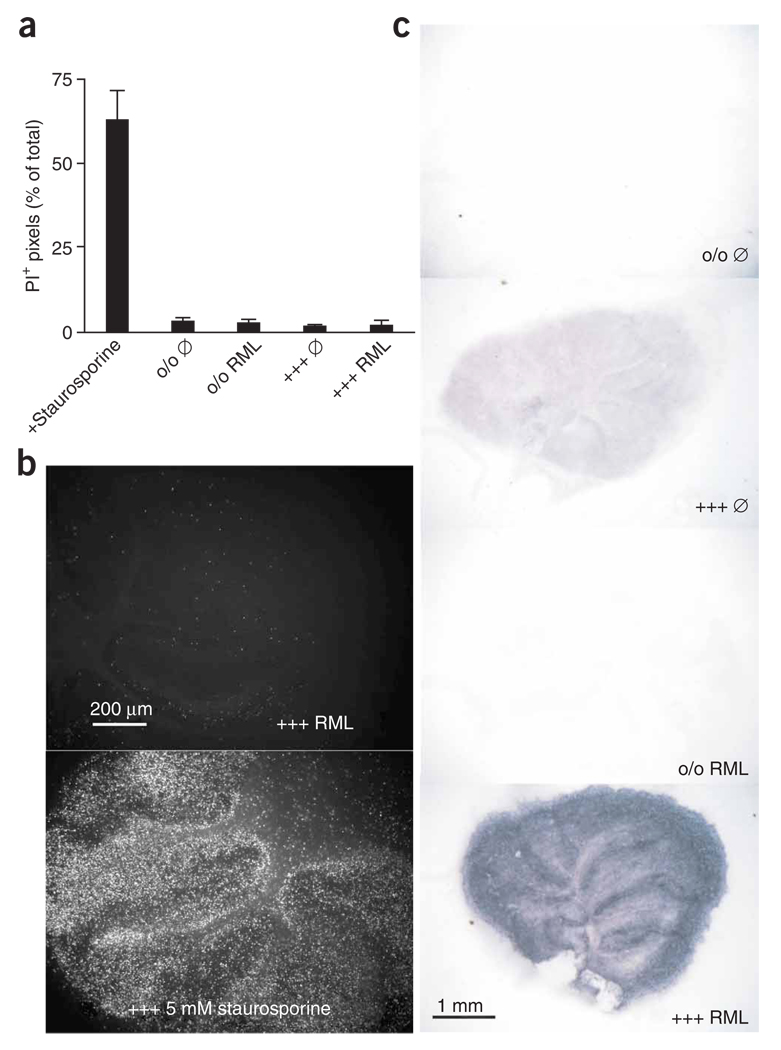
Unexpectedly, prion infection did not affect viability 5 weeks after prion infection (one-way analysis of variance (ANOVA) with Bonferroni multiple comparison test, P > 0.05, n = 12; Fig. 2a,b). We evaluated the localization of PrPSc replication and deposition using the histoblot technique15. We observed a strong PrPSc signal in the molecular and Purkinje cell layers, suggesting substantial PrPSc deposition in granule cell axons, Purkinje neurons and possibly astrocytes (Fig. 2c). An intermediate signal was observed in the granule cell layer, and a weak signal was present in the white matter.
Figure 2.
Localization and impact of prion replication. (a) PI incorporation at 35 dpi in Tga20TK and Prnpo/o cultures inoculated with 100 µg RML or with uninfected homogenate (Ø). Average of 12 slices ± s.d. (b) Representative examples of images used for PI incorporation. Owing to its high cellularity, the granule cell layer of staurosporine-treated slices is prominently stained by PI. (c) Histoblot analysis of PrPSc deposition in POSCA slices. Tga20TK and Prnpo/o cultures were inoculated with 100 µg RML or uninfected homogenate (Ø). PrPSc was detected by immunoblotting with POM1 antibody. PrPSc was found to predominantly accumulate in the molecular layer.
The POSCA is applicable to a broad range of prion strains
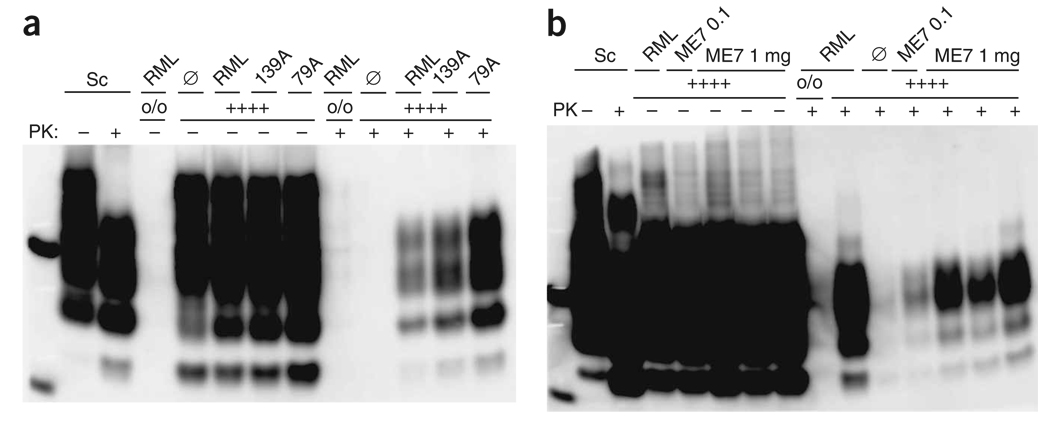
We exposed tga20+/+ slices to 100 µg of brain homogenate from mouse-adapted prion strains originally derived from scrapie-infected sheep, cows infected with bovine spongiform encephalopathy (BSE), or deer infected with chronic wasting disease (CWD) and analyzed PrPSc 5 weeks after inoculation (see Supplementary Table 1 online). Mouse-adapted scrapie strains 79A and 139A, which replicate in PK1 cells (data not shown), led to an accumulation of PrPSc comparable to or higher than that in RML-exposed slices (Fig. 3a). The mouse-adapted BSE strain 301C and, to a lesser extent, three scrapie isolates passaged once (5114/2) or twice in tga20 (5193/1) or wild-type mice (5192/2) also replicated in slices (Supplementary Fig. 5b and data not shown). PK1 cells are known to be resistant to infection with the mouse scrapie strain ME7 (ref. 4). In slices exposed to 100 µg ME7, we found only a faint PrPSc signal (Fig. 3b), but 1 mg of ME7 inoculum clearly induced PrPSc in all cultures (Fig. 3b).
Figure 3.
A broad range of strains is detectable by POSCA. (a,b) Immunoblots from cultures from 10-d-old tga20+/+ (++++) and Prnpo/o (o/o) pups, inoculated with various prion strains (100 µg) and harvested after 35 d. Ø, noninfectious brain homogenate. (a) Transmission of mouse-adapted scrapie strains RML, 139A, 79A and Ø. (b) Western blotting performed under less stringent conditions on 30 µg protein digested with 25 µg ml−1 PK (+) or 10 µg undigested protein (−) and detected with POM1. Cultures were from 10-d-old tga20+/+ and Prnpo/o pups, and were inoculated with 0.1 and 1 mg of RML, ME7 or Ø and cultured for 35 d.
Not all scrapie strains tested positive in the POSCA, and there was a correlation between short incubation times in vivo after intracerebral inoculation and the ability to replicate in the POSCA (Supplementary Fig. 5 and Supplementary Table 1). We also tested mouse-adapted CWD strains from a Colorado mule-deer herd16. Mouse-adapted CWD inoculum did not seem to replicate in tga20 slices, irrespective of in vivo incubation times (data not shown and Supplementary Table 1).
Conditional microglial depletion in organotypic slices
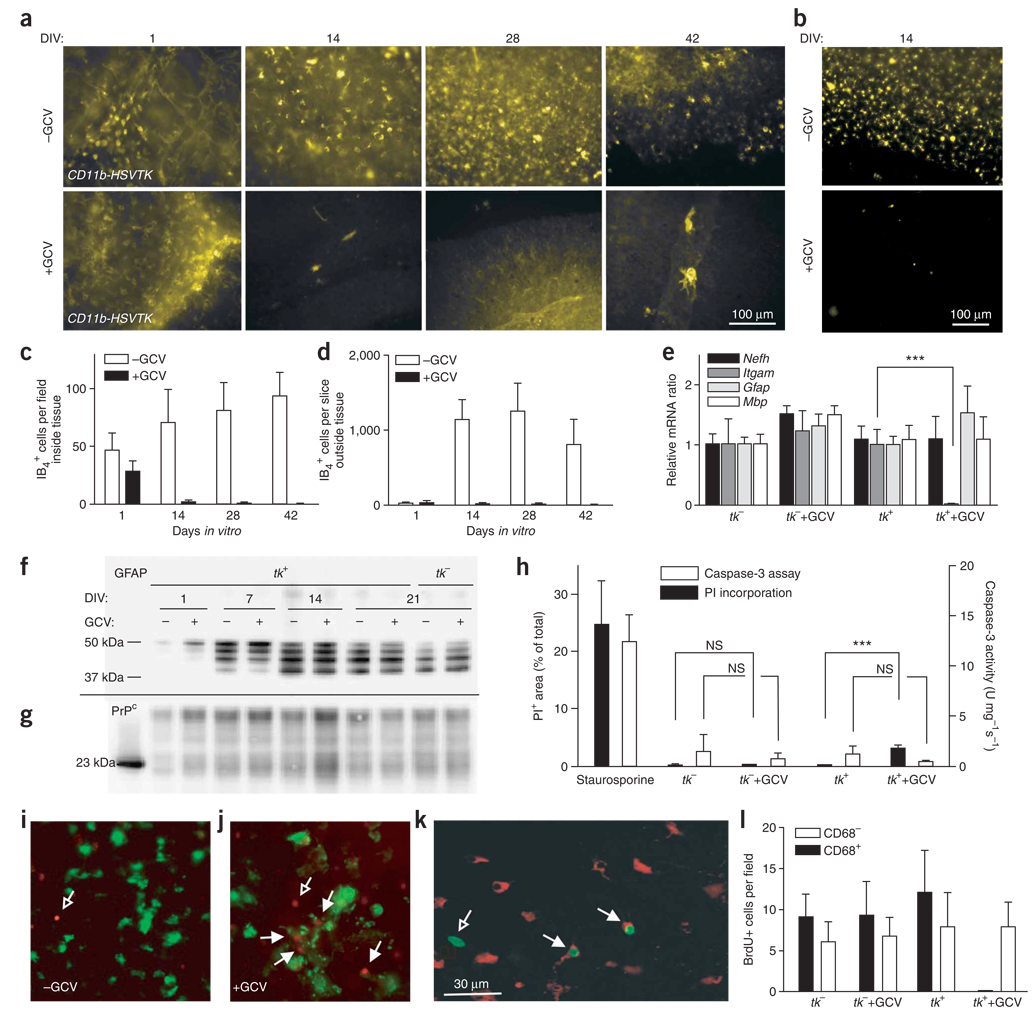
CD11b-HSVTK mice allow conditional ablation of microglia in vivo and in vitro with the cytotoxic prodrug ganciclovir (GCV)10. We cultured organotypic cerebellar slices from 10-d-old CD11b-HSVTK mice with GCV (5 µg ml−1). After 14 d, GCV led to an almost complete loss of cells positive for IB4 and CD68 (two macrophage-specific markers) in CD11b-HSVTK+ (tk+) slices (Fig. 4a,b). The few remaining IB4+ cells appeared fragmented or associated with IB4+ blood vessels (Fig. 4a). Time-lapse microscopy showed that microglial cells underwent apoptotic cell death (Supplementary Video 1 online). Neighboring microglia took up the dying cells but eventually also underwent apoptosis and were removed by other microglia.
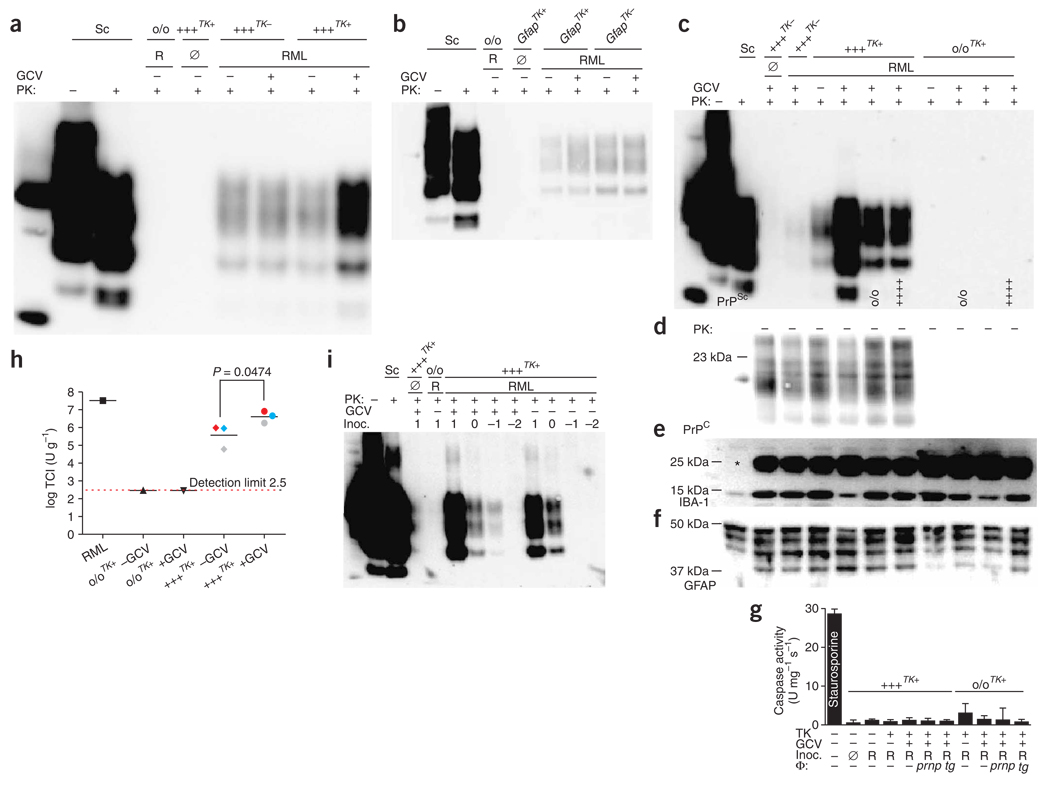
Figure 4.
Microglial depletion in organotypic slice cultures. (a,b) Slices from 10-d-old CD11b-HSVTK+ mice cultured for 1, 14, 28 or 42 d (DIV) in the optional presence of GCV and stained with IB4 (a) or anti-CD68 (b). (c,d) Quantification of cells resident within (c) or outside (d) of slices. (e) Real-time RT-PCR of CD11b-HSVTK+ (tk+) and CD11b-HSVTK− (tk−) slices cultured for 14 d in presence or absence of GCV (n = 3). mRNAs encoding neurofilament (Nefh), CD11b (Itgam), GFAP (Gfap) and MBP (Mbp) are shown. (f,g) Western blotting from cultures prepared from 10-d-old tk+ and tk− mice cultured with or without GCV, performed on 10 µg protein and detected with mouse IgG1 anti-GFAP (clone GA5) (f) or mouse IgG1 anti-PrPC (POM1) (g). (h) PI incorporation in cultures prepared as in e and treated with GCV for 5 weeks (n = 12). Positive control: tk− tissue treated with 5 µM staurosporine for 24 h. Caspase-3 enzymatic activity was measured and normalized to protein contents (n = 3 pools of four slices). (i,j) Tk+ slices untreated (i) or treated (j) with GCV for 10 d and then incubated while still alive with IB4 (green) and PI (red). Open arrows, IB4−PI+ cells; closed arrows, IB4+PI+ cells. Scale bar in k applies to i,j as well. (k,l) BrdU incorporation in samples prepared as in e after 13 dpi. (k) Example of stained cells. Green, IgG1 anti-BrdU; red, IgG2a anti-CD68. Closed arrows, CD68+BrdU+ cells; open arrows, BrdU+CD68− cells. (l) Counts of CD68+BrdU+ and CD68−BrdU+ cells (n = 6, each the average of five individual ×40 images). Averages of n replicas ± s.d.
We quantified microglial depletion from images acquired by confocal laser scanning microscopy at a depth of 35–40 µm (that is, underneath the glial scar). Tk+ slices showed reductions in microglia of 97%, 98% and 99% after 14, 28, and 42 d of GCV treatment, respectively (ANOVA, P < 0.001, n = 4; Fig. 4c). Few if any microglia were found outside the tissue in treated tk+ slices (Fig. 4d). Quantification of mRNA by quantitative RT-PCR showed a 98% reduction in CD11b transcript expression in tk+ slices treated with GCV for 14 d (P < 0.001, n = 3), whereas GFAP, MBP and neurofilament heavy-chain transcripts (markers for astrocytes, oligodendrocytes and neurons, respectively) were unaffected (ANOVA, P > 0.05) (Fig. 4e). Note that GCV treatment had no effect on tk− slices (Fig. 4e). GFAP protein expression increased over time, and isoforms of lower molecular masses could be observed after 7–21 d in culture (Fig. 4f). Depletion of microglia did not affect GFAP expression, suggesting that the astrogliosis induced by preparing and culturing the slices was independent of microglia activation. The concentration of PrPC was unaltered in microglia-depleted slices (Fig. 4g).
Lack of ‘bystander killing’ after microglia depletion
Phosphorylated GCV released from dying microglia may cause bystander toxicity. Thus we assessed the viability of CD11b-HSVTK+ slices treated with GCV for 14 d with respect to PI incorporation and DEVDase activity. Microglia-depleted slice cultures showed a small but significant increase in PI+ cells from approximately 1% up to 2% of the total surface area (ANOVA, P < 0.001, n = 12) (Fig. 4h). We observed no difference in caspase activity (n = 3 pools of four slices) (Fig. 4h).
To evaluate whether the increase in cell death was due to impaired corpse removal, we reconstituted microglia-depleted slices with peritoneal wild-type macrophages resistant to GCV. Macrophages were placed either directly onto the slices or separated by Millicell-CM membranes. Direct reconstitution of slices with wild-type macrophages placed onto slices partially reduced the GCV-induced increase in PI+ cells, suggesting a lack of removal of dead cells within microglia-depleted tissue (Supplementary Fig. 6 online). We speculated that the increased PI+ cells might represent dying or dead tk+ microglia. To test this, we performed dual labeling of unfixed, GCV-treated CD11b-HSVTK slices with PI and IB4. In GCV-treated slices, most PI+ cells also stained with IB4, indicating that the increase in PI+ cells was indeed due to dead microglia (Fig. 4i,j).
Phosphorylated GCV released from dying cells can also cause cell cycle arrest17. We therefore assayed bromodeoxyuridine (BrdU) incorporation into the DNA of dividing cells. GCV treatment of tk+ slices for 14 d abolished BrdU+CD68+ cells, as expected, whereas it did not affect the number of BrdU+CD68− cells (non-microglial BrdU+ cells characterized by a large nucleus) (Fig. 4k,l). Also, GCV treatment of tk− slices had no significant effect on BrdU incorporation. Therefore, depletion of CD68+ cells was selective and transgene-dependent, and did not elicit any significant bystander effects.
Microglial depletion enhances prion replication
To address the contribution of microglia to prion replication, we prepared slices from tga20TK CD11b-HSVTK− (tga20TK−) and tga20TK CD11b-HSVTK+ pups (tga20TK+). Tga20TK+ and tga20TK− slices infected with RML (100 µg RML, or 2.7 log SCI50) were split into two pools, one of which was treated with GCV. After 30 d, similar amounts of PrPSc were observed in untreated tga20TK+ and in both untreated and GCV-treated tga20TK− slices. Notably, microglial depletion by GCV treatment of tga20TK+ slices resulted in a fivefold increase in PK-resistant PrPSc (Fig. 5a). As this increase was independent of PrPC expression (Supplementary Fig. 7a online), microglia either directly affected the conversion of PrPC into PrPSc or, more likely, affected the half-life of PrPSc by phagocytosing and degrading prions. To test whether the observed increase in PrPSc was associated only with microglial expression of HSVTK, we infected slices prepared from 10-d-old GFAP-HSVTK mice allowing for conditional ablation of astrocytes18. In contrast to GCV treatment of CD11b-HSVTK+ cultures, GCV treatment of GFAP-HSVTK+ cultures had no major impact on PrPSc or PrPC (Fig. 5b and Supplementary Fig. 7b). Hence, increased PrPSc levels were selectively associated with microglial depletion.
Figure 5.
Impact of microglial depletion on prion replication. Cultures were prepared from 10-d-old mice either CD11b-HSVTK+ (tga20TK+ or Prnpo/o/TK+) or CD11b-HSVTK− (tga20TK−). (a) Western blot from infected tga20TK− and tga20TK+ slices treated with GCV. Samples were PK-digested and PrP detected with POM1. (b) Western blot from cultures from 10-d-old GFAP-HSVTK− (GfapTK−), GFAP-HSVTK+ (GfapTK+) or Prnpo/o mice treated as in a. (c–h) Macrophage reconstitution of microglia-depleted tissue. Antigen detected is indicated in lower left corner of immunoblots. (c) Experiment performed as in a, showing marked increase in PrPSc accumulation upon GCV treatment of RML-infected tga20TK+ slices, which was counteracted by Prnpo/o or tga20+/+ macrophages (genotype at bottom of lanes). (d–f) Undigested sample detected with POM1 (d), rabbit anti-Iba1 (e; *unidentified cross-reacting protein) or mouse anti-GFAP (f). (g) Caspase-3 enzymatic activity, normalized to protein content. Averages of four biological replicas ± s.d. (NS, P > 0.05). (h) SCEPA of homogenates of tga20TK+ or Prnpo/o/TK+ slices treated as in a. Three independent biological replicas of tga20TK+ and single replicas of Prnpo/o/TK+ slices or RML were analyzed in tenfold dilution steps using 6–12 replica PK1-containing wells per dilution. Data are indicated as the number of infectious tissue culture units per gram of slice culture protein and are the averages of biological replicas ± s.d. Slices from the same animal (pairs of −GCV and +GCV samples) are represented by the same color. (i) Immunoblot from cultures from 10-d-old tga20TK+ or Prnpo/o/TK+ mice uninfected (Ø) or infected with RML in tenfold dilution steps from 1.7 to −1.3 log SCI50; log(µg) inoculum is indicated above each lane. Each sample was divided into two pools (−GCV and +GCV) and harvested 35 dpi.
To further study whether the observed effect was due to GCV-mediated removal of microglia, we tested whether the enhanced accumulation of PrPSc could be counteracted by reconstituting the macrophage population. After RML inoculation, we separated tga20TK+ and Prnpo/o/TK+ slices into four pools each and treated three pools per condition with GCV to deplete microglia. We added 250,000 tga20+/+ or Prnpo/o macrophages to two GCV-treated cultures of each genotype immediately after RML infection and harvested and analyzed the tissues 30 d later. After microglial depletion, we observed a clear increase in PrPSc, as expected (Fig. 5c). This increase in PrPSc could be reduced upon reconstitution of slices with macrophages, irrespective of whether the macrophages expressed PrPC (Fig. 5c). PrPC amounts were equal in all samples (Fig. 5d). We verified microglial depletion and reconstitution by analyzing the expression levels of the macrophage marker Iba1 (Fig. 5e). We observed no differences in GFAP expression (Fig. 5f) or caspase activity (Fig. 5g) in either of the prion-infected tissues.
Next, we used SCEPA to analyze tga20TK+ samples, untreated or treated with GCV for 30 d, from three independent biological experiments performed on different days. Microglia-depleted cultures showed a significant 14.5 ± 13.2 fold increase over undepleted cultures in their prion titers (one-tailed paired Student’s t-test, P = 0.0474, n = 3; Fig. 5h), which corresponded very well to the difference observed by western blot analysis of microglia-depleted samples (9 ± 11.8 fold over that in undepleted slices, n = 6). Despite a high interexperimental variance, all six independent replicas showed a clear increase in PrPSc accumulation after microglial depletion (2–33 fold). No residual inoculum was observed in Prnpo/o-tk+ culture, irrespective of microglia status (Fig. 5h). These results confirmed that microglia negatively regulated both PrPSc and prion formation.
Microglia-depleted tissue accumulated prions to a higher degree, begetting the question of whether GCV-treated tk+ slices would be more sensitive to a prion infection. We therefore performed an endpoint titration experiment with RML on tga20TK+ slices. Tga20TK+ slices were infected with various concentrations of RML and separated into two pools, one of which was treated with GCV. After 5 weeks in vitro, we observed PK-resistant material in microglia-depleted tissue at a tenfold higher RML dilution. Hence, depletion of microglia rendered slices more sensitive to prion infections by 1 log (Fig. 5i and Supplementary Fig. 7c).
DISCUSSION
Analytical sensitivity of POSCA, SCEPA and other methods
As no certainty yet exists about the physical nature of the agent, transmission of serially diluted samples to susceptible hosts remains the gold standard for measuring prions. The POSCA strives to combine the versatility of the mouse bioassay, which can identify many strains of prions and take advantage of a large transgenic mouse repository, with the speed and precision of the SCEPA4. We evaluated the analytical sensitivity of the POSCA against the SCEPA4 and the mouse endpoint bioassay19. The sensitivity of SCEPA was <10-fold higher than that of POSCA, the limits of detection being 7.7 log ID50 per gram of brain for SCEPA, 9.9 log ID50 g−1 for the tga20 bioassay and >6.7 log ID50 g−1 for POSCA. Depletion of microglia led to a tenfold increase in analytical sensitivity, thereby approaching that of the SCEPA. As organotypic mouse slice cultures can be viable in culture for up to 26 weeks20, sensitivity might be enhanced by harvesting slices at later time points or by optimizing the infection and washing steps, particularly because residual inoculum does not seem to interfere with POSCA readings.
Only few cell lines, highly selective in their permissivity to various prion strains, are efficiently and chronically infectible with prions, and the crucial determinants of infectibility are unknown. In contrast, the transgenic expression of xenogeneic Prnp minigenes from various species has substantially broadened the range of strains measurable by mouse bioassay. The POSCA can fully exploit this broad range of hosts, as organotypic brain slice cultures can be prepared from mice of any desired genotype. This yields a fast, flexible and robust experimental system to study prion infection and the molecular basis of strain adaptation21 in a highly controlled environment. Toward this goal, we have successfully infected POSCA slices with POSCA-derived inoculum (data not shown). We found that not only the chronically mouse-adapted RML prions but also a variety of sheep scrapie and bovine BSE strains replicate in slices derived from tga20+/+ pups. The collection of POSCA-compatible strains includes ME7 prions, which do not replicate in PK1 cells4.
Compared to its in vivo counterparts, the POSCA has a very short incubation time. Intracerebral inoculation of tga20 mice with RML prions induces disease only after 60–150 d (ref. 22), yet we detected prion replication after 20 d in tga20TK slices. Analogously, we observed prion replication in Prnp+/o slices after 35 d, whereas Prnp+/o mice show incubation times of 225–300 d after intracerebral inoculation2. The rapid replication in slices indicates that there may be unidentified protective processes that operate in vivo but may be disabled in slices.
Unexpectedly, we did not observe any pathological changes in POSCA slices as a consequence of prion infection. Although neuronal death and astrogliosis are among the defining morphological characteristics of prion-infected brains, we saw no significant changes in slice viability and in GFAP expression. It is unlikely that any developing pathology was masked by continuous removal of dead cells by microglia, because there was no difference between microglia-depleted and undepleted prion-infected slices. It is more likely that gliosis induced by tissue preparation may mask any further prion-induced pathology. Inasmuch as the relevant neuroectodermal cell types are preserved, we do not view these changes as limiting for the deployment of the POSCA.
Microglia in prion disease
Microglia activation is an early hallmark of prion diseases. Many studies point to potentially deleterious effects of microglia. First, microglia can release inflammatory and neurotoxic factors associated with neurodegenerative diseases23–25. Second, microglial cells are often spatially associated with prion plaques, and infectivity was recovered from microglia purified from terminally ill mice6,8,26, leading to the conjecture that microglia act as a carrier of prions within the brain. This scheme predicts that microglia might be detrimental at several levels, but cannot be easily reconciled with the observation that peripheral macrophages can reduce prion infectivity in vitro and splenic PrPSc accumulation in vivo27–29. In addition, Prnp+/+ microglia do not support prion replication when grafted into Prnpo/o brains30.
Selective manipulation of microglial cells in vivo is hampered by their similarities to macrophages. In contrast, the POSCA is well suited to studying the impact of microglial manipulations on prion replication. We found that microglia could be completely ablated by treating CD11b-HSVTK+ cerebellar slice cultures10 with GCV without affecting the viability of other cell populations. Microglial removal drastically unleashed prion replication in organotypic slices, suggesting a role for microglia in containment of prion infections. Microglia may exert this effect by phagocytosing and degrading prions in situ27–30, possibly explaining the presence of prions in microglia acutely isolated from mice sick with scrapie26, despite the inability of microglia to replicate prions30.
The above phenomena may have practical ramifications. One intracerebral ID50 unit of prions has been reported to be equivalent to 104–105 PrP molecules31. This is in contrast to many viral diseases, in which 1–10 particles are equivalent to one ID50, and has been taken to question the identity of the prion with PrPSc (ref. 32). The priolytic activity of microglia uncovered here may contribute to increasing the minimal amount of PrPSc needed to infect individual organisms. By extension, one might speculate that medical conditions and iatrogenic interventions affecting microglia may heighten the susceptibility to prion infections.
METHODS
Culture preparation and prion inoculation
All mouse experiments conformed to Swiss law. The pups used for most experiments were F1 offspring of tga20+/+ Prnpo/o males on a 129Sv/BL6 background crossed to heterozygous CD11b-HSVTK females (tg620) on a C57BL/6 background10. We refer to the Prnp+/o tga20+ HSVTK transgene-negative offspring as tga20TK− and the transgene-positive littermates as tga20TK+. Prnpo/o, Prnp+/o and Prnpo/o tga20+ mice were all on a 129Sv/BL6 background. CD11b-HSVTK (tg620) and GFAP-HSVTK mice (line 7.1) were all on a C57BL/6 background and were genotyped as previously described10,33. Rocky Mountain Laboratory strain passage 6 (RML) was amplified in CD1 mice, ME7 in 129/Sv mice, 5193/1 in tga20 mice and 22F, 79A, 87A, 87V, 139V, 301V, 5192/2 and 301C (mouse-adapted BSE) were all amplified in C57BL/6 mice by intracerebral inoculation of 30 µl 10% wt/vol brain homogenate.
We prepared organotypic cerebellar slice cultures according to a modified version of a published protocol34. We obtained cerebella from 9- to 12-d-old pups. Brain tissue was embedded in 5 ml liquid (2% wt/vol) ultralow-melting-point agarose dissolved in Gey’s balanced salt solution (GBSS) (NaCl 8 g l−1, KCl 0.37 g l−1, Na2HPO4 0.12 g l−1, CaCl2·2H2O 0.22 g l−1, KH2PO4 0.09 g l−1, MgSO4·7H2O 0.07 g l−1, MgCl2·6H2O 0.210 g l−1, NaHCO3 0.227 g l−1) supplemented with the glutamate receptor antagonist kynurenic acid (1 mM) (GBSSK). We transferred liquid agarose (kept at 37 °C using a water bath) to a small container, submerged the isolated brain tissue and cooled on ice until the agarose solidified (10 min). The agarose block was mounted onto a specimen disc, and 350-µm-thick slices were cut on a vibratome (VT1000, Leica) while submerged in a cooled reservoir (4 °C) containing GBSSK. We recovered slices from the agarose and transferred them in a small volume of liquid to a 24-well plate using the broad end of a Pasteur pipette. Cultures were treated with various concentrations of prion-infected or uninfected brain homogenates (homogenized in PBS) diluted in 1 ml GBSSK. Tissues were incubated with brain homogenates as free-floating sections for 1 h at 4 °C and washed twice by transfer (in ~0.5 ml) to a reservoir containing 6 ml fresh GBSSK. Five to ten slices were placed on a 6-well Millicell-CM Biopore PTFE membrane insert (Millipore). Residual GBSS was removed and the inserts were transferred to a cell culture plate and cultured in slice culture medium (50% vol/vol MEM, 25% vol/vol basal medium Eagle and 25% vol/vol horse serum supplemented with 0.65% glucose (wt/vol), penicillin/streptomycin and Glutamax (Invitrogen). Cultures were kept in a standard cell incubator (37 °C, 5% CO2 and 95% humidity) and all of the culture medium was exchanged three times a week.
For reconstitution experiments, we obtained peritoneal macrophages by lavage. We killed mice and removed the abdominal skin without disturbing the peritoneal cavity, then injected 5 ml ice-cold PBS into the peritoneal cavity using a 25-gauge needle. After massaging the abdomen, we recovered macrophages and determined cells densities using 0.4% trypan blue. Slices were reconstituted at the time of preparation at a density of 250,000 macrophages per insert (ten slices per 6-well insert) and GCV treatment was initiated immediately. GCV was used at a concentration of 5 µg ml−1, a concentration previously shown to be optimal for microglia depletion in vitro10.
Western blot analyses
We washed cultures twice in PBS, removed residual PBS and scraped the tissue off the membrane using 10 µl lysis buffer per slice (0.5% wt/vol sodium deoxycholate and 0.5% vol/vol Nonidet P-40 in PBS). Tissue was lysed by three freeze-thaw cycles and triturated using a 200 µl pipette until homogeneous. For vol/vol SCEPA analysis, we harvested slices by scraping tissue off the membrane in PBS, triturated using a 29-gauge insulin syringe and sonicated for 30 s. Each sample was a pool of 5–10 slices grown on the same insert, yielding approximately 200–350 µg protein in total. We determined protein concentration using the bicinchoninic acid assay (Pierce) and digested the tissue with PK (Roche) in lysis buffer for 30 min at 37 °C. PK-resistant material was detected as a standard by digesting 20 µg protein lysate in a reaction volume of 20 µl (1 mg ml−1 total protein) with 25 µg ml−1 PK. This condition allowed specific detection of PrPSc. PK digestion was stopped by adding loading buffer (NuPAGE, Invitrogen) and boiling the samples at 95 °C for 5 min. Proteins were separated on a 12% Bis-Tris polyacrylamide gel (NuPAGE, Invitrogen) and blotted onto a nitrocellulose membrane. Membranes were blocked with 5% wt/vol Topblock (Fluka) in Tris-buffered saline supplemented with Tween (150 mM NaCl, 10 mM Tris HCl, 0.05% Tween 20 (vol/vol)) and incubated with primary antibodies in 1% Topblock. Primary antibodies used were POM1 mouse IgG1 antibody to PrPC (anti-PrPC) (200 ng ml−1), ascites fluid of mouse anti–glial fibrillary acidic protein (GFAP) IgG1 (clone GA5) (1:3,000, Sigma-Aldrich) and goat anti–human Iba1 (0.5 µg ml−1, Wako). Secondary antibodies used were horseradish peroxidase (HRP)-conjugated rabbit anti–mouse IgG1 (1:10,000, Zymed) and HRP-conjugated swine anti–rabbit IgG (260 ng ml−1, DAKO). The blots were developed using SuperSignal West Pico chemiluminescent substrate (Pierce) and detected using the VersaDoc system (model 3000, Bio-Rad). The molecular weight marker was spiked with recombinant PrPC, yielding a PrP signal at 23 kDa with a cleavage product at 15 kDa. We loaded 10 µl marker in the left lane on all blots.
Cell death measurements
We measured PI incorporation by incubating the slices with propidium iodide (10 µg ml−1, Molecular Probes) for 15 min. Images were acquired using a fluorescent microscope (Axiovert 200M equipped with Axiocam HRm, Zeiss) and analyzed using image analysis software analySIS vs5.0 (Olympus Soft Imaging Solutions GmbH). All images were acquired using the same exposure times and analyzed using the same software settings within a region of interest (the total area of tissue present in the image). After recording fluorescence images, we washed the tissue in PBS and lysed it in PBS containing 0.5% vol/vol Nonidet P-40, 0.5% wt/vol sodium deoxycholate and 5 mM 1,4-dithiothreitol. We used a caspase-3 fluorometric correlate assay (Assay Designs) to determine caspase-3 DEVDase activity: protein lysates were incubated with the fluorogenic caspase-3 substrate acetyl-Asp-Glu-Val-Asp-7-amino-4-methyl-coumarin, and enzymatic cleavage by caspase-3 was quantified by kinetic measurements (one measurement every 2 min for 2 h) using a fluorometer. The specific caspase activity was calculated from a standard curve generated using recombinant caspase-3 with a known enzymatic activity and normalized to the protein content. One unit of caspase-3 activity is defined as the amount of enzyme needed to convert 1 pmol of substrate per minute at 30 °C.
Immunocytochemistry
We performed histoblot analysis according to a standard protocol using 50 µg ml−1 PK (30 min, 37 °C)15. Proteins were transferred from slice cultures to nitrocellulose membranes by pressing the tissue onto membranes soaked in lysis buffer. After protein transfer, membranes were digested with PK and PrPSc was detected with POM1. For immunocytochemistry, the tissue was fixed in 2% wt/vol PFA overnight at 4 °C. Membrane inserts were washed and incubated for 1 h in blocking buffer (0.1% vol/vol Triton X-100 and 2% vol/vol goat serum dissolved in PBS) and incubated for 2 d with primary antibody diluted in blocking buffer. Primary antibodies and concentrations used were ascites fluid of anti–chicken calbindin IgG1 antibody (1:2,000, Swant), rabbit anti–mouse GFAP polyclonal antibody (1:1,000, DAKO), rat anti–mouse CD68 IgG2a (1 µg ml−1, Serotec) and ascites fluid of rat anti–bovine MBP IgG2a (1:250, Serotec). The primary antibodies were detected using Alexa-conjugated secondary antibodies (4 µg ml−1, Molecular Probes) and counterstained with 4,6-diamidino-2-phenylindole (DAPI) (1 µg ml−1). Microglia were detected in fixed and in live tissue by staining with IB4 (Griffonia simplicifolia) (2–4 µg ml−1, Molecular Probes). We removed the culture membranes from the inserts and mounted them directly onto a microscope slide using fluorescence mounting medium (DAKO). Images were acquired in a focal plane 35–40 mm below the tissue surface using a laser-scanning confocal microscope (SP 2, Leica), by fluorescence microscopy (Axiovert 200M, Zeiss) or, for live imaging studies, by using wide-field microscope (DM IRBE, Leica) equipped with a temperature controller and a CO2 box (37 °C, 5% CO2). We assessed incorporation of 5-BrdU into the tissue after 24 h incubation with 25 µg ml−1 BrdU. DNA was denatured by treatment with 1M HCl for 1 h at 37 °C, followed by two 30-min neutralization steps with 0.1 M borate, pH 8.5, and two washes in PBS. We then stained the tissue with 15 µg ml−1 mouse IgG1 anti-BrdU (clone BU-33, Sigma) and rat anti–mouse CD68 according to normal procedures. We counted cells manually, assisted by image analysis software analySIS vs5.0.
Quantitative PCR
Organotypic slice cultures were prepared and incubated as previously stated. We washed cultures once with PBS and extracted total RNA using TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer’s protocol. Before cDNA synthesis, residual genomic DNA was removed using the DNA-free kit (Ambion); cDNA was synthesized from 1 µg total RNA with the QuantiTect reverse transcription kit (Qiagen) using random hexamers according to the manufacturer’s protocol. We tested for successful cDNA synthesis (+ reverse transcriptase) and contamination of total RNA with genomic DNA (− reverse transcriptase) by PCR with primers specific for β-actin (Actb). Quantitative real-time PCR was performed using the SYBR Green PCR Master Mix (Applied Biosystems) on an ABI PRISM 7700 Sequence Detector (PerkinElmer). Regulation was calculated relative to untreated wild-type slices after normalization to the Actb signal. The following primer pairs were used: Actb sense (NM_007393), 5′-GACGGCCAGGTCATCACTAT-3′; antisense, 5′-ACATCTGCTGGAAGGTGGAC-3′. Itgam sense (NM_008401), 5′-GACTCAGTGAGCCCCATCAT-3′; antisense, 5′-AGATCGTCTTGGCAGATGCT-3′. Gfap sense (P03995), 5′-AAGGGACCATTCCCTGTC-3′; antisense, 5′-AGGTTAGCGGAGGTGGAA-3′. Mbp (NM_010777) and Nefh (NM_010904) we detected using the commercially available QuantiTect primer assay (Qiagen).
Scrapie cell assay in endpoint format (SCEPA)
Prion-susceptible neuroblastoma cells (subclone N2aPK1) were exposed to 300-µl brain homogenates in 96-well plates for 3 d. Cells were subsequently split three times 1:3 every 2 d, and three times 1:10 every 3 d. After they reached confluence, we filtered 25,000 cells from each well onto the membrane of an ELISPOT plate, treated them with PK (0.5 µg ml−1 for 90 min. at 37 °C), denatured, and detected individual infected (PrPSc-positive) cells by immunocytochemistry using alkaline phosphatase–conjugated POM1 mouse anti-PrP and an alkaline phosphatase–conjugated substrate kit (Bio-Rad). We performed serial tenfold dilutions in cell culture medium containing healthy mouse brain homogenate. Scrapie-susceptible PK1 cells were then exposed to dilutions of experimental samples ranging from 10−3 to 10−6, or to a 10−3 dilution of healthy mouse brain homogenate or RML. Samples were quantified by total luminescence per well or, in endpoint format, by counting positive wells according to established methods4.
Statistical analysis
We used one-way ANOVA with Bonferroni post-test for selected pairs of columns for statistical analysis of experiments involving the comparison of three or more samples, and paired Student’s t-test for comparing two samples. Results are displayed as the average of replicas ± s.d. For assay comparisons in Table 1, all values are given as the determined titer ± the standard error calculated within each single experiment (that is, reflecting technical rather than biological variations).
Supplementary Material
Note: Supplementary information is available on the Nature Neuroscience website.
ACKNOWLEDGMENTS
We thank L. Rietschin and B. Gähwiler for help with slice cultures, D. Marino and A. Marcel for technical help, and C.J. Sigurdson and M. Glatzel for prion strains. A.A. is supported by grants from the Sixth European Union Framework program (TSEUR, LSHB-CT-2005-018805), the Swiss National Foundation, the National Competence Center on Neural Plasticity and Repair, the Stammbach Foundation, and the UK Department for Environment, Food and Rural Affairs. J.F. is supported by a grant from Zentrum für Neurowissenschaften Zurich, the Desiree and Niels Yde foundation, the Swiss Center of Transgenic Expertise, the Henny Sophie Clausen og møbelarkitekt Axel Clausens Foundation and the Ivan Nielsens Foundation, and F.L.H. was supported by US National Institutes of Health grant R01 NS046006 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Aguzzi A, Polymenidou M. Mammalian prion biology. One century of evolving concepts. Cell. 2004;116:313–327. doi: 10.1016/s0092-8674(03)01031-6. [DOI] [PubMed] [Google Scholar]
- 2.Büeler H, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB, et al. Measurement of the scrapie agent using an incubation time interval assay. Ann. Neurol. 1982;11:353–358. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- 4.Klohn PC, Stoltze L, Flechsig E, Enari M, Weissmann C. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc. Natl. Acad. Sci. USA. 2003;100:11666–11671. doi: 10.1073/pnas.1834432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solassol J, Crozet C, Lehmann S. Prion propagation in cultured cells. Br. Med. Bull. 2003;66:87–97. doi: 10.1093/bmb/66.1.87. [DOI] [PubMed] [Google Scholar]
- 6.Manuelidis L, Fritch W, Xi YG. Evolution of a strain of CJD that induces BSE-like plaques. Science. 1997;277:94–98. doi: 10.1126/science.277.5322.94. [DOI] [PubMed] [Google Scholar]
- 7.Williams A, Lucassen PJ, Ritchie D, Bruce M. PrP deposition, microglial activation, and neuronal apoptosis in murine scrapie. Exp. Neurol. 1997;144:433–438. doi: 10.1006/exnr.1997.6424. [DOI] [PubMed] [Google Scholar]
- 8.Andreoletti O, et al. Phenotyping of protein-prion (PrPsc)-accumulating cells in lymphoid and neural tissues of naturally scrapie-affected sheep by double-labeling immunohistochemistry. J. Histochem. Cytochem. 2002;50:1357–1370. doi: 10.1177/002215540205001009. [DOI] [PubMed] [Google Scholar]
- 9.Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 10.Heppner FL, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 11.Büeler H, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 12.Polymenidou M, et al. Coexistence of multiple PrPSc types in individuals with Creutzfeldt-Jakob disease. Lancet Neurol. 2005;4:805–814. doi: 10.1016/S1474-4422(05)70225-8. [DOI] [PubMed] [Google Scholar]
- 13.Büeler H, et al. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol. Med. 1994;1:19–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Kaerber G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Arch. Exp. Pathol. Pharmakol. 1931;162:480–483. [Google Scholar]
- 15.Taraboulos A, et al. Regional mapping of prion proteins in brain. Proc. Natl. Acad. Sci. USA. 1992;89:7620–7624. doi: 10.1073/pnas.89.16.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigurdson CJ, et al. Strain fidelity of chronic wasting disease upon murine adaptation. J. Virol. 2006;80:12303–12311. doi: 10.1128/JVI.01120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubsam LZ, Davidson BL, Shewach DS. Superior cytotoxicity with ganciclovir compared with acyclovir and 1-β-d-arabinofuranosylthymine in herpes simplex virus-thymidine kinase-expressing cells: a novel paradigm for cell killing. Cancer Res. 1998;58:3873–3882. [PubMed] [Google Scholar]
- 18.Bush TG, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 19.Fischer M, et al. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 20.Gogolla N, Galimberti I, DePaola V, Caroni P. Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat Protoc. 2006;1:1165–1171. doi: 10.1038/nprot.2006.168. [DOI] [PubMed] [Google Scholar]
- 21.Weissmann C. The state of the prion. Nat. Rev. Microbiol. 2004;2:861–871. doi: 10.1038/nrmicro1025. [DOI] [PubMed] [Google Scholar]
- 22.Brandner S, et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 23.Dandoy-Dron F, et al. Gene expression in scrapie. Cloning of a new scrapie-responsive gene and the identification of increased levels of seven other mRNA transcripts. J. Biol. Chem. 1998;273:7691–7697. doi: 10.1074/jbc.273.13.7691. [DOI] [PubMed] [Google Scholar]
- 24.Baker CA, Manuelidis L. Unique inflammatory RNA profiles of microglia in Creutzfeldt-Jakob disease. Proc. Natl. Acad. Sci. USA. 2003;100:675–679. doi: 10.1073/pnas.0237313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker CA, Lu ZY, Manuelidis L. Early induction of interferon-responsive mRNAs in Creutzfeldt-Jakob disease. J. Neurovirol. 2004;10:29–40. doi: 10.1080/13550280490261761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker CA, Martin D, Manuelidis L. Microglia from Creutzfeldt-Jakob disease-infected brains are infectious and show specific mRNA activation profiles. J. Virol. 2002;76:10905–10913. doi: 10.1128/JVI.76.21.10905-10913.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carp RI, Callahan SM. Effect of mouse peritoneal macrophages on scrapie infectivity during extended in vitro incubation. Intervirology. 1982;17:201–207. doi: 10.1159/000149289. [DOI] [PubMed] [Google Scholar]
- 28.Michel B, Tamalet J, Bongrand P, Gambarelli D, Gastaut JL. Role of phagocytes in experimental scrapie in hamsters [in French with English abstract] Rev. Neurol. (Paris) 1987;143:526–531. [PubMed] [Google Scholar]
- 29.Beringue V, et al. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J. Pathol. 2000;190:495–502. doi: 10.1002/(SICI)1096-9896(200003)190:4<495::AID-PATH535>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 30.Priller J, et al. Early and rapid engraftment of bone marrow-derived microglia in scrapie. J. Neurosci. 2006;26:11753–11762. doi: 10.1523/JNEUROSCI.2275-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinley MP, Bolton DC, Prusiner SB. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983;35:57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- 32.Manuelidis L, Sklaviadis T, Manuelidis EE. Evidence suggesting that PrP is not the infectious agent in Creutzfeldt-Jakob disease. EMBO J. 1987;6:341–347. doi: 10.1002/j.1460-2075.1987.tb04760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bush TG, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 34.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Neuroscience website.