Abstract
Poly(ADP-ribose) polymerase-1 (PARP-1) is a key enzyme mediating the cellular response to DNA strand breaks. It plays a critical role in genomic stability and survival of proliferating cells in culture undergoing DNA damage. Intestinal epithelium is the most proliferative tissue in the mammalian body and its stem cells show extreme sensitivity to low-level genotoxic stress. We investigated the role of PARP-1 in the in vivo damage response of intestinal stem cells in crypts of PARP-1–/– and control mice following whole-body γ-irradiation (1 Gy). In the PARP-1–/– mice there was a significant delay during the first 6 h in the transient p53 accumulation in stem cells whereas an increased number of cells were positive for p21CIP1/WAF1. Either no or only marginal differences were noted in MDM2 expression, apoptosis, induction of or recovery from mitotic blockage, or inhibition of DNA synthesis. We further observed a dose-dependent reduction in crypt survival measured at 4 days post-irradiation in control mice, and this crypt-killing effect was significantly potentiated in PARP-1–/– mice. Our results thus establish that PARP-1 acts as a survival factor for intestinal stem cells in vivo and suggest a functional link with early p53 and p21CIP1/WAF1 responses.
INTRODUCTION
Poly(ADP-ribosyl)ation is a DNA strand break-driven enzymatic post-translational modification of nuclear proteins (1,2). This drastic modification, consisting of long, branched chains of poly(ADP-ribose), is catalysed mostly by the highly conserved poly(ADP-ribose) polymerase (PARP)-1, using NAD+ as substrate. Although this enzyme is very abundantly expressed in proliferating cells, its catalytic activity depends on DNA single- or double-strand breaks. Exposure of living cells to ionising radiation, alkylating agents or oxidants immediately triggers formation of protein-conjugated poly(ADP-ribose), which is followed by rapid degradation of the polymer catalysed by poly(ADP-ribose) glycohydrolase.
Considerable evidence from in vitro, cell culture and ex vivo studies shows that poly(ADP-ribosyl)ation plays a critical role in the survival and maintenance of genomic stability of proliferating cells exposed to low or moderate levels of DNA-damaging agents. This has been linked mechanistically with PARP-1 function in base-excision repair. Interestingly, the poly(ADP-ribosyl)ation capacity of peripheral blood lymphocytes is correlated with the life span of mammalian species (3), and an association was found between poly(ADP-ribosyl)ation capacity and longevity in humans (4), consistent with the cytoprotective functions of PARP-1 at low to moderate damage levels. In stark contrast, there is a risk of necrotic cell death due to severe NAD+ and ATP depletion when PARP-1 is acutely over-activated.
The ADP-ribose polymer binds specific domains of the tumour suppressor protein p53 in vitro and is suggested to modulate p53 DNA binding (5). Recently, a poly(ADP-ribose)-binding sequence motif was identified in several important DNA damage checkpoint and repair proteins, including p53, p21 CIP1/WAF1, XPA, MSH6, DNA ligase III, XRCC1, DNA polymerase ε, DNA-PKcs, NFκB, iNOS, caspase-activated DNase and telomerase (6). p53 accumulates to a high level in the nuclei of mammalian cells exposed to ionising radiation, ultraviolet, mitomycin C or restriction enzymes, as well as in cells infected with certain viruses (7). Promoters containing p53-binding sites showed massive increase in transcription in response to DNA damage. p53 can be co-immunoprecipitated from nuclear extracts by antibodies to PARP-1, suggesting that the two proteins may exist as a complex (8). Additionally, PARP-1 has been reported to poly(ADP-ribosyl)ate p53 (8,9), which occurs within 15 min after γ-irradiation of cells (10).
p21CIP1/WAF1 is a cyclin-dependent kinase (CDK) inhibitor that inhibits G1 cyclin/CDK complexes (11,12) and mediates p53-dependent cell cycle arrest (13). While p53 is not required to induce p21CIP1/WAF1 transcription during development and in most tissues of the adult mouse, p53-dependent regulation of p21CIP1/WAF1 is critical for the response to DNA damage (14). One of us has shown that whole-body irradiation (8 Gy) results in death of PARP-1 knockout (PARP–/–) mice within a few days, due to severe disruption of intestinal epithelial lining as compared to wild-type (wt) mice (15).
Intestinal epithelium is a highly proliferative organ in which stem cell positions are defined in terms of the spatial arrangement within the crypt (16). At the crypt base are 4–6 stem cells, which are highly sensitive to low-dose ionising radiation. Doses of radiation as low as 0.01–0.05 Gy induce apoptosis in these cells. This extreme sensitivity presumably serves to decrease risk of neoplasia by eliminating stem cells that suffer significant genome damage. The number of apoptotic cells increases with radiation dose up to a point (above ∼1 Gy) where all the true stem cells in a crypt are killed. If all true stem cells within a crypt die, their immediate daughters can assume the function of stem cells. These so-called clonogenic or potential stem cells are also radiosensitive but less so than stem cells, and they possess a high repair capacity. Doses in excess of ∼9 Gy will kill all the potential stem cells in some crypts, which as a consequence disappear from the tissue within a few days.
In spite of the intensive ‘quality assurance’ mechanisms that serve to eliminate damaged stem cells, these cells have been shown recently to undergo functional alteration with age. Martin et al. (17) reported a 2-fold increase in the number of stem cells undergoing apoptosis in older (29 months) mice following 1 Gy γ-irradiation, as compared to young (5 months) and middle aged (15–18 months) mice. The altered sensitivity of stem cells in older mice was associated with delayed expression of p53 and p21 in the early hours after irradiation (18). These data suggest the interesting possibility that the functional alteration seen with age is linked with the immediate cellular response to DNA damage.
Most of the work to date on PARP-1 provides evidence for poly(ADP-ribosyl)ation playing an important role in DNA repair, cell survival and maintenance of genomic stability, but has been obtained from work in vitro or on cultured cells. To study in vivo the effect of abrogating PARP-1 with regard to the network of cellular stress responses and the cellular phenotype, we have conducted the first comprehensive in vivo study on the role of poly(ADP-ribosyl)ation in response to ionising radiation in a stem cell system (intestinal epithelium).
MATERIALS AND METHODS
Experimental animals
PARP-1 null homozygous mice (PARP-1–/–) have been described previously (15) and PARP-1 wt (C57BL/ICRFa) mice were used as controls. Mice were aged 2–6 weeks and there were at least four mice in each experimental group. The mice were fed ad libitum. All animal experiments were performed in accordance with the relevant national legislation.
Irradiation procedure
Unanaesthetized mice were exposed to whole-body irradiation with air being pumped into the chamber during exposure. A 137Cs γ-irradiator was used with a dose rate of 3.8 Gy per minute. All irradiation treatments were begun between 9:00 and 10:00. For histochemical studies, a dose of 1 Gy was used and animals were killed at 40 min, 1 h, 2 h, 4 h 40 min or 6 h post-irradiation. Forty minutes prior to sacrifice, each mouse was intraperitoneally injected with 925 kBq of tritiated thymidine in 0.1 ml of physiological saline ([3H]TdR, 248 GBq/mmol). For the crypt survival study, doses were 1, 5, 8, 10, 12 or 14 Gy and animals were killed at 4 days after irradiation.
Tissue preparation and histological analysis
Following sacrifice, the entire small intestine was removed and fixed in 10% formal saline overnight. A length of 10 cm from the ileocecal valve was removed for analysis. This was then cut into five segments of ∼1 cm length. Each set of segments was bundled together using 3 M micropore tape prior to paraffin embedding. After processing, the segments were sectioned transversely at 3–5 µm. These sections were stained with haematoxylin and eosin.
Immunohistochemistry and autoradiography
Fixed sections were immunostained with specific antibodies for p53 (sheep polyclonal IgG, Ab-7, Calbiochem), p21 (rabbit polyclonal anti p21CIP1/WAF1 IgG, PC55, Calbiochem) or MDM2 (rabbit polyclonal IgG, SC-1022, SantaCruz). Immunohistochemistry was performed using biotin- conjugated rabbit anti-sheep or goat anti-rabbit secondary antibodies (Pierce), horse-radish peroxidase-linked avidin–biotin complex reagents (Vector Laboratories) and 3,3′-diaminobenzidine as immunodetection substrate, as previously described (19), and then counterstained with thionine blue. For autoradiography, sections were rehydrated and coated in K5 nuclear emulsion (Ilford). After the emulsion had dried, sections were boxed and exposed for 3 days at 4°C. Slides were developed using Kodak D-19 developer and fixed with Hypam fixative (Ilford). Sections were counterstained with thionine blue and the labelling index (i.e. proportion of cells in S phase as visualised by [3H]TdR uptake) was determined.
Scoring procedure
Apoptotic cells, mitotic cells, [3H]TdR-incorporating cells and cells stained with p21CIP1/WAF1, p53 and MDM2 antibody, respectively, were scored on a cell-positional basis within the crypts of the small intestine according to the method of Ijiri and Potten (20). For every staining procedure, 50 half-crypts were counted from each individual mouse in every group. Apoptosis was assessed on the evidence of morphological characteristics, such as cell shrinkage, chromatin condensation and cellular fragmentation (21). Mitotic cells were identified by virtue of chromatin condensation in the absence of cytoplasmic and nuclear shrinkage. In many mitotic cells, discrete chromosomal structure can be observed, and in addition, mitotic cells appear horizontally displaced from the other epithelial-lining cells, toward the lumen of the intestine. Data were analysed by modified median test according to Potten (22).
Crypt survival study
The parameters measured in the intestine were the number of surviving crypts and the width of the crypts. A surviving crypt was defined as containing 10 or more adjacent, healthy-looking, chromophilic non-Paneth cells, some Paneth cells and a lumen. The circumference of a transverse section of the intestine was used as a unit of length and the number of crypts was scored in each circumference. Usually, five circumferences per mouse and four mice per experimental group were assessed. Crypt width was measured at the middle of each longitudinal crypt section, using a Zeiss AxioHOME computer driven microscope system. Fifteen crypts were measured in this way for each mouse.
Data analysis for crypt survival
For the generation of the crypt survival curve, all data were corrected for crypt size changes following irradiation using previously established procedures (23,24). Measured numbers of surviving crypts were multiplied by a factor t0/t, where t0 is the mean crypt width measured in longitudinal crypt sections in the control group and t is the mean crypt width in the treated group, thus taking account of the fact that smaller surviving crypts would be less likely to be detected in a given tissue section.
Standard multi-target functions (25) were fitted to each data set giving estimates of the conventional parameters D0 (reciprocal of the slope of the exponential portion of the line, a measure of radiosensitivity) and N (the extrapolation number, obtained by extrapolating the straight line portion of the curve to the survival axis at zero dose). The multi-target function has the form:
SF = A{1 – [1 – exp(–D/D0)]N}
where SF denotes the fraction of crypts surviving. Estimates of D0 and N were obtained for each set of data using the DRFIT program (25), where A, the natural survival, should be 1, but as the control crypt number is not well estimated we fitted the parameter. Variance–ratio F-tests were used to compare the model with common D0 and N with that where they differ between the two genotypes, in order to test for any difference between the genotypes other than in crypt number.
Data on crypts per circumference and crypt width were averaged to give a single figure for each measure in the small intestine within each animal. Means and standard errors of the means for groups of animals were calculated on the basis of these figures. For comparison in each dose, significance was tested by Student’s t-test.
RESULTS
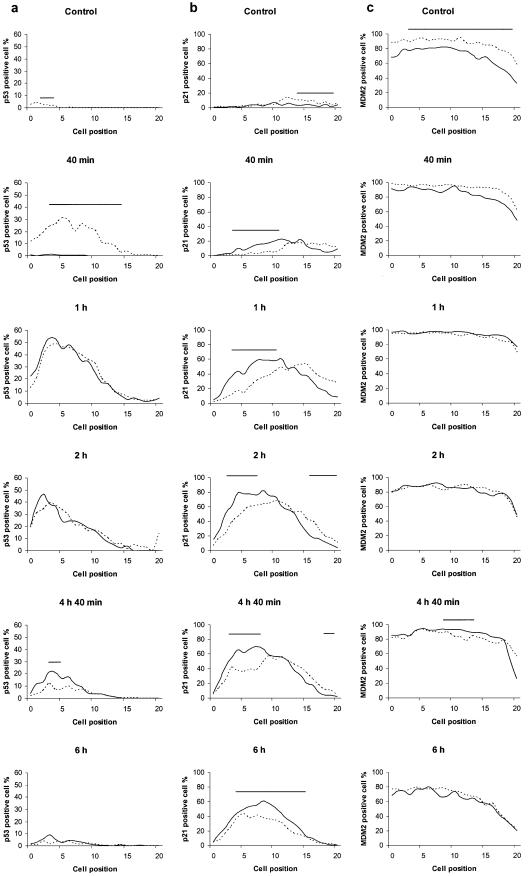
We assessed the response of p53, p21CIP1/WAF1, MDM2, apoptosis, mitosis and DNA replication of intestinal epithelial cells of PARP-1–/– and wt mice to low-dose γ-irradiation (Fig. 1a–f). In control animals (without irradiation) a few percent of intestinal epithelial cells expressed p53 spontaneously (Fig. 1a). In response to irradiation, a rapid increase in p53 positive cells was observed in wt mice, as expected. In contrast, almost no positive cells were detected in PARP-1–/– mice at 40 min after irradiation. However, the number of p53 positive cells in PARP-1–/– greatly increased by 1 h post-irradiation thus completely obliterating any difference between the two types of mice at 1 and 2 h post-irradiation, respectively. At 4 h 40 min, the number of p53 positive cells was significantly larger in PARP-1–/– than wt, although the area under the curve was decreased for either mouse type. Almost all p53 positivity had disappeared by 6 h post-irradiation. Taken together, abrogation of PARP-1 led to a delay in, but not loss of, the activation of p53 in intestinal clonogenic cells.
Figure 1.
Profiles of p53, p21 and MDM2 expression and of the fractions of apoptotic, mitotic and [3H]TdR-incorporating cells in small intestinal crypts of PARP-1–/– (continuous curves) and wt (dashed curves) mice following 1 Gy γ-irradiation. Cell positions are counted from the base of the crypt. The stem cells are concentrated at cell position 4–5. Each measure was determined as the average value for 50 half crypts taken from groups of at least four animals. Irradiated groups of mice were sacrificed at 40 min, 1 h, 2 h, 4 h 40 min and 6 h post-irradiation. (a) p53 positive cells; (b) p21 positive cells; (c) MDM2 positive cells; (d) apoptotic cells; (e) mitotic cells; (f) cells positive for [3H]TdR-incorporation. The range of cell positions where a statistically significant difference (P < 0.05) was found between PARP-1–/– and wt mice is indicated by a horizontal bar.
We also scored p21CIP1/WAF1 distribution in intestinal epithelial cells. This protein was also upregulated in both types of mice by irradiation (Fig. 1b). However, the time-course study clearly showed that the number of p21CIP1/WAF1 expressing cells was significantly higher in PARP-1–/– than wt in the stem cell positions through the post-irradiation time period studied. Interestingly this effect was most pronounced in the region centred on cell position 5, i.e. the stem cell positions.
Control of p53 stability is governed by MDM2, the expression of which is induced by p53 in response to irradiation. Over 80% of intestinal crypt cells expressed MDM2 even in unirradiated wt mice (Fig. 1c), while the fraction of positive cells was significantly lower in the PARP-1–/– mice. However, after irradiation the number of positive cells increased in both types of mice and the difference could no longer be observed.
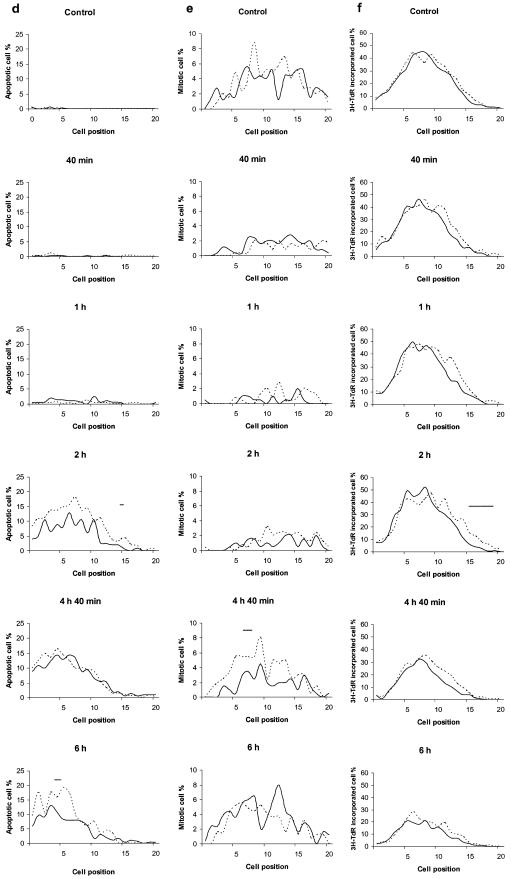
We also evaluated the frequency of apoptosis and mitosis, respectively, in the epithelial cells by haematoxylin and eosin staining (Fig. 1d and e). Apoptotic cells became detectable within a few hours after irradiation (Fig. 1d). In the stem cell area, the frequency of apoptotic cells tended to be lower in PARP–/– than wt at 6 h but the difference was not statistically significant. Mitosis was blocked immediately after irradiation (Fig. 1e) and completely recovered by 4 h 40 min in the control animals. By this time, but not by 6 h post-irradiation, PARP-1–/– displayed fewer mitotic cells. In contrast to the rapid effect on mitosis, it took several hours post-irradiation to reduce the cellular incorporation of [3H]TdR as a marker for DNA synthesis (Fig. 1f), and the data recorded in the two mouse types were very similar. Thus PARP-1 does not seem to have any measurable effect on inhibition of DNA synthesis under these experimental conditions.
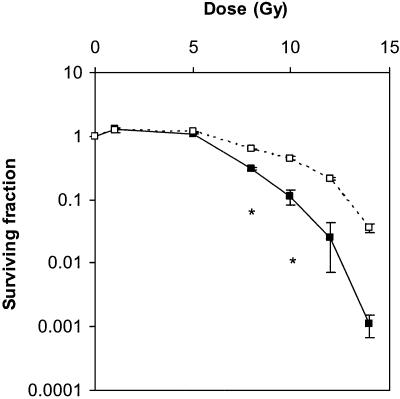
Finally we performed crypt survival studies to compare the sensitivity to radiation in both types of mice. We analysed the number of regenerating crypts of the small intestine at 4 days after various doses of irradiation (Fig. 2). The fitted D0 and N values are summarised in Table 1. There was a large difference between PARP-1–/– and wt in the number of surviving crypts in the intestine at doses of 8 Gy or higher, with the intestine of PARP-1–/– mice being significantly more sensitive than wt.
Figure 2.
Crypt survival curves for PARP-1–/– and wt mice measured 4 days post-irradiation. A marked reduction in crypt survival was observed in the PARP-1–/– mice (filled symbols) as the dose was raised. Means and the standard errors of the means are indicated. Statistically significant differences are indicated by asterisks (P < 0.05).
Table 1. Survival parameters for crypts on day 4 post-irradiation.
| PARP-1–/– | Wt | |
|---|---|---|
| A | 1.16 ± 0.01 | 0.81 ± 0.07 |
| D0 (Gy) | 1.14 ± 0.23 | 1.26 ± 0.35 |
| N | 149 ± 134 | 1182 ± 2579 |
| P | <0.001 | – |
A, natural survival; D0, reciprocal of the slope of the exponential portion of the line; N, extrapolation number. Data shown are mean ± standard error. P-value shows the overall difference in survival parameters for PARP-1–/– compared to wt mice.
DISCUSSION
In this study we found a delay in, but not loss of, transient p53 induction in intestinal crypt stem cells of PARP-1–/– mice compared to wt mice upon low-dose γ-irradiation (1 Gy). Therefore, PARP-1 is dispensable for p53 induction in vivo but supports a rapid p53 activation response. Within the first 6 h after treatment PARP-1–/– stem cells tended to display reduced incidence of apoptosis and delayed resumption of mitosis, but these effects were either not or only marginally significant. Surprisingly, despite the delayed p53 response, the percentage of p21CIP1/WAF1-positive stem cells was consistently increased in the PARP-1–/– mice after irradiation at all time points studied, whereas the percentage of MDM2-positive cells and the time-dependent inhibition of DNA synthesis were not significantly affected.
We previously reported a time-course study of radiation-induced p53 and p21CIP1/WAF1 expression in murine intestinal epithelium using specific antibodies (26). After 8 Gy of γ-irradiation, there was a time-dependent increase in the expression of both proteins and p53 accumulation persisted for at least 48 h. The response of p21CIP1/WAF1 was dependent on p53. In more recent work, some of us showed that after as little as 1 Gy of irradiation, p53 induction already occurred by 2 h post-irradiation (18), and in the present study, a significant p53 response could be seen in stem cells even by 40 min. Therefore, in murine intestinal epithelium, p53 activation is a very rapid response to even low doses of ionising irradiation, occurring within minutes rather than hours.
Several links between PARP-1 and p53 have been proposed. p53 has been shown to be a poly(ADP-ribose) acceptor protein (9,10,27,28). In addition, Pleschke et al. (6) reported a poly(ADP-ribose)-binding sequence motif of 20 amino acids present in several proteins, such as p53, p21CIP1/WAF1, XPA, MSH6, DNA ligase III, XRCC1, DNA polymerase-ε, DNA-PKcs, Ku70, NF-κB, iNOS, caspase-activated DNase and telomerase. This motif overlaps with several important functional domains of these proteins. Therefore PARP-1 might control not only p53 but also other specific signalling network proteins. That p53 is regulated somehow by PARP-1 activity is also made plausible by the fact that PARP-1 activation is triggered immediately after exposure to DNA strand breaks in vitro (29). This is reflected in living cells by poly(ADP-ribose) accumulation occurring within a few minutes after DNA-damaging treatment (30,31). The function of p53 in inhibiting potentially hazardous homologous recombination events in mammalian cells (32) is also fully compatible with a similar role assigned to PARP-1 (33).
Functionally, the effect of PARP-1 abrogation on p53 response to DNA damage has been studied by a number of groups in a variety of ways, yielding conflicting results. Simbulan-Rosenthal et al. (34) reported that p53 was not detectable in PARP-1–/– fibroblasts. Wesierska-Gadek et al. (35) reported reduced basal levels of p53 protein in PARP-1-deficient cells, which was not due to differences in the p53 gene transcription rate between wt and PARP-1-deficient cells. Moreover, PARP-1–/– cells failed to transactivate p53-responsive genes. In contrast, Agarwal et al. (36) reported normal transcriptional transactivation by p53 in PARP-1–/– fibroblasts treated with DNA-damaging agents. Furthermore, one of us showed that in splenocytes of the same PARP-1–/– model, there was rapid accumulation of p53 after N-methyl-N-nitrosourea (MNU) treatment, as observed by western blotting (15). However, addressing specifically the effects of γ-radiation, Valenzuela et al. (37) found that primary mouse embryonic fibroblasts derived from PARP-1–/– mice failed to accumulate p53 in response to 6 Gy irradiation, suggesting that the type of DNA damage influences the role of PARP-1 in the p53-mediated response to genotoxic stress. Finally, Beneke et al. (38) generated a new transgenic mouse system lacking PARP-1 activity in thymocytes via targeted overexpression of a dominant negative PARP-1 and reported a drastic increase of p53 expression and activity in thymocytes after DNA damage.
The present paper is the first to focus on PARP-1 in a stem cell system and our results reveal the absence of p53 accumulation in intestinal stem cells of PARP-1–/– mice in vivo by 40 min after irradiation (Fig. 1a). In contrast, at 4 h 40 min post-irradiation, numbers of p53-immunoreactive epithelial cells were even higher in PARP-1–/– than wt mice, revealing a striking difference in the kinetics of p53 activation and highlighting the need for careful time-course studies when investigating the interaction of PARP-1 and p53 activation.
p21CIP1/WAF1 inhibits cell cycle progression by binding to and inhibiting the cyclin-dependent kinases and proliferating cell nuclear antigen (12,39). The expression of p21CIP1/WAF1 protein mediates p53-dependent cell cycle arrest (40–43). Wilson et al. (26) found that the position of the p21CIP1/WAF1 immunoreactive cells in intestinal crypts did not entirely overlap with that of p53, with p21CIP1/WAF1-positive cells being found at relatively higher cell positions. A similar difference in location between p21CIP1/WAF1 and p53-positive intestinal epithelial cells was reported after 5-fluorouracil treatment (44). Interestingly, in the present work we found that upon irradiation the p21CIP1/WAF1-positive cells in PARP-1–/– tissue were located nearer the bottom of the crypt than in wt cells (Fig. 1b). The underlying mechanism remains to be elucidated.
In the present study, survival of intestinal crypts in PARP-1–/– mice was drastically reduced even at irradiation doses that are sublethal for wt mice (Fig. 2). By 4 days after exposure to 8 Gy irradiation, the number of surviving crypts of PARP-1–/– mice was approximately half of those in unirradiated controls. This is consistent with the previous observation by one of us that PARP-1–/– (exon 4 disruption) mice are highly sensitive to 8 Gy of irradiation (15). Wang et al. (45) (exon 2 disruption) and Masutani et al. (46) (exon 1 disruption) independently confirmed this hypersensitivity of PARP-1–/– mice to radiation. Lethality was due to massive disruption of the intestinal epithelium which became apparent a few days after irradiation. A reduction of crypt survival as drastic as shown here has not been observed in the small intestine of either p53-deficient (47) or bcl-2-deficient mice (48,49) after sublethal irradiation doses. These observations underscore the notion that PARP-1 plays a crucial role in stem cell survival after DNA damage and that its contribution may well exceed those of p53 or Bcl-2.
Exposure of p53 null mice to 8 Gy of γ-radiation resulted in massive reduction of apoptosis in intestinal epithelial cells with some residual fraction of cells undergoing p53- independent apoptosis (50). In contrast, irradiation with 1 Gy failed to induce a p53-independent apoptosis in vivo (51). Therefore, apoptosis of intestinal epithelial cells in this study (1 Gy) is very likely to be p53-dependent. Whether or not the massive reduction of intestinal crypts shown in this study at doses of 8 Gy or higher (Fig. 2) is due to apoptosis or necrosis is as yet unclear. However, it should be noted that in the immediate response to 1 Gy irradiation, apoptosis appeared to be slightly reduced in the PARP-1–/– tissue, making it unlikely that this is directly related to the phenotype of reduced crypt survival. It is tempting to speculate that increased p21 upregulation in the stem cell compartment of the knockout tissue (Fig. 1b) is causally linked with reduced crypt survival via terminal growth arrest of the cells.
The ATM protein, the gene of which is mutated in Ataxia telangiectasia patients, is a well known key player in DNA-damage signalling upstream of the p53 response and has been shown to act synergistically with PARP-1 in pathways that either monitor or repair DNA damage during mouse development (52). It remains to be studied whether or not some difference in the regulation of ATM might underlie the differences in the p53 response of PARP-1–/– intestinal stem cells versus other cells such as cultured fibroblasts.
In conclusion, we observed that PARP-1 abrogation leads to a significant delay in the transient p53 accumulation in intestinal stem cells occurring during the first 6 h after 1 Gy of irradiation, but to an increased fraction of cells staining positive for p21CIP1/WAF1. During the same period there were only marginal differences, if any, in apoptosis, in the induction of or rapid recovery from mitotic blockage, or in the inhibition of DNA synthesis. We further observed a dose-dependent reduction in crypt survival measured at 4 days post-irradiation in control mice, and this crypt-killing effect was significantly potentiated in PARP-1–/– mice, demonstrating that PARP-1 acts as a survival factor for stem cells in vivo at low DNA damage levels. Since it is becoming increasingly clear that the ageing of several tissues, especially those with high cell turnover, is linked with disturbances at the level of stem cells, further studies on the role of PARP-1 activity in ageing of intestinal and other tissues are warranted.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank Steve Roberts (Paterson Institute for Cancer Research) for statistical analysis of crypt survival study. This work was supported by Research into Ageing, the Digestive Disorders Foundation and the Cancer Research Campaign.
REFERENCES
- 1.Bürkle A. (2001) Physiology and pathophysiology of poly(ADP-ribosyl)ation. Bioessays, 23, 795–806. [DOI] [PubMed] [Google Scholar]
- 2.Shall S. and de Murcia,G. (2000) Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat. Res., 460, 1–15. [DOI] [PubMed] [Google Scholar]
- 3.Grube K. and Bürkle,A. (1992) Poly (ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proc. Natl Acad. Sci. USA, 89, 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muiras M.-L., Müller,M., Schächter,F. and Bürkle,A. (1998) Increased poly(ADP-ribose) polymerase activity in lymphoblastoid cell lines from centenarians. J. Mol. Med., 76, 346–354. [DOI] [PubMed] [Google Scholar]
- 5.Malanga M., Pleschke,J.M., Kleczkowska,H.E. and Althaus,F.R. (1998) Poly(ADP-ribose) binds to specific domains of p53 and alters its DNA binding functions. J. Biol. Chem., 273, 11839–11843. [DOI] [PubMed] [Google Scholar]
- 6.Pleschke J.M., Kleczkowska,H.E., Strohm,M. and Althaus,F.R. (2000) Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem., 275, 40974–40980. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y., Hall,T., Gay,L.S. and Donelson,J.E. (1993) Point mutations are associated with a gene duplication leading to the bloodstream reexpression of a trypanosome metacyclic VSG. Cell, 72, 397–406. [DOI] [PubMed] [Google Scholar]
- 8.Kumari S.R., Mendoza-Alvarez,H. and Alvarez-Gonzalez,R. (1998) Functional interactions of p53 with poly(ADP-ribose) polymerase (PARP) during apoptosis following DNA damage: covalent poly(ADP-ribosyl)ation of p53 by exogenous PARP and noncovalent binding of p53 to the M(r) 85,000 proteolytic fragment. Cancer Res., 58, 5075–5078. [PubMed] [Google Scholar]
- 9.Wesierska-Gadek J., Bugajska-Schretter,A. and Cerni,C. (1996) ADP-ribosylation of p53 tumor suppressor protein: mutant but not wild-type p53 is modified. J. Cell. Biochem., 62, 90–101. [DOI] [PubMed] [Google Scholar]
- 10.Smith H.M. and Grosovsky,A.J. (1999) PolyADP-ribose-mediated regulation of p53 complexed with topoisomerase I following ionizing radiation. Carcinogenesis, 20, 1439–1443. [DOI] [PubMed] [Google Scholar]
- 11.Harper J.W., Adami,G.R., Wei,N., Keyomarsi,K. and Elledge,S.J. (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell, 75, 805–816. [DOI] [PubMed] [Google Scholar]
- 12.Xiong Y., Hannon,G.J., Zhang,H., Casso,D., Kobayashi,R. and Beach,D. (1993) p21 is a universal inhibitor of cyclin kinases. Nature, 366, 701–704. [DOI] [PubMed] [Google Scholar]
- 13.Gartel A.L. and Tyner,A.L. (1999) Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp. Cell Res., 246, 280–289. [DOI] [PubMed] [Google Scholar]
- 14.Macleod K.F., Sherry,N., Hannon,G., Beach,D., Tokino,T., Kinzler,K., Vogelstein,B. and Jacks,T. (1995) p53-dependent and independent expression of p21 during cell growth, differentiation and DNA damage. Genes Dev., 9, 935–944. [DOI] [PubMed] [Google Scholar]
- 15.Ménissier-de Murcia J.M., Niedergang,C., Trucco,C., Ricoul,M., Dutrillaux,B., Mark,M., Oliver,F.J., Masson,M., Dierich,A., LeMeur,M., Walztinger,C., Chambon,P. and de Murcia,G. (1997) Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc. Natl Acad. Sci. USA, 94, 7303–7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potten C.S. (1998) Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos. Trans. R. Soc. Lond. B Biol. Sci., 353, 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin K., Kirkwood,T.B.L. and Potten,C.S. (1998) Age changes in stem cells of murine small intestinal crypts. Exp. Cell Res., 241, 316–323. [DOI] [PubMed] [Google Scholar]
- 18.Martin K., Potten,C.S. and Kirkwood,T.B.L. (2000) Age-related changes in irradiation-induced apoptosis and expression of p21 and p53 in crypt stem cells of murine intestine. Ann. N. Y. Acad. Sci., 908, 315–318. [DOI] [PubMed] [Google Scholar]
- 19.Wilson J.W. and Potten,C.S. (1996) Immunohistochemical localization of BAX and BAD in the normal and BCL-2 null gastrointestinal tract. Apoptosis, 1, 183–190. [Google Scholar]
- 20.Ijiri K. and Potten,C.S. (1983) Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br. J. Cancer, 47, 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerr J.F., Wyllie,A.H. and Currie,A.R. (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer, 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potten C.S. (1990) A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int. J. Radiat. Biol., 58, 925–973. [DOI] [PubMed] [Google Scholar]
- 23.Potten C.S. and Hendry,J.H. (1985) The microcolony assay in mouse small intestine. In Potten,C.S. and Hendry,J.H. (eds), Cell Clones: Manual of Mammalian Cell Techniques. Churchill Livingstone, Edinburgh, UK, pp. 50–60. [Google Scholar]
- 24.Potten C.S., Rezvani,M., Hendry,J.H., Moore,J.V. and Major,D. (1981) The correction of intestinal microcolony counts for variation in size. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med., 40, 321–326. [DOI] [PubMed] [Google Scholar]
- 25.Roberts S.A. (1990) DRFIT: a program for fitting radiation survival models. Int. J. Radiat. Biol., 57, 1243–1246. [DOI] [PubMed] [Google Scholar]
- 26.Wilson J.W., Pritchard,D.M., Hickman,J.A. and Potten,C.S. (1998) Radiation-induced p53 and p21WAF-1/CIP1 expression in the murine intestinal epithelium: apoptosis and cell cycle arrest. Am. J. Pathol., 153, 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaziri H., West,M.D., Allsopp,R.C., Davison,T.S., Wu,Y.S., Arrowsmith,C.H., Poirier,G.G. and Benchimol,S. (1997) ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J., 16, 6018–6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wesierska-Gadek J., Schmid,G. and Cerni,C. (1996) ADP-ribosylation of wild-type p53 in vitro: binding of p53 protein to specific p53 consensus sequence prevents its modification. Biochem. Biophys. Res. Commun., 224, 96–102. [DOI] [PubMed] [Google Scholar]
- 29.Berger N.A. and Petzold,A.J. (1985) Identification of minimal size requirements of DNA for activation of poly(ADP-ribose) polymerase. Biochemistry, 24, 4352–4355. [DOI] [PubMed] [Google Scholar]
- 30.Bürkle A., Chen,G., Küpper,J.H., Grube,K. and Zeller,W.J. (1993) Increased poly(ADP-ribosyl)ation in intact cells by cisplatin treatment. Carcinogenesis, 14, 559–561. [DOI] [PubMed] [Google Scholar]
- 31.Juarez-Salinas H., Sims,J.L. and Jacobson,M.K. (1979) Poly(ADP-ribose) levels in carcinogen-treated cells. Nature, 282, 740–741. [DOI] [PubMed] [Google Scholar]
- 32.Janz C. and Wiesmüller,L. (2002) Wild-type p53 inhibits replication-associated homologous recombination. Oncogene, 21, 5929–5933. [DOI] [PubMed] [Google Scholar]
- 33.Meyer R., Müller,M., Beneke,S., Küpper,J.H. and Bürkle,A. (2000) Negative regulation of alkylation-induced sister-chromatid exchanges by poly(ADP-ribose) polymerase-1 activity. Int. J. Cancer, 88, 351–355. [DOI] [PubMed] [Google Scholar]
- 34.Simbulan-Rosenthal C.M., Haddad,B.R., Rosenthal,D.S., Weaver,Z., Coleman,A., Luo,R., Young,H.M., Wang,Z.Q., Ried,T. and Smulson,M.E. (1999) Chromosomal aberrations in PARP(–/–) mice: genome stabilization in immortalized cells by reintroduction of poly(ADP-ribose) polymerase cDNA. Proc. Natl Acad. Sci. USA, 96, 13191–13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesierska-Gadek J., Wang,Z.Q. and Schmid,G. (1999) Reduced stability of regularly spliced but not alternatively spliced p53 protein in PARP-deficient mouse fibroblasts. Cancer Res., 59, 28–34. [PubMed] [Google Scholar]
- 36.Agarwal M.L., Agarwal,A., Taylor,W.R., Wang,Z.Q., Wagner,E.F. and Stark,G.R. (1997) Defective induction but normal activation and function of p53 in mouse cells lacking poly-ADP-ribose polymerase. Oncogene, 15, 1035–1041. [DOI] [PubMed] [Google Scholar]
- 37.Valenzuela M.T., Guerrero,R., Nunez,M.I., Ruiz De Almodovar,J.M., Sarker,M., de Murcia,G. and Oliver,F.J. (2002) PARP-1 modifies the effectiveness of p53-mediated DNA damage response. Oncogene, 21, 1108–1116. [DOI] [PubMed] [Google Scholar]
- 38.Beneke R., Geisen,C., Zevnik,B., Bauch,T., Müller,,W.U., Küpper,J.H. and Moroy,T. (2000) DNA excision repair and DNA damage-induced apoptosis are linked to Poly(ADP-ribosyl)ation but have different requirements for p53. Mol. Cell. Biol., 20, 6695–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waga S., Hannon,G.J., Beach,D. and Stillman,B. (1994) The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature, 369, 574–578. [DOI] [PubMed] [Google Scholar]
- 40.Brugarolas J., Chandrasekaran,C., Gordon,J.I., Beach,D., Jacks,T. and Hannon,G.J. (1995) Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature, 377, 552–557. [DOI] [PubMed] [Google Scholar]
- 41.Del Sal G., Murphy,M., Ruaro,E., Lazarevic,D., Levine,A.J. and Schneider,C. (1996) Cyclin D1 and p21/waf1 are both involved in p53 growth suppression. Oncogene, 12, 177–185. [PubMed] [Google Scholar]
- 42.Dulic V., Kaufmann,W.K., Wilson,S.J., Tlsty,T.D., Lees,E., Harper,J.W., Elledge,S.J. and Reed,S.I. (1994) p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell, 76, 1013–1023. [DOI] [PubMed] [Google Scholar]
- 43.Waldman T., Kinzler,K.W. and Vogelstein,B. (1995) p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res., 55, 5187–5190. [PubMed] [Google Scholar]
- 44.Pritchard D.M., Jackman,A., Potten,C.S. and Hickman,J.A. (1998) Chemically-induced apoptosis: p21 and p53 as determinants of enterotoxin activity. Toxicol. Lett., 102–103, 19–27. [DOI] [PubMed] [Google Scholar]
- 45.Wang X., Ohnishi,K., Takahashi,A. and Ohnishi,T. (1998) Poly(ADP-ribosyl)ation is required for p53-dependent signal transduction induced by radiation. Oncogene, 17, 2819–2825. [DOI] [PubMed] [Google Scholar]
- 46.Masutani M., Nozaki,T., Nakamoto,K., Nakagama,H., Suzuki,H., Kusuoka,O., Tsutsumi,M. and Sugimura,T. (2000) The response of Parp knockout mice against DNA damaging agents. Mutat. Res., 462, 159–166. [DOI] [PubMed] [Google Scholar]
- 47.Hendry J.H., Cai,W.B., Roberts,S.A. and Potten,C.S. (1997) p53 deficiency sensitizes clonogenic cells to irradiation in the large but not the small intestine. Radiat. Res., 148, 254–259. [PubMed] [Google Scholar]
- 48.Hendry J.H., Broadbent,D.A., Roberts,S.A. and Potten,C.S. (2000) Effects of deficiency in p53 or bcl-2 on the sensitivity of clonogenic cells in the small intestine to low dose-rate irradiation. Int. J. Radiat. Biol., 76, 559–565. [DOI] [PubMed] [Google Scholar]
- 49.Hoyes K.P., Cai,W.B., Potten,C.S. and Hendry,J.H. (2000) Effect of bcl-2 deficiency on the radiation response of clonogenic cells in small and large intestine, bone marrow and testis. Int. J. Radiat. Biol., 76, 1435–1442. [DOI] [PubMed] [Google Scholar]
- 50.Merritt A.J., Potten,C.S., Kemp,C.J., Hickman,J.A., Balmain,A., Lane,D.P. and Hall,P.A. (1994) The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res., 54, 614–617. [PubMed] [Google Scholar]
- 51.Merritt A.J., Allen,T.D., Potten,C.S. and Hickman,J.A. (1997) Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene, 14, 2759–2766. [DOI] [PubMed] [Google Scholar]
- 52.Ménissier-de Murcia J., Mark,M., Wendling,O., Wynshaw-Boris,A. and de Murcia,G. (2001) Early embryonic lethality in PARP-1 Atm double-mutant mice suggests a functional synergy in cell proliferation during development. Mol. Cell Biol., 21, 1828–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]