Abstract
KRN7000 is an important ligand identified for CD1d protein of APC, and KRN7000/CD1d complex can stimulate NKT cells to release Th1 and Th2 cytokines. In an effort to understand the structure-activity relationships, we have carried out the synthesis of a complete set of the KRN7000 stereoisomers, and their biological activities examined.
In the early 1990s potent antitumor activity was located in the agelasphins extracted from Okinawan sponge Agelas mauritianus by Kirin Pharmaceuticals, and α-galactosyl ceramide (α-GalCer) named KRN7000 was found to have the best activity in the SAR (structure-activity relationship) studies of related synthetic glycolipids.1 Further studies have revealed that T cell receptors (TCR) of NKT cells recognize KRN7000 presented by major histocompatibility complex (MHC) class I-like protein CD1d of antigen presenting cells (APC). Upon recognition of KRN7000/CD1d complex, NKT cells rapidly release large amounts of a broad range of cytokines including pro-inflammatory T helper 1 (Th1) cytokines such as interferon-γ (IFNγ) and tumor necrosis factor-α, and anti-inflammatory Th2 cytokines such as interleukin-4, -10, and -13 (IL-4, 10, 13).2 Th1 cytokines are thought to correlate with the antitumor, antiviral and antibacterial effects of KRN7000, whereas Th2 cytokine are believed to either delay or prevent the onset of autoimmune diseases such as type1 diabetes.3
Due to the biological significance, many α-GalCer analogues have been synthesized and biologically evaluated to understand the structure-activity relationships (SAR). Studies focused on the sugar moiety of α-GalCer indicated that the α-anomeric galactose group is important for α-GalCer to bind CD1d and to active NKT cells through their TCR.4 It was also found that the equatorial C2-OH group of the galactose is crucial for the activity of α-GalCer, any modification at this position largely abolishes activity.5 Modifications at the C3- and C6-OH groups of the galactose was found to be tolerated.6 The SAR studies involving the ceramide moiety of α-GalCer can be separated into modifications of the acyl- and phytosphingosine chain, and the polar portion of the ceramide. It was found that truncations of either the phytosphingosine or and acyl chain resulted in α-GalCer that biased NKT cells toward release of Th2 cytokines (IL-4).7 Insertion of double bonds into the acyl chain of α-GalCer (C20:2) seemed to bias toward Th2 responses.8 Introduction of an aromatic residue to the acyl chain or phytosphingosine chain enhanced the Th1 cytokine profiles.9 The α-C-GalCer, in which the glycosidic oxygen was replaced by a methylene group stimulated strong Th1 responses in vivo from NKT cells.10 The C3- and C4-OH groups of the phytosphingosine was reported essential for the activity of α-GalCer, although C3-OH group being more important than C4-OH group.4
These results were supported by crystal structural studies. Two X-ray crystallography studies of the CD1d/ α-GalCer complexes have revealed that the lipid chains of α-GalCer fits tightly into the CD1d binding groove (A′ and F′ pockets). The key hydrogen-bonding interations were observed between CD1d and α-GalCer [2-OH group of the galactose group and Asp151 (mouse Asp153) of the CD1d; 3-OH group of the phytosphingsine and Asp80 of CD1d]. These bonds serve to anchor α-GalCer in a distinct orientation and position it in the lipid binding groove.11,12 Recently, the crystal structure of human NKT TCR-CD1d-α-GalCer complex showed that α-GalCer protrudes minimally from the CD1d cleft with only the galactose group exposed for recognition by the NKT TCR. There are several hydrogen-bonding interactions between the CDR1α and CDR3α loops of TCR and α-GalCer [Phe29α of the CDR1α loops and 4-OH group of the galactose group; Ser30α of the CDR1α loops and 3-OH group of the galactose group; Arg95α of the CDR3α loops and 3-OH group of the phytosphingosine; Gly96α of the CDR3α loops and 2-OH group of the galactose group].13
A variety of sugar and chain modified analogues of KRN7000 have been prepared and their biological properties evaluated. However, a similar systematical study of phytosphingosine core modified KRN7000 stereoisomers has not yet been carried out, probably because the stereoisomers of phytosphingosine are not readily available (Figure 1). Although phytosphingosine stereoisomers have been targets of much synthetic efforts, the complete set of all stereoisomers of phytosphingosine has not been readily available. Previously, we developed practical synthetic methods for all four stereoisomers of sphingosine,14 and extended them to generate all eight phytosphingosine stereoisomers in a divergent manner. These stereoisomers of sphingosine and phytosphingosine were also utilized in the construction of ceramide libraries, each library consisting of more than 500 compounds.15 Now we wish to report herein the complete synthesis of KRN7000 stereoisomers from the phytosphingosine stereoisomers and preliminary results on their biological activities in mouse and human iNKT cells.
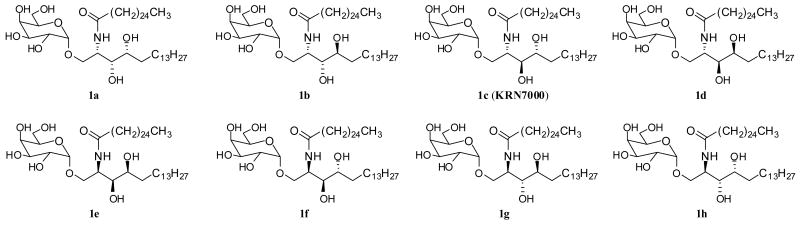
Figure 1.
Structure of eight KRN7000 stereoisomers.
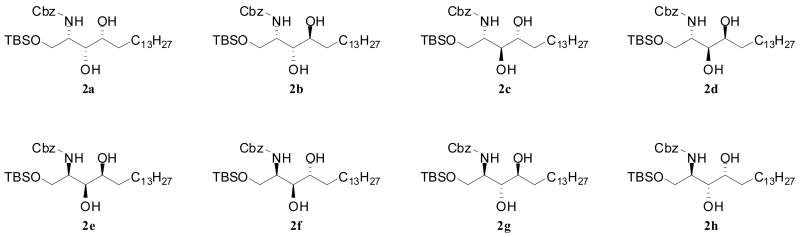
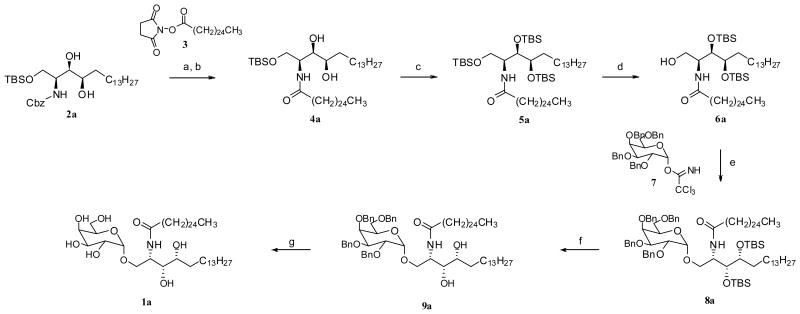
Our syntheses of all eight KRN7000 stereoisomers entailed 1) preparation of appropriately protected derivatives of all phytosphingosine, 2) N-acylation to ceramides, and 3) α-glycosidation with protected galactose of the terminal hydroxyl group of the phytosphingosine residue after removal of the protecting group. Thus, we first prepared substantial quantities of protected derivatives of the eight stereoisomers of phytosphingosine (Figure 2: 2a, 2b, 2c, 2d, 2e, 2f, 2g and 2h) from L- and D-serine via four sphingosine stereoisomers as previously reported.14,15 Briefly, both the L- and D-serine were converted to the four stereoisomers of N,O-diprotected sphingosine. The double bond of each sphingosine isomer was epoxidized to provide the ‘up’ and ‘down’ epoxides, which after separation was regioselectively reduced to give the appropriately protected phytosphingosine isomers respectively. In this way each sphingosine stereoisomer was converted to two phytosphingosine isomers shown in Figure 2. Transformation of each phytosphingosine stereoisomer to α-galactosylceramide is illustrated for the case of isomer 2a, and all the other isomers were similarly transformed to provide a complete set of all 8 stereoisomers of KRN7000. Hydrogenolysis of N-Cbz protected 2a over Pd/C generated the free amine, which was conveniently acylated with the N-hydroxysuccinimide activated ester 3 in the presence of triethylamine16 to give ceramide 4a. The two hydroxyl groups of 4a were protected with TBSOTf and 2,6-lutidine in CH2Cl2 to yield the O-TBS protected ceramide 5a. Now the regioselective removal of the TBS group from the primary hydroxyl in 5a was needed, and it was accomplished by treatment with HF·py in THF-pyridine17 to afford the intermediate 6a. The glycosylation of 6a with the perbenzylated galactosyl trichloroacetimidate 718 in the presence of BF3·OEt2 gave α-galactoside 8a in modest yield. All protecting groups in 8a were sequentially removed by treatment with TBAF in THF, and the resulting diol 9a with Pd(OH)2/C in EtOH-CHCl3 to provide the target compound, KRN7000 stereoisomer 1a (Scheme 1). By employing the identical procedures on the other stereoisomers of phytosphingosine (2b-2h), the corresponding KRN7000 stereoisomers (1b-1h) were uneventfully prepared. The melting point and specific rotation data for all stereoisomers of KRN7000 are listed in Table 1.
Figure 2.
Structure of eight phytosphingosine stereoisomers derivatives.
Scheme 1.
Reagents and conditions: (a) Pd/C, H2, THF, rt; (b) 3, Et3N, THF, 50°C, 85% (2 steps); (c) TBSOTf, 2,6-lutidine, CH2Cl2, rt, 90%; (d) HF·py, THF-pyridine, rt, 87% (e) 7, BF3·OEt2, 4Å molecular sieves, THF-Et2O, -20°C, 43%; (f) TBAF, THF, rt, 97%; (g) Pd(OH)2/C, H2, EtOH-CHCl3, rt, 88%.
Table 1.
Physical properties of KRN7000 stereoisomers.
| Compound | (pyridine) | mp (°C) |
|---|---|---|
| 1a | + 35.3 (c 0.30) | 187 |
| 1b | + 21.7 (c 0.51) | 189 |
| 1c | + 42.4 (c 0.50) | 190 |
| 1d | + 46.2 (c 0.51) | 191 |
| 1e | + 46.6 (c 0.43) | 182 |
| 1f | + 53.4 (c 0.35) | 197 |
| 1g | + 42.1 (c 0.49) | 194 |
| 1h | + 35.3 (c 0.36) | 183 |
The biological activities of these isomers have been examined in both mouse and human iNKT cells in terms of in vitro proliferation and induction of IFNγ, IL-4 and IL-13. The preliminary data indicate the following trends for the stereoisomers of KRN7000; 1) differential activity was observed between mouse and human iNKT cells with mouse cells being more sensitive, and 2) differential activity was also observed for different isomers. More specifically, with mouse iNKT cells the following observations have been made; 1) good in vitro proliferation with 1c (KRN7000) > 1a, 1d, 1g, and much weaker proliferation with 1b, 1f, and 1h, and 2) good induction of IFNγ, IL-4/IL-13 with 1c and 1d, and weak IFNγ and IL-13 at a high dose of Ia, and induction of IL-4 at high dosage of 1b, 1a, 1h. With human iNKT cells, the following trends have been observed; 1) the stereoisomers (2S) derived from L-serine, namely 1a, 1b, 1c, and 1d show high potency (IFNγ, IL-4 and IL-13 production), while the isomers (2R) derived from D-serine, i.e. 1e, 1g, 1h exhibit weak potency (IFNγ and IL-4 production), but decent IL-13 production, and 2) isomer 1f showes virtually no activity except IL-13 production at high dose. The isomer 1d, having the inverted C4-OH group stereochemistry when compared with 1c, displays comparable potency (proliferation and cytokine secretion) with those for 1c (KRN7000). Thus, the 3-D spatial orientations of C2-NH2 group and C3-OH group of phytosphingosine appear crucial for the activity, and the configuration of C2-NH2 group is much more important than C3-OH group; the stereochemical variation of C4-OH group seems minimal. The more extensive studies of the biological activity of KRN7000 stereoisomers are in progress and will be reported in due course.19
Acknowledgments
This work was supported by the Korea Research Foundation grant funded from the Korean Government (MOEHRD: KRF-2005-070-C00078).
References and notes
- 1.Morita M, Motoki K, Akimoto K, Natori T, Skai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. J Med Chem. 1995;38:2176. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kronenberg M. Annu Rev Immunol. 2005;23:877. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]; (b) Savage PB, Teyton L, Bendelac A. Chem Soc Rev. 2006;35:771. doi: 10.1039/b510638a. [DOI] [PubMed] [Google Scholar]; (c) Tsuji M. Cell Mol Life Sci. 2006;63:1889. doi: 10.1007/s00018-006-6073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Stronge VS, Salio M, Jones EY, Cerundolo V. Trends Immunol. 2007;28:455. doi: 10.1016/j.it.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. J Exp Med. 2000;192:921. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fuji N, Ueda Y, Fujiwara H, Toh T, Yoshimura T, Yamagishi H. Clin Cancer Res. 2000;6:3380. [PubMed] [Google Scholar]; (c) Wang B, Geng YB, Wang CR. J Exp Med. 2001;194:313. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Van Kaer L. Nature Review. 2005;5:31. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Konodo E, Koseki H, Taniguchi M. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]; (b) Brossay L, Naidenko O, Burdin N, Matsuda J, Sakai T, Kronengerg M. J Immunol. 1998;161:5124. [PubMed] [Google Scholar]; (c) Sidobre S, Hammond KJL, Sidobre LB, Maltsev SD, Richardson SK, Ndonye RM, Howell AR, Sakai T, Besra GS, Porcelli SA, Kronenberg M. Proc Natl Acad Sci USA. 2004;101:12254. doi: 10.1073/pnas.0404632101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Barbieri L, Costantino V, Fattorusso E, Mangoni A, Aru E, Parapini S, Taramelli D. Eur J Org Chem. 2004:468. [Google Scholar]; (b) Barbieri L, Costantino V, Fattorusso E, Mangoni A, Basilico N, Mondani M, Taramelli D. Eur J Org Chem. 2005:3279. [Google Scholar]
- 6.(a) Zhou XT, Forestier C, Goff RD, Li C, Teyon L, Bendelac A, Savage PB. Org Lett. 2002;4:1267. doi: 10.1021/ol025565+. [DOI] [PubMed] [Google Scholar]; (b) Xing GW, Wu D, Poles MA, Horowitz A, Tsuji M, Ho DD, Wong CH. Bioorg Med Chem. 2005;13:2907. doi: 10.1016/j.bmc.2005.02.018. [DOI] [PubMed] [Google Scholar]; (c) Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong CH. Proc Natl Acad Sci USA. 2005;102:1351. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Miyamoto K, Miyake S, Yamamura T. Nature. 2001;413:531. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]; (b) Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, III, Teyton L, Bendelac A, Savage PB. J Am Chem Soc. 2004;126:13602. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 8.Yu KOA, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, Chang YT, Besra GS, Porcelli SA. Proc Natl Acad Sci USA. 2005;102:3383. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. J Am Chem Soc. 2006;128:9022. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]; (b) Toba T, Murata K, Nakanishi K, Takahashi B, Takemoto N, Akabane M, Nakatsuka T, Imajo S, Yamamura T, Miyake S, Annoura H. Bioorg Med Chem Lett. 2007;17:2781. doi: 10.1016/j.bmcl.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 10.Frank RW, Tsuji M. Acc Chem Rev. 2006;39:692. doi: 10.1021/ar050006z. [DOI] [PubMed] [Google Scholar]
- 11.Zajonc DM, Cantu C, III, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Nat Immunol. 2005;6:810. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. Nat Immunol. 2005;6:819. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 13.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MCJ, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. Nature. 2007;448:44. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 14.(a) Chung SK, Lee JM. Tetrahedron: Asymmetry. 1999;10:1441. [Google Scholar]; (b) Lee JM, Lim HS, Chung SK. ibid. 2002;13:343. [Google Scholar]; (c) Lee JM, Lim HS, Seo KC, Chung SK. ibid. 2003;14:3639. [Google Scholar]
- 15.(a) Chang YT, Choi J, Ding S, Prieschl EE, Baumruker T, Lee JM, Chung SK, Schultz PG. J Am Chem Soc. 2002;124:1856. doi: 10.1021/ja017576o. [DOI] [PubMed] [Google Scholar]; (b) Park JJ, Lee JH, Li Q, Diaz K, Chang YT, Chung SK. Bioor Chem. 2008 doi: 10.1016/j.bioorg 2007.12.004. available online 14 February 2008. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Song S, Lee T, Jung S, Kim D. Synthesis. 2004;6:847. [Google Scholar]
- 17.Nocolau KC, Hepworth D, King NP, Finlay MRV, Scarpelli R, Pereira MMA, Bollbuck B, Bigot A, Werschkun B, Winssinger N. Chem Eur J. 2000;6:2783. doi: 10.1002/1521-3765(20000804)6:15<2783::aid-chem2783>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.(a) Figueroa S, Schmidt RR. Carbohydr Res. 2000;328:95. doi: 10.1016/s0008-6215(00)00092-6. [DOI] [PubMed] [Google Scholar]; (b) Plettenburg O, Bodmer-Narkevitch V, Wong CH. J Org Chem. 2002;67:4559. doi: 10.1021/jo0201530. [DOI] [PubMed] [Google Scholar]
- 19.Porcelli SA. unpublished results. 2008 [Google Scholar]