Abstract
Stress can affect the brain and lead to depression; however, the molecular pathogenesis is unclear. An association between stress and stress-induced hypersecretion of glucocorticoids occurs during stress. Dexamethasone (a synthetic glucocorticoid steroid) has been reported to induce apoptosis and increase the activity of monoamine oxidase (MAO) (Youdim et al. 1989). MAO is an enzyme for the degradation of aminergic neurotransmitters; dopamine, noradrenaline and serotonin and dietary amines and MAO inhibitors are classical antidepressant drugs. In this study, we have compared the ability of rasagiline (Azilect) and its main metabolite, R-aminoindan with selegiline (Deprenyl) in prevention of dexamethasone-induced brain cell death employing human neuroblastoma SH-SY5Y cells and glioblastoma 1242-MG cells. Dexamethasone reduced cell viability as measured by MTT test, but rasagiline, selegiline, and 1-R-aminoindan could significantly prevent dexamethasone-induced brain cell death. Among three drugs, rasagiline had the highest neuroprotective effect. Furthermore, the inhibitory effects of these drugs on MAOB catalytic activity and on apoptotic DNA damage (TUNEL staining) were examined. Rasagiline exhibited highest inhibition on MAO B enzymatic activity and prevention on DNA damage as compared to selegiline and 1-R-aminoindan. In summary, the greater neuroprotective effect of rasagiline may be associated with the combination of the parent drug and its metabolite 1-R-aminoindan.
Introduction
The brain is the key organ in the response to stress. Long-term stressful situations can affect the brain and lead to depression. For example, environmental stressors related to job or family situations are important triggers of depressive episodes. Major depression is one of the world’s greatest public health burdens. However, the development of novel antidepressants is based upon an improved neurobiological understanding on the cellular changes that take place in the brain during the long-term stress. The major hormonal response to stress is in the form of glucocorticoids. Glucocorticoids are the steroid hormones secreted from adrenal gland during stress. Abnormal increased levels of glucocorticoids are associated with atrophy in the hippocampus (Lee et al. 2002) and also associated with major depression (Duval et al. 2006). Thus, glucocorticoids may play a contributing role toward neuronal death and depression. Anti-glucocorticoid drugs appear to be an effective therapy for anti-depression (Murphy 1997). Steroid hormones are involved in the regulation of many functions in which monoamine oxidase (MAO) also plays important roles such as response to stress, behavioral adaptation, and mood (de Kloet et al. 1990). MAO is an enzyme located on the outer membrane of the mitochondria. The synthetic glucocorticoid, dexamethasone, increases MAO A activity in the human neuroblastoma (Ou et al. 2006a), the human fibroblasts (Edelstein and Breakefield 1986), and rat brain frontal cortex (Slotkin et al. 1998). Both anti-glucocorticoid agents (Wolkowitz and Reus 1999) and MAO A inhibitors (Kato et al. 1998; Volz and Gleiter 1998) have been used in the treatment of depression.
MAO exists in two isoforms, MAO A and MAO B (Shih et al. 1999). However, within the human brain, MAO B is much more prevalent in the striatum. MAO B catalyzes biogenic and dietary amines including neurotransmitters (such as phenylethylamine and dopamine), and produces hydrogen peroxide (H2O2), a toxic product that induces cell apoptosis (Ou et al. 2006b; Johnson et al. 2007).
Apoptosis can be prevented with the use of propargylamine-derived MAO B inhibitors (Malorni et al. 1998; Youdim et al. 2001b; Youdim et al. 2005b). Two such inhibitors, rasagiline (Azilect) and selegiline (1-Deprenyl or Emsam), were developed in the treatment of Parkinson’s disease. Both of these drugs are irreversible inhibitors of MAO B, where rasagiline is a restricted analog of selegiline. Rasagiline is significantly more active in vivo than selegiline (Youdim et al. 2001a). They differ, however, by their metabolic products. Whereas selegiline (1-Deprenyl) is metabolized to amphetaminic metabolites, rasagiline is metabolized to neuroprotective aminoindan. l-methampehtamine, the metabolite of selegiline, produces neurotoxic effects that may counterbalance the neuroprotective effects of selegiline. On the other hand, aminoindan has no neurotoxic effects and has also been shown to have its own neuroprotective activity (Youdim et al. 2001a; Bar-Am et al. 2007). Dexamethasone also has been shown to induce apoptosis at concentrations of 100 nM–100 µM (Glick et al. 2000; Dolmatov et al. 2004; Jacobs et al. 2006). In this study, we have employed dexamethasone (10 µM) to induce apoptosis in order to evaluate the neuroprotective properties of rasagiline, selegiline (deprenyl), and rasagiline’s major metabolite, 1-R-aminoindan, in protection against cell death-induced by toxicity of dexamethasone.
Materials and Methods
Cell Lines and Reagents
A human neuroblastoma SH-SY5Y cell line was used. This line was purchased from ATCC. The cells were grown and cultured in RPMI 1640 media and supplemented with 10% fetal bovine serum, and a human glioblastoma 1242-MG cell line was also used. It was obtained as a gift from Dr. B. Westermark (Dept. of Pathology, University Hospital, Uppsala, Sweden). They were grown and cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum. Rasagiline was synthesized by a Ph.D student, Hailin Zheng, in the laboratory of Dr. Youdim’s (Haifa, Israel). 1-R-aminoindan and selegiline (deprenyl) were purchased from Sigma-Aldrich USA. In Situ Cell Death Detection Kit (for TUNEL staining) was purchased from Roche (Indianapolis, IN).
Cell Culture and Treatments
The SH-SY5Y and 1242-MG cells were seeded into 6-well plates and cultured overnight in medium. Cells were supplemented with charcoal-stripped, steroid-free fetal calf serum for ~6 h. The medium was then replaced with medium treated with 10 µM of dexamethasone, 0.25 nM of rasagiline, 0.25 nM of selegiline, or 1 µM of 1-R-aminoindan in the presence of charcoal-stripped fetal calf serum. The treatments were performed every other day for 4 days.
MAO B Catalytic Activity Assay
SH-SY5Y and 1242-MG cells were grown to confluence, harvested, and washed with phosphate-buffered saline. One hundred micrograms of total proteins were incubated with 10 µM 14C-labeled phenylethylamine (Amersham Biosciences) in the assay buffer (50 mM sodium phosphate buffer, pH 7.4) at 37°C for 20 min and terminated by the addition of 100 µl of 6 N HCl. The reaction products were then extracted with ethyl acetate/toluene (1:1) and centrifuged at 4°C for 10 min. The organic phase containing the reaction product was extracted, and its radioactivity was obtained by liquid scintillation spectroscopy (Geha et al. 2001).
MTT Assay
The survival and proliferation of the cells were measured using MTT assays. MTT, (3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyl tetrazolium bromide), is a yellow tetrazolium salt that is metabolized within the cell to form a purple formazan crystal, which can then be dissolved using a detergent in order to measure the solution’s light absorbance. First, the MTT solution (5 mg/ml) was diluted with PBS to form a 1× solution (0.5 mg/ml). For the 6-well plates, 400 µl of MTT dye was added to each well. Then, the cells were left to incubate for 4–5 h. During this incubation period, the yellow dye was converted by the mitochondria of the viable cells into a purple formazan crystal, which were then dissolved by 1.2 ml of DMSO. The NanoDrop Spectrophotometer was used to determine the optical density at 572 nm of each well.
TUNEL Assay
The terminal deoxynucleotidyl transferase (TdT)-mediated dUTP Nick End Labeling (TUNEL) assay was used to assess the extent of apoptosis in treated cells. Briefly, cells were plated on a four-well chamber slide on the day preceding the experiment, and treated with or without 10 µM dexamethasone, 0.25 nM of rasagiline, 0.25 nM of selegiline, or 1 µM of 1-R-aminoindan for 2 days. Cells were then washed with PBS and fixed using 4% paraformaldehyde in PBS. The slides were again washed with PBS, and fragmented DNA was detected in apoptotic cells by adding fluorescein 12-dUTP to nicked ends of DNA (In Situ Cell Death Detection Kit, Roche). Slides were incubated for 1 h at 37°C in the dark and washed in PBS three times and then visualized with a fluorescent light microscope. Green fluorescence was correlated with DNA fragmentation. Experiments were done in duplicate for three times, and the percentage of TUNEL-positive cells was determined.
Statistical Analysis
The statistical significance was evaluated using one-way ANOVA to test for differences between groups and followed by post-hoc t-tests. A value of P<0.05 was considered to be significant.
Results
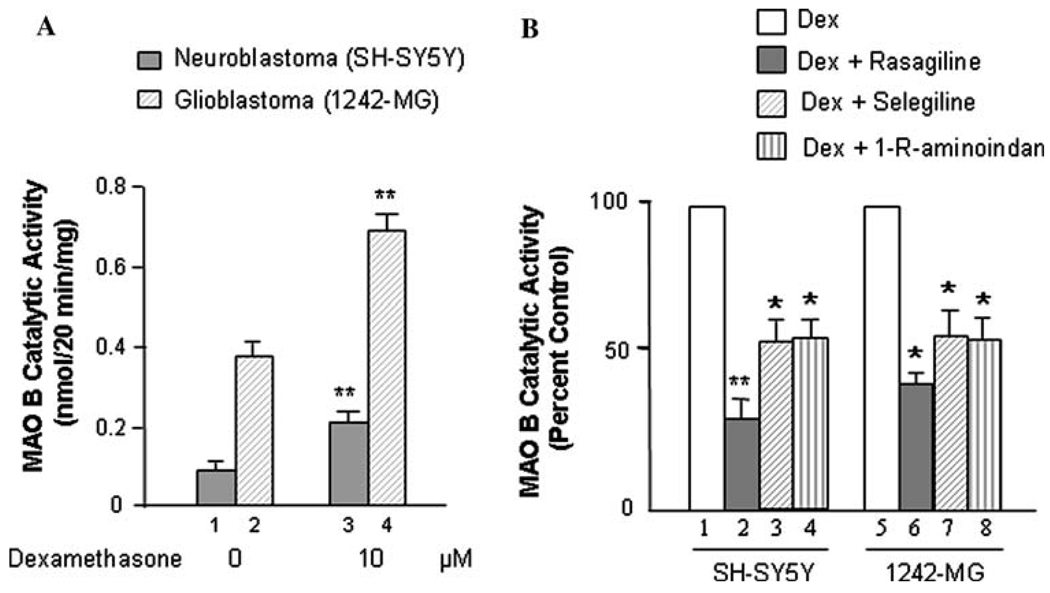
The expression of MAO B was investigated in two human brain cell lines, neuroblastoma SH-SY5Y, and glioblastoma 1242-MG. Cells were seeded in 10-cm dishes. After 24 h, cells were treated with dexamethasone (0 or 10 µM) and/or 0.25 nM rasagiline or selegiline or 1 µM 1-R-aminoindan for 96 h. The MAO B catalytic activities were determined (Fig. 1A, B). The results indicated MAO B enzymatic activity was significantly increased with the dexamethasone treatment in both cell lines, confirming previous studies in endothelial and chromaffin cells (Youdim et al. 1989). In Fig. 1A (lanes 3 vs. 1 and 4 vs. 2), MAO B catalytic activity significantly increases by twofold after treatment with dexmethasone in both SH-SY5Y and 1242-MG (P<0.02). In Fig. 1B, rasagiline lowers MAO B catalytic activity by ~70% in SH-SY5Y (Fig. 1B, lane 2 vs. 1) and~60% 1242-MG (Fig. 1B, lane 6 vs. 5)—more significantly in SH-SY5Y (P<0.02). Selegiline and 1-R-aminoindan also significantly (P<0.05) lower the catalytic activity of MAO B by about 50% in both SH-SY5Y and 1242-MG cells (Fig. 1B, lanes 3 vs. 1, 4 vs. 1, 7 vs. 5, and 8 vs. 5, respectively).
Fig. 1.
Effects of dexamethasone and MAO B inhibitors on MAO B catalytic activity. A. Dexamethasone increases the MAO B catalytic activity in both SH-SY5Y and 1242-MG. Cells were treated with dexamethasone (0 or 10 µM) for 96 h, and the MAO B catalytic activities were determined. *P<0.02 compared with control cells (without treatment). B. The MAO B inhibitors, rasagiline, selegiline, and 1-R-aminoidan (rasagiline’s metabolite) decrease MAO B catalytic activity induced by dexamethasone in both SH-SY5Y and 1242-MG. Cells were treated with dexamethasone (0 or 10 µM) and/or 0.25 nM rasagiline or selegiline or 1 µM 1-R-aminoindan for 96 h, and the MAO B catalytic activities were determined. *P<0.02 and **P<0.01 compared to cells treated with dexamethasone alone
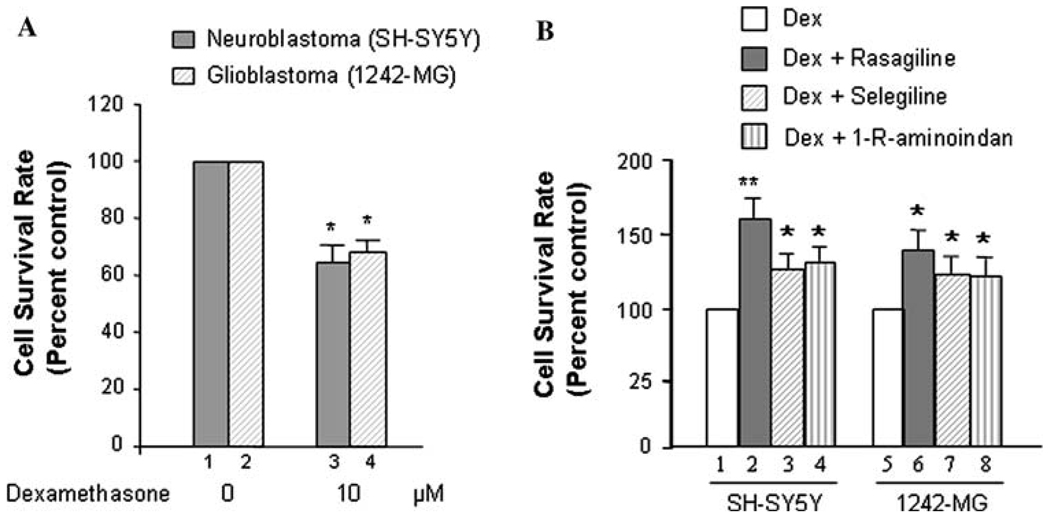
Furthermore, the evaluation of cell viability was performed by determining cell proliferation rates among the different treatment groups with MTT Assay (Fig. 2A, B). The results indicated that the proliferation of SH-SY5Y and 1242-MG significantly (P<0.05) decreased with the addition of 10 µM Dexamethasone (Fig. 2A, lanes 3 vs. 1 and 4 vs. 2, respectively). The survival rate of SH-SH5Y is decreased by~35%; subsequently, the proliferation rate of 1242-MG is decreased by ~32% after dexamethasone treatment. Whereas, the addition of rasagiline, selegiline, and 1-R-aminoindan significantly increased the proliferation rates of SH-SY5Y and 1242-MG upon dexamethasone treatment. Rasagiline had the greatest effect on both SH-SY5Y and 1242-MG (Fig. 2B, lanes 2 vs. 1 and 6 vs. 5, respectively). Rasagiline caused ~60% increase in the cell proliferation rate for SH-SY5Y cells treated with dexamethasone. Selegiline and 1-R-aminoidan cause ~25% increase in the proliferation of SH-SY5Y cells treated with dexamethasone (Fig. 2B, lanes 3 vs. 1 and 4 vs. 1, respectively). In 1242-MG, rasagiline causes ~35% increase in cell proliferation rates (Fig. 2B, lanes 6 vs. 5) while both selegiline and 1-R-aminoidan cause ~20% increase (Fig. 2B, lanes 7 vs. 5 and 8 vs. 5, respectively) in viability of dexamethasone-treated cells.
Fig. 2.
Effects of dexamethasone and MAO B inhibitors on cell proliferation rates. A. Dexamethasone lowers the proliferation rates of both SH-SY5Y and 1242-MG cells. Cells were treated with dexamethasone (0 or 10 µM) for 96 h, and the proliferation rates were determined by MTT assay. *P<0.05 compared with control cells (without treatment). B. The MAO B inhibitors, rasagiline, selegiline, and 1-R-aminoidan (rasagiline’s metabolite) increase the proliferation rates in both SH-SY5Y and 1242-MG. Cells were treated with dexamethasone (0 or 10 µM) and/or 0.25 nM rasagiline or selegiline or 1 µM 1-R-aminoindan for 96 h, and the proliferation rates were determined by MTT assay. *P<0.05 and **P<0.02 compared to cells treated with dexamethasone alone
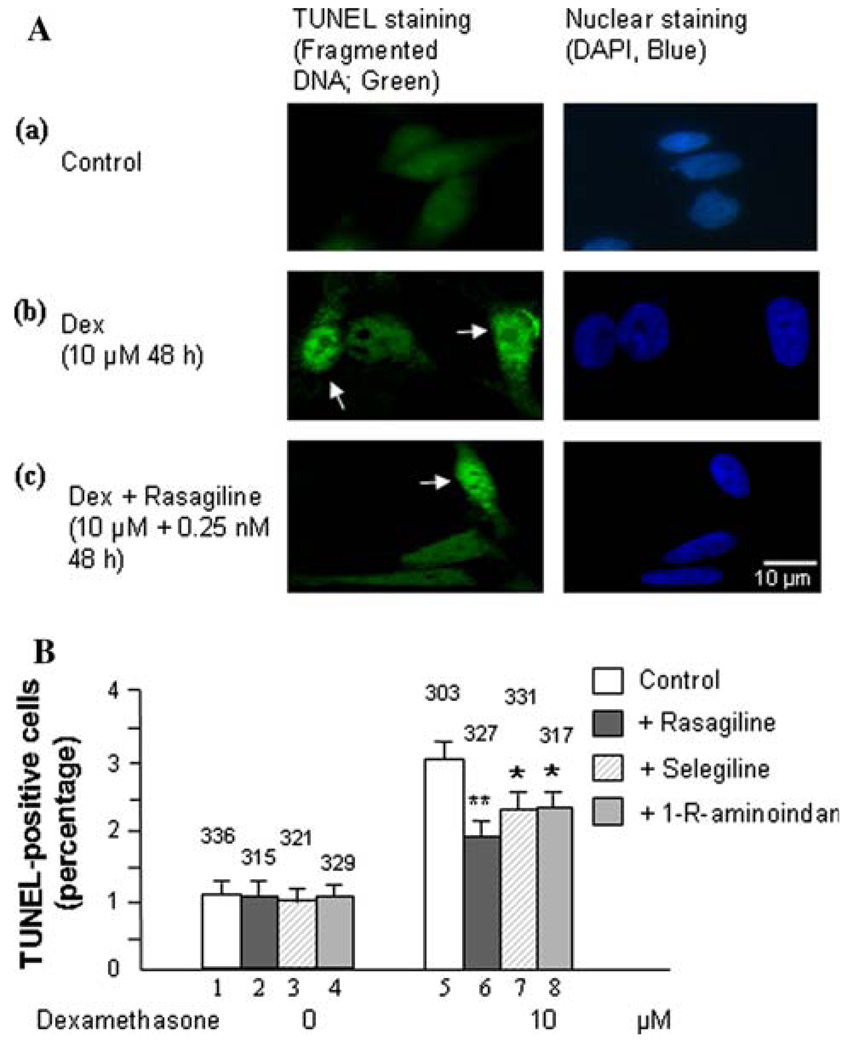
Results from the MAO B activity assay (Fig. 1A and B) and MTT assay (Fig. 2A and B) were further supported by the TUNEL assay (Fig. 3), since an increase in MAO B catalytic activity may produce more H2O2. H2O2 can cause cell apoptosis which can be detected by measuring fragmented DNA using TUNEL staining (Phillips et al. 2003). As shown in Fig. 3A, green fluorescent-labeled DNA fragmentation in SH-SY5Y after treatment with dexamethasone significantly increased compared to untreated cells (Fig. 3A, 2 vs. 1). Additionally, Fig. 3 also indicates that treatment with rasagiline, selegiline, or 1-R-aminoidan significantly decreased the percentage of TUNEL-positive cells present in the dexamethasone-treated group (Fig. 3A, 3 vs. 2). Rasagiline causes a 30% decrease in fragmented DNA of dexamethasone-treated SH-SY5Y cells (P<0.02; Fig. 3B, lanes 6 vs. 5). Selegiline and 1-R-aminoidan cause a 20% decrease in the amount of fragmented DNA present in SH-SY5Y cells treated with dexamethasone (P<0.05; Fig. 3B, lanes 7 vs. 5 and 8 vs. 5, respectively). Similar results were exhibited by 1242-MG cells (data not shown).
Fig. 3.
Effects of dexamethasone and MAO B inhibitors on cell apoptosis in SH-SY5Y cells. A. Fluorescence showing TUNEL(+) cells and TUNEL(−) cells in (a) control cells, (b) cells treated with dexamethasone or (c) cells treated with 10 µM dexamethasone and 0.25 nM rasagiline for 48 h. Scale bar is 10 µm. Photomicrographs show representative cells from each treatment group, and the arrows indicate apoptotic cells. B. Percentage of cells that contain damaged DNA as revealed by the TUNEL assay in each group. TUNEL-labeled DNA fragmentation is correlated with green fluorescence. Experiments were done in duplicates for three times. Bar graph indicating the average percentage of TUNEL-positive cells counted from each group. The counted cell numbers are shown at the top of each group. *P<0.05 and **P<0.02 compared to cells treated with dexamethasone alone
Discussion
The results of this study indicated that dexamethasone reduces cell proliferation rates but increases MAO B catalytic activity. The aberrant increase of MAO B activity has been implicated in neurodegenerative diseases, such as Alzheimer’s disease (Saura et al. 1994), Parkinson’s disease (Schneider et al. 1981), Huntington’s disease (Mann et al. 1986; Senatorov et al. 2003), and alcoholism (Carlsson et al. 1980). Dexamethasone has been reported to induce MAO B expression and activity in endothelial and astrocytes in a dose- and time-dependent manner (Carlo et al. 1996). The abnormally increased MAO B catalytic activity may produce toxic products, such as H2O2, therefore these data support an important role for glucocorticoids in the increase in brain MAO B associated with neurodegenerative diseases and mental disorders. In contrast, the MAO B inhibitors, deprenyl (selegiline) and Azilect® (rasagiline), have been used in the therapy of neurodegenerative diseases such as Parkinson’s Disease (Paterson et al. 1997; Tatton et al. 2000; Maruyama et al. 2001; Fernandez and Chen 2007), depression (Goodnick 2007; Robinson and Amsterdam 2007), and senile dementia (Tariot et al. 1987; Youdim et al. 2005a; Youdim 2006). However, whether these drugs can also protect neurons from glucocorticoids-induced toxicity has never been studied.
We report here for the first time that rasagiline, selegiline, and 1-R-aminoindan significantly prevent dexamethasone-induced brain cell death involving in both neuroblastoma and glioblastoma cells. Among the three compounds, rasagiline has the highest neuroprotective effect compared to either selegiline or 1-R-aminoindan. Rasagiline (Azilect) and selegiline (1-deprenyl or Emsam) are irreversible inhibitors of MAO B. The greater neuroprotective quality of rasagiline may in part be due to the effects of the parent compound and its major metabolite, 1-R-aminoindan. Furthermore, the inhibitory effects of these drugs on MAO B catalytic activity and on apoptotic DNA fragment damage (observed by TUNEL staining) were examined. Rasagiline has shown the highest inhibition on MAO B enzymatic activity (Youdim et al. 2001a) and also has shown the highest prevention on apoptosis compared to selegiline and 1-R-aminoindan. The mechanism by which rasagiline and selegiline initiate their anti-apoptotic effect can be summarized by their up regulation of anti-apoptotic Bcl-2 and Bcl-Xl and down regulation of propaoptotic Bad, Bax, PARP, and caspase 3 (see Youdim et al. 2005a) and Youdim et al. 2006) for reviews). Because Bcl-2 and caspase 3 are key factors for preventing or mediating the mitochondrial-involved apoptosis (Lakhani et al. 2006), it suggests that the MAO inhibitors may protect cells from apoptosis through a mechanism involving the maintenance of mitochondrial homeostasis (Malorni et al. 1998). In addition, structure activity studies with rasagiline have shown that it is propargylamine moiety that produces this effect, since propargylamine which has little or no MAO inhibitory activity has a similar mechanism of neuroprotective activity with similar potency (Bar-Am et al. 2005). Furthermore, both rasagiline and proppargylamine activate neuroprotective protein kinase C (PKCα and PKCε), while down-regulating propaoptotic PKCδ and γ. Inhibition of PKC by GF109203X prevents their neuroprotective activity (Weinreb et al. 2005; Youdim et al. 2005a). The mechanism by which aminoindan has been fully elucidated. The neuroprotective properties of 1-R-aminoindan have been assessed employing a cytotoxic model of human neuroblastoma SKN-SH cells in high-density culture-induced neuronal death and in response to 6-hydroxydopamine. We show that 1-R-aminoindan (0.1–1 µM) significantly reduced the apoptosis-associated phosphorylated protein, H2A.X (Ser139), decreased the cleavage of caspase 9 and caspase 3, while increasing the anti-apoptotic proteins, Bcl-2 and Bcl-xl. Protein kinase C (PKC) inhibitor, GF109203X, prevented the neuroprotection, indicating the involvement of PKC in aminoindan-induced cell survival. Aminoindan markedly elevated pPKC (pan) and specifically that of the pro-survival PKC isoform, PKC epsilon (Bar-Am et al. 2007).
In summary, the neurorptoective activity seen with rasagiline and its major metabolite, 1-R-aminoindan in the present and previous studies, may have relevance to the recent prospective clinical study in Parkinsonian subjects, ADAGIO, where rasagiline indicated that early treatment with rasagiline provided benefits that were not obtained with later initiation of the drug. This is the first time that a prospective large-scale, randomized, double-blind trial has provided evidence that a drug might slow down PD progression via neuroprotection (Hughes 2008).
Acknowledgments
This study was supported by Public Health Service Grants P20 RR17701, a NARSAD Young Investigator Award and an Intramural Research Support grant from The University of Mississippi Medical Center. Shawna Tazik and Shakevia Johnson were supported by the Neuroscience Summer Scholars Program (Department of Psychiatry, University of Mississippi Medical Center) We acknowledge Teva Pharmaceutical Co. (Israel) and Hailin Zheng for synthesizing rasagiline and Dr. B. Westermark for providing 1242-MG cells.
Contributor Information
Shawna Tazik, Division of Neurobiology and Behavioral Research, Department of Psychiatry and Human Behavior (G-109), University of Mississippi Medical Center, 2500N. State Street, Jackson, MS 39216, USA.
Shakevia Johnson, Division of Neurobiology and Behavioral Research, Department of Psychiatry and Human Behavior (G-109), University of Mississippi Medical Center, 2500N. State Street, Jackson, MS 39216, USA.
Deyin Lu, Division of Neurobiology and Behavioral Research, Department of Psychiatry and Human Behavior (G-109), University of Mississippi Medical Center, 2500N. State Street, Jackson, MS 39216, USA.
Chandra Johnson, Division of Neurobiology and Behavioral Research, Department of Psychiatry and Human Behavior (G-109), University of Mississippi Medical Center, 2500N. State Street, Jackson, MS 39216, USA.
Moussa B. H. Youdim, Eve Topf Center, Technion-Rappaport Family Faculty of Medicine, Haifa, Israel
Craig A. Stockmeier, Division of Neurobiology and Behavioral Research, Department of Psychiatry and Human Behavior (G-109), University of Mississippi Medical Center, 2500N. State Street, Jackson, MS 39216, USA Department of Psychiatry, Case Western Reserve University, Cleveland, OH, USA.
Xiao-Ming Ou, Email: xou@psychiatry.umsmed.edu, Division of Neurobiology and Behavioral Research, Department of Psychiatry and Human Behavior (G-109), University of Mississippi Medical Center, 2500N. State Street, Jackson, MS 39216, USA.
References
- Bar-Am O, Weinreb O, Amit T, Youdim MB. Regulation of Bcl-2 family proteins, neurotrophic factors, and APP processing in the neurorescue activity of propargylamine. FASEB J. 2005;19:1899–1901. doi: 10.1096/fj.05-3794fje. [DOI] [PubMed] [Google Scholar]
- Bar-Am O, Amit T, Youdim MB. Aminoindan and hydroxyaminoindan, metabolites of rasagiline and ladostigil, respectively, exert neuroprotective properties in vitro. J Neurochem. 2007;103:500–508. doi: 10.1111/j.1471-4159.2007.04777.x. [DOI] [PubMed] [Google Scholar]
- Carlo P, Violani E, Del Rio M, Olasmaa M, Santagati S, Maggi A, Picotti GB. Monoamine oxidase B expression is selectively regulated by dexamethasone in cultured rat astrocytes. Brain Res. 1996;711:175–183. doi: 10.1016/0006-8993(95)01353-9. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Adolfsson R, Aquilonius SM, Gottfries CG, Oreland L, Svennerholm L, Winblad B. Biogenic amines in human brain in normal aging, senile dementia, and chronic alcoholism. Adv Biochem Psychopharmacol. 1980;23:295–304. [PubMed] [Google Scholar]
- de Kloet ER, Reul JM, Sutanto W. Corticosteroids and the brain. J Steroid Biochem Mol Biol. 1990;37:387–394. doi: 10.1016/0960-0760(90)90489-8. [DOI] [PubMed] [Google Scholar]
- Dolmatov IY, Dolmatova LS, Shitkova OA, Kovaleva AL. Dexamethasone-induced apoptosis in phagocytes of holothurians. London: Taylor & Francis Group; 2004. [Google Scholar]
- Duval F, Mokrani MC, Monreal-Ortiz JA, Fattah S, Champeval C, Schulz P, Macher JP. Cortisol hypersecretion in unipolar major depression with melancholic and psychotic features: dopaminergic, noradrenergic and thyroid correlates. Psychoneuroendocrinology. 2006;31:876–888. doi: 10.1016/j.psyneuen.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Edelstein SB, Breakefield XO. Monoamine oxidases A and B are differentially regulated by glucocorticoids and “aging” in human skin fibroblasts. Cell Mol Neurobiol. 1986;6:121–150. doi: 10.1007/BF00711066. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, Chen JJ. Monamine oxidase inhibitors: current and emerging agents for Parkinson disease. Clin Neuropharmacol. 2007;30:150–168. doi: 10.1097/01.wnf.0000240956.49315.be. [DOI] [PubMed] [Google Scholar]
- Geha RM, Rebrin I, Chen K, Shih JC. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276:9877–9882. doi: 10.1074/jbc.M006972200. [DOI] [PubMed] [Google Scholar]
- Glick RD, Medary I, Aronson DC, Scotto KW, Swendeman SL, La Quaglia MP. The effects of serum depletion and dexamethasone on growth and differentiation of human neuroblastoma cell lines. J Pediatr Surg. 2000;35:465–472. doi: 10.1016/s0022-3468(00)90216-1. [DOI] [PubMed] [Google Scholar]
- Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8:59–64. doi: 10.1517/14656566.8.1.59. [DOI] [PubMed] [Google Scholar]
- Hughes New hope for Parkinson’s disease progression delay. Nat Rev Drug Discov. 2008;7:791. [Google Scholar]
- Jacobs CM, Trinh MD, Rootwelt T, Lomo J, Paulsen RE. Dexamethasone induces cell death which may be blocked by NMDA receptor antagonists but is insensitive to Mg2+ in cerebellar granule neurons. Brain Res. 2006;1070:116–123. doi: 10.1016/j.brainres.2005.10.093. [DOI] [PubMed] [Google Scholar]
- Johnson S, Williams AN, Johnson C, Ou XM. The effects of antidepressant drug on ethanol-induced cell death. Drug Discov Ther. 2007;1:130–135. [PubMed] [Google Scholar]
- Kato M, Katayama T, Iwata H, Yamamura M, Matsuoka Y, Narita H. In vivo characterization of T-794, a novel reversible inhibitor of monoamine oxidase-A, as an antidepressant with a wide safety margin. J Pharmacol Exp Ther. 1998;284:983–990. [PubMed] [Google Scholar]
- Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- Malorni W, Giammarioli AM, Matarrese P, Pietrangeli P, Agostinelli E, Ciaccio A, Grassilli E, Mondovi B. Protection against apoptosis by monoamine oxidase A inhibitors. FEBS Lett. 1998;426:155–159. doi: 10.1016/s0014-5793(98)00315-9. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Kaplan RD, Bird ED. Elevated postmortem monoamine oxidase B activity in the caudate nucleus in Huntington’s disease compared to schizophrenics and controls. J Neural Transm. 1986;65:277–283. doi: 10.1007/BF01249088. [DOI] [PubMed] [Google Scholar]
- Maruyama W, Akao Y, Youdim MB, Davis BA, Naoi M. Transfection-enforced Bcl-2 overexpression and an anti-Parkinson drug, rasagiline, prevent nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase induced by an endogenous dopaminergic neurotoxin, N-methyl(R)salsolinol. J Neurochem. 2001;78:727–735. doi: 10.1046/j.1471-4159.2001.00448.x. [DOI] [PubMed] [Google Scholar]
- Murphy BE. Antiglucocorticoid therapies in major depression: a review. Psychoneuroendocrinology. 1997;22:S125–S132. [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by R1 and Sp1. J Biol Chem. 2006a;281:21512–21525. doi: 10.1074/jbc.M600250200. [DOI] [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci U S A. 2006b;103:10923–10928. doi: 10.1073/pnas.0601515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson IA, Barber AJ, Gelowitz DL, Voll C. (−)Deprenyl reduces delayed neuronal death of hippocampal pyramidal cells. Neurosci Biobehav Rev. 1997;21:181–186. doi: 10.1016/s0149-7634(96)00008-5. [DOI] [PubMed] [Google Scholar]
- Phillips AJ, Sudbery I, Ramsdale M. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc Natl Acad Sci USA. 2003;100:14327–14332. doi: 10.1073/pnas.2332326100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DS, Amsterdam JD. The selegiline transdermal system in major depressive disorder: a systematic review of safety and tolerability. J Affect Disord. 2007;105:15–23. doi: 10.1016/j.jad.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Saura J, Luque JM, Cesura AM, Da Prada M, Chan-Palay V, Huber G, Loffler J, Richards JG. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience. 1994;62:15–30. doi: 10.1016/0306-4522(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Schneider G, Oepen H, von Wedel HR. Monoamine oxidase activity in brain regions and organs of patients with Parkinson’s disease and Huntington’s disease and serum MAO activity of patients with Huntington’s disease as compared with neurologically healthy individuals. Arch Psychiatr Nervenkr. 1981;230:5–15. doi: 10.1007/BF00343762. [DOI] [PubMed] [Google Scholar]
- Senatorov VV, Charles V, Reddy PH, Tagle DA, Chuang DM. Overexpression and nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase in a transgenic mouse model of Huntington’s disease. Mol Cell Neurosci. 2003;22:285–297. doi: 10.1016/s1044-7431(02)00013-1. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Ritchie JC. Effects of aging and glucocorticoid treatment on monoamine oxidase subtypes in rat cerebral cortex: therapeutic implications. Brain Res Bull. 1998;47:345–348. doi: 10.1016/s0361-9230(98)00111-7. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Cohen RM, Sunderland T, Newhouse PA, Yount D, Mellow AM, Weingartner H, Mueller EA, Murphy DL. L-deprenyl in Alzheimer’s disease. Preliminary evidence for behavioral change with monoamine oxidase B inhibition. Arch Gen Psychiatry. 1987;44:427–433. doi: 10.1001/archpsyc.1987.01800170041007. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Chalmers-Redman RM, Elstner M, Leesch W, Jagodzinski FB, Stupak DP, Sugrue MM, Tatton NA. Glyceraldehyde-3-phosphate dehydrogenase in neurodegeneration and apoptosis signaling. J Neural Transm Suppl. 2000;60:77–100. doi: 10.1007/978-3-7091-6301-6_5. [DOI] [PubMed] [Google Scholar]
- Volz HP, Gleiter CH. Monoamine oxidase inhibitors. A perspective on their use in the elderly. Drugs Aging. 1998;13:341–355. doi: 10.2165/00002512-199813050-00002. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Bar-Am O, Chillag-Talmor O, Youdim MB. Novel neuroprotective mechanism of action of rasagiline is associated with its propargyl moiety: interaction of Bcl-2 family members with PKC pathway. Ann NY Acad Sci. 2005;1053:348–355. doi: 10.1196/annals.1344.030. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI. Treatment of depression with antiglucocorticoid drugs. Psychosom Med. 1999;61:698–711. doi: 10.1097/00006842-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Youdim MB. The path from anti Parkinson drug selegiline and rasagiline to multifunctional neuroprotective anti Alzheimer drugs ladostigil and m30. Curr Alzheimer Res. 2006;3:541–550. doi: 10.2174/156720506779025288. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Banerjee DK, Kelner K, Offutt L, Pollard HB. Steroid regulation of monoamine oxidase activity in the adrenal medulla. FASEB J. 1989;3:1753–1759. doi: 10.1096/fasebj.3.6.2495232. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Gross A, Finberg JP. Rasagiline [N-propargyl-1R(+)-aminoindan], a selective and potent inhibitor of mitochondrial monoamine oxidase B. Br J Pharmacol. 2001a;132:500–506. doi: 10.1038/sj.bjp.0703826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Wadia A, Tatton W, Weinstock M. The anti-Parkinson drug rasagiline and its cholinesterase inhibitor derivatives exert neuroprotection unrelated to MAO inhibition in cell culture and in vivo. Ann NY Acad Sci. 2001b;939:450–458. doi: 10.1111/j.1749-6632.2001.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Maruyama W, Naoi M. Neuropharmacological, neuroprotective and amyloid precursor processing properties of selective MAO-B inhibitor antiparkinsonian drug, rasagiline. Drugs Today (Barc) 2005a;41:369–391. doi: 10.1358/dot.2005.41.6.893613. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Bar-Am O, Yogev-Falach M, Weinreb O, Maruyama W, Naoi M, Amit T. Rasagiline: neurodegeneration, neuroprotection, and mitochondrial permeability transition. J Neurosci Res. 2005b;79:172–179. doi: 10.1002/jnr.20350. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]