Abstract
The orphan G protein coupled receptor, GPR30, has the characteristics of a high affinity, specific estrogen membrane receptor on Atlantic croaker oocytes and mediates estrogen inhibition of oocyte maturation in this perciform fish. In order to determine the broad applicability of these findings to other teleosts, similar experiments were conducted in a cyprinid fish, zebrafish, in the present study. GPR30 mRNA expression was detected in zebrafish oocytes but not in the ovarian follicular cells. Both spontaneous and 17, 20β-dihyroxy-4-pregnen-3-one (DHP)-induced maturation of follicle-enclosed zebrafish oocytes was significantly decreased when they were incubated with either estradiol-17β, or the GPR30 agonists, ICI 182 780 and tamoxifen, or with the GPR30 specific agonist G-1. On the other hand spontaneous oocyte maturation increased two-fold when zebrafish ovarian follicles were incubated with an aromatase inhibitor, ATD. Moreover, the stimulatory effects of ATD on germinal vesicle breakdown (GVBD) were partially reversed by co-treatment with 100 nM of E2 or G-1. These results suggest that endogenous estrogens acting through GPR30 are involved in maintaining meiotic arrest of zebrafish oocytes.
Keywords: G protein-coupled receptor 30, GPR30, oocyte maturation, aromatase inhibitor, meiosis arrest
Introduction
Recent studies show that the orphan G protein coupled receptor, GPR30, originally cloned from human and rat tissues (O’Dowd et al., 1998), is involved in estrogen signaling in breast cancer cells (Filardo et al., 2000; Filardo et al., 2002) and may function as a membrane estrogen receptor (Filardo & Thomas, 2005). Studies in our laboratory, subsequently confirmed by another group, showed that recombinant human GPR30 displays high affinity, specific estrogen binding typical of an estrogen membrane receptor (Revankar et al., 2005; Thomas et al., 2005). Moreover, we showed that estrogen binding to GPR30 caused activation of a stimulatory G protein resulting in increased cAMP production (Thomas et al., 2005). Subsequently we cloned and characterized GPR30 from a representative of the distantly-related teleost fishes, Atlantic croaker, to test the hypothesis that estrogen receptor binding and signal transduction is a fundamental and shared function of the GPR30 protein in the vertebrates. Atlantic croaker GPR30 had similar estrogen receptor binding and signaling characteristics as human GPR30 and showed high expression in croaker oocytes (Pang et al., 2008). In addition preliminary evidence was obtained for an involvement of GPR30 in maintaining oocyte meiotic arrest in croaker oocytes (Pang et al., 2008). Furthermore, GPR30 was also identified in zebrafish oocytes and microinjection of antisense mopholino oligo nucleotides to the GPR30 mRNA sequence into zebrafish oocytes resulted in an increase in oocyte maturation (Pang et al., 2008), indicating that GPR30 also may play a role in controlling oocyte maturation in this fish species. To further examine the roles of estrogens acting through GPR30 in the regulation of meiotic arrest in zebrafish, in the present study the effects of inhibiting endogenous estrogen production with an aromatase inhibitor as well as incubation with GPR30 agonists on oocyte maturation were investigated in this second fish model belonging to a different teleost Superorder, Ostariophysi.
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Steraloids Inc. (Newport, RI) unless otherwise stated. G-1, the GPR30 specific agonist, was purchased from EMD Chemicals (San Diego, CA). The nuclear estrogen receptor antagonist, ICI 182 780 was purchased from Tocris Biosciences (Ellisville, MO).
Animals
Mature zebrafish (Danio rerio) were purchased from a local pet store and maintained at a ratio of 1 male to 5 females in 50 L aquaria at 26°C on a 14-h light: 10-h dark photoperiod. The fish were fed twice a day with commercial tropical fish food and frozen brine shrimp and given a supplement of live brine shrimp twice a week.
Isolation of zebrafish ovarian follicles and GVBD bioassay
Ovarian follicles were isolated and incubated according to procedures described previously (Pang & Ge, 2002). Briefly, gravid female zebrafish were anaesthetized in 0.01% tricaine methanesulfonate solution for 2 min and humanely killed by severing the spinal cord using a protocol approved by the University of Texas at Austin Animal Care and Use Committee. The ovaries were then removed, placed in a 35-mm culture dish and washed several times with 60% Leibovitz L-15 medium. The follicles were carefully separated with the aid of a fine scalpel blade without damaging the ovarian membrane and their diameters measured with an ocular micrometer under a dissecting microscope. Intact vitellogenic and full-grown follicles 0.50–0.55 mm in diameter were selected, pooled, and randomly distributed in wells of a 24-well plate (Falcon) for the in vitro GVBD experiments (30–40 follicles/1 ml medium/well). The follicles were incubated for 6–16 h in the presence or absence of 17, 20β-dihydroxy-4-pregnen-3-one (DHP), estrogens, and the aromatase inhibitor, 1,4,6-Androstatrien-3,17-dione (ATD). The follicles were scored at the end of incubation for GVBD, an easily identifiable marker for oocyte maturation. All experiments were repeated three times to confirm the results.
Preparation of ovarian follicle cell and denuded oocyte fractions
Isolated ovarian follicles were washed several times with 5 ml 60% L-15 medium followed by a 30 minute treatment at room temperature with 0.1 mg/ml collagenase (Sigma). The ovarian preparation was subsequently forced through a narrow pipette (~1 mm diameter) 10–20 times to break up the tissue. The resulting mixture of follicle cells and denuded oocytes was transferred to a new tube and allowed to settle for 1 minute. The supernatant containing the follicle cells was then transferred to 1.5 ml tubes and washed 2 times with L-15 medium by centrifugation (1000 rpm, 2 min). The effectiveness of the separation procedure was confirmed by examination of the denuded oocytes and follicle cell suspensions under a microscope, followed by DAPI staining of the oocytes to confirm they were completely denuded of follicle cells. The denuded oocyte and follicle cell fractions were either used for extraction of total RNA immediately, or stored at −80C for later analysis.
RT-PCR
Total RNA was extracted with Tri-reagent (Sigma-Aldrich) from zebrafish oocytes and ovarian follicle cells according to the protocol provided by the manufacturer. The integrity of mRNA was confirmed by electrophoresis on a denaturing agarose gel followed by staining with ethidium bromide. Total RNA (1–3 μg) was reverse transcribed into cDNA at 50°C for 1 h in 10 μl reaction solution containing 1 × First Strand Buffer, 10 mM dithiothreitol, 0.5 mM each dNTP, 0.5 μg oligo-dT, and 100 U SuperScript III (Invitrogen). Each PCR reaction was carried out in 20 μl reaction mix diluted from 2 × PCR master mix (Promega) with 0.5 μl of the RT product. The zebrafish GPR30 primers, designed according to the sequence of zebrafish GPR30 (GenBank accession No. XM_688551), were: sense, 5′CAT CGG CCT GTT TCT CTC AT; antisense, 5′GTA GCA CAG GCC GAT AAT, with an expected PCR product size of 534 base pairs. After an initial denaturation for 4 min at 94°C, the cycling reaction was performed on a thermal cycler (Eppendorf) with a profile of 30 seconds at 94°C, 30 seconds at 55°C, and 1 min at 72°C for 35 cycles, followed by a 10-min extension at 72°C. The PCR reaction mix (10 μl) was electrophoresed on an agarose gel containing ethidium bromide to visualize the products. β-actin gene (primers: sense, 5′ GAG CAG GAG ATG GGA ACC; antisense, GAT GGA GTT GAA GGT GGT CT, with an expected PCR product size of 176 base pairs ) was chosen for an internal control to normalize the mRNA concentration in the RT reaction.
Results
Detection of GPR30 mRNA in zebrafish oocytes and ovarian follicle cells
GPR30 mRNA was detected in zebrafish oocytes but not in the surrounding follicle cells after 35 PCR cycles (Fig. 1). The β-actin RT-PCR confirmed the presence of equivalent amounts of mRNA in the oocyte and follicle cell preparations.
Fig. 1.
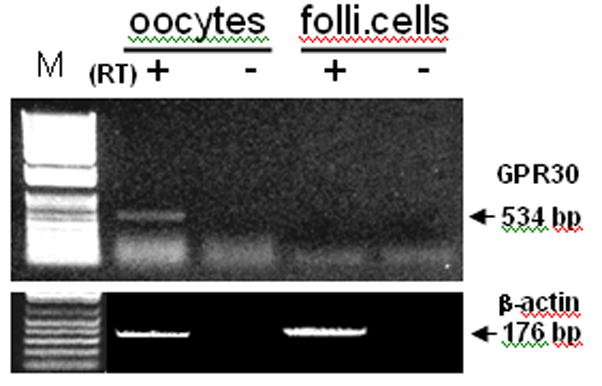
RT-PCR detection of GPR30 mRNA in oocytes and ovarian follicle cells. RT +, -: PCR with or without reverse transcript of total RNA samples. M: DNA marker.
Effects of E2, ICI 182780 and tamoxifen on DHP–induction of GVBD
DHP (10 nM) significantly increased the percentage of fully-grown oocytes (500–550 μm in diameter) that had completed GVBD (63.9±2.5%) compared to vehicle treated controls (34.2±5.8%) after 16 h incubation. Co-treatment with E2 (20 nM), ICI 182780, or tamoxifen significantly attenuated the GVBD in response to DHP and the oocytes that completed GVBD (35.8±8.7%, 30.50±11.1% and 26.90±4.27%, respectively) were not significantly different than that of the vehicle-treated controls (Fig. 2)
Fig. 2.
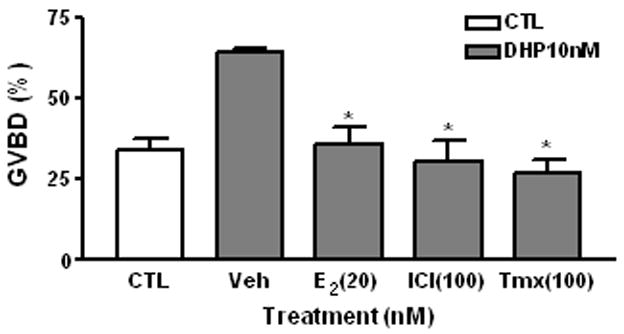
Effects of E2, ICI182 780 and tamoxifen on DHP-induced GVBD of zebrafish oocytes. CTL, control group. Veh, vehicle. ICI, ICI 182 780. Tmx, Tamoxifen. Incubation period: 16 hrs. *: P<0.05 compared to vehicle (Veh) group. The experiment was repeated 3 times and similar results were obtained each time.
Effects of the GPR30 specific agonist G-1 on DHP induction of GVBD
G-1 (20 nM) exhibited a significant inhibitory effect on DHP-induced oocyte maturation (GVBD G-1: 37.7±4.5%, DHP: 56.1±3.5%), similar to that observed with E2 and the other GPR30 agonists. The percent of oocytes that completed GVBD in the G-1 group was low (5.4±2.8%) but was not significantly different from controls (9.0±2.20 %) (Fig. 3).
Fig. 3.
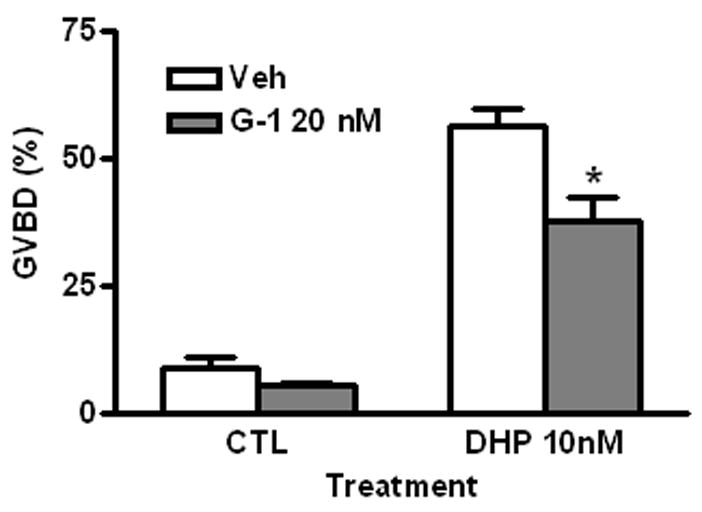
Effects of G-1 on spontaneous and DHP-induced oocyte GVBD. Veh, vehicle. CTL, control. Incubation period: 16 hrs. *: P<0.05 compared to Veh group. The experiment was repeated 3 times and similar results were obtained each time.
Effects of the aromatase inhibitor ATD on oocyte maturation
Ovarian follicles were treated with the aromatase inhibitor, ATD, to determine whether endogenous estrogens are produced by ovarian follicles in sufficient amounts to influence oocyte maturation. Treatment with 10 μg/ml of ATD increased the percent of oocytes that completed GVBD five-fold over control levels GVBD to (ATD: 65.9±4.5%, compared to controls: 12.3±7.1%, P<0.05). Co-treatment with 20 nM E2 or G-1 reversed the effects of ATD on GVBD (42.5±6.6% and 47.3±7.8%, respectively, P<0.05) (Fig. 4).
Fig. 4.
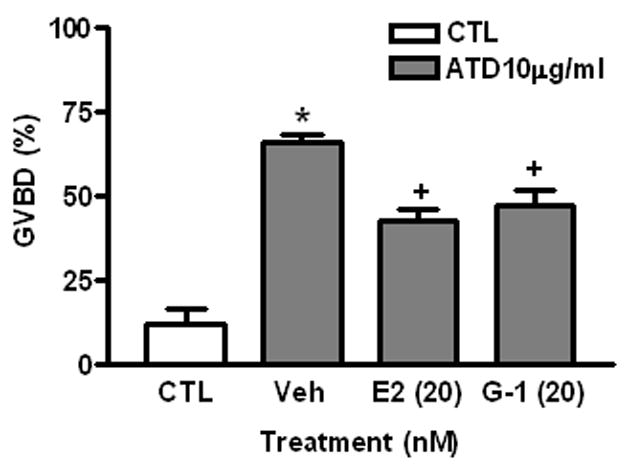
Effects of E2 and G-1 on ATD-induced oocyte GVBD. CTL, control. Veh, vehicle. Incubation period: 6 hrs. *: P<0.05 compared to CTL; +: P<0.05 compared to Veh. The experiment was repeated 3 times and similar results were obtained each time.
Discussion
The present results support our previous preliminary findings suggesting estrogens inhibit the meiotic maturation of zebrafish oocytes through activation of the 7-transmembrane estrogen receptor, GPR30. The RT-PCR demonstrated the presence of GPR30 mRNA in zebrafish oocytes. The bioassay results showed that treatment with E2, as well as the GPR30 agonists tamoxifen and ICI 182 780, and the specific GPR30 agonist G-1 caused a significant inhibition of DHP-induced oocyte maturation. On the other hand, suppression of endogenous estrogen production by treatment with the aromatase inhibitor, ATD, caused a marked increase in the percent of oocytes that matured. Finally it was shown that the ATD-induced increase in oocyte maturation could be partially reversed by co-treatment with E2 and G-1. The finding that estrogens act via the same receptor mechanism to inhibit the resumption of oocyte maturation in two distantly-related teleost species, representing two major teleosts superorders, Acanthopterigii and Ostariophysi, suggests a widespread role for GPR30 in regulating oocyte maturation amongst teleost fishes.
The possibility that GPR30 has a similar physiological role in the regulation of oocyte maturation in other vertebrate groups remains to be investigated. GPR30 has been identified in hamster oocytes and its expression is regulated by gonadotropins (Wang et al., 2007). In contrast to the findings in the Atlantic croaker ovary, where GPR30 is only expressed in the oocytes, GPR30 is also present in the follicle cells of the hamster ovary (Pang et al., 2008, Wang et al., 2008), which suggests that GPR30 has additional physiological functions in mammalian ovaries.
The finding that the nuclear receptor antagonists, ICI 182 780 and tamoxifen, as well as the specific GPR30 agonist, G-1 (Bologa et al., 2006), mimic the inhibitory effects of E2 on DHP-induced maturation of zebrafish oocytes is in agreement with previous results with Atlantic croaker oocytes (Pang et al., 2008) and suggests this estrogen action is mediated through GPR30 in zebrafish. Both ICI 182 780 and tamoxifen bind to human and croaker GPR30 and act as agonists, causing G protein activation and increases in cAMP production (Pang et al., 2008; Thomas et al., 2005). The estrogen agonist activities of ICI 182 780, tamoxifen and G-1 at concentrations of 20–100 nM observed in the present study suggests that these compounds have relatively high binding affinities for zebrafish GPR30, similar to their binding affinities for croaker and human GPR30.
A major finding of the present study was that inhibition of aromatase activity in the zebrafish ovarian follicle by ATD caused a marked increase in the rate of spontaneous oocyte maturation, similar to the findings with croaker oocytes (Pang et al., 2008). These results showing that endogenous estrogen production by the ovarian follicle is involved in regulating the resumption of oocyte meiotic maturation in representatives of two teleost Suborders suggests this estrogen action is of widespread importance in teleost fish ovarian physiology. Moreover, the demonstration that the stimulatory effect of ATD can be partially reversed by co-treatment with G-1 in zebrafish as well as in croaker suggests GPR30 is the common mediator of this novel estrogen action on teleost oocytes.
A central dogma in teleost ovarian physiology is that estrogen production declines after the oocyte has completed the vitellogenic growth phase and that a periovulatory surge in luteinizing hormone (LH) causes a switch in the steroidogenic pathway in the follicle cells from the production of androgens and estrogens to the production of progestin hormones (Khan and Thomas, 1998; Nagahama, 1994). The progestin hormones, 17, 20β-dihydroxy-4-pregnen-3-one and 17,20β, 21-trihydroxy-4-pregnen-3-one, induce meiotic maturation on fish oocytes by activating membrane progestin receptors (mPRs) on the surface of oocytes and subsequently induce ovulation through binding to a nuclear progestin receptor in the ovarian follicle cells (Thomas et al., 2002). Although E2 can frequently be detected in the circulation of fish during the periovulatory period, especially in species with asynchronous oocyte development with developing ovarian follicles containing secondary growth phase (vitellogenic) oocytes, estrogens have not until now been proposed to have a role in regulating the onset of oocyte maturation. There is evidence from studies in several teleost species, including croaker, that gonadotropin can cause an increase in E2 production by fully grown ovarian follicles both in vivo and in vitro that immediately precedes the increase in progestin secretion during oocyte maturation (Sakai et al., 1987; Trant & Thomas, 1989). However, it is uncertain whether a transient increase in estrogen production associated with the periovulatory surge in LH secretion is widespread amongst teleosts, because it has not been reported in a variety of fish species that have been investigated extensively. Even if LH does not increase E2 production during the periovulatory period in a particular fish species, basal estrogen production by the ovarian follicle could be sufficient to influence the initiation of oocyte meiosis. Our current hypothesis is that the powerful inhibitory effects of endogenous estrogens on the resumption of meiosis in fully grown oocytes immediately prior to maturation prevents some of them from maturing precociously, maintaining meiotic arrest until the stimulatory progestin system is fully developed, thereby synchronizing meiotic maturation and ovulation of each batch of oocytes to be spawned. Additional studies with aromatase inhibitors in a wider range of teleost species will be required to test this hypothesis.
Acknowledgments
This research was funded by NIH grant ESO ESO12961 to P.T
This manuscript is affiliated with 6th International Symposium in Fish Endocrinology (6ISFE)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Khan IA, Thomas P. Ovarain cycle, Teleost Fish. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. Vol. 3. Acdemic Press; 1998. pp. 552–564. [Google Scholar]
- Nagahama Y. Endocrine regulation of gametogenesis in fish. Int J Dev Biol. 1994;38:217–229. [PubMed] [Google Scholar]
- O’Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH, Kolakowski LF, Jr, George SR. Discovery of three novel G-protein-coupled receptor genes. Genomics. 1998;47:310–313. doi: 10.1006/geno.1998.5095. [DOI] [PubMed] [Google Scholar]
- Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149:3410–3426. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Ge W. Gonadotropin regulation of activin betaA and activin type IIA receptor expression in the ovarian follicle cells of the zebrafish, Danio rerio. Mol Cell Endocrinol. 2002;188:195–205. doi: 10.1016/s0303-7207(01)00719-5. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Sakai N, Iwamatsu T, Yamauchi K, Nagahama Y. Development of the steroidogenic capacity of medaka (Oryzias latipes) ovarian follicles during vitellogenesis and oocyte maturation. Gen Comp Endocrinol. 1987;66:333–342. doi: 10.1016/0016-6480(87)90242-5. [DOI] [PubMed] [Google Scholar]
- Thomas P, Zhu Y, Pace M. Progestin membrane receptors involved in the meiotic maturation of teleost oocytes: A review with some new findings. Steroids. 2002;67:511–517. doi: 10.1016/s0039-128x(01)00180-5. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Trant JM, Thomas P. Changes in ovarian steroidogenesis in vitro associated with final maturation of Atlantic croaker oocytes. Gen Comp Endocrinol. 1989;75:405–412. doi: 10.1016/0016-6480(89)90175-5. [DOI] [PubMed] [Google Scholar]
- Wang C, Prossnitz ER, Roy SK. Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology. 2007;148:4853–4864. doi: 10.1210/en.2007-0727. [DOI] [PubMed] [Google Scholar]
- Wang C, Prossnitz ER, Roy SK. GPR30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology. 2008 doi: 10.1210/en.2008-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]


