Summary
The vast majority of peripheral T cells exist as resting lymphocytes until a signal for activation has been received. In response to antigen, this involves ligation of the T cell receptor (TCR) and signal transmission through the CD3 complex, which then initiates a cascade of intracellular events that leads to the expression of genes used in T cell activation. T cell activation also requires soluble mediators in the form of cytokines and chemokines that regulate the process in both positive and negative ways, and costimulatory signals received in conjunction with TCR/CD3 signaling are important in the activation of T cells. Unlike T cells in other peripheral immune compartments, small and large intestinal intraepithelial lymphocytes (IELs) bear some but not all properties of activated T cells, suggesting that they constitute a large population of ‘partially-activated’ effector cells. Because of that, regulation of the IEL activation process must be held in tight check, yet it must be ready to respond to foreign antigen rapidly and effectively. We discuss how costimulatory molecules may hold the key to controlling IEL activation through a multi-phase process beginning with cells that have already entered into the early stage of activation.
Keywords: costimulation, IEL, T cell, epithelium, regulation
Introduction
The intention of this article is to address issues pertaining to the underlying mechanisms of IEL activation with special emphasis on the role played by costimulatory molecules and molecular mediators. Although costimulation is now widely regarded as a major contributing event in the activation of peripheral T cells, considerably less attention has been placed on costimulation as a factor involved in IEL activation. The view presented here will be from that of the IELs themselves and only peripherally from the perspective of the cells that participate in the activation process, i.e., professional antigen-presenting cells and the allied costimulatory molecules and ligands that drive T cell activation. The discussion of IEL costimulation will be limited to that which occurs in conjunction with TCR/CD3 signaling, and will not include costimulation of alternate receptor forms, such as IEL natural killer (NK) receptors, despite their obvious importance to the activation of some types of IELs. Emphasis will be placed on groups of IEL-borne costimulatory molecules and receptors in an effort to place the activation process within a contextual framework of events that deliver IELs into a state of full activation.
Are IELs activated T cells?
Intestinal IELs are a phenotypically, developmentally, and functionally complex populations of cells (1-4). The question of whether IELs are resting T cells, activated T cells, or are T cells that exist in some intermediate stage of activation, has been debated for many years. Early studies into the function of IELs demonstrated that they consist of a high proportion of NK cells (5-8). Subsequent studies revealed that both TCRαβ and TCRγδ are cytolytic upon isolation from the intestinal epithelium in the absence of overt stimulation (9-12), and that most IELs express some markers of activated T cells, such as the CT antigen (in mice) (10), CD69 (13-16), or CD11c, the latter being a marker of activated intestinal CD8+ T cells (17). Those observations are paralleled by studies using OT-1 mice, which express a TCR transgene for an MHC-I-restricted ovalbumin peptide antigen, and have cytolytic activity in the absence of antigen priming (18). Similar findings have been reported for KN6 TCRγδ transgenic mice, in which IELs but not spleen cells from either germ-free or conventional mice were found to be cytolytic in redirected cytotoxicity assays (19). Upon activation following CD3ε ligation, murine CD8+ IELs produce IFN-γ more rapidly and to greater levels than CD8+ lymph node cells (15). Moreover, the activation state of CD4+ intestinal lamina propria cells also appears to reflect the nature of the intestinal response to oral antigens (20).
However, despite the presence of cytotoxicity — a hallmark of CD8+ effector T cells — IELs do not behave as a population of activated T cells in most other ways. For example, they do not express many of the markers commonly present on activated T cells such as CD25 or CD122; they do not spontaneously elaborate Th1 or Th2 cytokines; and they are not actively proliferating cells (15). Consistent with those observations, gene microarray studies of murine TCRαβ and/or TCRγδ IELs point to a population of cells with characteristics of both activated and resting T cells (21, 22). Thus, a picture emerges in which IELs, in both the small and large intestine, exist in a novel state of intermediate or partial T cell activation. Though IELs have been described as being ‘activated yet resting’ T cells (21), as just described, they lack many of the basic properties of an activated T cells population. Accordingly, the discussion in this paper will follow the premise that IELs are ‘partially-activated’ T cells waiting for appropriate signals to thrust them into a state of full activation.
T cell costimulation holds the key to maintaining the activation status of IELs
T cell activation can be viewed as a process that is regulated through an on-off switching mechanism. While this works well for most peripheral T cells, the IELs do not fit well into that model for the reasons just described. Hence, metaphorically, it might be more appropriate to view IELs as a cell population controlled by a ‘dimmer’ switch, in that they already possess some of the initial properties of activation and are awaiting external signals for full charging. In that scenario there would be a need for mechanisms to control both suppression and rapid-activation. The conundrum, of course, is how to sustain the IELs in the partially-activated state. Costimulation could serve both purposes in that regard. Many molecules and soluble molecular mediators now have been identified that may function as regulatory elements for suppression or activation. These include but are not limited to CD43, CD59 (Ly-6C), CD69, CD134 (OX40), CD137 (4-1BB), CD160 (By55), CD278 (ICOS), interleukin (IL)-18, and transforming growth factor (TGF)β1. It should be noted that with the exception of some CD4+ IELs, the prototypical T cell costimulatory molecule, CD28, is not widely expressed on IELs (23).
In general, IEL costimulatory signals can be viewed as belonging to two groups:
costimulatory surface antigens or receptors that are constitutively expressed on IELs,
costimulatory surface antigens or receptors that are upregulated following immune activation via stimulation through the TCR/CD3 complex.
Moreover, each type of costimulatory molecule will exert differential effects on the activation process, dictated by the time of expression of the costimulatory element, the subset(s) of IELs which bear it, and the types of immune responses that are elicited by it.
Constitutively-expressed costimulatory molecules
CD69/TGFβ1 — holding the brakes on IEL activation
The constitutively expressed costimulatory molecules/receptors discussed here include CD69, CD43, CD160, and the IL-18 receptor (IL-18R); however, only CD43 is probably expressed on T cells prior to entry into the intestinal mucosa.
CD69 is a type-II membrane glycoprotein that is expressed on >95% of all murine IELs (24, 25). Although CD69 expression might be assumed to reflect the activated cytotoxic nature of IELs (9-12), and thus would be consistent with a population of T cells in the early throws of activation (26), its function on IELs remains elusive. In fact, a surprising feature of CD69 expression is its lack of association with a proinflammatory state as seen from studies in mice deficient in CD69, which have exacerbated autoimmune and anti-tumor responses (27, 28), and given that CD69 transgenic mice show few T cell abnormalities (29).
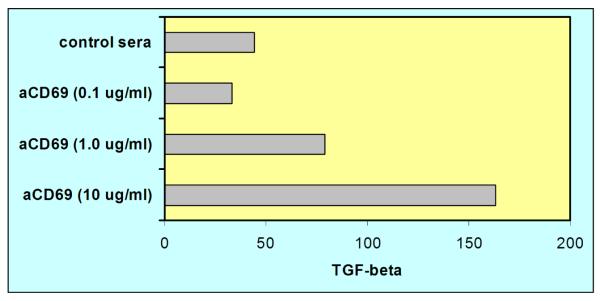
Recent studies suggest that CD69 is probably acquired rapidly on T cells upon entry into the lamina propria and epithelium (30), and that its expression may be influenced by local glucocorticoid production (31). However, given that all entering IELs would not be expected to have received a TCR/CD3-mediated activation signal, the presence of CD69 may be emblematic of functions not specifically linked to IEL activation. One possibility is that, even as IELs are beginning their foray in the activation process, CD69 serves as a suppressive signal. A case that can be made for this comes from recent studies demonstrating TGFβ1 secretion from T cells following CD69 stimulation (27, 28). This also has been demonstrated in our laboratory for murine small intestinal IELs as shown in Fig. 1. Note the low level of TGFβ1 production in the absence of CD69 stimulation, possibly due to synthesis by CD4+CD25+ T cells (32), and the ~3-fold increase in synthesis following direct ligation with functional-grade anti-CD69 mAb. IELs thus bathed in low levels of TGFβ1 may be resistant to wanton tissue destruction from indiscriminate lytic activity or cytokine synthesis. Assuming that the (as yet unidentified) ligand for CD69 is routinely available for CD69+ IELs, this would provide a direct mechanism for retaining those cells in a semi-quiescent, partially-activated state. This appears to be feasible given present knowledge of the role of TGFβ1 in maintaining both immunological and epithelial cell health as witnessed by intestinal tissue damage in TGFβ−/− mice (33), and by the beneficial effects of TGFβ1 gene therapy in modulating intestinal inflammation in an experimental model of trinitrobenzene sulfonic acid (TNBS)—induced colitis in rats (34). Hence, TGFβ1, through CD69 signaling, may play a key role in providing a level of self control for IEL activation.
Fig. 1. CD69 stimulation of murine small intestinal IELs results in the secretion of TGFβ1.
Small intestinal IELs from normal C57BL/6 mice were cultured in vitro with 0.1, 1.0, or 10 μg/ml of functional grade anti-CD69 mAb and cell-free supernatants were assayed for TGFβ1 activity after 24 hrs. Note the dose-dependent synthesis of TGFβ1 production following by CD69 stimulation, and the low level of TGFβ1 activity in cultures without stimulation. These findings suggest that the presence of CD69 on intestinal IELs may serve to maintain a level of TGFβ1 that is used to retain those cells in a quiescent, partially-activated state until a signal for full activation has been received.
CD160 (By55)
CD160 is a glycosylphosphatidylinositol (GPI)-anchored cell surface molecule that has received only modest attention on human and murine IELs. In humans, CD160 is expressed on NK cells (35), as well as most CD8+ intestinal IELs (36). Perhaps the most interesting aspect of CD160 expression is its association with CD28− cells (37), a property that may hold particular significance for T cells of the intestinal epithelium given that most IELs are CD28− cells (23). Interestingly, the costimulatory properties of CD160 have been tied to activated but not resting T cells (38) — a feature that is compatible with the partially-activated nature of IELs. In mice, expression of CD160 can be modestly upregulated following T cell activation, it may be released in a soluble form (13), and CD160+ CD8+ T cells produced IFN-γ more rapidly than CD160− CD8+ T cells (13). It has been reported that expression of CD160 reflects memory T cell status (13). Regardless, costimulation through CD160 in conjunction with CD3 triggering results in enhanced cell proliferation (37), thus making it an ideal alternative to CD28 for costimulation of IELs.
CD43 and its related glycoforms
CD43 is a third constitutively-expressed costimulatory molecule of small intestinal IELs; however, unlike CD160 and CD69, CD43 is present on T cells prior to their entry into the intestinal mucosa. CD43 expression is by no means restricted to IELs since it is a ubiquitous molecule that is present on bone marrow cells, thymocytes, and peripheral T cells and B cells (39-42). It is expressed at high density on lymphoid cells, and has multiple O-linked glycosylation sites (43, 44), thereby allowing for the generation of numerous potential glycoforms made by post-translational modification of the core protein. Many functions, including both positive and negative immunoregulatory effects, have been ascribed to CD43 on peripheral T cells (45, 46). The saialoadhesin molecule on macrophages is a potential ligand for CD43 (47); however, it is likely that other ligands exist that selectively react with specific CD43 glycoforms.
Most work into the function of CD43 on IELs comes from studies in mice. Costimulatory activity of CD43 leading to enhanced proliferation has been demonstrated through in vitro studies involving anti-CD43 crosslinking in conjunction with TCR/CD3 stimulation (48). More importantly, significant differences have been demonstrated with regard to cytokine profiles elicited following CD43 costimulation compared to CD3 stimulation alone or non-stimulated cultures (49). Of twenty-two analytes tested, CCL5 was the only cytokine detected in non-stimulated cultures (49). CD3 crosslinking on IELs resulted in upregulation of granulocyte-monocyte colony stimulating factor (GM-CSF), IL-2, IL-6, IL-12p70, IL-17, interferon (IFN)-γ, and CCL5, while costimulation via CD43 resulted in significantly increased levels of IL-2, IFN-γ and CCL5, and modestly-elevated levels of GCSF, and GM-CSF compared to direct CD3 stimulation alone (49). Unexpectedly, IL-17 secretion was suppressed following CD43 costimulation (49). IL-17 has been shown to down-regulate CCL5 synthesis in the presence of tumor necrosis factor (TNF)α (50). If a similar pattern holds true for CD43 costimulation, it is possible that the lack of an IL-17 response following CD43 costimulation may reflect a regulatory effect designed to enhance CCL5 synthesis, an outcome that would have significance to the disease process of inflammatory bowel disease (IBD) (51, 52).
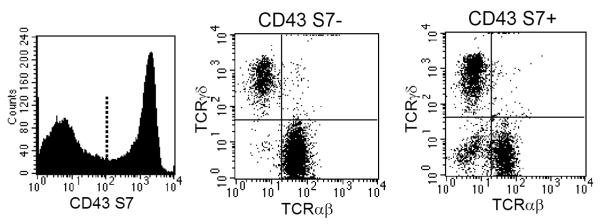
Recent studies demonstrate that expression of CD43 is important for IELs not only in terms of its function-related activities, but also due to its capacity to demarcate IELs according to subsets based on whether they express the neuraminidase-sensitive determinant recognized by the S7 monoclonal antibody (41). That property of CD43 is particularly interesting since it separates IELs into two major populations (S7+ and S7− cells) independent of TCR (TCRαβ vs. TCRγδ) or CD8 (CD8αα vs. CD8αβ) expression. Consequently, S7+ and S7− IELs consist of both TCRαβ and TCRγδ cells as shown in Fig. 2, yet all IELs in mice express the CD43 core molecule recognized by anti-CD43 mAb R2/60 (53, 54). For reasons that remain unclear, intestinal IELs appear to be the only peripheral T cell population with a large proportion of cells lacking the S7 determinant.
Fig. 2. Murine small intestinal IELs consist of equivalent proportions of CD43 S7+ and S7− cells, with each population consisting of both TCRαβ and TCRγδ IELs.
Unlike T cells in other murine peripheral immune compartments, which uniformly express the CD43 neuraminidase-sensitive CD43 S7 determinant, only about half of the IELs are S7+ cells. The S7+ and S7− IELs display important functional differences as discussed in the article.
The distribution of CD43 S7 expression on IELs is more than a phenotypic distinction, however, since S7+ and S7− cells display prominent functional differences. In normal mice, CD43 S7+ IELs have been shown to undergo more in situ homeostatic proliferation as witnessed by bromodeoxyuridine (BrdU) incorporation compared to S7− IELs (55). However, in mice infected with reovirus serotype 3, both S7+ and S7− IELs progressed through an in situ cell expansion phase based on BrdU incorporation that peaked on day 4 of infection (56), suggesting that both populations of IELs are responsive to virus antigenic challenge. S7+ IELs secrete significantly higher levels of Th1 cytokines (IL-2 and IFN-γ), Th2 and regulatory cytokines (IL-4 and IL-10), and IL-17, upon in vitro CD3 stimulation (55). CD43 S7+ IELs also are more cytolytic than S7− IELs — both upon immediate isolation from the intestine and following in vitro CD3 stimulation (55). Importantly, gene array studies demonstrate that S7+ IELs expressed genes typical of cells that mediate adaptive immunity, or cells that are associated with T cell activation and/or inflammation (e.g., CD6, CD44, CD97, CCR2, CXCR3, MIP-1β). Although CD44 is expressed on both S7+ and S7− IELs, flow cytometric analysis indicated that S7+ IELs were primarily CD44high cells (55), while S7− IELs consisted of cells ranging from CD44low to CD44high expression (55). The presence of CD6 and CD97 on S7+ IELs is consistent with a population of cytolytic cells, and may contribute to the effector response by augmenting cell adhesion (55). In that context, increased CCR2, CXCR2, and MIP-1β expression has been demonstrated in a number of experimental models of IBD (57, 58), as well as in humans with ulcerative colitis or Crohn’s disease (59, 60). In contrast to S7+ IELs, S7− cells express genes of innate immunity including receptors involved in positive and negative regulation of NK activity (e.g., Ly49E-G, Ly49G.2, Ly49H, Ly49C, CD94/NKG2) (55). Adoptive transfer experiments demonstrate that CD43 S7+ IELs are predominantly involved in small intestine tissue destruction during graft vs. host disease (GVHD) (61). Moreover, S7+ IELs in the terminal ileum of IL-10−/− mice spontaneously produced 10-fold more IFN-γ than S7− IELs (55). These findings thus point to substantial functional differences in IELs according to whether they express a glycoform of CD43.
IL-18
Originally noted for its ability to promote IFN-γ production (62), IL-18 is now regarded to be a highly-potent proinflammatory cytokine that is capable of influencing a broad range of immunobiological activities, including cell-mediated immunity, both Th1 and Th2 responses, and NK activity (63). Although IL-18 is perhaps best known for its ability to promote IFN-γ production, either alone or synergistically with IL-12 (64), it also has the ability to independently induce IL-4 and IL-13 production (65-67). The importance of IL-18 in terms of host immune protection has been demonstrated in many experimental systems of bacterial, viral, fungal, and protozoal infections; these are discussed in detail in the review by Nakanishi, et. al., (63). Interestingly, studies in IL-10−/− mice reveal a role for IL-10 in suppressing IL-12 but not IL-18 activity (68), thus providing evidence for some degree of non-synergistic activity between IL-12 and IL-18. That dichotomy is also evident in studies of dextran sodium sulfate (DSS)-induced experimental murine colitis; surprisingly, pathology was more severe in IL-12p35−/− mice than in either IL-18−/− or IL-18R−/− mice (69).
IL-18 also has been shown to increase OX40L expression on dendritic cells (DCs) (70), and has been shown to induce nitric oxide synthesis (71). A wide range of cells — macrophages, DCs, and epithelial cells — can serve as a source of IL-18 (72). An IL-18 isoform arising from an alternatively spliced variant, termed IL-18s (IL-18 short), has recently been identified and characterized in mice (73). That form has no IFN-γ promoting activity independently, but significantly increased IFN-γ activity in combination with conventional IL-18 (73), implying a potential role for IL-18s as an immune modulator of IL-18 activity. The significance of this to human IL-18 remains to be determined, but could have bearing on the relationship between IL-18 restriction fragment length polymorphism variations and CARD15/NOD2 status noted in Crohn’s disease patients (74).
The IL-18R, formerly referred to as the IL-1 receptor related protein, belongs to the IL-1R/toll-like receptor superfamily and consists of an α-chain molecule used for extracellular IL-18 binding, and a β-chain molecule used for intracellular signaling (75, 76). For functional activity, the IL-18R requires the presence of the IL-18 accessory protein (IL-18 AcP) (77). Studies in our laboratory indicate that genes for both the IL-18R and IL-18 AcP are expressed in IELs, and that there is a preference for expression of both in the CD43 S7+ subset (Wang HC and Klein JR, unpublished observations). Utilization of IL-18 by leukocytes is susceptible to manipulation by soluble IL-18 binding protein (IL-18BP) (78), which interferes with the IL-18/IL-18R interaction, and thus acts as a negative regulator of IL-18 function (79). IL-12 may serve as a cofactor for increasing IL-18R expression as seen from studies of murine peripheral T cells (80). In humans, most colonic IELs express IL-18Rα (72, 81). Moreover, the costimulatory effects of IL-18 have been documented in in vitro cultures of CD3-stimulated human IELs in which there was a significant increase in proliferation when costimulated with 50-100 ng/ml IL-18. Although stimulation also occurred in the presence of IL-2 without CD3 stimulation, there was approximately a 10-fold increase in proliferation in IL-18 costimulated cultures in conjunction with anti-CD3 stimulation (72).
The reduction in the tissue destructive potential mediated by IL-18 is strikingly evident from the large number of pathologies that lend themselves to treatment by anti-IL-18 or anti-IL-18BP therapy, or are ameliorated in IL-18−/− mice. This includes endotoxin-induced liver disease (82-84), ischemic renal and myocardial diseases (85), GVHD (86), and collagen-induced arthritis (87), among others. Additionally, at least two experimental models of murine colitis have been shown to benefit from anti-IL-18 or anti-IL-18BP treatment, which in both cases concomitantly suppressed TNFα activity (88). In human conditions of intestinal inflammation, IL-18 appears to contribute significantly to the pathology of Crohn’s disease and to a lesser extent to ulcerative colitis (89-92), and may be involved in celiac disease pathology (93). This is consistent with the general Th1 polarizing properties of IL-18 and the Th1 nature of the inflammatory response in Crohn’s disease. Additionally, serum IL-18 levels generally parallel disease severity in Crohn’s disease patients, and may serve as predictors of the overall progression of disease (91, 92).
Up-regulated costimulatory molecules
A rather large group of surface costimulatory molecules can be expressed on IELs following an initial activation event. In general, upregulation of these occurs quickly — usually within 24 hrs — thereby offering a rapid response through the costimulatory signal. In the case of CD59, at least two non-immune pathways leading to the expression of CD59 (as will be discussed further in this section) may enter into the regulatory process.
CD137 (4-1BB)
CD137 is a 30 kDa glycoprotein molecule belonging to the TNF receptor superfamily (94). It is expressed on NK cells, CD4+ and CD8+ T cells, and intestinal IELs (95-98). Given that >85% of small intestinal IELs express CD8, this molecule would be expected to have costimulatory activity for most IELs. Moreover, as with costimulatory molecules such as CD43 (54), CD137 operates independently of CD28 (99-101). CD137 is upregulated on IEL following immune stimulation or by IL-2 enhanced cytotoxicity in in vitro cultures (96).
Despite an extensive number of studies on the role of CD137 in inflammation and autoimmunity, the involvement of CD137 within the intestine in health and disease has not been extensively explored. In one study using an experimental model of large intestine inflammation consisting of CD4+ CD137−/− T cell transfer into SCID mice, animals were found to develop colitis in a manner similar from control animals, although both Th1 and Th2 cytokine responses were elicited rather than the usual polarization toward a Th1 response (102). Moreover, regulatory CD4+ CD25+ T cells from CD137−/− mice were as capable of averting colitis as regulatory T cells from wild-type animals, thus suggesting that the CD137 molecule may be dispensable for mediating that immunoprotective effect (102). Patients with Crohn’s disease but not ulcerative colitis were shown to have elevated expression of CD137 in the lamina propria of inflamed tissues (103), and agonist CD137 antibodies promoted higher levels of IFN-γ synthesis from lamina propria T cells in vitro when costimulated with anti-CD3 antibody (103). Collectively, these findings implicate CD137 as a potentially-important costimulatory molecule used in the activation of intestinal T cells.
CD59 (Ly-6C)
CD59 is a GPI-anchored membrane glycoprotein that is variously expressed on hematopoietic cells and is constitutively expressed on a small proportion of peripheral T cells, possibly including memory cells (104, 105). On normal IELs, CD59 expression rarely exceeds 5-10% of the CD8+ cells (15). Once expressed on T cells, CD59 can act as a costimulatory molecule in conjunction with a CD3-mediated signal, or direct crosslinking of CD59 can lead to T cell activation resulting in proliferation and IL-2 synthesis (106). CD59 is unique from other regulated costimulatory molecules in that there are at least three modalities through which it can be upregulated. The prototypical mode involves stimulation through the TCR/CD3 complex in response to antigen (15). However, a significant degree of CD59 upregulation can occur on murine small intestinal IELs either by exposure to type-I interferon (IFN-α), or by direct ligation of CD43. In each case, CD59 expression on IELs is upregulated by 30-60%. Because the CD43 core molecule is expressed on almost all murine small intestinal IELs (55), direct stimulation of CD43 via sialoadhesin on macrophages, a ligand for CD43 (47), would result in CD59 expression within 24-48 hours regardless of TCR/CD3 signaling. Similarly, the presence of IFN-α released from virus-infected enterocytes would drive IELs to express CD59. In both cases this could occur in the absence of TCR/CD3 signaling. Subsequent stimulation of CD59 by its ligand expressed on CD11b+ cells or B cells in the intestinal mucosa then would serve to induce IEL proliferation and IL-2 secretion. This pathway, therefore, could provide a mechanism for generating an early response of intestinal IELs to infectious agents by expanding the pool of IELs independent of TCR specificity. Since this would be expected to occur in a polyclonal manner, the effect would be to increase the potential for selection of antigen-specific T cells.
CD134 (OX40)
CD134 is a member of the tumor necrosis receptor superfamily (107). CD134 was originally identified on rat lymphocytes (OX40) (108), was subsequently characterized as an activation marker on mouse CD4+ T cells, and now been shown to be expressed on activated CD8 T cells (14, 109-111). The CD134 receptor belongs to the TNF family and is expressed at low levels on B cells, T cells, professional APCs and some endothelial cells, and is upregulated following activation (112-114). In the small intestine of mice, expression of CD134 is expressed within 24 hr of CD3 ligation (14), or ~48 hrs sooner than occurs for similarly activated CD8+ lymph node T cells (14), implying a more sensitive response of CD134 on intestinal T cells. Expression of CD134 on small intestinal IELs following CD3 stimulation accompanies IFN-γ synthesis and increased cytotoxicity (14). Costimulation of IELs via CD134 significantly suppresses IL-10 production (14). Taken together, these findings indicate that the function of CD134 during the activation scheme of IELs is to augment Th1 responses and cell-mediated immunity, possibly by curtailing the synthesis of IL-10.
Increased expression of CD134 has been described in small and/or large intestine tissues from patients with IBD and celiac disease (115, 116). CD134-CD134L interactions have been shown to contribute to disease in a murine model of GVHD (117), while blockade reduced GVHD tissue destruction (118). The therapeutic effects of targeting CD134 or CD134L also have been documented in experimental colitis (119, 120).
CD278 (ICOS)
CD278, a member of the CD28 superfamily (121). The CD278 ligand (B7RP-1) is expressed on macrophages, DCs and some intestinal epithelial cells (122). CD278 is not expressed on naïve T cells (123). Its expression has been shown to be upregulated on human, mouse, and rat colonic leukocytes during induced or natural inflammation (124-127), and CD278 may serve as a therapeutic target for treatment of IBD (128). CD278 is critical for CD4+ T cell activation as seen from studies in CD278−/− mice (129, 130). Moreover, a temporal relationship between CD278 costimulation and disease expression has been demonstrated in a model of experimental allergic encephalitis, in which treatment of mice with CD278-Ig was shown to exacerbate pathology when administered during the early phase of disease development, and to suppress disease during the effector phase (129), thus suggesting a biphasic nature to CD278 signaling with regard to the T cell response elicited.
In an experimental model of reovirus serotype 3 infection in mice, virus infection, which peaked at day 7 post-infection as determined by virus-specific real-time PCR, was preceded by in situ expansion of both the CD43 S7+ and S7− IEL populations (56). CD278 expression also peaked on IELs at day 7 post-infection (56) and, as determined from multi-color flow cytometric analysis, CD278+ cells localized within the CD43 S7+, CD4+, CD8− IEL subset, of which roughly half of those cell expressed CD278 (56). Additionally, at the time of peak virus infection, expression of the B7RP-1 CD278 ligand doubled on intestinal enterocytes (from 5.4% to 9.3%) (56), suggesting that in addition to the expression of B7RP-1 on APCs in the intestine, there may be a role for intestinal enterocyte/epithelial cells as stimulatory signal for CD278.
To define the involvement of CD278 in the IEL immune response to reovirus, IELs from non-infected mice and from day 7 reovirus-infected mice were cultured in vitro with mouse PM cells, a macrophage line that expresses the B7RP-1 ligand; secreted IFN-γ was measured in 24 hr culture supernatants. Although low-level IFN-γ activity was detected from IELs of non-infected mice (a finding that would be predicted due to expression of CD278 on a small proportion of normal IELs), there was a significant increase in IFN-γ activity from IELs of virus-infected mice (56); reduction in IFN-γ synthesis was achieved by blockade using anti-CD278 antibody. Consistent with those findings, there was an increase in IFN-γ mRNA in cell-sorted CD4+ IELs (the population with the greatest CD278 expression) at day 6 post-infection as measured by real-time PCR (56).
IEL activation — a synthesis
Perhaps even more than T cells in other peripheral immune compartments, the activation status of IELs must be perpetually monitored and controlled
So how might costimulation be integrated into the process of IELs activation? Clearly, a compendium of multiple signals delivered to IELs simultaneously would result in a level of chaos that would be counter-productive for the development of an effective immune response. Hence, under natural physiological conditions, the process would need to be tightly regulated. In that context, it is important to bear in mind that under most circumstances, the IELs likely perform their task of host immune defense effectively and that conditions of non-physiological inflammation reflect perturbations in the homeostatic balance needed to deliver an effective immune response. However, as alluded to in the beginning of this article, this may pose a greater challenge for the intestinal immune system compared to that of peripheral immunological tissues that are not submerged in a milieu of foreign replicating and non-replicating antigens. Therefore, we will begin with our initial presumption that IELs are partially-activated T cells — a beneficial condition that would facilitate rapid response to potentially dangerous threats to the host. The availability of a level of pre-armed cytotoxicity would eliminate the time for the development of that effector function, thus rendering IELs already prepared to destroy many types of bacteria, viruses, and parasites. Clearly, clonal expansion of antigen-specific CD8+ effector IELs would be required to complete the response. Although IELs are not known for their proliferative potential (131, 132) and, in fact, under normal circumstances many may have already entered into apoptosis, there are nonetheless mechanisms available for a considerable degree of cell proliferation. Cytokines such as IL-2, IL-15, and stem-cell factor, (although not covered in this article) are critical for IEL development and clonal expansion (133-140). NK cells also may contribute to the regulatory process, particularly in the context of CD1d-restricted T cells (141).
Prior to antigenic stimulation, it would be critical for IELs to be maintained in a state of quiescence despite their status as partially-activated effector cells. This would be achieved at least in part through the elaboration of TGFβ1 (26-28, 142), as discussed earlier. However, in as much as TGFβ1 is actively produced in humans with IBD (143), TGBβ alone is probably not sufficient to control the activation process of IELs once it has moved toward full activation. The other prime candidate for controlling IEL activation would be IL-10, a cytokine that also is known to have strong immunosuppressive activity. The IL-10−/− mouse model is particularly revealing since, although most IL-10−/− mice develop intestinal inflammation by the time they are young adults, inflammation is absent in IL-10−/− mice raised under germ-free conditions (144, 145). This implies a continual ‘balancing-act’ between the intestinal inflammatory response and the presence of pathogenic microorganisms, and it suggests that in the absence of the latter, the immune response in the intestinal mucosa can be effectively managed without IL-10. However, it also suggests that once the full activation event has begun, it cannot be controlled in the absence of IL-10.
Thus, we can view suppression as acting at two levels: one that is regulated by TGFβ1, the other that is regulated by IL-10. According to this, TGFβ1 would function primarily to maintain the broad population of partially-activated IELs in a quiescent state. Upregulation of CD69 on IELs upon entry into intestinal sites would facilitate this process by promoting the secretion of TGFβ1 (Fig. 1). Certainly, a better understanding of the role of CD69/TGFβ1 may become evident as more is learned about the ligand for CD69 and its expression and distribution among intestinal tissues. Once immune activation has been initiated either by direct engagement and signaling through the TCR/CD3 complex, or upon activation with one of the costimulatory molecules described here, we predict that IL-10 and not TGFβ1 is the key element involved in immunological control. Unpublished studies in our laboratory using cytokine multiplex panels and IL-10−/− mice indicate that there is massive and early dysregulation of many cytokines and chemokines in IL-10−/− mice, particularly those that drive and perpetuate the inflammatory process, suggesting that IL-10 is especially important in restraining intestinal inflammation.
IEL activation — let the process begin
Using the model shown in Fig. 3, the process of IEL activation can be viewed as occurring in multiple discrete stages. It is important to note that as IELs are activated in the initial stage, costimulation need not be involved since direct ligation of TCR to peptide antigens presented by classical or non-classical MHC would be sufficient for low level activation. Though already cytotoxic, CD8+ IELs (whether TCRαβ or TCRγδ) would acquire an additional level of effector activity, i.e., modest cell proliferation and synthesis of cytokines. Under mild infectious situations, this may be sufficient to provide a protective edge for the host. However, also immediately available would be the constitutively-expressed CD43 and CD160 costimulatory molecules and the IL-18R. CD160 costimulation would enhance the proliferative process, thus expanding out a population of antigen-reactive IELs.
Fig. 3. Two-phase model of IEL costimulation involving constitutively-expressed and upregulated costimulatory molecules and receptors.
The constitutively-expressed molecules consist of CD43, CD69, CD160, and the IL-18R. CD69, which is acquired on IELs upon entry into the intestinal mucosa, serves to maintain partially-activated (cytotoxic) IELs, through the secretion of low levels of TGFβ1, from entering into a state of full activation until a signal has been received for activation via the TCR/CD3 complex alone, or upon TCR/CD3 stimulation in conjunction with one of the constitutively-expressed costimulatory molecules. Costimulation through CD43 or CD160 leads to enhanced proliferation and/or cytokine synthesis, and overrides the immunosuppressive effects mediated by CD69/TGFβ1. Costimulation of IELs by IL-18, a powerful proinflammatory cytokine, increases both Th1 and Th2 cytokine activities and augments cell-mediated immunity and NK responses. Direct stimulation of IELs via the TCR/CD3 complex in the absence of costimulation results in selective upregulation of CD134, CD137, CD59, and/or CD278, depending upon the type of IEL that has been activated. These will drive the IEL response into a heightened state of activation consisting of greater cell proliferation, enhanced cytotoxicity, and/or the synthesis of regulatory and effector cytokines, while concomitantly overriding the effects of TGFβ1. The immunological consequences of activation through the costimulatory molecules will depend largely upon the IEL subset(s) that expresses them, as delineated in Table 1.
A similar effect would occur upon CD43 costimulation, although, as discussed earlier, the situation for CD43 is more complex due to the expression of several possible CD43 glycoforms. Studies in our laboratory have explored the role of CD43 costimulation in murine experimental systems. Costimulation via the core CD43 molecule in conjunction with TCR/CD3 signaling would increase cell proliferation (48). However, the more dominant effect may be that mediated by cells which express the S7 glycoform. Since the S7+ subset of IELs already bears many properties of ‘high-responder’ effector cells (55), costimulation of that population either by trigger of a core CD43 epitope, or by direct stimulation of the S7 carbohydrate residue itself, would be expected to induce high levels of cytokine secretion and to considerably augment cytotoxic activity. Experiments are currently underway to determine the effect of CD43 S7 stimulation relative to CD43 core stimulation. It is interesting that, at present, we have found little evidence for regulated changes in the S7 glycoform on murine small intestinal IELs, resulting in either a loss or a gain of S7 expression, although IL-18 costimulation of IELs with CD3 ligation has been found to increase the proportion of S7+ IELs as a consequence of preferential cell expansion of that population (Montufar-Solis D and Klein JR, unpublished observation). Therefore, among murine small intestinal IELs, expression of the CD43 S7 glycoform has relevance for biological as well as empirical purposes. First, S7+ IELs reflect a population of ‘high-responder’ effector cells. Secondly, if a population of IELs similar to the CD43 S7+ cells exists in humans, it that may have value for vaccine development strategies, and it also may define a population of IELs that can be targeted in efforts for control non-physiological inflammation.
Accessibility to IL-18 would depend upon the local manufacture of that cytokine by macrophages and DCs, and possibly also from epithelial cells, particularly following stress, infection, or tissue damage. The presence of IL-18 would have profound effects on the activational process, generally polarizing it in a Th1 direction. Moreover, the effect of IL-18 may be further amplified by the local synthesis of IL-12 from DCs and macrophages. Importantly, all of the early costimulatory signals (CD43, CD160, and IL-18) would be expected to occur rapidly given the constitutive presence of those molecules or receptors on most IELs. The rate-limiting factor in this process would be governed at the level of ligand expression.
Upregulated costimulatory molecules selectively activate specific IEL subsets
Following the initial activation signal delivered to IELs through the TCR/CD3 complex, the upregulated costimulatory molecules described here would be expressed within 12-24 hrs. Depending upon the availability of ligands for those, IELs would then enter into a second wave of activation. A particularly important aspect of the four upregulated costimulatory molecules (CD59, CD134, CD137, and CD278) is their selective expression on specific IEL subsets, as described in Table 1. Note that CD137 and CD59 are affiliated primarily with CD8+ IELs, CD278 are affiliated primarily with CD4+ IELs, and CD134 IELs may be either CD4+ or CD8+ cells. Moreover, it is reasonable to assume that even within a costimulatory subset, subtle but significant differences exist. This was evident from our studies of reovirus infection and CD278 expression, which was selectively upregulated on CD4+8−, but not on CD4+8+, CD4−8+, or CD4−8− IELs (56). This implies that during the course of typical reovirus infection, the role for CD278 is centered on CD4+ T helper rather than CD8+ effector IELs, and suggests that CD278 participates in the expansion phase of the immune response. It is likely, therefore, that the events that promote the upregulation of costimulatory molecules reflect the type of immunological challenge confronting the host.
Table 1.
IELs expressing constitutive or upregulated costimulatory molecules
| Costimulatory molecule/receptor | Subset | Reference |
|---|---|---|
| Constitutively expressed | ||
| CD69 | CD4+ and CD8+ | 13-16 |
| CD43 S7+a | Some TCRαβ+ and TCRγδ+ | 55,56,61 |
| CD43 S7−a | Some TCRαβ+ and TCRγδ+ | 55,56,61 |
| CD160 | Primarily CD8+ | 36 |
| IL-18R | TCRαβ+ and TCRγδ+ | 72,81 |
| Upregulated | ||
| CD59 | Primarily CD8+ | 15 |
| CD134 | CD4+ and CD8+ | 14 |
| CD137 | Primarily CD8+ | 96,97 |
| CD278 | Primarily CD4+ | 56 |
Defined only on mouse IELs
In summary, although costimulation is known to be an important aspect of the activation process of peripheral T cells, knowledge of the contributing role for costimulation in the activation of intestinal T cells is scant by comparison. Moreover, owing to the fact that IELs exist in a novel state of activation, a better understanding of the factors that control activation will be paramount for elucidating mechanisms of intestinal biology in health and disease. Clearly, much work is yet to be done to decipher the intracellular signaling pathways and gene activating elements that are employed by IELs following costimulation, with regard to both immunosuppression and activation.
References
- 1.Poussier P, Julius M. Thymus independent T cell development and selection in the intestinal epithelium. Annu Rev Immunol. 1994;12:521–553. doi: 10.1146/annurev.iy.12.040194.002513. [DOI] [PubMed] [Google Scholar]
- 2.Rocha B, Vassalli P, Guy-Grand D. The Vβ repertoire of mouse gut homodimeric α CD8+ intraepithelial T cell receptor α/β + lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991;173:483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocha B, Guy-Grand D, Vassalli P. Extrathymic T cell differentiation. Curr Opin Immunol. 1995;7:235–242. doi: 10.1016/0952-7915(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 4.Cheroutre H. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol Rev. 2005;206:114–131. doi: 10.1111/j.0105-2896.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 5.Parrott DM, Tait C, MacKenzie S, Mowat AM, Davies MD, Micklem HS. Analysis of the effector functions of different populations of mucosal lymphocytes. Ann N Y Acad Sci. 1983;409:307–320. doi: 10.1111/j.1749-6632.1983.tb26879.x. [DOI] [PubMed] [Google Scholar]
- 6.Tagliabue A, Befus AD, Clark DA, Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982;155:1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petit A, Ernst PB, Befus AD, Clark DA, Rosenthal KL, Ishizaka T, Bienenstock J. Murine intestinal intraepithelial lymphocytes I. Relationship of a novel Thy-1-,Lyt-1-,Lyt-2+, granulated subpopulation to natural killer cells and mast cells. Eur J Immunol. 1985;15:211–215. doi: 10.1002/eji.1830150302. [DOI] [PubMed] [Google Scholar]
- 8.Cerf-Bensussan N, Guy-Grand D, Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985;26:81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viney JL, Kilshaw PJ, MacDonald TT. Cytotoxic α/β+ and γδ+ T cells in murine intestinal epithelium. Eur J Immunol. 1990;20:1623–1626. doi: 10.1002/eji.1830200734. [DOI] [PubMed] [Google Scholar]
- 10.Klein JR. Ontogeny of the Thy-1-, Lyt-2+ murine intestinal intraepithelial lymphocyte. Characterization of a unique population of thymus-independent cytotoxic effector cells in the intestinal mucosa. J Exp Med. 1986;164:309–314. doi: 10.1084/jem.164.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefrancois L, Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science. 1989;243:1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- 12.Mosley RL, Styre D, Klein JR. Differentiation and functional maturation of bone marrow-derived intestinal epithelial T cells expressing membrane T cell receptor in athymic radiation chimeras. J Immunol. 1990;145:1369–1375. [PubMed] [Google Scholar]
- 13.Tsujimura K, Obata Y, Matsudaira Y, Nishida K, Akatsuka Y, Ito Y, Demachi-Okamura A, et al. Characterization of murine CD160+ CD8+ T lymphocytes. Immunol Lett. 2006;106:48–56. doi: 10.1016/j.imlet.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Wang HC, Klein JR. Multiple levels of activation of murine CD8+ intraepithelial lymphocytes defined by OX40 (CD134) expression: effects on cell-mediated cytotoxicity, IFN-γ, and IL-10 regulation. J Immunol. 2001;167:6717–6723. doi: 10.4049/jimmunol.167.12.6717. [DOI] [PubMed] [Google Scholar]
- 15.Wang HC, Zhou Q, Dragoo J, Klein JR. Most murine CD8+ intestinal intraepithelial lymphocytes are partially but not fully activated T cells. J Immunol. 2002;169:4717–4722. doi: 10.4049/jimmunol.169.9.4717. [DOI] [PubMed] [Google Scholar]
- 16.Pahar B, Lackner AA, Veazey RS. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur J Immunol. 2006;36:583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 17.Huleatt JW, Lefrancois L. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo. J Immunol. 1995;154:5684–5693. [PubMed] [Google Scholar]
- 18.Kim SK, Reed DS, Heath WR, Carbone F, Lefrancois L. Activation and migration of CD8 T cells in the intestinal mucosa. J Immunol. 1997;159:4295–4306. [PubMed] [Google Scholar]
- 19.Kawaguchi-Miyashita M, Shimada S, Matsuoka Y, Ohwaki M, Nanno M. Activation of T-cell receptor-γδ+ cells in the intestinal epithelia of KN6 transgenic mice. Immunology. 2000;101:38–45. doi: 10.1046/j.1365-2567.2000.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HO, Cooper CJ, Choi JH, Alnadjim Z, Barrett TA. The state of CD4+ T cell activation is a major factor for determining the kinetics and location of T cell responses to oral antigen. J Immunol. 2002;168:3833–3838. doi: 10.4049/jimmunol.168.8.3833. [DOI] [PubMed] [Google Scholar]
- 21.Shires J, Theodoridis E, Hayday AC. Biological insights into TCRγγ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 22.Fahrer AM, Konigshofer Y, Kerr EM, Ghandour G, Mack DH, Davis MM, Chien YH. Attributes of γγ intraepithelial lymphocytes as suggested by their transcriptional profile. Proc Natl Acad Sci U S A. 2001;98:10261–10266. doi: 10.1073/pnas.171320798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohteki T, MacDonald HR. Expression of the CD28 costimulatory molecule on subsets of murine intestinal intraepithelial lymphocytes correlates with lineage and responsiveness. Eur J Immunol. 1993;23:1251–1255. doi: 10.1002/eji.1830230609. [DOI] [PubMed] [Google Scholar]
- 24.Sillett HK, Southgate J, Howdle PD, Trejdosiewicz LK. Expression of activation and costimulatory elements by human intestinal intraepithelial lymphocytes. Scand J Immunol. 1999;50:52–60. doi: 10.1046/j.1365-3083.1999.00561.x. [DOI] [PubMed] [Google Scholar]
- 25.O’Keeffe J, Doherty DG, Kenna T, Sheahan K, O’Donoghue DP, Hyland JM, O’Farrelly C. Diverse populations of T cells with NK cell receptors accumulate in the human intestine in health and in colorectal cancer. Eur J Immunol. 2004;34:2110–2119. doi: 10.1002/eji.200424958. [DOI] [PubMed] [Google Scholar]
- 26.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Sancho D, Gomez M, Viedma F, Esplugues E, Gordon-Alonso M, Garcia-Lopez MA, de la Fuente H, et al. CD69 downregulates autoimmune reactivity through active TGF-β production in collagen-induced arthritis. J Clin Invest. 2003;112:872–882. doi: 10.1172/JCI19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esplugues E, Sancho D, Vega-Ramos J, Martinez C, Syrbe U, Hamann A, Engel P, et al. Enhanced antitumor immunity in mice deficient in CD69. J Exp Med. 2003;197:1093–1106. doi: 10.1084/jem.20021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauzurica P, Sancho D, Torres M, Albella B, Marazuela M, Merino T, Bueren JA, et al. Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice. Blood. 2000;95:2312–2320. [PubMed] [Google Scholar]
- 30.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, et al. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 31.Cima I, Corazza N, Dick B, Fuhrer A, Herren S, Jakob S, Ayuni E, et al. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J Exp Med. 2004;200:1635–1646. doi: 10.1084/jem.20031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-β 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 33.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giladi E, Raz E, Karmeli F, Okon E, Rachmilewitz D. Transforming growth factor-beta gene therapy ameliorates experimental colitis in rats. Eur J Gastroenterol Hepatol. 1995;7:341–347. [PubMed] [Google Scholar]
- 35.Maiza H, Leca G, Mansur IG, Schiavon V, Boumsell L, Bensussan A. A novel 80-kD cell surface structure identifies human circulating lymphocytes with natural killer activity. J Exp Med. 1993;178:1121–1126. doi: 10.1084/jem.178.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anumanthan A, Bensussan A, Boumsell L, Christ AD, Blumberg RS, Voss SD, Patel AT, et al. Cloning of BY55, a novel Ig superfamily member expressed on NK cells, CTL, and intestinal intraepithelial lymphocytes. J Immunol. 1998;161:2780–2790. [PubMed] [Google Scholar]
- 37.Nikolova M, Marie-Cardine A, Boumsell L, Bensussan A. BY55/CD160 acts as a co-receptor in TCR signal transduction of a human circulating cytotoxic effector T lymphocyte subset lacking CD28 expression. Int Immunol. 2002;14:445–451. doi: 10.1093/intimm/14.5.445. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal S, Marquet J, Freeman GJ, Tawab A, Bouteiller PL, Roth P, Bolton W, et al. Cutting edge: MHC class I triggering by a novel cell surface ligand costimulates proliferation of activated human T cells. J Immunol. 1999;162:1223–1226. [PubMed] [Google Scholar]
- 39.Moore T, Huang S, Terstappen LW, Bennett M, Kumar V. Expression of CD43 on murine and human pluripotent hematopoietic stem cells. J Immunol. 1994;153:4978–4987. [PubMed] [Google Scholar]
- 40.Baecher CM, Infante AJ, Semcheski KL, Frelinger JG. Identification and characterization of a mouse cell surface antigen with alternative molecular forms. Immunogenetics. 1988;28:295–302. doi: 10.1007/BF00364226. [DOI] [PubMed] [Google Scholar]
- 41.Gulley ML, Ogata LC, Thorson JA, Dailey MO, Kemp JD. Identification of a murine pan-T cell antigen which is also expressed during the terminal phases of B cell differentiation. J Immunol. 1988;140:3751–3757. [PubMed] [Google Scholar]
- 42.Rosenstein Y, Santana A, Pedraza-Alva G. CD43, a molecule with multiple functions. Immunol Res. 1999;20:89–99. doi: 10.1007/BF02786465. [DOI] [PubMed] [Google Scholar]
- 43.Jones AT, Federsppiel B, Ellies LG, Williams MJ, Burgener R, Duronio V, Smith CA, et al. Characterization of the activation-associated isoform of CD43 on murine T lymphocytes. J Immunol. 1994;153:3426–3439. [PubMed] [Google Scholar]
- 44.Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat Immunol. 2002;3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- 45.Woodman RC, Johnston B, Hickey MJ, Teoh D, Reinhardt P, Poon BY, Kubes P. The functional paradox of CD43 in leukocyte recruitment: a study using CD43-deficient mice. J Exp Med. 1998;188:2181–2186. doi: 10.1084/jem.188.11.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, et al. Dynamic regulation of T cell immunity by CD43. J Immunol. 2002;168:6022–6031. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- 47.van den Berg TK, Nath D, Ziltener HJ, Vestweber D, Fukuda M, van Die I, Crocker PR. Cutting edge: CD43 functions as a T cell counterreceptor for the macrophage adhesion receptor sialoadhesin (Siglec-1) J Immunol. 2001;166:3637–3640. doi: 10.4049/jimmunol.166.6.3637. [DOI] [PubMed] [Google Scholar]
- 48.Bagriacik EU, Tang M, Wang HC, Klein JR. CD43 potentiates CD3-induced proliferation of murine intestinal intraepithelial lymphocytes. Immunol Cell Biol. 2001;79:303–307. doi: 10.1046/j.1440-1711.2001.01007.x. [DOI] [PubMed] [Google Scholar]
- 49.Montufar-Solis D, Garza T, Klein JR. Selective upregulation of immune regulatory and effector cytokine synthesis by intestinal intraepithelial lymphocytes following CD43 costimulation. Biochem Biophys Res Commun. 2005;338:1158–1163. doi: 10.1016/j.bbrc.2005.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andoh A, Fujino S, Bamba S, Araki Y, Okuno T, Bamba T, Fujiyama Y. IL-17 selectively down-regulates TNF-α-induced RANTES gene expression in human colonic subepithelial myofibroblasts. J Immunol. 2002;169:1683–1687. doi: 10.4049/jimmunol.169.4.1683. [DOI] [PubMed] [Google Scholar]
- 51.McCormack G, Moriarty D, O’Donoghue DP, McCormick PA, Sheahan K, Baird AW. Tissue cytokine and chemokine expression in inflammatory bowel disease. Inflamm Res. 2001;50:491–495. doi: 10.1007/PL00000223. [DOI] [PubMed] [Google Scholar]
- 52.Mazzucchelli L, Hauser C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, Mueller C. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996;178:201–206. doi: 10.1002/(SICI)1096-9896(199602)178:2<201::AID-PATH440>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 53.Mosley RL, Hamad M, Whetsell M, Klein JR. A novel marker of murine bone marrow hematopoietic stem cells that is expressed on peripheral T cells and is associated with a functionally important molecule on activated cytotoxic T lymphocytes. Hybridoma. 1994;13:353–358. doi: 10.1089/hyb.1994.13.353. [DOI] [PubMed] [Google Scholar]
- 54.Sperling AI, Green JM, Mosley RL, Smith PL, DiPaolo RJ, Klein JR, Bluestone JA, et al. CD43 is a murine T cell costimulatory receptor that functions independently of CD28. J Exp Med. 1995;182:139–146. doi: 10.1084/jem.182.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang HC, Montufar-Solis D, Teng BB, Klein JR. Maximum immunobioactivity of murine small intestinal intraepithelial lymphocytes resides in a subpopulation of CD43+ T cells. J Immunol. 2004;173:6294–6302. doi: 10.4049/jimmunol.173.10.6294. [DOI] [PubMed] [Google Scholar]
- 56.Montufar-Solis D, Garza T, Teng BB, Klein JR. Upregulation of ICOS on CD43+ CD4+ murine small intestinal intraepithelial lymphocytes during acute reovirus infection. Biochem Biophys Res Commun. 2006;342:782–790. doi: 10.1016/j.bbrc.2006.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheerens H, Hessel E, de Waal-Malefyt R, Leach MW, Rennick D. Characterization of chemokines and chemokine receptors in two murine models of inflammatory bowel disease: IL-10−/− mice and Rag-2−/− mice reconstituted with CD4+CD45RBhigh T cells. Eur J Immunol. 2001;31:1465–1474. doi: 10.1002/1521-4141(200105)31:5<1465::AID-IMMU1465>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 58.Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, Dawson T, Kuziel WA, et al. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164:6303–6312. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- 59.Agace WW, Roberts AI, Wu L, Greineder C, Ebert EC, Parker CM. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur J Immunol. 2000;30:819–826. doi: 10.1002/1521-4141(200003)30:3<819::AID-IMMU819>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 60.Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol. 2003;199:28–35. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- 61.Bagriacik EU, Armstrong MD, Okabe M, Klein JR. Differential expression of CD43 isoforms on murine T cells and their relationship to acute intestinal graft versus host disease: studies using enhanced-green fluorescent protein transgenic mice. Int Immunol. 1999;11:1651–1662. doi: 10.1093/intimm/11.10.1651. [DOI] [PubMed] [Google Scholar]
- 62.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 63.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 64.Lochner M, Forster I. Anti-interleukin-18 therapy in murine models of inflammatory bowel disease. Pathobiology. 2002;70:164–169. doi: 10.1159/000068149. [DOI] [PubMed] [Google Scholar]
- 65.Yoshimoto T, Tsutsui H, Tominaga K, Hoshino K, Okamura H, Akira S, Paul WE, et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci U S A. 1999;96:13962–13966. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoshino T, Wiltrout RH, Young HA. IL-18 is a potent coinducer of IL-13 in NK and T cells: a new potential role for IL-18 in modulating the immune response. J Immunol. 1999;162:5070–5077. [PubMed] [Google Scholar]
- 67.Yoshimoto T, Mizutani H, Tsutsui H, Noben-Trauth N, Yamanaka K, Tanaka M, Izumi S, et al. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol. 2000;1:132–137. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- 68.Zediak VP, Hunter CA. IL-10 fails to inhibit the production of IL-18 in response to inflammatory stimuli. Cytokine. 2003;21:84–90. doi: 10.1016/s1043-4666(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 69.Takagi H, Kanai T, Okazawa A, Kishi Y, Sato T, Takaishi H, Inoue N, et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38:837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- 70.Maxwell JR, Yadav R, Rossi RJ, Ruby CE, Weinberg AD, Aguila HL, Vella AT. IL-18 bridges innate and adaptive immunity through IFN-γ and the CD134 pathway. J Immunol. 2006;177:234–245. doi: 10.4049/jimmunol.177.1.234. [DOI] [PubMed] [Google Scholar]
- 71.Ueno N, Kashiwamura S, Ueda H, Okamura H, Tsuji NM, Hosohara K, Kotani J, et al. Role of interleukin 18 in nitric oxide production and pancreatic damage during acute pancreatitis. Shock. 2005;24:564–570. doi: 10.1097/01.shk.0000184285.57375.bc. [DOI] [PubMed] [Google Scholar]
- 72.Okazawa A, Kanai T, Nakamaru K, Sato T, Inoue N, Ogata H, Iwao Y, et al. Human intestinal epithelial cell-derived interleukin (IL)-18, along with IL-2, IL-7 and IL-15, is a potent synergistic factor for the proliferation of intraepithelial lymphocytes. Clin Exp Immunol. 2004;136:269–276. doi: 10.1111/j.1365-2249.2004.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang YJ, Wang ZY, Chen SH, Ge XR. Cloning and characterization of a new isoform of mouse interleukin-18. Acta Biochim Biophys Sin (Shanghai) 2005;37:826–834. doi: 10.1111/j.1745-7270.2005.00116.x. [DOI] [PubMed] [Google Scholar]
- 74.Glas J, Torok HP, Tonenchi L, Kapser J, Schiemann U, Muller-Myhsok B, Folwaczny M, et al. Association of polymorphisms in the interleukin-18 gene in patients with Crohn’s disease depending on the CARD15/NOD2 genotype. Inflamm Bowel Dis. 2005;11:1031–1037. doi: 10.1097/01.mib.0000187574.41290.b1. [DOI] [PubMed] [Google Scholar]
- 75.Subramaniam S, Stansberg C, Cunningham C. The interleukin 1 receptor family. Dev Comp Immunol. 2004;28:415–428. doi: 10.1016/j.dci.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 76.Kato Z, Jee J, Shikano H, Mishima M, Ohki I, Ohnishi H, Li A, et al. The structure and binding mode of interleukin-18. Nat Struct Biol. 2003;10:966–971. doi: 10.1038/nsb993. [DOI] [PubMed] [Google Scholar]
- 77.Born TL, Thomassen E, Bird TA, Sims JE. Cloning of a novel receptor subunit, AcPL, required for interleukin-18 signaling. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 78.Fantuzzi G, Banda NK, Guthridge C, Vondracek A, Kim SH, Siegmund B, Azam T, et al. Generation and characterization of mice transgenic for human IL-18-binding protein isoform a. J Leukoc Biol. 2003;74:889–896. doi: 10.1189/jlb.0503230. [DOI] [PubMed] [Google Scholar]
- 79.Dinarello CA, Novick D, Rubinstein M, Lonnemann G. Interleukin 18 and interleukin 18 binding protein: possible role in immunosuppression of chronic renal failure. Blood Purif. 2003;21:258–270. doi: 10.1159/000070699. [DOI] [PubMed] [Google Scholar]
- 80.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 81.Kanai T, Watanabe M, Okazawa A, Nakamaru K, Okamoto M, Naganuma M, Ishii H, et al. Interleukin 18 is a potent proliferative factor for intestinal mucosal lymphocytes in Crohn’s disease. Gastroenterology. 2000;119:1514–1523. doi: 10.1053/gast.2000.20260. [DOI] [PubMed] [Google Scholar]
- 82.Takeuchi D, Yoshidome H, Kato A, Ito H, Kimura F, Shimizu H, Ohtsuka M, et al. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. Hepatology. 2004;39:699–710. doi: 10.1002/hep.20117. [DOI] [PubMed] [Google Scholar]
- 83.Tsutsui H, Matsui K, Kawada N, Hyodo Y, Hayashi N, Okamura H, Higashino K, et al. IL-18 accounts for both TNF-α and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J Immunol. 1997;159:3961–3967. [PubMed] [Google Scholar]
- 84.Faggioni R, Cattley RC, Guo J, Flores S, Brown H, Qi M, Yin S, et al. IL-18-binding protein protects against lipopolysaccharide-induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. J Immunol. 2001;167:5913–5920. doi: 10.4049/jimmunol.167.10.5913. [DOI] [PubMed] [Google Scholar]
- 85.Raeburn CD, Dinarello CA, Zimmerman MA, Calkins CM, Pomerantz BJ, McIntyre RC, Jr., Harken AH, et al. Neutralization of IL-18 attenuates lipopolysaccharide-induced myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2002;283:H650–657. doi: 10.1152/ajpheart.00043.2002. [DOI] [PubMed] [Google Scholar]
- 86.Min CK, Maeda Y, Lowler K, Liu C, Clouthier S, Lofthus D, Weisiger E, et al. Paradoxical effects of interleukin-18 on the severity of acute graft-versus-host disease mediated by CD4+ and CD8+ T-cell subsets after experimental allogeneic bone marrow transplantation. Blood. 2004;104:3393–3399. doi: 10.1182/blood-2004-02-0763. [DOI] [PubMed] [Google Scholar]
- 87.Plater-Zyberk C, Joosten LA, Helsen MM, Sattonnet-Roche P, Siegfried C, Alouani S, van De Loo FA, et al. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J Clin Invest. 2001;108:1825–1832. doi: 10.1172/JCI12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wirtz S, Becker C, Blumberg R, Galle PR, Neurath MF. Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J Immunol. 2002;168:411–420. doi: 10.4049/jimmunol.168.1.411. [DOI] [PubMed] [Google Scholar]
- 89.Monteleone G, Fina D, Caruso R, Pallone F. New mediators of immunity and inflammation in inflammatory bowel disease. Curr Opin Gastroenterol. 2006;22:361–364. doi: 10.1097/01.mog.0000231808.10773.8e. [DOI] [PubMed] [Google Scholar]
- 90.Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol. 2004;34:2347–2355. doi: 10.1002/eji.200425351. [DOI] [PubMed] [Google Scholar]
- 91.Ludwiczek O, Kaser A, Novick D, Dinarello CA, Rubinstein M, Tilg H. Elevated systemic levels of free interleukin-18 (IL-18) in patients with Crohn’s disease. Eur Cytokine Netw. 2005;16:27–33. [PubMed] [Google Scholar]
- 92.Wiercinska-Drapalo A, Flisiak R, Jaroszewicz J, Prokopowicz D. Plasma interleukin-18 reflects severity of ulcerative colitis. World J Gastroenterol. 2005;11:605–608. doi: 10.3748/wjg.v11.i4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salvati VM, MacDonald TT, Bajaj-Elliott M, Borrelli M, Staiano A, Auricchio S, Troncone R, et al. Interleukin 18 and associated markers of T helper cell type 1 activity in coeliac disease. Gut. 2002;50:186–190. doi: 10.1136/gut.50.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alderson MR, Smith CA, Tough TW, Davis-Smith T, Armitage RJ, Falk B, Roux E, et al. Molecular and biological characterization of human 4-1BB and its ligand. Eur J Immunol. 1994;24:2219–2227. doi: 10.1002/eji.1830240943. [DOI] [PubMed] [Google Scholar]
- 95.Wilcox RA, Tamada K, Strome SE, Chen L. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol. 2002;169:4230–4236. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- 96.Zhou Z, Pollok KE, Kim KK, Kim YJ, Kwon BS. Functional analysis of T-cell antigen 4-1BB in activated intestinal intra-epithelial T lymphocytes. Immunol Lett. 1994;41:177–184. doi: 10.1016/0165-2478(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Z, Kim S, Hurtado J, Lee ZH, Kim KK, Pollok KE, Kwon BS. Characterization of human homologue of 4-1BB and its ligand. Immunol Lett. 1995;45:67–73. doi: 10.1016/0165-2478(94)00227-i. [DOI] [PubMed] [Google Scholar]
- 98.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 99.Bukczynski J, Wen T, Watts TH. Costimulation of human CD28- T cells by 4-1BB ligand. Eur J Immunol. 2003;33:446–454. doi: 10.1002/immu.200310020. [DOI] [PubMed] [Google Scholar]
- 100.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003;14:265–273. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 101.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28- T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- 102.Maerten P, Kwon BS, Shen C, De Hertogh G, Cadot P, Bullens DM, Overbergh L, et al. Involvement of 4-1BB (CD137)-4-1BBligand interaction in the modulation of CD4 T cell-mediated inflammatory colitis. Clin Exp Immunol. 2006;143:228–236. doi: 10.1111/j.1365-2249.2005.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maerten P, Geboes K, De Hertogh G, Shen C, Cadot P, Bullens DM, Van Assche G, et al. Functional expression of 4-1BB (CD137) in the inflammatory tissue in Crohn’s disease. Clin Immunol. 2004;112:239–246. doi: 10.1016/j.clim.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 104.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 105.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Havran WL, Fitch FW. Characterization of murine cytolytic-helper hybrid T cell clones. Nature. 1987;325:65–67. doi: 10.1038/325065a0. [DOI] [PubMed] [Google Scholar]
- 107.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. Embo J. 1990;9:1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, Williams AF. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 109.Fujita T, Ukyo N, Hori T, Uchiyama T. Functional characterization of OX40 expressed on human CD8+ T cells. Immunol Lett. 2006;106:27–33. doi: 10.1016/j.imlet.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 110.al-Shamkhani A, Birkeland ML, Puklavec M, Brown MH, James W, Barclay AN. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur J Immunol. 1996;26:1695–1699. doi: 10.1002/eji.1830260805. [DOI] [PubMed] [Google Scholar]
- 111.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 112.Weinberg AD, Vella AT, Croft M. OX-40: life beyond the effector T cell stage. Semin Immunol. 1998;10:471–480. doi: 10.1006/smim.1998.0146. [DOI] [PubMed] [Google Scholar]
- 113.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- 114.Lane P. Role of OX40 signals in coordinating CD4 T cell selection, migration, and cytokine differentiation in T helper (Th)1 and Th2 cells. J Exp Med. 2000;191:201–206. doi: 10.1084/jem.191.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, α4β7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–863. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stuber E, Buschenfeld A, Luttges J, Von Freier A, Arendt T, Folsch UR. The expression of OX40 in immunologically mediated diseases of the gastrointestinal tract (celiac disease, Crohn’s disease, ulcerative colitis) Eur J Clin Invest. 2000;30:594–599. doi: 10.1046/j.1365-2362.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 117.Stuber E, Von Freier A, Marinescu D, Folsch UR. Involvement of OX40-OX40L interactions in the intestinal manifestations of the murine acute graft-versus-host disease. Gastroenterology. 1998;115:1205–1215. doi: 10.1016/s0016-5085(98)70092-7. [DOI] [PubMed] [Google Scholar]
- 118.Tsukada N, Akiba H, Kobata T, Aizawa Y, Yagita H, Okumura K. Blockade of CD134 (OX40)-CD134L interaction ameliorates lethal acute graft-versus-host disease in a murine model of allogeneic bone marrow transplantation. Blood. 2000;95:2434–2439. [PubMed] [Google Scholar]
- 119.Totsuka T, Kanai T, Uraushihara K, Iiyama R, Yamazaki M, Akiba H, Yagita H, et al. Therapeutic effect of anti-OX40L and anti-TNF-α MAbs in a murine model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G595–603. doi: 10.1152/ajpgi.00450.2002. [DOI] [PubMed] [Google Scholar]
- 120.Obermeier F, Schwarz H, Dunger N, Strauch UG, Grunwald N, Scholmerich J, Falk W. OX40/OX40L interaction induces the expression of CXCR5 and contributes to chronic colitis induced by dextran sulfate sodium in mice. Eur J Immunol. 2003;33:3265–3274. doi: 10.1002/eji.200324124. [DOI] [PubMed] [Google Scholar]
- 121.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 122.Subudhi SK, Alegre ML, Fu YX. The balance of immune responses: costimulation verse coinhibition. J Mol Med. 2005;83:193–202. doi: 10.1007/s00109-004-0617-1. [DOI] [PubMed] [Google Scholar]
- 123.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 124.Sato T, Kanai T, Watanabe M, Sakuraba A, Okamoto S, Nakai T, Okazawa A, et al. Hyperexpression of inducible costimulator and its contribution on lamina propria T cells in inflammatory bowel disease. Gastroenterology. 2004;126:829–839. doi: 10.1053/j.gastro.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 125.Totsuka T, Kanai T, Iiyama R, Uraushihara K, Yamazaki M, Okamoto R, Hibi T, et al. Ameliorating effect of anti-inducible costimulator monoclonal antibody in a murine model of chronic colitis. Gastroenterology. 2003;124:410–421. doi: 10.1053/gast.2003.50050. [DOI] [PubMed] [Google Scholar]
- 126.Ishii K, Kanai T, Totsuka T, Uraushihara K, Ishikura T, Yamazaki M, Okamoto R, et al. Hyperexpression of inducible costimulator on lamina propria mononuclear cells in rat dextran sulfate sodium colitis. J Gastroenterol Hepatol. 2004;19:174–181. doi: 10.1111/j.1440-1746.2004.03202.x. [DOI] [PubMed] [Google Scholar]
- 127.Nakazawa A, Dotan I, Brimnes J, Allez M, Shao L, Tsushima F, Azuma M, et al. The expression and function of costimulatory molecules B7H and B7-H1 on colonic epithelial cells. Gastroenterology. 2004;126:1347–1357. doi: 10.1053/j.gastro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 128.Kanai T, Totsuka T, Tezuka K, Watanabe M. ICOS costimulation in inflammatory bowel disease. J Gastroenterol. 2002;37(Suppl 14):78–81. doi: 10.1007/BF03326419. [DOI] [PubMed] [Google Scholar]
- 129.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 130.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 131.Mosley RL, Whetsell M, Klein JR. Proliferative properties of murine intestinal intraepithelial lymphocytes (IEL): IEL expressing TCR αβ or TCR γδ are largely unresponsive to proliferative signals mediated via conventional stimulation of the CD3-TCR complex. Int Immunol. 1991;3:563–569. doi: 10.1093/intimm/3.6.563. [DOI] [PubMed] [Google Scholar]
- 132.Sydora BC, Mixter PF, Holcombe HR, Eghtesady P, Williams K, Amaral MC, Nel A, et al. Intestinal intraepithelial lymphocytes are activated and cytolytic but do not proliferate as well as other T cells in response to mitogenic signals. J Immunol. 1993;150:2179–2191. [PubMed] [Google Scholar]
- 133.Wang T, Langley KE, Gourley WK, Klimpel GR. Stem cell factor (SCF) can regulate the activation and expansion of murine intraepithelial lymphocytes. Cytokine. 2000;12:272–280. doi: 10.1006/cyto.1999.0551. [DOI] [PubMed] [Google Scholar]
- 134.Laky K, Lefrancois L, Puddington L. Age-dependent intestinal lymphoproliferative disorder due to stem cell factor receptor deficiency: parameters in small and large intestine. J Immunol. 1997;158:1417–1427. [PubMed] [Google Scholar]
- 135.Hirose K, Suzuki H, Nishimura H, Mitani A, Washizu J, Matsuguchi T, Yoshikai Y. Interleukin-15 may be responsible for early activation of intestinal intraepithelial lymphocytes after oral infection with Listeria monocytogenes in rats. Infect Immun. 1998;66:5677–5683. doi: 10.1128/iai.66.12.5677-5683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ebert EC. Interleukin 15 is a potent stimulant of intraepithelial lymphocytes. Gastroenterology. 1998;115:1439–1445. doi: 10.1016/s0016-5085(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 137.Puddington L, Olson S, Lefrancois L. Interactions between stem cell factor and c-Kit are required for intestinal immune system homeostasis. Immunity. 1994;1:733–739. doi: 10.1016/s1074-7613(94)80015-4. [DOI] [PubMed] [Google Scholar]
- 138.Wang T, Alam R, Langley KE, Klimpel GR. Stem cell factor and IL-2 act synergistically in inducing intraepithelial lymphocyte proliferation and cytokine production: upregulation of the IL-2 receptor gamma-chain and signaling via JAK-3. Cell Immunol. 2000;205:62–71. doi: 10.1006/cimm.2000.1707. [DOI] [PubMed] [Google Scholar]
- 139.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sperber K, Silverstein L, Brusco C, Yoon C, Mullin GE, Mayer L. Cytokine secretion induced by superantigens in peripheral blood mononuclear cells, lamina propria lymphocytes, and intraepithelial lymphocytes. Clin Diagn Lab Immunol. 1995;2:473–477. doi: 10.1128/cdli.2.4.473-477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaser A, Nieuwenhuis EE, Strober W, Mayer L, Fuss I, Colgan S, Blumberg RS. Natural killer T cells in mucosal homeostasis. Ann N Y Acad Sci. 2004;1029:154–168. doi: 10.1196/annals.1309.032. [DOI] [PubMed] [Google Scholar]
- 142.Ebert EC. Inhibitory effects of transforming growth factor-β (TGF-β) on certain functions of intraepithelial lymphocytes. Clin Exp Immunol. 1999;115:415–420. doi: 10.1046/j.1365-2249.1999.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- 144.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schwerbrock NM, Makkink MK, van der Sluis M, Buller HA, Einerhand AW, Sartor RB, Dekker J. Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm Bowel Dis. 2004;10:811–823. doi: 10.1097/00054725-200411000-00016. [DOI] [PubMed] [Google Scholar]