Abstract
Gold nanorods (AuNRs) have unique optical properties for numerous biomedical applications, but the interactions between AuNRs and proteins, particularly those of the extracellular matrix (ECM), are poorly understood. Here the effects of AuNRs on the self-assembly, mechanics, and remodeling of type I collagen gels were examined in vitro. AuNRs were modified with polyelectrolyte multilayers (PEMs) to minimize cytotoxicity, and AuNRs with different terminal polymer chemistries were examined for their interactions with collagen by turbidity assays, rheological tests, and microscopy. Gel contraction assays were used to examine the effects of the PEM-coated AuNRs on cell-mediated collagen remodeling. Polyanion-terminated AuNRs significantly reduced the lag (nucleation) phase of collagen self-assembly and significantly increased the dynamic shear modulus of the polymerized gels, whereas polycation-terminated AuNRs had no effect on the mechanical properties of the collagen. Both polyanion- and polycation-terminated AuNRs significantly inhibited collagen gel contraction by cardiac fibroblasts, and the nanoparticles were localized in intra-, peri-, and extracellular compartments, suggesting that PEM-coated AuNRs influence cell behavior via multiple mechanisms. These results demonstrate the significance of nanoparticle-ECM interactions in determining the bioactivity of nanoparticles.
1.0 Introduction
Gold nanorods (AuNRs) are under investigation for use in biomedical applications including photothermal therapies [1], imaging [2], biochemical sensing [3], and drug delivery [4]. In many cases the target physiologic milieu is rich in extracellular matrix (ECM), a mixture of fibrillar and globular proteins, proteoglycans, and glycosaminoglycans. The most abundant component of the ECM in connective tissues is the structural protein collagen. Therefore, it is likely that the performance, toxicity, and bioactivity of these particles will be affected by their interactions with ECM proteins such as type I collagen. Several studies describe complex empirical relationships between nanoparticle chemistry and serum protein adsorption [5-8], and polymeric nanoparticles have been shown to promote the fibrillation (i.e., polymerization) of proteins, such as β2-microglobulin [9] and amyloid β [10]. Little is known, however, about how nanoparticles interact with ECM proteins.
Several groups have reported the facile surface modification of nanoparticles with polyelectrolyte multilayers (PEMs) composed of alternating layers of polyanions and polycations [11-13]. By virtue of their electrostatics-based assembly, PEMs typically confer a net charge on the substrate and are therefore expected to influence protein adsorption to nanoparticles. Indeed, the PEM composition and deposition conditions can alter the kinetics and extent of protein adsorption on planar surfaces [14]. Biophysical properties of the polyelectrolytes, such as charge and charge density, can also influence cytotoxicity [15], ECM synthesis [16], and inflammation [17]. We have shown previously that AuNRs coated with a polycation are more readily endocytosed than particles coated with a polyanion while the two preparations have similar, minimal cytotoxicity [18]. PEMs have demonstrated their utility in regulating cell phenotype and suppressing the immune response, but our understanding of how such surface modifications influence nanoparticle-protein interactions is in the nascent stages of development.
The effects of nanoparticles on cell-ECM interactions have received little attention to date. MacDonald and colleagues showed that single-walled carbon nanotubes with carboxylated surfaces delayed the contraction of collagen gels by smooth muscle cells [19]. Ultrafine carbon black particles (∼14nm in diameter) inhibited collagen gel contraction by lung fibroblasts, and the effect was attributed to the particles binding fibronectin and transforming growth factor-β1 [20]. Recently, we reported that AuNRs coated with PEMs terminated with poly(styrene sulfonate) (PSS) also inhibited cell-mediated contraction of type I collagen gels[21]. We observed that cardiac fibroblasts suspended in collagen doped with the PSS-terminated AuNRs had lower expression of mRNAs for alpha smooth muscle actin and type I collagen than controls, indicating that the composite 3D scaffold altered the phenotype of the cells. We also noted qualitative differences in the mechanical properties between control gels and collagen gels doped with AuNRs. Others have demonstrated an important role for ECM stiffness in the regulation of progenitor, cardiac, and muscle cell phenotypes [22], so our results led us to speculate that PSS-terminated AuNRs altered the assembly and mechanics of the collagen scaffold to yield the differences in gel contraction.
Cardiac fibroblasts constitute a major population of cells within the heart [23] and are responsible for the production and remodeling of the collagen network within the heart, both in normal development and in response to pathological injury. The three-dimensional collagen gel contraction model has long been used to examine fibroblast behavior [24] and is commonly used to model wound healing and ECM remodeling. The objective of the current study is to quantitatively examine the interactions of PEM-coated gold nanorods on the self-assembly and properties of type I collagen. We hypothesized that PEM-coated AuNRs, by altering the assembly and stiffness of type I collagen networks, regulate the transformation of fibroblasts into a more contractile myofibroblast phenotype. The ability to modulate cell phenotype and thus control wound contraction would be useful in regulating scar formation. Using PEMs of various compositions and end-groups, we investigate the effects of different surface chemistries on the self-assembly of collagen and cell-mediated contraction of collagen-AuNR composites in vitro.
2.0 Materials and methods
2.1 Materials
All chemicals were obtained from Sigma (St. Louis, MO), unless otherwise noted. High glucose Dulbecco’s Modified Eagle’s Medium (DMEM), rhodamine phalloidin, 4′,6-diamidino-2-phenylindole (DAPI), and the stock penicillin/streptomycin solution were from Invitrogen (Carlsbad, CA).
2.2 AuNR synthesis and layer-by-layer deposition of PEMs
High aspect ratio gold nanorods were synthesized by a seed-mediated, surfactant-directed approach as previously described [25,26]. Briefly, ∼4 nm spherical (seed) particles were prepared via reduction of chloroauric acid (250 μM) by 0.01 M ice-cold sodium borohydride in water in the presence of 0.1 M ultrapure hexadecyltrimethylammonium bromide (CTAB). After 15 min., the seed particles were diluted by a 3 step sequence (∼15 s / step) of 10-fold dilutions in solutions containing 250 μM chloroauric acid, 0.1 M CTAB, and 500 μM ascorbic acid and allowed to react overnight at room temperature. The supernatant was decanted, and the remaining particles were resuspended in 18 MΩ/cm water. These reactions yielded polydisperse populations of nanoparticles, with the predominant morphologies being rods of length 392 ± 93 nm and width 22 ± 3 nm. The concentration of gold in preparations of AuNRs was determined using a Model 720-ES inductively coupled plasma optical emission spectrometer (ICP-OES) (Varian, Palo Alto, CA).
A positively charged CTAB bilayer was present at the surface of the as-prepared AuNRs and served as the basis for layer-by-layer deposition of polyelectrolytes. Assembly of PEMs on the AuNRs was performed as previously described [12] and illustrated in Figure 1A. Briefly, AuNRs were first incubated with 1.7 mg/mL PSS (70 kDa) or poly(acrylic acid) (PAA, 15 kDa) in 1 mM NaCl for 30 min at room temperature. The rods were then washed by centrifugation [4000 relative centrifugal force (RCF), 5 min.] and resuspended in 18 MΩ/cm water. PSS-and PAA-coated rods were then incubated with 1.7 mg/mL poly(diallyldimethylammonium) chloride (PDADMAC, <100 kDa) or poly(allylamine) hydrochloride (PAH, 15kDa), respectively, for 30 min at room temperature. After washing as described above, portions of PDADMAC- and PAH-coated AuNRs were incubated with 1.7 mg/mL PSS or PAA, respectively, for 30 min at room temperature and washed. Multilayer assembly was monitored by measurement of the zeta potential (ζ) of the AuNRs in 18 MΩ/cm water (ZetaPALS, Brookhaven Instruments Corp., Holtsville, NY). The zeta potential, an approximation of the effective surface charge, reversed polarity with the addition of each polyelectrolyte layer, and the final zeta potential for each preparation of AuNRs is given in Table 1. We estimate that the 3-layer coatings used in this study added 4-5 nm to both the radius and the length of the as-prepared AuNRs.
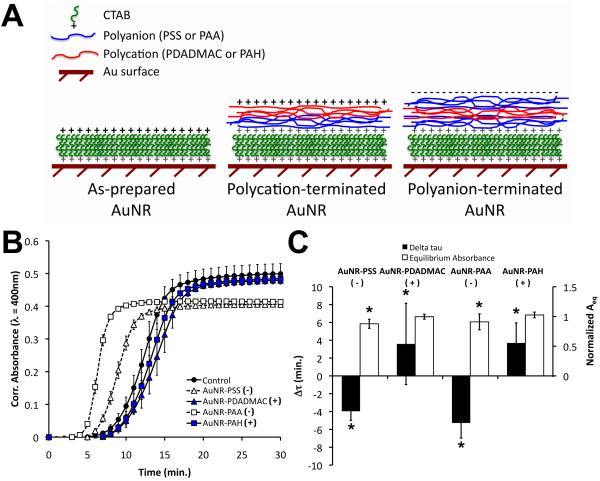
Figure 1.
Polyelectrolyte multilayer assembly on the surfaces of AuNRs and the effects of polyelectrolyte-coated AuNRs on the self-assembly of type I collagen. (A) Schematic of gold nanomaterial surfaces presenting the CTAB bilayer and the 2-layer (for polycation—terminated AuNRs) or 3-layer (for polyanion-terminated AuNRs) polyelectrolyte coatings. Layer thicknesses are not drawn to scale. (B) Cell-free turbidity assays revealed charge-dependent perturbations in the polymerization of collagen doped with polyelectrolyte-coated AuNRs. Plot legend indicates the identity and polarity of the terminal polyelectrolyte for each PEM-coated AuNR. Data are mean ± S.D. with n = 3 from a representative experiment. Absorbances were corrected by subtraction of the initial (t=0) absorbance. (C) A kinetic parameter, Δτ = τ50 - τ50,control, and the equilibrium absorbance, Aeq, were determined from the polymerization curves. AuNR surfaces terminated with negatively charged polyelectrolytes had negative Δτ and lower Aeq than controls, whereas AuNRs with a positively charged surface had positive Δτ. Aeq values were normalized by control values. Data are mean ± S.D. with n = 8 from 3 independent experiments. * indicates p<0.05 vs. controls.
Table 1. Zeta potentials (ζ) of asprepared and polyelectrolytecoated gold nanorods suspended in 18M Ω/cm water. The label of each AuNR preparation used in the figure legends is also given.
| Polyelectrolyte Multilayer Composition | Figure Legend Label | ζ (mV) |
|---|---|---|
| None | AuNR | + 44.14 ± 1.35 |
| PSS-PDADMAC-PSS | AuNR-PSS | - 44.08 ± 1.25 |
| PSS-PDADMAC | AuNR-PDADMAC | + 56.26 ± 1.06 |
| PAA-PAH-PAA | AuNR-PAA | - 28.37 ± 1.05 |
| PAA-PAH | AuNR-PAH | + 52.63 ± 1.64 |
2.3 Turbidity Assays
We used a well established turbidity assay [27] modified for a microplate reader to examine the effects of AuNRs on the polymerization of type I collagen. Acid-solubilized bovine type I collagen from a commercial source (3.1 mg/mL, Inamed, Santa Barbara, CA) was neutralized on ice with 0.2 N 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 9.0 and 10-fold concentrated phosphate buffered saline (PBS) in the volumetric ratio of 8:1:1. The neutralized collagen was then mixed with as-prepared AuNRs (no PEM), or PSS-, PDADMAC-, PAA-, or PAH-terminated AuNRs. The collagen-AuNR solutions were vortexed briefly, sonicated for 30 s, and kept on ice for no more than 10 min prior to polymerization. The final concentrations of collagen and gold were 2.2 mg/mL (∼22 μM) and 337 ng/mL (∼200 pM in particles), respectively. Portions of each solution (100 μL) were moved to the wells of a microplate in duplicate or triplicate for each experiment. The microplate was then moved to a temperature-controlled (37 °C) microplate reader (Bio-Rad, Hercules, CA) and the absorbance (λ = 400 nm) was measured every 60 s for 1 h. Absorbances were corrected by subtraction of the initial (t = 0) absorbance, to account for the extinction due to the nanoparticles. Kinetic parameters, including τ50 and Δτ, and the equilibrium absorbance, Aeq, were calculated from each turbidity curve to quantify AuNR-induced changes in polymerization. Aeq was calculated as the mean absorbance over the last 5 minutes of the experiment. τ50 was defined as the time for each sample to reach 50% of its Aeq, and Δτ was calculated as the difference between τ50 of the sample and the mean τ50 of controls within each experiment. Aeq values were normalized by dividing by the average Aeq of controls within each experiment.
2.4 Rheology
The effects of AuNRs on the mechanical properties of cell-free type I collagen gels were examined by dynamic torsional shear tests. Tests were performed on an RFS II Fluid Spectrometer (Rheometrics Scientific, Piscataway, NJ) with a 25-mm parallel plate measurement geometry and a temperature-controlled lower plate, and the data were analyzed with Rhios version 4.2.2 software (Rheometrics Scientific).
Type I collagen gels with and without AuNRs were prepared as described above for the turbidity assays, and 285 μL of ice-cold neutralized collagen solution was placed on the lower plate at a temperature of ∼14 °C. The upper plate was lowered to a gap of 500 μm, and low viscosity mineral oil was applied around each sample to minimize evaporation. The complex shear modulus (G*) was measured for 1 h as the temperature of the lower plate was increased to 37 °C and the sample polymerized; during this time each sample was subjected to sinusoidal shear strain at 1 rad/s and 2% strain. These tests also afforded computation of the phase angle, δ, between the applied strain wave and measured stress wave. The tangent of the phase angle, tan δ, was calculated as the ratio of the loss modulus to storage modulus (G”/G’) and was used to describe the viscoelastic behavior of each material. Following phase transition and equilibration for 1 h at 37 °C, each sample was subjected to sinusoidal shear strain (10 rad/s) over a range of strains (γ = 1-100%) to examine the strain-stiffening behavior characteristic of polymerized type I collagen [28].
2.5 Cell isolation and culture
Cardiac fibroblasts were isolated from 4-day-old neonatal rats as previously described [29] and according to a protocol approved by the University of South Carolina Institutional Animal Care and Use Committee. Briefly, whole hearts were aseptically harvested from the animals, rinsed and minced in Krebs-Ringer bicarbonate buffer, and digested with 100 U/mL collagenase (Invitrogen). The digests were mechanically dissociated by passing through a 14 gauge cannula and moved to T75 flasks for 2 h in a standard tissue culture incubator (37 °C, 5% CO2, ∼95% relative humidity), after which a fibroblast-rich population of cells had adhered to the flasks. Cells were expanded through passage 2 or 3 in culture medium composed of DMEM supplemented with 10% newborn bovine serum, 5% fetal bovine serum, 100 U/mL penicillin G, 100 μg/mL streptomycin, and 10 μg/mL gentamicin (Invitrogen). Prior to suspension in 3D collagen or collagen-AuNR gels, cells were rinsed with Moscona’s saline, trypsinized [0.25% trypsin with 1 mM ethylenediaminetetraacetic acid (EDTA), Invitrogen] for 3 min in a tissue culture incubator, quenched with serum-containing culture medium, centrifuged, and resuspended in serum-free medium composed of DMEM with 0.5% BSA, 1mg/mL insulin, 0.55 mg/ml transferrin, 0.5 μg/mL sodium selenite, 82 μg/mL ascorbic acid 2-PO4, and antibiotics as listed above. Cell number and viability were determined with a hemocytometer by counting and erythrosin B exclusion.
Neutralized collagen and collagen-AuNR composites were prepared as described above for the turbidity experiments. The concentration of AuNRs used in these culture experiments was based on the results of a previously reported dose-response study [21]. The concentration of collagen is consistent with several studies implementing the collagen gel contraction assay as a model of myocardial wound remodeling [30-32]. The collagen gel constructs serve as model tissues in which the cells can interact with an ECM and the nanoparticles in 3 dimensions. Cardiac fibroblasts were suspended in the neutralized collagen solutions at a final cell density of 200,000 cells/mL, and the constructs were cast in the wells of a 24-well plate (500 μL/well) preadsorbed with 5% BSA. The plates were moved to a tissue culture incubator where the constructs were allowed to polymerize for 1 h. The constructs were then detached from the surface of the wells and floated in serum-free culture medium. Serum-free medium was used to minimize the effects of exogenous growth factors and fibronectin on AuNR-collagen and AuNR-cell interactions. Collagen gel contraction over 48 h was quantified by analysis of images captured with a digital camera (Canon USA, Lake Success, NY) using ImageJ software [33]. The culture medium was collected and replaced every 24 h. After 48 h, some constructs were incubated with Alamar Blue (Invitrogen) according to the manufacturer’s instructions for another 24 h. Some 8 h and 24 h constructs were fixed in 4% paraformaldehyde overnight at 4 °C for confocal microscopy, while additional 24 h constructs were fixed overnight in 2.5% glutaraldehyde, 1% tannic acid, and 1% OsO4 in 0.1 M sodium cacodylate for transmission electron microscopy (TEM).
2.6 Darkfield, confocal, and transmission electron microscopy
The morphology of the collagen network in the absence and presence of AuNRs was examined through dark field microscopy of thin films. Collagen and collagen-AuNR composites were prepared as described above for the turbidity assays and diluted with ice-cold PBS to a final concentration of 0.5 mg/mL collagen; the molar ratio of collagen to AuNRs was similar to that of the samples used in turbidity assays, rheological measurements, and culture experiments. The films were prepared by applying a small volume of sample (∼100 μL) to the surface of an uncharged microscope slide and then placing a 22 mm × 40 mm × 1.5 mm coverslip over the sample. The slides were warmed in a tissue culture incubator for 30 min at 37 °C, and the coverslips were sealed with nail polish prior to imaging. The polymerized collagen networks were imaged through 20x air darkfield optics on a Nikon E600 with a mercury vapor lamp light source (EXFO, Quebec, Canada). Images were captured with a Spot Insight camera and Spot software (both from Diagnostic Instruments, Sterling Heights, MI), and the gamma values were manually adjusted to enhance contrast in ImageJ.
Constructs fixed for confocal microscopy were rinsed with PBS, permeabilized with 0.01% Triton X-100/0.1 M glycine/PBS for 30 min at room temperature, and stained whole mount with 1 U/mL rhodamine-phalloidin and 1 μg/mL DAPI in PBS for 4 h at room temperature. The constructs were rinsed three times with PBS, mounted in 1 mg/mL 1,4-diazabicyclo[2.2.2]octane in 3:1 glycerol:PBS (v/v), and set in CoverWell chambers (Electron Microscopy Sciences, Hatfield, PA) on microscope slides. The constructs were optically sectioned on a 510LSM confocal laser scanning microscope (Carl Zeiss Microimaging, Thornwood, NY) with 40x and 63x oil immersion objectives. Rhodamine phalloidin and DAPI were excited by 543 nm and 405 nm lasers, respectively, and detected via appropriate emission filters. The collagen network and AuNRs were detected by confocal reflectance microscopy with the 488 nm and 633 nm laser lines. The collagen efficiently reflected 488 nm light, whereas the AuNRs efficiently reflected both 488 nm and 633 nm light, and this difference afforded distinction between the two components of the scaffold material. The 488 nm reflectance tracks in each image were post-processed using an anisotropic diffusion algorithm [34] in ImageJ to reduce noise.
Constructs fixed for TEM were dehydrated in graded alcohols, embedded in Polybed 812 in acetonitrile, and sectioned at 110 nm on a Leica Ultracut R. Each section was placed on a copper grid, stained with Hanaichi’s lead stain, dried at room temperature, and imaged on a 200CX TEM at 120kV (JEOL, Tokyo, Japan).
2.6 Statistics
Turbidity assays and culture experiments were examined in 3 independent experiments, with 2-3 replicates in each experiment, and rheology tests were performed on 3 samples per condition. The darkfield, confocal, and TEM images are representative of 4-5 fields. Data were analyzed by the general linear model and Dunnett’s post-hoc comparisons using Minitab version 15 (State College, PA), and differences were deemed significant for p < 0.05.
3.0 Results
3.1 Polymerization of collagen in the absence and presence of AuNRs
We examined the self-assembly of collagen in the absence and presence of PEM-coated AuNRs by turbidity assays. Neutralized type I collagen exhibits a sol-gel phase transition at ∼37 °C characterized by a lag phase in which monomers coalesce into nuclei and a growth phase during which nuclei undergo anisotropic longitudinal and lateral accretion [35]. During the growth phase, the turbidity of the collagen solution increases as the growing fibrils increasingly scatter and absorb light. The diffusion and concentration of monomer eventually become limiting, fibril formation (i.e., fibrillogenesis) slows, and the optical density of the solution approaches a plateau (Aeq).
In our experiments, controls composed of neutralized collagen in the absence of AuNRs exhibited a lag phase of approximately 5 min and a τ50 of approximately 12 min (Figure 1B). The addition of AuNRs terminated with negatively charged polyelectrolytes, PSS or PAA, accelerated the self-assembly of collagen whereas cationic particles, terminated with PDADMAC or PAH, delayed fibrillogenesis. Quantification of the kinetic and equilibrium turbidity parameters revealed statistically significant reductions in τ50 (indicated by negative Δτ) and Aeq with the addition of PSS- or PAA-presenting AuNRs compared to controls (Figure 1C), and PAA-terminated AuNRs accelerated fibrillogenesis more potently than PSS-terminated AuNRs. In contrast, AuNRs with positively charged end groups significantly increased τ50 over that of controls but had no effect on Aeq. The addition of as-prepared τAuNRs presenting the positively charged CTAB end group had no significant effects on τ50 or Aeq (not shown). These results indicate that PEM-coated AuNRs influenced collagen self-assembly in charge- and end-group-dependent manners and suggest that polyanionic surface chemistries promoted fibril nucleation in collagen-AuNR composites.
3.2 Rheology of collagen and collagen-AuNR composites
The mechanical properties of collagen and collagen-AuNR composite gels were examined by rheological methods during and following polymerization. A time sweep during which oscillatory torsion was applied to a warming sample revealed that controls exhibited an increase in the complex shear modulus (G*) beginning approximately 10 min after the start of the test and within 1 min of the plate reaching 37 °C (Figure 2A). Consistent with the results of the turbidity assays, collagen doped with polyanion-terminated AuNRs exhibited a reduced lag time between the start of the test and the increase in G* as compared to controls. In contrast, addition of AuNRs terminated with the polycation PDADMAC modestly delayed the increase in G*. PAH-terminated and as-prepared AuNRs, also positively charged, had no significant effects on the change in G* associated with the collagen phase transition.
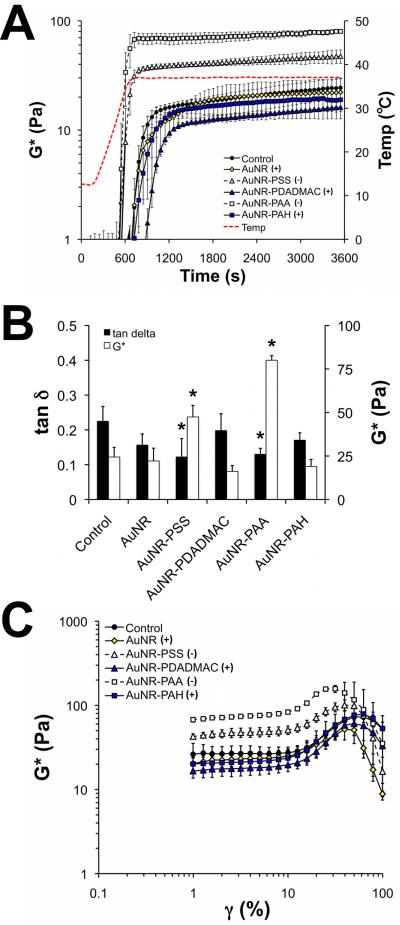
Figure 2.
The dynamic shear properties of type I collagen doped with various preparations of AuNRs. (A) Low amplitude oscillatory torsion tests were performed on neutralized collagen gels as they underwent a temperature-dependent phase change. Gels containing PSS- or PAA-terminated AuNRs polymerized more rapidly than controls, whereas polycation-terminated AuNRs modestly delayed increases in G*. The detection limit of the instrument was G* ≈ 2 Pa in these experiments. (B) After 1 h at 37 °C, collagen gels doped with PSS- or PAA-functionalized AuNRs had lower tan δ and higher G* than controls (p<0.05). (C) The AuNR-induced differences in G* were detected over a range of shear strains. Data are mean ± S.D. with n = 3.
AuNR-induced differences in the mechanical properties of the collagen were evaluated in terms of the equilibrium G* and tan δ, calculated as the means of these parameters over the last 5 min of the time sweep (Figure 2B). Collagen doped with PSS- or PAA-terminated AuNRs had significantly higher G* (+94% and +228%, respectively) and lower tan δ (-45% and -42%, respectively) than controls, indicating that these composite materials are stiffer and more elastic (i.e., capable of storing kinetic energy) than controls. In contrast, collagen doped with positively charged AuNRs had equilibrium G* and tan δ values statistically similar to controls. Several reports describe a role for substrate stiffness in regulating progenitor cell maturation [22], so AuNR-induced increases in the stiffness of the collagen gel may regulate the phenotype of cardiac fibroblasts. AuNR-induced reductions in the viscous behavior (i.e., lower tan δ) of the collagen may perturb short time-scale events know to be important for cell-mediated contraction, such as formation of podosomes [36].
Type I collagen and other biopolymers, such as fibrin and several cytoskeletal proteins, exhibit strain-stiffening behavior [28], and we implemented strain sweeps to explore the effects of PEM-coated AuNRs on this property of polymerized collagen (Figure 2C). In our experiments, the G* of control gels was steady for γ = 1-10%, increased for strains of 10∼73%, and decreased at higher strains. Collagen gels doped with polyanionic (PAA- or PSS-terminated) AuNRs had higher G* values than controls for most strains, but otherwise exhibited similar strain-stiffening behavior to controls. Polycation-terminated AuNRs had G* values and strain-stiffening behavior similar to controls. Interestingly, collagen containing PAA-terminated AuNRs had a significantly lower failure strain, defined as the strain at which the peak G* was measured, than controls (∼31% vs. ∼63%, p < 0.05). These results indicate that PEM-coated AuNRs do not substantially perturb the strain stiffening behavior of type I collagen, but suggest that the nanoparticles can adversely affect the failure properties of collagen gels.
3.3 Collagen network morphology
The morphology of collagen fibrils and the pore space between them in 3D gels and thin films were qualitatively examined using confocal reflectance and darkfield microscopy, respectively (Figure 3). AuNRs and aggregates thereof were also readily detected by these methods.
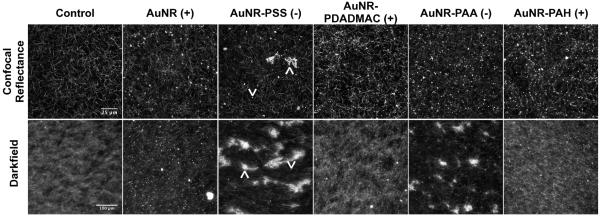
Figure 3.
The morphology of cell-free collagen and collagen-AuNR composites. Upper panels are representative images of 3D constructs 100μm from the gel surface captured via confocal reflectance microscopy (488nm laser line, optical section thickness ∼800nm). Lower panels are images of thin films captured by darkfield microscopy. In images of collagen doped with AuNR-PSS, upward arrowheads point to aggregates of AuNRs and downward arrowheads point to individual AuNRs.
Optical sections of 3D gels approximately 100 μm from the gel surface (Figure 3, upper panels) illustrate the meshwork of fibrils and high porosity characteristic of polymerized collagen. Collagen gels doped with as-prepared AuNRs had a network morphology similar to that of controls with intense points of reflected light indicative of AuNRs and their aggregates. As we have observed in previous studies [21], the addition of negatively charged PSS-coated AuNRs did not substantially alter the collagen network morphology, although large clusters of nanoparticles were identified by confocal reflectance in the midsubstance and on the surfaces (not shown) of the 3D gels. PAA-terminated AuNRs were found to be dispersed throughout the 3D gels as individual and/or relatively small aggregates closely associated with fibrils and fibril-fibril junctions. Collagen gels prepared with PDADMAC- or PAH-terminated AuNRs had network morphologies similar to controls, and both varieties of polycationic AuNRs were dispersed as individual particles or small aggregates in the collagen. For all AuNR-doped gels, the nanoparticles and aggregates thereof were detected primarily along, within, or at the junction of collagen fibrils. These results show that AuNRs have subtle effects on the micro-scale organization of collagen in 3D gels, but suggest that the nanoparticles are localized in the insoluble compartment of the network, intimately associated with the collagen fibers.
Analysis of thin collagen films by darkfield microscopy revealed striking differences in network morphology by the addition of AuNRs (Figure 3, lower panels). Controls exhibited a fine meshwork of fibrils amid a diffuse background arising from out-of-plane fibrils. Films containing as-prepared AuNRs had lower background than controls, and, as in the 3D gels, the AuNRs were dispersed throughout the film as individual particles or small aggregates. Films prepared with PSS-terminated AuNRs formed large, heterogeneous aggregates, and PAA-terminated AuNRs induced formation of similar, although generally smaller, aggregates. Interestingly, fibrils of collagen extended radially from the surfaces of the aggregates. In contrast, films prepared with polycationic (PDADMAC- or PAH-terminated) AuNRs exhibited collagen network morphologies qualitatively similar to controls. These data suggest that polyanion-terminated AuNRs promote nucleation (i.e., aggregate formation) in thin films of type I collagen.
3.4 Gel contraction and cell morphology in collagen and collagen-AuNR composites
We used gel contraction culture studies to quantitatively compare the cell-mediated remodeling of collagen and collagen-AuNR composites. Control constructs, composed of AuNR-free collagen seeded with neonatal cardiac fibroblasts, contracted to approximately 46% of the initial gel area over 48 h (Figure 4A). Constructs doped with as-prepared AuNRs exhibited minimal contraction over the same period, and PEM-coated AuNRs reduced contraction by 13-28%. The kinetics of contraction were similar between controls and composites containing PEM-coated AuNRs. AuNR-induced differences in gel contraction after 48 h were statistically significant for all groups except the PAHterminated AuNRs (Figure 4B).
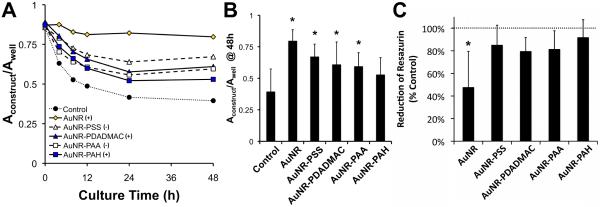
Figure 4.
The contraction of collagen and collagen-AuNR scaffolds by cardiac fibroblasts. (A) Contraction of free-floating collagen gels was measured over 48 h. Data are means with n = 8-9 from 3 independent experiments; error bars are omitted for clarity. (B) After 48 h, gels containing AuNRs were contracted less than controls. Data are means + S.D. (C) As-prepared AuNRs, but not polyelectrolyte-coated AuNRs, significantly reduced mitochondrial activity as measured by the Alamar Blue assay. Data are means + S.D. with n = 6 from 2 independent experiments. For B and C, * indicates p < 0.05 vs. controls.
The contribution of cytotoxicity in the gel contraction studies was examined using the Alamar Blue assay. The reactive component of Alamar Blue, resazurin, undergoes reduction to a different-colored product in the presence of viable cells. We observed a statistically significant decrease in resazurin reduction in constructs doped with as-prepared, uncoated AuNRs compared to controls (Figure 4C), indicating lower metabolic activity and suggesting that these nanoparticles are cytotoxic. Previous work in our lab has shown that free CTAB in the AuNR preparation is responsible for this cytotoxicity [18]. In contrast, AuNRs terminated with PSS, PDADMAC, PAA, or PAH exhibited resazurin reductions statistically similar to that of controls, suggesting that AuNR-induced reductions in collagen gel contraction were not due to cytotoxicity.
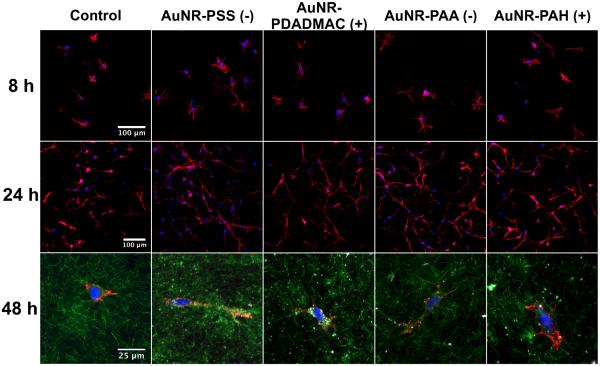
We examined cell spreading in the collagen and collagen-AuNR composite constructs by confocal microscopy. After 8 h, an early time by which substantial inhibition of gel contraction was observed, cells in constructs doped with PEM-coated AuNRs were spread similar to cells in control gels (Figure 5, top row). Cells seeded with as-prepared AuNRs had failed to spread by this time (not shown), as indicated by the round shape of most cells and consistent with the cytotoxicity noted in these cultures by the Alamar blue assay. After 24 h, cells in control gels and gels doped with poylelectrolyte-coated AuNRs were again similarly well spread (Figure 5, middle row). Using confocal reflectance microscopy with the 633 nm laser line, we observed PEM-coated AuNRs in the peri- and/or intra-cellular compartments and in the interstitial compartment among collagen fibrils (Figure 5, bottom row). These results indicate PEM-coated AuNRs do not alter superficial aspects of cell-collagen interactions (e.g., spreading) regardless of surface charge, and that the AuNRs are in close proximity to the cell surface.
Figure 5.
The morphologies of cells embedded in collagen and collagen-AuNR composites. The top and middle row panels are representative images of cells after 8 h and 24 h of culture, respectively. Actin was stained with rhodamine-phalloidin (red) and nuclei were stained with DAPI (blue). The bottom row panels are representative images of cells after 48 h of culture. Actin and nuclei were stained as in upper panels. Confocal reflectance images reveal the morphology of the collagen network (green, 488nm) and the locations of AuNRs and their aggregates (white, coincident reflection of 488nm and 633nm).
3.5 TEM localization of AuNRs in collagen and cells
Images of AuNR-collagen and AuNR-cell interactions were generated by TEM of constructs cultured for 24 h; we focused on the interactions of PSS- and PAA-terminated AuNRs due to their potent effects on collagen self-assembly, mechanics, and cell-mediated gel contraction. Both PSS- and PAA-terminated AuNRs were observed as individual particles and as aggregates surrounded by disorganized collagen (Figure 6A, B). In addition, polyanion-terminated AuNRs were detected within cells as individual particles and aggregates (Figure 6C, D). These results indicate that the AuNRs became entrained within the collagen and were taken up by the fibroblasts via endocytosis.
Figure 6.
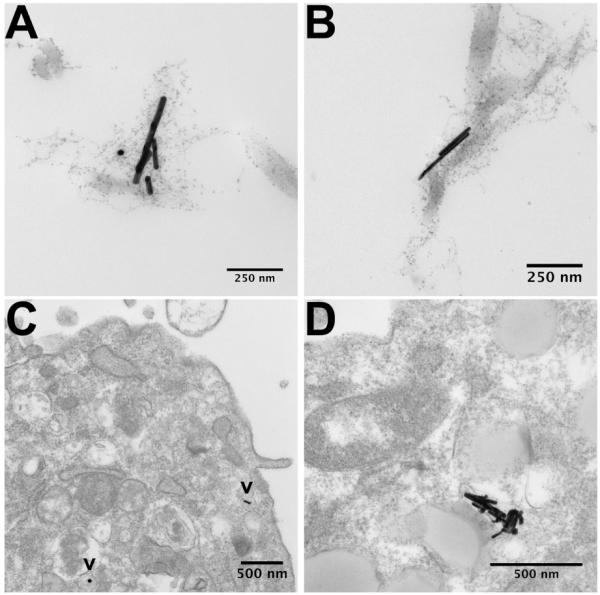
TEM images of polyanion-terminated AuNRs localized in the collagen matrix and in intracellular compartments. PSS-terminated (A) and PAA-terminated AuNRs (B) were often surrounded by disorganized collagen. After 24 h of culture, the AuNRs were detected as single particles (C, indicated by “v”) and as aggregates (D) within the cells.
Discussion
AuNRs have great potential for use in high resolution imaging of tissues and cells [37], biochemical sensing [3], photothermal therapies [1,38], and delivery of therapeutic siRNA [39], but questions remain about how proteins and cells interact with these nanoparticles. Indeed, the toxicity and in vivo fate of nanoparticles are of increasing interest in the burgeoning fields of nanomedicine and nanobiotechnology, and a recent report describes the accumulation of IV-injected gold nanoparticles in the myocardium of rats [40]. Cardiac fibroblasts assume a contractile myofibroblast phenotype following myocardial infarction and are primary mediators of ECM remodeling and scar formation in the heart [41]. The mature scar is characterized by high concentrations of fibrous ECM (e.g., type I collagen) and can adversely affect cardiac function, thereby contributing to the progression of heart disease [42]. The localized, timely delivery of antifibrotic agents may permit functional wound healing while limiting the accumulation of excessive scar tissue and thus tailoring the ultimate mechanical properties of the scar. We observed previously that PSS-terminated AuNRs inhibited cardiac fibroblast-mediated contraction of type I collagen gels in vitro and that this effect was in part due to reduced expression of genes associated with the contractile myofibroblast (scar-forming) phenotype[21]. The current study represents an initial approach to elucidating the mechanisms by which the AuNRs elicited these differences in collagen remodeling and cell phenotype.
PEM-coated AuNRs terminated with PSS or PAA potently accelerated the polymerization of type I collagen. Natural polyanions in solution, including sulfated glycosaminoglycans and sodium alginate, are known to influence the kinetics of polymerization of type I collagen gels [43-46]. There are few reports describing the effects of synthetic polyanions on collagen, but high molecular weight PAA (Mw ∼ 150 kDa) did not interact with type I collagen in solution at physiologic pH and salt concentration [47], whereas PSS (Mw ∼ 1.4 MDa) had dose-dependent effects on the conformation of denatured collagen (gelatin) [48]. In our experiments the PAA- and PSS-terminated AuNRs significantly reduced the lag phase of polymerization, indicating that these nanoparticles promoted aggregation of collagen monomers (nucleation of fibrils). Polymeric nanoparticles accelerated fibrillation of the serum proteins β2-microglobulin and amyloid-β in vitro, and the effect was attributed to recruitment of monomers to the particle surface via electrostatic interactions [9,10,49]. The pI of type I collagen in PBS is ∼7.4 as determined by zeta potential titration [50]. However, collagen monomers contain numerous charged residues capable of interacting with the multivalent PEM coatings on the nanoparticles used in this study, and confocal and electron microscopy confirmed that the PSS- and PAA-terminated AuNRs interacted directly with the collagen. In addition, darkfield images of collagen-AuNR thin films revealed that the polyanion-terminated AuNRs induced formation of large, stable aggregates of collagen. These results support the hypothesis that polyanion-terminated AuNRs interact directly with collagen monomers to accelerate the aggregation and polymerization of collagen in vitro. Such interactions may be significant in the contexts of delivering these nanoparticles to collagen-rich tissues (e.g., fibrotic myocardium) or to collagen-synthesizing cells such as fibroblasts.
Collagen gels doped with polyanion-terminated AuNRs were significantly stiffer than controls under oscillatory shear. The shear stiffness of collagen gels has previously been shown to be sensitive to the presence of the natural polyanion chondroitin sulfate [51], and collagen-rich tissues with high concentrations of glycosaminoglycans (e.g., articular cartilage) exhibit shear properties dependent on the concentration of immobilized polyelectrolytes and arising in part from the osmotic swelling associated with Donnan equilibrium [52]. Using the measured dimensions of the AuNRs and calculated polymer chain lengths, we estimated the bulk concentrations of “available” polyelectrolyte (polyelectrolytes in the outermost layer of the nanoparticle surface coatings) in our collagen-AuNR solutions to be 1-5 μg/mL. At such low concentrations of polyelectrolyte we would expect osmotic swelling effects and electrostatic interactions between polyelectrolyte chains to contribute little to the bulk mechanical properties of the collagen gel. It is likely, however, that within aggregates of PAA- or PSS-terminated AuNRs - which were readily observed by confocal, darkfield, and electron microscopy - the local concentration of polyelectrolytes was substantially higher than the bulk concentration and that these pockets of high charge density were responsible for changes in the bulk shear properties of the collagen gels. Such charged structures may locally alter the interactions between collagen fibrils and/or the network mesh size (i.e., distance between fibril-fibril junctions) to alter the mechanics of the gel. Indeed, there was evidence of increased mesh size in the thin films of collagen gels doped with polyanion-terminated AuNRs. Further studies are necessary to resolve the biophysical origin of AuNR-induced stiffening of the collagen.
PAA-terminated AuNRs had more potent effects on collagen polymerization and mechanics than PSS-terminated AuNRs. In addition, collagen gels doped with PAA-terminated AuNRs uniquely exhibited a lower failure strain than controls. PAA is a weak polyanion (pKa ∼ 5) whereas PSS is a strong polyanion (pKa ∼ 1), and the magnitude of the zeta potential of PAA-terminated AuNRs was substantially lower than that of the PSS-terminated AuNRs. We speculate that the distinct biophysical properties of the carboxylate and sulfonate functional groups on these polyelectrolytes contributed to different affinities of PAA- and PSS-terminated AuNRs for collagen monomers and/or aggregates. Further work is ongoing to elucidate the origin of the differences between PAA- and PSS-terminated AuNRs and their effects on collagen assembly and mechanics, and our results underscore the important role that surface chemistry (i.e., PEM composition) plays in determining particle-protein interactions.
PEM-coated AuNRs inhibited cell-mediated contraction of collagen gels independent of PEM composition. Whereas PSS- and PAA-terminated AuNRs inhibited contraction in part by increasing the stiffness of the collagen, it is less clear how PDADMAC- and PAH-terminated AuNRs inhibited contraction since these nanoparticles had no detectable effects on the mechanical or morphological properties of the collagen scaffold. It is possible that the polycation-coated AuNRs were preferentially internalized by the cardiac fibroblasts in our studies and that intracellular accumulation of the AuNRs inhibited cell contractility. In MDCK and HeLa cells, polymeric nanoparticles with a cationic surface charge were more readily endocytosed than similar particles with an anionic surface charge [53,54]. Previous work by our group has also shown that PAH-terminated AuNRs accumulated within HT-29 colon cancer cells to a greater extent (by nearly 10 fold) than PAA-terminated AuNRs [18]. These charge effects may be attributed to electrostatic interactions between the PEM nanoparticle coating and the negatively charged phospholipids of the cell membrane. Little is known, however, about the functional implications of intracellular nanoparticle accumulation and transport. PEM composition-dependent differences in AuNR uptake, particularly in 3D culture environments, will benefit from future studies.
The effects of PEM-coated AuNRs on cell-ECM interactions require further investigation. Several recent reports show that cells are sensitive to nanoscale features of the ECM. Spherical albumin nanocarriers functionalized with fragments of fibronectin enhanced de novo deposition of fibronectin by human fibroblasts [55]. Zinc oxide nanorods inhibited cell adhesion and lamellapodia formation in 2D cell cultures [56], and nanoscale clustering of the adhesion ligand RGD regulated cell adhesion, motility, and response to substrate stiffness [57,58]. AuNRs were readily detected at and near the cell surface in our experiments; it is possible that the nanoparticles altered the availability of collagen in sufficient proximity to cell surface receptors to alter ligand binding or clustering and downstream signaling events.
Type I collagen is an abundant protein that is just one important candidate for evaluating nanoparticle-protein interactions. Other soluble and insoluble factors are also likely to interact with PEM-coated AuNRs in our culture system. Basic fibroblast growth factor (bFGF) and fibronectin, for example, contain heparin-binding domains that are rich in acidic residues and are likely to bind immobilized polyanions. There is precedent for this concept, as the polyanions alginate and alginate-sulfate were recently shown to have affinities for growth factors equal to or greater than those of heparin[59]. Significantly, fibronectin assumes different conformations when bound to PEMs terminated with polyanions and polycations [14], suggesting that interaction with PEM-coated AuNRs alters the activity and/or availability of fibronectin in a surface chemistry-dependent manner. In addition, both bFGF and fibronectin regulate the cell-mediated remodeling and contraction of collagen scaffolds [60,61], and fibronectin and transforming growth factor β1 bound carbon black nanoparticles in the context of a collagen scaffold [20]. Thus, the biophysical properties of PEM-coated AuNRs - like other nanoparticles - are favorable for binding numerous soluble and insoluble proteins in the ECM, and such in teractions likely to influence the physiologic effects of the AuNRs both in vitro and in vivo.
Conclusions
The results of this study provide evidence that AuNRs coated with negatively charged polyelectrolytes interact directly with type I collagen to alter the polymerization and mechanical properties of this ECM component. Both polyanion- and polycation-terminated AuNRs inhibited contraction of collagen gels by cardiac fibroblasts in vitro. . Understanding the non-specific interactions of PEM-coated AuNRs with ECM proteins such as type I collagen will contribute to the development of nanoparticles for use in cardiac imaging and as vehicles for drug/gene delivery. In addition, the effects of the PEM-coated AuNRs on collagen contraction and mechanical properties may be therapeutic in the context of limiting consolidation of provisional ECM into dense scar in the infarcted heart, but further studies are required to elucidate the mechanisms by which these nanoparticles exert their effects on cells.
Acknowledgements
The authors would like to thank Cheryl Cook for assisting with the isolation and culture of the cardiac fibroblasts, Jeffery Davis for help with the TEM studies, and Kole Hexel for assisting with the ICP-OES measurements. These studies were supported by funds from NIH grant number HL73937. CGW was supported by a fellowship administered through NIH grant P20 RR-016461 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–20. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 2.Stone JW, Sisco PN, Goldsmith EC, Baxter SC, Murphy CJ. Using gold nanorods to probe cell-induced collagen deformation. Nano Lett. 2007;7:116–9. doi: 10.1021/nl062248d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy CJ, Gole AM, Hunyadi SE, Stone JW, Sisco PN, Alkilany A, et al. Chemical sensing and imaging with metallic nanorods. Chem Commun (Camb) 2008:544–57. doi: 10.1039/b711069c. [DOI] [PubMed] [Google Scholar]
- 4.Das M, Sanson N, Fava D, Kumacheva E. Microgels loaded with gold nanorods: Photothermally triggered volume transitions under physiological conditions. Langmuir. 2007;23:196–201. doi: 10.1021/la061596s. [DOI] [PubMed] [Google Scholar]
- 5.Blunk T, Hochstrasser DF, Sanchez JC, Müller BW, Müller RH. Colloidal carriers for intravenous drug targeting: Plasma protein adsorption patterns on surface-modified latex particles evaluated by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1993;14:1382–7. doi: 10.1002/elps.11501401214. [DOI] [PubMed] [Google Scholar]
- 6.Lück M, Schröder W, Harnisch S, Thode K, Blunk T, Paulke BR, et al. Identification of plasma proteins facilitated by enrichment on particulate surfaces: Analysis by two-dimensional electrophoresis and n-terminal microsequencing. Electrophoresis. 1997;18:2961–7. doi: 10.1002/elps.1150181538. [DOI] [PubMed] [Google Scholar]
- 7.Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green ML, Sim RB. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43:193–201. doi: 10.1016/j.molimm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Cedervall T, Lynch I, Lindman S, Berggård T, Thulin E, Nilsson H, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A. 2007;104:2050–5. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linse S, Cabaleiro-Lago C, Xue WF, Lynch I, Lindman S, Thulin E, et al. Nucleation of protein fibrillation by nanoparticles. Proc Natl Acad Sci U S A. 2007;104:8691–6. doi: 10.1073/pnas.0701250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu WH, Sun X, Yu YP, Hu J, Zhao L, Liu Q, et al. Tio2 nanoparticles promote beta-amyloid fibrillation in vitro. Biochem Biophys Res Commun. 2008;373:315–8. doi: 10.1016/j.bbrc.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Gittins DI, Caruso F. Tailoring the polyelectrolyte coating of metal nanoparticles. J Phys Chem B. 2001;105:6846–52. [Google Scholar]
- 12.Gole A, Murphy CJ. Polyelectrolyte-Coated gold nanorods: Synthesis, characterization and immobilization. Chemistry of Materials. 2005;17:1325–30. [Google Scholar]
- 13.Dorris A, Rucareanu S, Reven L, Barrett CJ, Lennox RB. Preparation and characterization of polyelectrolyte-coated gold nanoparticles. Langmuir. 2008;24:2532–8. doi: 10.1021/la703003m. [DOI] [PubMed] [Google Scholar]
- 14.Ngankam AP, Mao G, Van Tassel PR. Fibronectin adsorption onto polyelectrolyte multilayer films. Langmuir. 2004;20:3362–70. doi: 10.1021/la035479y. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald RA, Laurenzi BF, Viswanathan G, Ajayan PM, Stegemann JP. Collagen-Carbon nanotube composite materials as scaffolds in tissue engineering. J Biomed Mater Res A. 2005;74:489–96. doi: 10.1002/jbm.a.30386. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Liu X, Kobayashi T, Kohyama T, Wen FQ, Romberger DJ, et al. Ultrafine carbon black particles inhibit human lung fibroblast-mediated collagen gel contraction. Am J Respir Cell Mol Biol. 2003;28:111–21. doi: 10.1165/rcmb.4796. [DOI] [PubMed] [Google Scholar]
- 17.Sisco PN, Wilson CG, Mironova E, Baxter SC, Murphy CJ, Goldsmith EC. The effect of gold nanorods on cell-mediated collagen remodeling. Nano Lett. 2008;8:3409–12. doi: 10.1021/nl802142h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 19.Jana NR, Gearheart L, Murphy CJ. Seed-Mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template*. Adv Mater. 2001;13:1389–93. [Google Scholar]
- 20.Jana NR, Gearheart L, Murphy CJ. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J Phys Chem B. 2001;105:4065–7. [Google Scholar]
- 21.Wood GC, Keech MK. The formation of fibrils from collagen solutions. 1. The effect of experimental conditions: Kinetic and electron-microscope studies. Biochemical Journal. 1960;75:588–98. doi: 10.1042/bj0750588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435:191–4. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 23.Borg TK, Rubin K, Lundgren E, Borg K, Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol. 1984;104:86–96. doi: 10.1016/0012-1606(84)90038-1. [DOI] [PubMed] [Google Scholar]
- 24.Abramoff MA, Magelhaes PJ, Ram SJ. Image processing with imagej. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 25.Tschumperlé D, Deriche R. Vector-Valued image regularization with pdes: A common framework for different applications. IEEE Trans Pattern Anal Mach Intell. 2005;27:506–17. doi: 10.1109/TPAMI.2005.87. [DOI] [PubMed] [Google Scholar]
- 26.Trelstad RL, Hayashi K, Gross J. Collagen fibrillogenesis: Intermediate aggregates and suprafibrillar order. Proc Natl Acad Sci U S A. 1976;73:4027–31. doi: 10.1073/pnas.73.11.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alkilany AM, Nagaria PK, Hexel CR, Shaw TJ, Murphy CJ, Wyatt MD. Cellular uptake and cytotoxicity of gold nanorods: Molecular origin of cytotoxicity and surface effects. Small. 2009 doi: 10.1002/smll.200801546. [DOI] [PubMed] [Google Scholar]
- 28.Javier DJ, Nitin N, Roblyer DM, Richards-Kortum R. Metal-Based nanorods as molecule-specific contrast agents for reflectance imaging in 3D tissues. J Nanophotonics. 2008;2:23506. doi: 10.1117/1.2927370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman RS, Stone JW, Gole A, Murphy CJ, Sabo-Attwood TL. Targeted photothermal lysis of the pathogenic bacteria, pseudomonas aeruginosa, with gold nanorods. Nano Lett. 2008;8:302–6. doi: 10.1021/nl0727056. [DOI] [PubMed] [Google Scholar]
- 30.Wood GC. The formation of fibrils from collagen solutions. 3. Effect of chondroitin sulphate and some other naturally occurring polyanions on the rate of formation. Biochemical Journal. 1960;75:605–12. doi: 10.1042/bj0750605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathews MB. The interaction of collagen and acid mucopolysaccharides. A model for connective tissue. Biochemical Journal. 1965;96:710–6. doi: 10.1042/bj0960710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathews MB, Decker L. The effect of acid mucopolysaccharides and acid mucopolysaccharide-proteins on fibril formation from collagen solutions. Biochemical Journal. 1968;109:517–26. doi: 10.1042/bj1090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brightman AO, Rajwa BP, Sturgis JE, McCallister ME, Robinson JP, Voytik-Harbin SL. Time-Lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers. 2000;54:222–34. doi: 10.1002/1097-0282(200009)54:3<222::AID-BIP80>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.Nezu T, Winnik FM. Interaction of water-soluble collagen with poly(acrylic acid) Biomaterials. 2000;21:415–9. doi: 10.1016/s0142-9612(99)00204-5. [DOI] [PubMed] [Google Scholar]
- 35.Gillmor JR, Connelly RW, Colby RH, Tan JS. Effect of sodium poly(styrene sulfonate) on thermoreversible gelation of gelatin. Journal of Polymer Science Part B-Polymer Physics. 1999;37:2287–95. [Google Scholar]
- 36.Cabaleiro-Lago C, Quinlan-Pluck F, Lynch I, Lindman S, Minogue AM, Thulin E, et al. Inhibition of amyloid beta protein fibrillation by polymeric nanoparticles. J Am Chem Soc. 2008;130:15437–43. doi: 10.1021/ja8041806. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Asadi A, Monroe MR, Douglas EP. Ph effects on collagen fibrillogenesis in vitro: Electrostatic interactions and phosphate binding. Materials Science and Engineering C. 2009 In Press. [Google Scholar]
- 38.Stuart K, Panitch A. Influence of chondroitin sulfate on collagen gel structure and mechanical properties at physiologically relevant levels. Biopolymers. 2008;89:841–51. doi: 10.1002/bip.21024. [DOI] [PubMed] [Google Scholar]
- 39.Jin M, Grodzinsky AJ. Effect of electrostatic interactions between glycosaminoglycans on the shear stiffness of cartilage: A molecular model and experiments. Macromolecules. 2001;34:8330–9. [Google Scholar]
- 40.Harush-Frenkel O, Rozentur E, Benita S, Altschuler Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules. 2008;9:435–43. doi: 10.1021/bm700535p. [DOI] [PubMed] [Google Scholar]
- 41.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105:11613–8. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira M, Sharma RI, Penkala R, Gentzel TA, Schwarzbauer JE, Moghe PV. Engineered cell-adhesive nanoparticles nucleate extracellular matrix assembly. Tissue Eng. 2007;13:567–78. doi: 10.1089/ten.2006.0228. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Kang BS, Hicks B, Chancellor TF, Chu BH, Wang HT, et al. The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials. 2008;29:3743–9. doi: 10.1016/j.biomaterials.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 44.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113(Pt 10):1677–86. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 45.Koo LY, Irvine DJ, Mayes AM, Lauffenburger DA, Griffith LG. Co-Regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J Cell Sci. 2002;115:1423–33. doi: 10.1242/jcs.115.7.1423. [DOI] [PubMed] [Google Scholar]
- 46.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29:3260–8. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 47.Assouline M, Chew SJ, Thompson HW, Beuerman R. Effect of growth factors on collagen lattice contraction by human keratocytes. Invest Ophthalmol Vis Sci. 1992;33:1742–55. [PubMed] [Google Scholar]
- 48.Sethi KK, Yannas IV, Mudera V, Eastwood M, McFarland C, Brown RA. Evidence for sequential utilization of fibronectin, vitronectin, and collagen during fibroblast-mediated collagen contraction. Wound Repair Regen. 2002;10:397–408. doi: 10.1046/j.1524-475x.2002.10609.x. [DOI] [PubMed] [Google Scholar]