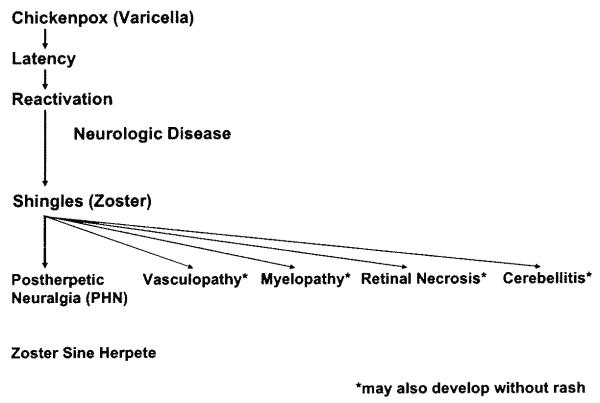
Varicella zoster virus (VZV) is an exclusively human neurotropic alpha-herpesvirus. Primary infection causes varicella (chickenpox), after which virus becomes latent in cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia along the entire neuraxis. Years later, in association with a decline in cell-mediated immunity in elderly and immunocompromised individuals, VZV reactivates and causes a wide range of neurologic disease, including herpes zoster, postherpetic neuralgia, vasculopathy, myelopathy, retinal necrosis, cerebellitis and zoster sine herpete (Fig. 1). Importantly, many of these complications occur without rash. This article discusses the clinical manifestations, treatment, and prevention of VZV infection and reactivation; pathogenesis of VZV infection; and current research focusing on VZV latency, reactivation, and animal models.
Fig. 1.
The neurologic complications of varicella zoster virus reactivation.
Clinical manifestations of primary varicella zoster virus infection
Varicella
Initial infection with VZV results in chickenpox (varicella), which is typically seen in children 1 to 9 years of age [1]. Primary infection in adults is usually more severe and may be accompanied by interstitial pneumonia. Infection in immunocompromised individuals often causes severe, disseminated disease. Climate seems to affect the epidemiology of varicella. In most temperate climates, more than 90% of people are infected before adolescence [2-5] with an incidence of 13 to 16 cases per 1000 people per year [6-8]. In tropical climates, VZV infection occurs later in life and adults are more susceptible than children [9-11]. Varicella has a peak incidence in the late winter and spring [10,12-14], and epidemics tend to occur every 2 to 5 years [12-14].
Varicella is characterized by fever concurrent with a self-limiting rash on the skin and sometimes mucosa. Headache, malaise, and loss of appetite are also seen. The rash begins as macules, rapidly progresses to papules, followed by a vesicular stage and crusting of lesions. Crusts slough off after 1 to 2 weeks. VZV is highly infectious and transmission occurs by direct contact with skin lesions or by respiratory aerosols from infected individuals. Central nervous system complications include self-limiting cerebellar ataxia in 1 in 4000 cases [15], meningitis, meningoencephalitis, and vasculopathy [16]. Strokes may occur months after varicella secondary to VZV vasculopathy and are not always easy to diagnose (see section on VZV vasculopathy).
Diagnosis of varicella is based on the characteristic vesicular rash. Treatment is aimed at symptomatic relief. Acetaminophen is used to control fever, fluids are given for hydration, and topical medications are given for the pruritic rash. Treatment with intravenous acyclovir is mandatory in patients at risk for or with clinical evidence of disseminated disease, or in newborns who were exposed to VZV shortly after birth. In otherwise healthy children, antiviral treatment is not mandatory, but Dunkle and colleagues [17] have shown that treatment with oral acyclovir within 24 hours of illness results in a 1-day reduction in the duration of fever and a reduced severity of cutaneous and systemic symptoms and signs.
Clinical manifestations of varicella zoster virus reactivation
Herpes zoster
Zoster affects approximately 1 million individuals in the United States per year. Most patients are over age 60 [18] or immunocompromised [19]. The annual incidence of zoster is approximately 5 to 6.5 per 1000 individuals at age 60, increasing to 8 to 11 per 1000 at age 70 [19]. Unlike varicella, which occurs primarily in the spring, there is no seasonal predilection for zoster. The development of zoster may be viewed in the context of a continuum in immunodeficient individuals, ranging from a natural decline in VZV-specific cell-mediated immunity with age, to more serious immune deficits seen in cancer patients and transplant recipients, and ultimately in patients with AIDS [20]. Not surprisingly, zoster in otherwise young, healthy individuals may be the first manifestation of HIV infection [21]. Interestingly, varicella in infancy predisposes to zoster earlier in life [22].
Herpes zoster usually begins with a prodromal phase characterized by pain, itching, paresthesias (numbness or tingling), dysesthesias (unpleasant sensations), or sensitivity to touch (allodynia) in one to three dermatomes. A few days later, a unilateral maculopapular rash appears on the affected area, which then evolves into vesicles. These vesicles usually scab over in 10 days, after which the lesions are not contagious. Dissemination may occur in immunosuppressed patients, such as patients with a hematologic malignancy or iatrogenic immunosuppression. In most patients, the disappearance of skin lesions is accompanied by decreased pain and complete resolution of pain in 4 to 6 weeks. In zoster, MRI has shown enhancement of dorsal root ganglia and affected nerve roots [23].
Zoster affects any level of the neuraxis. The most common site is the chest, followed by lesions on the face, typically in the ophthalmic distribution of the trigeminal nerve. In immunocompromised patients, multidermatomal involvement is common and may be the first clue to the immunodeficient condition. Herpes zoster ophthalmicus is often accompanied by zoster keratitis, which can lead to blindness if unrecognized and not treated. If visual symptoms are present in these patients, an immediate slit-lamp examination by an ophthalmologist is imperative, especially if skin lesions extend to the medial side of the nose (Hutchinson’s sign). Involvement of the optic nerves with subsequent optic neuritis and neuropathy has occurred rarely in association with herpes zoster ophthalmicus and other cutaneous zoster eruptions [24,25]. Ophthalmoplegia after zoster most frequently involves cranial nerves III and VI, and less frequently cranial nerve IV [24,26-28]; in addition, involvement of the maxillary and mandibular distribution of the trigeminal nerve can produce osteonecrosis and spontaneous tooth exfoliation [29-31].
Zoster affecting the seventh cranial nerve (geniculate) ganglion causes weakness or paralysis of ipsilateral facial muscles, with rash in the external auditory canal (zoster oticus) or tympanic membrane, or on the ipsilateral anterior two thirds of the tongue or hard palate. Lesions in these areas are often missed. The combination of peripheral facial weakness and zoster oticus constitutes the Ramsay Hunt syndrome [32]. Although the Ramsay Hunt syndrome is traditionally defined as lower motor neuron facial palsy with zoster oticus, many of these patients also have tinnitus, hearing loss, nausea, vomiting, vertigo, and nystagmus, indicating involvement not only of the geniculate ganglion, but also the eighth cranial nerve within the bony facial canal. Rarely, cranial nerves V, VI, IX, and X may also be involved [33]. Compared with Bell’s palsy (peripheral facial paralysis without rash), individuals with the Ramsay Hunt syndrome often have more severe facial paralysis at onset and are less likely to recover completely [34]. In addition, peripheral facial paralysis caused by VZV may develop in the absence of rash as demonstrated by a four-fold rise in antibody to VZV or the presence of VZV DNA in auricular skin, mononuclear cells (MNCs), middle ear fluid, or saliva [35]. Some patients with idiopathic facial weakness actually represent another variant of zoster sine herpete (pain without rash).
Cervical or lumbar distribution zoster may be followed by lower motor neuron type weakness in the arm or leg, respectively [36,37]. Cervical zoster may rarely be followed by diaphragmatic weakness [38]. Rare cases of thoracic zoster have been associated with abdominal muscle weakness, which can result in abdominal herniation [39].
Zoster is presumed to develop by retrograde transport of virus from ganglia to skin in a host partially immune to VZV. VZV has also been isolated from the blood of immunocompromised patients with localized and disseminated zoster [40], suggesting a role for hematogenous spread. Cardinal pathologic features of zoster are inflammation and hemorrhagic necrosis with associated neuritis, localized leptomeningitis, unilateral segmental poliomyelitis, and degeneration of related motor and sensory roots [41,42]. Demyelination has also been observed in areas with MNC infiltration and microglial proliferation. Intranuclear inclusions, viral antigen, and herpesvirus particles have been detected in acutely infected ganglia [43-46].
Treatment for zoster should consider the patient’s immune status and age. In immunocompetent individuals under age 50, analgesics are used to relieve pain. Antivirals (famciclovir, 500 mg orally three times daily, or valacyclovir, 1 g three times daily for 7–10 days) are not required, but speed healing of rash. In immunocompetent individuals age 50 and older, treatment with both analgesics and antivirals is recommended and is essential in patients with ophthalmic distribution zoster. Similarly, treatment of patients with the Ramsay Hunt syndrome within 7 days of onset reportedly improves recovery [47,48], although prospective randomized treatment trials remain to be conducted. The authors also use prednisone (1 mg/kg body weight once a day for 5 days) to reduce the inflammatory response, although double-blind placebo-controlled studies to prove additional efficacy are lacking. In immunocompromised patients, intravenous acyclovir (10 mg/kg three times per day for no less than 7 days) is recommended.
Postherpetic neuralgia
About 40% of zoster patients over age 60 experience postherpetic neuralgia (PHN) [49,50]. PHN is characterized by constant, severe, stabbing or burning, dysesthetic pain that persists for at least 3 months and sometimes years after resolution of rash. The cause and pathogenesis of PHN are unknown. Two nonmutually exclusive theories are that excitability of ganglionic or even spinal cord neurons is altered, and persistent or low-grade productive virus infection exists in ganglia. The concept that PHN is produced by low-level ganglionitis is supported by the detection of VZV DNA and proteins in blood MNCs of many patients with PHN [51-53], and by a favorable response of some PHN patients to antiviral treatment [54-56].
Although not life-threatening, PHN is difficult to manage. Treatment is supportive with use of neuroleptic drugs and various analgesics, including opiates to alleviate pain, but no universally accepted treatment exists. Gabapentin (300 mg daily with gradually increasing doses, up to a maximum of 3600 mg/day in three doses) is one of the most widely accepted treatments [57,58]. Lidocaine is administered as 5% patches with up to three patches applied topically at one time for up to 12 hours within a 24-hour period. Pregabalin is given initially at a dose of 75 mg orally twice a day or 50 mg orally three times a day, then gradually increased to a maximum dose of 300 mg per day based on efficacy and tolerability. If minimal relief is obtained at 300 mg per day for 2–4 weeks, the dose can be increased to 600 mg per day in two or three divided doses, although dosing needs to be adjusted based on side effects of the drug as well as the patient’s renal function.
In addition, oxycodone (controlled release, 10–40 mg orally every 12 hours) or controlled-release morphine sulfate and tricyclic antidepressants are used [59]. Levorphanol produces morphine-like analgesia, at a dose of 2 mg orally every 6 to 8 hours as needed with maximum doses of 6 to 12 mg daily [60]. Combination treatment with morphine and gabapentin also decreases pain more than either drug alone or placebo [61]. Tricyclic antidepressants, including amitriptyline (10–25 mg orally at bedtime with a maximum dose of 150–200 mg/day), nortriptyline, mapotriline, and despramine, lessen the pain of PHN.
Numerous studies indicate that antiviral therapy with oral acyclovir, famcyclovir, or valacyclovir may reduce the duration and severity of pain after zoster [62-64]. A recent prospective, open-label phase I/II clinical trial treated 15 patients with moderate to severe PHN with intravenous acyclovir for 2 weeks, followed by oral valacyclovir for 1 month; 8 (53%) of 15 patients reported improvement of pain [56].
Varicella zoster virus vasculopathy
VZV vasculopathy results from productive virus infection in large or small cerebral arteries, or both. Patients present with headache; fever; mental status changes; transient ischemic attacks; and focal deficits (stroke). The clinical spectrum of VZV vasculopathy is protean. For example, one case of VZV vasculopathy was characterized by posterior ischemic optic neuropathy with a normal cerebral angiogram and MRI [65]. Cerebral aneurysms and hemorrhage can also develop from viral invasion of vessels [66,67]. VZV vasculopathy often occurs without rash [68,69].
The cerebrospinal fluid (CSF) usually, but not always, reveals a mononuclear pleocytosis and oligoclonal bands. The oligoclonal IgG has been shown to be antibody directed against VZV [70]. Brain imaging usually reveals ischemic or hemorrhagic infarcts, more deep-seated than cortical lesions and at gray-white matter junctions. Cerebral angiography also reveals areas of focal arterial stenosis or occlusion. Macroscopically, a predominance of gray-white matter junction lesions is seen. Microscopically, virus is present in affected cerebral arteries [71] but not in areas of infarction, although in chronic cases virus may be seen in brain parenchyma, usually close to arteries and veins. The primary site of VZV is in cerebral arteries, which contain multinucleated giant cells, Cowdry A inclusion bodies, and herpes virus particles. Postmortem virologic analysis has revealed not only VZV DNA, but also VZV antigen in cerebral vessels [71].
Confirmation of VZV vasculopathy requires virologic analysis to detect amplifiable VZV DNA or anti-VZV IgG antibodies or both in the CSF. PCR is the diagnostic test of choice for herpes simplex virus (HSV) encephalitis, with HSV DNA present early in the course of acute disease, whereas antiviral antibody is detected only in the second week [72]. In cases of VZV vasculopathy, the CSF does not always contain PCR-amplifiable VZV DNA, but does contain anti-VZV IgG [73]. The detection of anti-VZV IgG, but not VZV DNA, likely reflects the chronic, protracted course of disease. Testing for both VZV DNA and anti-VZV IgG must be done, and only when both are negative can the diagnosis of VZV vasculopathy be excluded. Also, because VZV vasculopathy can occur without rash, all vasculopathies of unknown etiology should be evaluated for VZV. Rapid diagnosis of VZV vasculopathy is important because the mortality without treatment is 25% [74], whereas treatment with intravenous acyclovir, even after neurologic disease has been present for months, can be curative [65].
Varicella zoster virus myelopathy
Two clinical presentations of VZV myelitis predominate. The first is a self-limiting, monophasic spastic paraparesis, with or without sensory features and sphincter problems. This so-called “postinfectious myelitis” usually occurs in immunocompetent patients, days to weeks after acute varicella or zoster. Its pathogenesis is unknown. The CSF usually reveals a mild mononuclear pleocytosis with a normal or slightly elevated protein. Steroids are used to treat these patients [75], although some improve spontaneously [76].
The second presentation is an insidious, progressive, and sometimes fatal myelitis, seen mostly in immunocompromised individuals. Because AIDS is so prevalent, this has become the most common condition associated with VZV myelitis. CSF examination reveals a mild, predominantly mononuclear pleocytosis with elevated protein. MRI reveals longitudinal serpiginous enhancing lesions. Diagnosis is confirmed by the presence of VZV DNA or anti-VZV IgG or both in CSF [77]. Pathologic and virologic analyses of the spinal cord from fatal cases have shown frank invasion of VZV in the parenchyma [78] and, in some instances, spread of virus to adjacent nerve roots [79]. Not surprisingly, several patients have responded favorably to antiviral therapy [80-82]. Importantly, VZV myelitis may develop with rash. Early diagnosis and aggressive treatment with intravenous acyclovir has been helpful, even in immunocompromised patients [80]. The benefit of steroids in addition to antiviral agents is unknown.
Most recently, a case of VZV spinal cord infarction was identified by diffusion-weighted MRI and confirmed virologically [83]. This indicates that VZV vasculopathy can cause stroke in the spinal cord and the brain, and that abnormalities on diffusion-weighted MRI is crucial for diagnosis.
Varicella zoster virus retinal necrosis
VZV-induced necrotizing retinitis manifests as two clinical syndromes: acute retinal necrosis (ARN) and progressive outer retinal necrosis (PORN).
Acute retinal necrosis
ARN is seen in both immunocompetent and immunocompromised hosts. Patients present with periorbital pain and floaters with hazy vision and loss of peripheral vision. ARN is a full-thickness retinal necrosis characterized by focal, well-demarcated areas of necrosis in the retina located beyond the major temporal vascular arcades. Distinguishing features of this occlusive vasculopathy are prominent intraocular inflammation in the anterior chamber and vitreous [84]. In addition to VZV as the causal agent [85], both HSV-1 and -2 can induce ARN [86,87]. Patients are typically treated with intravenous acyclovir, steroids, and aspirin followed by oral acyclovir [88]. Intravitreal injections of foscarnet and oral acyclovir have been used in early, milder cases. In ARN caused by VZV, brivudine (not available in the United States) and valgancyclovir have demonstrated good results [89].
Progressive outer retinal necrosis
PORN is caused almost exclusively by VZV. After cytomegalovirus, VZV-associated PORN is the second most common opportunistic retinal infection among AIDS patients in North America [90]. PORN occurs primarily in AIDS patients with CD4 counts typically less than 10 cells/mm3 of blood [91], and in other immunosuppressed individuals [92]. PORN may be preceded by retrobulbar optic neuritis and aseptic meningitis [93], central retinal artery occlusion, or ophthalmic distribution zoster [94], and may occur together with multifocal vasculopathy or myelitis. Patients present with sudden painless loss of vision, floaters, and constricted visual fields with resultant retinal detachment. Unlike ARN, there is little or no inflammation in the anterior chamber or vitreous and no occlusive vasculitis. Multifocal, discrete opacified lesions begin in the outer retinal layers peripherally or posterior pole; only late in disease are inner retinal layers involved. Diffuse retinal hemorrhages and whitening with macular involvement bilaterally are characteristic findings.
VZV was shown to be the causative agent of PORN based on the detection of VZV DNA, VZV antigen, and virus particles in aqueous-vitreous biopsies [95], and in vitreous-retinal cultures [96], and by histologic examination of necropsy specimens from eyes and brains combined with in situ hybridization [97]. There have also been rare reports of cytomegalovirus and HSV-1 antigens detected in the retina of patients with PORN [98], and cytomegalovirus DNA has been amplified in the vitreous of patients with PORN [99]. Nevertheless, multiple studies have shown that VZV is the most common cause.
The immediate recognition and treatment of PORN is essential because of its destructive nature and high likelihood of retinal detachment. Unfortunately, multiple combinations of antiviral medications and other experimental treatments have not successfully treated all cases of PORN. Treatment with intravenous acyclovir has given poor or inconsistent results [100], and even when acyclovir helped, VZV retinopathy recurred when drug was tapered or stopped. PORN patients treated with a combination of ganciclovir and foscarnet or with ganciclovir alone had a better final visual acuity than those treated with acyclovir or foscarnet [101]. In one instance, oral bromovinyldeoxyuridine treatment was successful when acyclovir failed [102]. Aggressive combined antiviral treatment over a prolonged period with repair of retinal detachment may save the patient’s vision. The best treatment for PORN in AIDS patients may be prevention with highly active antiretroviral therapy, which seems to have decreased the incidence of this syndrome [103].
Zoster sine herpete
Zoster sine herpete (pain without rash) is caused by reactivation of VZV [104], a concept first supported by the description of dermatomal distribution radicular pain in areas distinct from pain with rash in zoster patients [105]. Currently, most clinicians regard zoster sine herpete exclusively as the rare occurrence of chronic radicular pain without rash with virologic confirmation of VZV reactivation. In recent years, the detection of VZV DNA and anti-VZV antibody in patients with meningoencephalitis, vasculopathy, myelitis, cerebellar ataxia, and polyneuritis cranialis, all without rash, has expanded the spectrum of VZV infection without rash.
The first verification of zoster sine herpete was in a physician who developed acute trigeminal distribution pain without rash, associated with a four-fold rise in serum antibody specific to VZV [106]. Schott [107] reported four patients who developed trigeminal distribution zoster followed years later by zoster sine herpete in the same distribution of the trigeminal nerve as their previous zoster; unfortunately, none were studied virologically. Further virologic verification of zoster sine herpete came from PCR analysis of CSF from two men with prolonged thoracic distribution radicular pain without rash; amplifiable VZV DNA, but not HSV DNA, was found in their CSF and blood MNCs, and pain resolved after treatment with intravenous acyclovir [104]. An additional virologically confirmed case demonstrated electromyographic fibrillation potentials restricted to chronically painful thoracic roots [108]. MRI of another patient with virologically verified active VZV infection revealed inflammation in ganglia and nerve roots corresponding to persistent pain [23].
Virologic analyses have demonstrated the association of VZV with meningoencephalitis, vasculopathy, myelitis, cerebellar ataxia, and polyneuritis cranialis, all without rash. Powell and coworkers [109] reported a patient with meningoencephalitis without rash in whom VZV DNA was detected in the CSF, and Mancardi and coworkers [110] described a patient with encephalomyelitis without rash in whom anti-VZV antibody was found in the CSF. Kleinschmidt-DeMasters and coworkers [111] reported an HIV-positive patient with a fatal encephalomyelitis and necrotizing vasculitis, pathologically verified to be caused by VZV without rash. Cases of unifocal and multifocal VZV vasculopathy in the absence of zoster rash resulting in stroke have been verified virologically [68,69]. Two patients with myelopathy in the absence of rash have been described: one developed myelopathy 5 months after zoster rash, at which time amplifiable VZV DNA was detected in the CSF; the second patient developed myelopathy concurrent with zoster and the myelopathy recurred 6 months later in the absence of rash, at which time both VZV DNA and VZV antibody were detected in the CSF [104].
Although it is well recognized that cerebellar ataxia may complicate childhood varicella [112], there is one report of a child who became ataxic 5 days before chickenpox developed [113]. Most recently, acute cerebellar ataxia without rash has been reported in adults whose CSF revealed VZV DNA and VZV antibody [114,115]. Polyneuritis cranialis without rash caused by VZV infection has also been described in a patient with involvement of cranial nerves IX, X, and XI, and upper cervical nerve roots without rash, and with anti-VZV antibody in the CSF [116]. There is also a growing body of literature on ocular abnormalities associated with zoster sine herpete. Goon and coworkers [117] reported a case of severe, unremitting eye pain without rash proved to be caused by VZV infection by the detection of VZV DNA in nasal and conjunctival samples. In addition, cases of third cranial nerve palsies [118], retinal periphlebitis [119], uveitis [118,120], iridocyclitis [121], and disciform keratitis [122], all without rash and confirmed virologically to be caused by VZV, have been reported.
Two remarkable cases of VZV infection without rash deserve mention. The first was a 77-year-old man with T-cell lymphoma and no history of zoster rash who developed an acute fatal meningoradiculitis of cranial nerve roots and cauda equina, pathologically and virologically confirmed to be caused by VZV [123]. The second case was an immunocompetent adult who had experienced relentless trigeminal distribution pain for more than a year with no history of zoster rash; pathologic and virologic analysis of a trigeminal ganglionic mass confirmed chronic active VZV ganglionitis [124].
Prevalence estimates of VZV-induced pathology without rash await virologic analysis of additional patients with prolonged radicular pain or other neurologic symptoms and signs. Analysis should include both a test for anti-VZV IgG and PCR to amplify VZV DNA in CSF, and examination of blood MNCs for VZV DNA. Finally, the nosologic entity of zoster sine herpete has considerable implications for analysis and treatment of patients with PHN. Overall, VZV reactivation from latency in ganglia produces a variety of neurologic disorders all caused by the same pathogen and in the absence of zoster rash.
Vaccination
Widespread, aggressive VZV vaccination has reduced the total number of varicella cases by approximately 85% and the number of moderate-to-severe cases by 95% to 100% [125]. Now, like the live childhood varicella vaccine, there is a live zoster vaccine that seems to be safe and effective clinically. The results of a prospective, double-blind, placebo-controlled trial of attenuated VZV vaccine designed to prevent zoster and PHN in men and women over the age of 60 were recently reported [126]. Otherwise healthy adults age 60 years or older (median 69 years) were vaccinated with placebo or an attenuated Oka/Merck-VZV vaccine containing 18,700 to 60,000 plaque-forming units of virus, considerably greater than the approximately 1350 plaque-forming units in the Oka/Merck-VZV vaccine administered to American children since 1995. More than 38,000 recipients of the “zoster vaccine” were followed closely for 3 years. The incidence of zoster in the placebo group was 11.1 per 1000-person years, approximating the results of an epidemiologic survey performed a decade ago, which revealed zoster exceeding 10 cases per 1000-person years in individuals older than 75 years [19]. The effect of zoster vaccine was impressive; compared with placebo, vaccination reduced the incidence of shingles by 51%, the incidence of PHN by 66%, and the burden of illness by 61%.
Overall, serious adverse effects and deaths occurred in 1.4% of both vaccine and placebo recipients. In more than 6000 subjects who kept daily diaries of minor adverse effects for 42 days, 48% of vaccine recipients reported injection site erythema, pain or tenderness, swelling, and pruritis, compared with 16% of placebo recipients. In the same 6000 subjects, serious adverse effects were significantly more frequent (P = 0.03) in vaccine recipients (1.9%) compared with placebo recipients (1.3%), although no specific serious effects emerged. The relative impact of these side effects on the elderly (age ≥70) compared with younger patients was not examined but might be important in future analyses, because the at-risk population over age 70 years is projected to increase substantially in the coming decades. Although the Oka/Merck VZV vaccine on rare occasions unmasks a childhood immunodeficiency disorder, no cases of disseminated zoster that might have been attributed to zoster vaccine in a person with undiagnosed lymphoma, leukemia, or the like were reported.
In 2006, zoster vaccine received FDA approval for healthy VZV-seropositive adults over age 60. Zoster vaccine increases cell-mediated immunity to VZV in such individuals, and the boost is likely to last for decades. Because zoster and its attendant neurologic complication of PHN are common and serious, it seems prudent to recommend zoster vaccine. The Census Bureau projects that by the year 2050, there will be more than 21 million Americans 85 years of age or older (http://www.census.gov/ipc/www/usinterimproj/natprojtab02a.pdf).
Despite the development of a vaccine to prevent zoster, even if every healthy adult in the United States over age 60 years is vaccinated, there would still be approximately 500,000 zoster cases annually, about 200,000 of whom will experience PHN, and stroke, blindness, and myelopathy caused by VZV reactivation. Furthermore, because zoster vaccine is not approved for immunocompromised individuals, neurologic disease produced by VZV reactivation in this population is a continuing problem.
Physical and molecular properties of varicella zoster virus
VZV is one of the eight human herpesviruses and is morphologically indistinguishable from HSV-1, the prototype alphaherpesvirus. The VZV genome is a linear, double-stranded DNA molecule 124,884 nucleotides in length [127] with close homology to the HSV genome. The lipid envelope encloses the icosahedral nucleocapsid, which consists of 162 capsomeres. Virions are pleomorphic with a 150- to 200-nm diameter. In tissue culture, VZV produces a cytopathic effect in approximately 3 days, characterized by the formation of large multinucleated syncytia without the release of significant quantities of stable infectious virions. VZV is highly cell-associated making it difficult to raise sufficient quantities of cell-free virus for molecular analysis.
The entire virus genome from 13 independent isolates has been sequenced. The VZV prototype consists of a long and short region, each bounded by inverted and terminal repeat sequences [127]. The long segment contains 104,836 base pairs (bp) of unique DNA flanked by 88-bp terminal repeat sequences. The short segment consists of 5232 bp of unique DNA flanked by 7320-bp repeat sequences. Analysis of the 124,884-bp VZV genome has identified 71 predicted open reading frames (ORF) numbered consecutively from the leftward end of the virus genome. ORFs 62, 63, and 64 map within the internal repeat region of the short segment of the VZV genome and are duplicated (although in opposite orientation) as ORFs 71, 70, and 69, respectively, within the terminal repeat region. ORFs 42 and 45 may be exons from the same approximately 5.7-kbp primary transcript. There are 68 predicted unique VZV genes. Additionally, two novel VZV genes (ORFs 9A and 33.5) have been identified experimentally, indicating a potential coding capacity of 70 unique genes.
Alphaherpesvirus gene transcription is classified into three distinct kinetic groups: (1) immediate-early, (2) early, and (3) late [128-130]. Immediate-early genes are transcribed in the absence of de novo protein synthesis and regulate transcription of early and late virus genes. The onset of early gene transcription precedes virus DNA replication. Transcription of early genes is induced by immediate-early proteins and early proteins, which are predominately involved in virus DNA replication and accumulate in the presence of inhibitors of DNA synthesis. Late proteins, which include the major virus structural proteins, are transcribed from progeny viral DNA, and their transcription is blocked by inhibitors of virus DNA synthesis.
During productive infection of cells in culture, transcripts mapping to all predicted VZV genes have been detected. PCR-based macroarrays developed to detect VZV transcription showed that major regions of gene transcription are not clustered, and instead are located throughout the virus genome [131]. These results were confirmed in a study using oligonucleotide-based microarrays [132]. Slight differences that were noted between the two array analyses are most likely caused by differences in virus strain and host cells.
Latency
The hallmark of herpesviruses is their ability to establish a life-long latent infection punctuated by periods of virus recrudescence. Alphaherpesvirus latency is characterized by the ability to reactivate infectious virus, the presence of virus DNA in ganglionic neurons, and limited virus gene transcription.
Virologic features in latently infected human ganglia
The hypothesis that VZV can establish a latent infection in sensory ganglia was first proposed by Head and Campbell [41], who noticed dermatomal distribution, varicella-like lesions in zoster cases. Serologic data indicated that varicella and zoster were caused by the same virus [133], but it was not until restriction endonuclease analysis of DNA from varicella and zoster lesions in the same individual that VZV was confirmed to cause both diseases [134].
VZV becomes latent in neurons [135-138] in cranial nerve, dorsal root, and autonomic ganglia along the entire human neuraxis. Latent VZV DNA assumes a circular or concatameric (end-to-end) state [139] and is present at a frequency of two to nine copies in 1% to 7% of individual neurons, which correlates to a virus burden of 30 to 3500 VZV DNA copies per 100 ng of total ganglionic DNA [137,138,140-144]. The wide range of VZV copy number during latent infection may reflect the degree of primary infection. For example, during varicella, the VZV DNA burden in blood ranges from 200 to 500 copies per 15,000 peripheral blood MNCs, 100 to 1000 copies per milliliter of whole blood, and 100 to more than 10,000 copies per milliliter of serum [145,146]. Furthermore, during the many decades from the time of infection until death, the amount of virus in latently infected ganglia is likely to be affected by exposure of adults to children with varicella or to other adults with zoster, or by spontaneous subclinical reactivation of VZV [147-149].
Varicella zoster virus gene expression in latently infected human ganglia
Transcripts corresponding to VZV genes 21, 29, 62, 63, and 66 have been identified in latently infected human ganglia [150-152]. The VZV gene 63 transcript is the most prevalent and abundant detected [153]. IE63, the immediate-early protein encoded by ORF 63, was the first VZV protein to be detected in latently infected human ganglia [154]. Subsequently, immunohistochemistry detected proteins encoded by VZV genes 62 and 66 [155] and by VZV genes 4, 21, and 29 [156,157]. The detection of protein encoded by VZV gene 4 requires confirmation because VZV gene 4 transcripts have not yet been found in latently infected human ganglia.
Animal models of varicella zoster virus infection
Development of an experimental animal model that recapitulates the pathogenesis of VZV seen in humans has been a goal ever since the realization that VZV infects only humans. Important criteria for any animal model of VZV latency include: (1) ability to reactivate the virus; (2) presence of virus nucleic acids in ganglia, but not in nonganglionic tissues; (3) presence of virus exclusively in neurons; and (4) limited transcription of virus genes.
Experimental inoculation of VZV into small animals including rabbits, mice, and rats leads to seroconversion in the absence of clinical signs [158-163]. After corneal inoculation of VZV in mice, viral DNA is detected in ganglionic neurons and nonneuronal cells, and in nonganglionic tissues 1 month after infection [164]. Intramuscular inoculation of guinea pigs with VZV produces a papular exanthem without vesicles [165], and VZV DNA has been detected by PCR in ganglia of guinea pigs 80 days after subcutaneous inoculation [166]. VZV RNA has also been detected in ganglia of guinea pigs by in situ hybridization 5 weeks after ocular inoculation [167]. It is difficult, however, to evaluate the usefulness of the guinea pig model because the absence of virus nucleic acids in nonganglionic tissues has not been shown. In addition, reactivation of latent VZV has not been demonstrated in guinea pigs.
Sadzot-Delvaux and coworkers [168] inoculated VZV subcutaneously into adult rats. Although no clinical signs developed, virus nucleic acids and proteins were detected in dissociated ganglionic neurons up to 9 months after experimental infection. Because the ganglia were cultured for 3 to 12 days, in vitro reactivation could not be excluded. Latent VZV infection has been reported in rats inoculated by footpad and sacrificed 1 month later [169,170]. The detection of VZV DNA in both neurons and nonneuronal cells [171] of dorsal root ganglia harvested 1 to 3 months after footpadinoculation, however, questions the validity of the rat model. In addition, in vivo VZV reactivation in rats has not been reported.
Direct inoculation of VZV into human thymus and liver implants under the kidney capsule of severe combined immunodeficient mice results in virus infection as evidenced by the detection of virus proteins for 3 weeks after infection in CD4+ and CD8+ T cells [172]. Similar studies using human ganglionic implants have been used to demonstrate virus infection [173]. The absence of an intact immune system in these animals makes it difficult, however, to study latency or reactivation. Overall, although VZV reaches ganglia after experimental infection of small animals, nonganglionic tissues have not been studied and reactivation has not been demonstrated.
Oral-nasal-conjunctival application of the attenuated vaccine strain of VZV in marmosets results in mild pneumonia and an immune response without clinical disease [174]. Subcutaneous inoculation of the Oka VZV (vaccine strain) into the breast of chimpanzees produces viremia and a mild rash restricted to the site of inoculation [175]. VZV latency or reactivation has not been studied, however, in chimpanzees.
Simian varicella virus as a model for human varicella zoster virus disease
Immunologic, virologic, and pathologic features of simian varicella virus (SVV) infection of nonhuman primates closely resemble those of human VZV infection. Like VZV in humans, primary infection of primates with SVV leads to varicella followed by virus latency and spontaneous reactivation [176]. Intratracheal inoculation of SVV into nonhuman primates results in persistence of virus DNA in multiple organs, including blood MNCs for 2 years [177,178]. SVV DNA can be detected in ganglia 6 to 7 days after intratracheal or intravenous inoculation before varicella rash, pointing to hematogenous spread of the virus [179]. In monkeys intratracheally inoculated with SVV, virus DNA has been detected in both neurons and nonneuronal cells 9 to 10 months after infection, and exclusively in neurons 2 years after infection [180].
The authors developed a model of natural SVV infection in monkeys by exposing SVV-seronegative monkeys to other monkeys that had been inoculated intratracheally with SVV [181]. These naturally infected monkeys harbor latent SVV DNA in ganglionic neurons [182] at multiple levels of the neuraxis [181], and both clinical and subclinical reactivation of SVV were induced by immunosuppression and stress [183]. Subclinical reactivation of latent SVV also occurs after irradiation in rhesus monkeys [184].
The model of SVV infection of nonhuman primates is well-suited for studies to dissect the relative role of immunosuppression in varicella reactivation. Such studies assume importance in light of the association between VZV reactivation frequency and extent of immunosuppression, with a higher incidence in patients receiving chemotherapy and radiotherapy than in those receiving either alone [185]. Moreover, varicella reactivation results in serious neurologic complications in immunosuppressed individuals.
Summary
VZV is an exclusively human, highly neurotropic alphaherpesvirus. Primary infection causes chickenpox (varicella), after which virus becomes latent in cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia along the entire neuraxis. Decades later, VZV may reactivate to cause herpes zoster (shingles), pain, and rash in one to three dermatomes. Multiple neurologic complications after VZV reactivation include PHN; vasculopathy; myelitis; necrotizing retinitis; and zoster sine herpete (pain without rash). Many may occur without rash and are difficult to recognize. Virologic confirmation requires testing the CSF for VZV DNA and anti-VZV IgG. Immediate treatment with antiviral agents may be warranted. The relative role of immunosuppression in the frequency and consequences of VZV reactivation awaits elucidation in the animal model of infection of monkeys with SVV, the only system to date that closely recapitulates the human disease. Successful mass vaccination against herpes zoster will have a profound impact on the health and quality of life of a steadily growing elderly population. Even if every healthy adult in the United States over age 60 years is vaccinated, however, there would still be approximately 500,000 zoster cases annually, about 200,000 of whom will experience PHN, and stroke, loss of vision, and myelopathy caused by VZV reactivation.
Acknowledgments
The authors thank Marina Hoffman for editorial review and Cathy Allen for preparing the manuscript.
This work was supported in part by Public Health Service grants NS32623 and AG06127 from the National Institutes of Health. Drs. Maria Nagel and Niklaus Mueller are supported by Public Health Service grant NS07321 from the National Institutes of Health.
References
- [1].Finger R, Hughes JP, Meade BJ, et al. Age-specific incidence of chickenpox. Public Health Rep. 1994;109:750–5. [PMC free article] [PubMed] [Google Scholar]
- [2].Heininger U, Braun-Fahrländer C, Desgrandchamps D, et al. Seroprevalence of varicellazoster virus immunoglobulin G antibodies in Swiss adolescents and risk factor analysis for seronegativity. Pediatr Infect Dis J. 2001;20:775–8. doi: 10.1097/00006454-200108000-00011. [DOI] [PubMed] [Google Scholar]
- [3].Wutzler P, Farber I, Wagenpfeil S, et al. Seroprevalence of varicella-zoster virus in the German population. Vaccine. 2001;20:121–4. doi: 10.1016/s0264-410x(01)00276-6. [DOI] [PubMed] [Google Scholar]
- [4].Kudesia G, Partridge S, Farrington CP, et al. Changes in age related seroprevalence of antibody to varicella zoster virus: impact on vaccine strategy. J Clin Pathol. 2002;55:154–5. doi: 10.1136/jcp.55.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003;70(Suppl 1):S111–8. doi: 10.1002/jmv.10364. [DOI] [PubMed] [Google Scholar]
- [6].Choo PW, Donahue JG, Manson JE, et al. The epidemiology of varicella and its complications. J Infect Dis. 1995;172:706–12. doi: 10.1093/infdis/172.3.706. [DOI] [PubMed] [Google Scholar]
- [7].Boelle PY, Hanslik T. Varicella in non-immune persons: incidence, hospitalization and mortality rates. Epidemiol Infect. 2002;129:599–606. doi: 10.1017/s0950268802007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gershon AA, Hambleton S. Varicella vaccine for susceptible adults: do it today. Clin Infect Dis. 2004;39:1640–1. doi: 10.1086/425618. [DOI] [PubMed] [Google Scholar]
- [9].Ooi PL, Goh KT, Doraisingham S, et al. Prevalence of varicella-zoster virus infection in Singapore. Southeast Asian J Trop Med Public Health. 1992;23:22–5. [PubMed] [Google Scholar]
- [10].Lee BW. Review of varicella zoster seroepidemiology in India and Southeast Asia. Trop Med Int Health. 1998;3:886–90. doi: 10.1046/j.1365-3156.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- [11].Mandal BK, Mukherjee PP, Murphy C, et al. Adult susceptibility to varicella in the tropics is a rural phenomenon due to the lack of previous exposure. Infect Dis. 1998;178(Suppl 1):S52–4. doi: 10.1086/514262. [DOI] [PubMed] [Google Scholar]
- [12].Deguen S, Chau NP, Flahault A. Epidemiology of chickenpox in France (1991–1995) J Epidemiol Community Health. 1998;52(Suppl l):46–9. [PubMed] [Google Scholar]
- [13].Tobias M, Reid S, Lennon D, et al. Chickenpox immunisation in New Zealand. N Z Med J. 1998;111:274–81. [PubMed] [Google Scholar]
- [14].Bramley JC, Jones IG. Epidemiology of chickenpox in Scotland:1981 to 1998. Commun Dis Public Health. 2000;3:282–7. [PubMed] [Google Scholar]
- [15].Guess HA, Broughton DD, Melton LJ. Population-based studies of varicella complications. Pediatrics. 1986;78(Suppl 4):723–7. [PubMed] [Google Scholar]
- [16].Askalan R, Laughlin S, Mayank S, et al. Chickenpox and stroke in childhood: a study of frequency and causation. Stroke. 2001;32:1257–62. doi: 10.1161/01.str.32.6.1257. [DOI] [PubMed] [Google Scholar]
- [17].Dunkle LM, Arvin AM, Whitley RJ, et al. A controlled trial of acyclovir for chickenpox in normal children. N Engl J Med. 1991;325:1539–44. doi: 10.1056/NEJM199111283252203. [DOI] [PubMed] [Google Scholar]
- [18].Harnisch JP. Zoster in the elderly: clinical, immunologie and therapeutic considerations. J Am Geriatr Soc. 1984;32:789–93. doi: 10.1111/j.1532-5415.1984.tb06298.x. [DOI] [PubMed] [Google Scholar]
- [19].Donahue JG, Choo PW, Manson JE, et al. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–9. [PubMed] [Google Scholar]
- [20].Gilden DH, Cohrs RJ, Mahalingam R. Clinical and molecular pathogenesis of varicella virus infection. Viral Immunol. 2003;16:243–58. doi: 10.1089/088282403322396073. [DOI] [PubMed] [Google Scholar]
- [21].Leppard B, Naburi AE. Herpes zoster: an early manifestation of HIV infection. Afr Health. 1998;21:5–6. [PubMed] [Google Scholar]
- [22].Kakourou T, Theodoridou M, Mostrou G, et al. Herpes zoster in children. J Am Acad Dermatol. 1998;39:207–10. doi: 10.1016/s0190-9622(98)70076-3. [DOI] [PubMed] [Google Scholar]
- [23].Blumenthal DT, Salzman KL, Baringer JR, et al. MRI abnormalities in chronic active varicella zoster infection. Neurology. 2004;63:1538–9. doi: 10.1212/01.wnl.0000141855.67420.73. [DOI] [PubMed] [Google Scholar]
- [24].Carroll WM, Mastaglia FL. Optic neuropathy and ophthalmoplegia in herpes zoster oticus. Neurology. 1979;29:726–9. doi: 10.1212/wnl.29.5.726. [DOI] [PubMed] [Google Scholar]
- [25].Meenken C, van den Horn GJ, de Smet MD, et al. Optic neuritis heralding varicella zoster virus retinitis in a patient with acquired immunodeficiency syndrome. Ann Neurol. 1998;43:534–6. doi: 10.1002/ana.410430420. [DOI] [PubMed] [Google Scholar]
- [26].Archambault P, Wise JS, Rosen J, et al. Herpes zoster ophthalmoplegia: report of six cases. J Clin Neuroophthalmol. 1988;8:185–93. [PubMed] [Google Scholar]
- [27].Sodhi PK, Goel JL. Presentations of cranial nerve involvement in two patients with herpes zoster ophthalmicus. J Commun Dis. 2001;33:130–5. [PubMed] [Google Scholar]
- [28].Karmon Y, Gadath N. Delayed oculomotor nerve palsy after bilateral cervical zoster in an immunocompetent patient. Neurology. 2005;65:170. doi: 10.1212/01.wnl.0000167287.02490.76. [DOI] [PubMed] [Google Scholar]
- [29].Garty B-Z, Dinari G, Sarnat H, et al. Tooth exfoliation and osteonecrosis of the maxilla after trigeminal herpes zoster. J Pediatr. 1985;106:71–3. doi: 10.1016/s0022-3476(85)80469-8. [DOI] [PubMed] [Google Scholar]
- [30].Manz HJ, Canter HG, Melton J. Trigeminal herpes zoster causing mandibular osteonecrosis and spontaneous tooth exfoliation. South Med J. 1986;79:1026–8. doi: 10.1097/00007611-198608000-00028. [DOI] [PubMed] [Google Scholar]
- [31].Volvoikar P, Patil S, Dinkar A. Tooth exfoliation, osteonecrosis and neuralgia following herpes zoster of trigeminal nerve. Indian J Dent Res. 2002;13:11–4. [PubMed] [Google Scholar]
- [32].Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:149–54. doi: 10.1136/jnnp.71.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Asnis DS, Micic L, Giaccio D. Ramsay Hunt syndrome presenting as a cranial polyneuropathy. Cutis. 1996;57:421–4. [PubMed] [Google Scholar]
- [34].Robillard RB, Hilsinger RL, Jr, Adour KK. Ramsay Hunt facial paralysis: clinical analyses of 185 patients. Otolaryngol Head Neck Surg. 1986;95:292–7. doi: 10.1177/01945998860953P105. [DOI] [PubMed] [Google Scholar]
- [35].Furuta Y, Ohtani F, Aizawa H, et al. Varicella-zoster virus reactivation is an important cause of acute peripheral facial paralysis in children. Pediatr Infect Dis J. 2005;24:97–101. doi: 10.1097/01.inf.0000151032.16639.9c. [DOI] [PubMed] [Google Scholar]
- [36].Merchet MP, Gruener G. Segmental zoster paresis of limbs. Electromyogr Clin Neurophysiol. 1996;36:369–75. [PubMed] [Google Scholar]
- [37].Yoleri O, Olmez N, Oztura I, et al. Segmental zoster paresis of the upper extremity: a case report. Arch Phys Med Rehabil. 2005;86:1492–4. doi: 10.1016/j.apmr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- [38].Anderson JP, Keal EE. Cervical herpes zoster and diaphragmatic paralysis. Br J Dis Chest. 1969;63:222–6. doi: 10.1016/s0007-0971(69)80022-7. [DOI] [PubMed] [Google Scholar]
- [39].Tjandra J, Mansel RE. Segmental abdominal herpes zoster paresis. Aust N Z J Surg. 1986;56:807–8. doi: 10.1111/j.1445-2197.1986.tb02331.x. [DOI] [PubMed] [Google Scholar]
- [40].Gold E. Serologic and virus-isolation studies of patients with varicella or herpes-zoster infection. N Engl J Med. 1966;274:181–5. doi: 10.1056/NEJM196601272740403. [DOI] [PubMed] [Google Scholar]
- [41].Head H, Campbell AW. The pathology of herpes zoster and its bearing on sensory localization. Brain. 1900;23:353–523. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- [42].Denny-Brown D, Adams RD, Fitzgerald PJ. Pathologic features of herpes zoster: a note on geniculate herpes. Arch Neurol Psychiatry. 1944;51:216–31. [Google Scholar]
- [43].Cheatham WJ, Weller TH, Dolan TF, et al. Varicella: report on two fatal cases with necropsy, virus isolation, and serologic studies. Am J Pathol. 1956;32:1015–35. [PMC free article] [PubMed] [Google Scholar]
- [44].Esiri MM, Tomlinson AH. Herpes zoster: demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- [45].Ghatak NR, Zimmerman HM. Spinal ganglion in herpes zoster. Arch Pathol. 1973;95:411–5. [PubMed] [Google Scholar]
- [46].Nagashima K, Nakazawa M, Endo H. Pathology of the human spinal ganglia in varicellazoster virus infection. Acta Neuropathol. 1975;33:105–17. doi: 10.1007/BF00687537. [DOI] [PubMed] [Google Scholar]
- [47].Murakami S, Hato N, Horiuchi J, et al. Treatment of Ramsay Hunt syndrome with acyclovir-prednisone: significance of early diagnosis and treatment. Ann Neurol. 1997;41:353–7. doi: 10.1002/ana.410410310. [DOI] [PubMed] [Google Scholar]
- [48].Furuta Y, Ohtani F, Mesuda Y, et al. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology. 2000;55:708–10. doi: 10.1212/wnl.55.5.708. [DOI] [PubMed] [Google Scholar]
- [49].Rogers RS, III, Tindall JP. Herpes zoster in the elderly. Postgrad Med. 1971;50:153–7. doi: 10.1080/00325481.1971.11697705. [DOI] [PubMed] [Google Scholar]
- [50].Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitripty-line: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327–31. doi: 10.1016/s0885-3924(97)00077-8. [DOI] [PubMed] [Google Scholar]
- [51].Vafai A, Wellish M, Gilden DH. Expression of varicella-zoster virus in blood mononuclear cells of patients with postherpetic neuralgia. Proc Natl Acad Sci USA. 1988;85:2767–70. doi: 10.1073/pnas.85.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Devlin ME, Gilden DH, Mahalingam R, et al. Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J Infect Dis. 1992;165:619–22. doi: 10.1093/infdis/165.4.619. [DOI] [PubMed] [Google Scholar]
- [53].Mahalingam R, Wellish M, Brucklier J, et al. Persistence of varicella-zoster virus DNA in elderly patients with postherpetic neuralgia. J Neurovirol. 1995;1:130–3. doi: 10.3109/13550289509111018. [DOI] [PubMed] [Google Scholar]
- [54].Terada K, Niizuma T, Kawano S, et al. Detection of varicella-zoster virus DNA in peripheral mononuclear cells from patients with Ramsay Hunt syndrome or zoster sine herpete. J Med Virol. 1998;56:359–63. [PubMed] [Google Scholar]
- [55].Gilden DH, Cohrs RJ, Hayward AR, et al. Chronic varicella zoster virus ganglionitis: a possible cause of postherpetic neuralgia. J Neurovirol. 2003;9:404–7. doi: 10.1080/13550280390201722. [DOI] [PubMed] [Google Scholar]
- [56].Quan D, Hammack BN, Kittelson J, et al. Improvement of postherpetic neuralgia after treatment with intravenous acyclovir followed by oral valacyclovir. Arch Neurol. 2006;63:940–2. doi: 10.1001/archneur.63.7.noc60049. [DOI] [PubMed] [Google Scholar]
- [57].Rowbotham M, Harden N, Stacey B, et al. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–42. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- [58].Rice AS, Maton S. Postperpetic Neuralgia Study Group. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–24. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- [59].Dubinsky RM, Kabbani H, El-Chami Z, et al. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;63:959–65. doi: 10.1212/01.wnl.0000140708.62856.72. [DOI] [PubMed] [Google Scholar]
- [60].Rowbotham MC, Manville NS, Ren J. Pilot tolerability and effectiveness study of levetiracetam for postherpetic neuralgia. Neurology. 2003;61:866–7. doi: 10.1212/01.wnl.0000079463.16377.07. [DOI] [PubMed] [Google Scholar]
- [61].Gilron I, Bailey JM, Tu D, et al. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:1324–34. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- [62].Huff JC. Antiviral treatment in chickenpox and herpes zoster. J Am Acad Dermatol. 1988;18:204–6. doi: 10.1016/s0190-9622(88)70029-8. [DOI] [PubMed] [Google Scholar]
- [63].Beutner KR, Friedman DJ, Forszpaniak C, et al. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546–53. doi: 10.1128/aac.39.7.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia: a randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89–96. doi: 10.7326/0003-4819-123-2-199507150-00002. [DOI] [PubMed] [Google Scholar]
- [65].Gilden DH, Lipton HL, Wolf JS, et al. Two patients with unusual forms of varicella-zoster virus vasculopathy. N Engl J Med. 2002;347:1500–3. doi: 10.1056/NEJMoa020841. [DOI] [PubMed] [Google Scholar]
- [66].Fukumoto S, Kinjo M, Hokamura K, et al. Subarachnoid hemorrhage and granulomatous angiitis of the basilar artery: demonstration of the varicella-zoster-virus in the basilar artery lesions. Stroke. 1986;17:1024–8. doi: 10.1161/01.str.17.5.1024. [DOI] [PubMed] [Google Scholar]
- [67].O’Donohue JM, Enzmann DR. Mycotic aneurysm in angiitis associated with herpes zoster ophthalmicus. AJNR Am J Neuroradiol. 1987;8:615–9. [PMC free article] [PubMed] [Google Scholar]
- [68].Gilden DH, Bennett JL, Kleinschmidt-DeMasters BK, et al. The value of cerebrospinal fluid antiviral antibody in the diagnosis of neurologic disease produced by varicella zoster virus. J Neurol Sci. 1998;159:140–4. doi: 10.1016/s0022-510x(98)00153-1. [DOI] [PubMed] [Google Scholar]
- [69].Nau R, Lantsch M, Stiefel M, et al. Varicella zoster virus-associated focal vasculitis without herpes zoster: recovery after treatment with acyclovir. Neurology. 1998;51:914–5. doi: 10.1212/wnl.51.3.914. [DOI] [PubMed] [Google Scholar]
- [70].Burgoon MP, Hammack BN, Owens GP, et al. Oligoclonal immunoglobulins in cerebro-spinal fluid during varicella zoster virus (VZV) vasculopathy are directed against VZV. Ann Neurol. 2003;54:459–63. doi: 10.1002/ana.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, et al. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5-1995 revisited. Neurology. 1996;47:1441–6. doi: 10.1212/wnl.47.6.1441. [DOI] [PubMed] [Google Scholar]
- [72].Aurelius E, Johansson B, Skoldenberg B, et al. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet. 1991;337:189–92. doi: 10.1016/0140-6736(91)92155-u. [DOI] [PubMed] [Google Scholar]
- [73].Nagel MA, Forghani B, Mahalingam R, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68:1069–73. doi: 10.1212/01.wnl.0000258549.13334.16. [DOI] [PubMed] [Google Scholar]
- [74].Hilt DC, Buchholz D, Krumholz A, et al. Herpes zoster ophthalmicus and delayed contra-lateral hemiparesis caused by cerebral angiitis: diagnosis and management approaches. Ann Neurol. 1983;14:543–53. doi: 10.1002/ana.410140509. [DOI] [PubMed] [Google Scholar]
- [75].Pina MA, Ara JR, Capablo JL, et al. Myelitis and optic neuritis caused by varicella. Rev Neurol. 1997;25:1575–6. [PubMed] [Google Scholar]
- [76].Celik Y, Tabak F, Mert A, et al. Transverse myelitis caused by varicella. Clin Neurol Neurosurg. 2001;103:260–1. doi: 10.1016/s0303-8467(01)00166-4. [DOI] [PubMed] [Google Scholar]
- [77].Gilden DH, Beinlich BR, Rubinstien EM, et al. Varicella-zoster virus myelitis: an expanding spectrum. Neurology. 1994;44:1818–23. doi: 10.1212/wnl.44.10.1818. [DOI] [PubMed] [Google Scholar]
- [78].Kleinschmidt-DeMasters BK, Gilden DH. Varicella-zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–80. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- [79].Devinsky O, Cho ES, Petito CK, et al. Herpes zoster myelitis. Brain. 1991;114:1181–96. doi: 10.1093/brain/114.3.1181. [DOI] [PubMed] [Google Scholar]
- [80].de Silva SM, Mark AS, Gilden DH, et al. Zoster myelitis: improvement with antiviral therapy in two cases. Neurology. 1996;47:929–31. doi: 10.1212/wnl.47.4.929. [DOI] [PubMed] [Google Scholar]
- [81].Chua HC, Tjia H, Sitoh YY. Concurrent myelitis and Guillain-Barré syndrome after varicella infection. Int J Clin Pract. 2001;55:643–4. [PubMed] [Google Scholar]
- [82].Schvoerer E, Frechin V, Warter A, et al. Persistent multiple pulmonary nodules in a nonimmunocompromised woman after varicella-related myelitis treated with acyclovir. J Clin Microbiol. 2003;41:4904–5. doi: 10.1128/JCM.41.10.4904-4905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Orme HT, Smith G, Nagel MA, et al. VZV spinal cord infarction identified by diffusion-weighted magnetic resonance imaging (DWI) Neurology. 2007;69:398–400. doi: 10.1212/01.wnl.0000266390.27177.7b. [DOI] [PubMed] [Google Scholar]
- [84].Holland GN. The progressive outer retinal necrosis syndrome. Int Ophthalmol. 1994;18:163–5. doi: 10.1007/BF00915966. [DOI] [PubMed] [Google Scholar]
- [85].Soushi S, Ozawa H, Matsuhashi M, et al. Demonstration of varicella-zoster virus antigens in the vitreous aspirates of patients with acute retinal necrosis syndrome. Ophthalmology. 1988;95:1394–8. doi: 10.1016/s0161-6420(88)33012-5. [DOI] [PubMed] [Google Scholar]
- [86].Thompson WS, Culbertson WW, Smiddy WE, et al. Acute retinal necrosis caused by reactivation of herpes simplex virus type 2. Am J Ophthalmol. 1994;118:205–11. doi: 10.1016/s0002-9394(14)72900-9. [DOI] [PubMed] [Google Scholar]
- [87].Tan JCH, Byles D, Stanford MR, et al. Acute retinal necrosis in children caused by herpes simplex virus. Retina. 2001;21:344–7. doi: 10.1097/00006982-200108000-00008. [DOI] [PubMed] [Google Scholar]
- [88].Bonfioli AA, Eller AW. Acute retinal necrosis. Semin Ophthalmol. 2005;20:155–60. doi: 10.1080/08820530500232027. [DOI] [PubMed] [Google Scholar]
- [89].Savant V, Saeed T, Denniston A, et al. Oral valganciclovir treatment of varicella zoster virus acute retinal necrosis. Eye. 2004;18:544–5. doi: 10.1038/sj.eye.6700703. [DOI] [PubMed] [Google Scholar]
- [90].Engstrom RE, Jr, Holland GN, Margolis TP, et al. The progressive outer retinal necrosis syndrome: a variant of necrotizing herpetic retinopathy in patients with AIDS. Ophthalmology. 1994;101:1488–502. doi: 10.1016/s0161-6420(94)31142-0. [DOI] [PubMed] [Google Scholar]
- [91].Guex-Crosier Y, Rochat C, Herbort CP. Necrotizing herpetic retinopathies: a spectrum of herpes virus-induced diseases determined by the immune state of the host. Ocul Immunol Inflamm. 1997;5:259–65. doi: 10.3109/09273949709085066. [DOI] [PubMed] [Google Scholar]
- [92].Lewis JM, Nagae Y, Tano Y. Progressive outer retinal necrosis after bone marrow transplantation. Am J Ophthalmol. 1996;122:892–5. doi: 10.1016/s0002-9394(14)70391-5. [DOI] [PubMed] [Google Scholar]
- [93].Franco-Paredes C, Bellehemeur T, Merchant A, et al. Aseptic meningitis and optic neuritis preceding varicella-zoster progressive outer retinal necrosis in a patient with AIDS. AIDS. 2002;16:1045–9. doi: 10.1097/00002030-200205030-00011. [DOI] [PubMed] [Google Scholar]
- [94].Menerath JM, Gerard M, Laurichesse H, et al. Bilateral acute retinal necrosis in a patient with acquired immunodeficiency syndrome. J Fr Ophthalmol. 1995;18:625–33. [PubMed] [Google Scholar]
- [95].Tran TH, Rozenberg F, Cassoux N, et al. Polymerase chain reaction analysis of aqueous humour samples in necrotising retinitis. Br J Ophthalmol. 2003;87:79–83. doi: 10.1136/bjo.87.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Margolis TP, Lowder CY, Holland GN, et al. Varicella-zoster virus retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1991;112:119–31. doi: 10.1016/s0002-9394(14)76690-5. [DOI] [PubMed] [Google Scholar]
- [97].van den Horn GJ, Meenken C, Troost D. Association of progressive outer retinal necrosis and varicella zoster encephalitis in a patient with AIDS. Br J Ophthalmol. 1996;80:982–5. doi: 10.1136/bjo.80.11.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kashiwase M, Sata T, Yamauchi Y, et al. Progressive outer retinal necrosis caused by herpes simplex virus type 1 in a patient with acquired immunodeficiency syndrome. Ophthalmology. 2000;107:790–4. doi: 10.1016/s0161-6420(99)00143-8. [DOI] [PubMed] [Google Scholar]
- [99].Biswas J, Choudhry S, Priya K, et al. Detection of cytomegalovirus from vitreous humor in a patient with progressive outer retinal necrosis. Indian J Ophthalmol. 2002;50:319–21. [PubMed] [Google Scholar]
- [100].Johnston WH, Holland GN, Engstrom RE, Jr, et al. Recurrence of presumed varicellazoster virus retinopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;116:42–50. doi: 10.1016/s0002-9394(14)71742-8. [DOI] [PubMed] [Google Scholar]
- [101].Moorthy RS, Weinberg DV, Teich SA, et al. Management of varicella zoster virus retinitis in AIDS. Br J Ophthalmol. 1997;81:189–94. doi: 10.1136/bjo.81.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dullaert H, Maudgal PC, Leys A, et al. Bromovinyldeoxyurdine treatment of outer retinal necrosis due to varicella-zoster virus: a case-report. Bull Soc Beige Ophtalmol. 1996;262:107–13. [PubMed] [Google Scholar]
- [103].Austin RB. Progressive outer retinal necrosis syndrome: a comprehensive review of its clinical presentation, relationship to immune system status, and management. Clin Eye Vis Care. 2000;12:119–29. doi: 10.1016/s0953-4431(00)00052-7. [DOI] [PubMed] [Google Scholar]
- [104].Gilden DH, Wright RR, Schneck SA, et al. Zoster sine herpete, a clinical variant. Ann Neurol. 1994;35:530–3. doi: 10.1002/ana.410350505. [DOI] [PubMed] [Google Scholar]
- [105].Lewis GW. Zoster sine herpete. Br Med J. 1958;34:418–21. doi: 10.1136/bmj.2.5093.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Easton HG. Zoster sine herpete causing acute trigeminal neuralgia. Lancet. 1970;2:1065–6. doi: 10.1016/s0140-6736(70)90291-6. [DOI] [PubMed] [Google Scholar]
- [107].Schott GD. Triggering of delayed-onset postherpetic neuralgia. Lancet. 1998;351:419–20. doi: 10.1016/s0140-6736(05)78359-8. [DOI] [PubMed] [Google Scholar]
- [108].Amlie-Lefond C, Mackin GA, Ferguson M, et al. Another case of virologically confirmed zoster sine herpete, with electrophysiologic correlation. J Neurovirol. 1996;2:136–8. doi: 10.3109/13550289609146547. [DOI] [PubMed] [Google Scholar]
- [109].Powell KF, Wilson HG, Corxson MO, et al. Herpes zoster meningoencephalitis without rash: varicella zoster virus DNA in CSF. J Neurol Neurosurg Psychiatry. 1995;59:198–9. doi: 10.1136/jnnp.59.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mancardi GL, Melioli G, Traverso F, et al. Zoster sine herpete causing encephalomyelitis. Ital J Neurol Sci. 1987;8:67–70. doi: 10.1007/BF02361439. [DOI] [PubMed] [Google Scholar]
- [111].Kleinschmidt-DeMasters BK, Mahalingam R, Shimek C, et al. Profound cerebrospinal fluid pleocytosis and Froin’s syndrome secondary to widespread necrotizing vasculitis in an HIV-positive patient with varicella zoster virus encephalomyelitis. J Neurol Sci. 1998;159:213–8. doi: 10.1016/s0022-510x(98)00171-3. [DOI] [PubMed] [Google Scholar]
- [112].Connolly AM, Dodson WE, Prensky AL, et al. Course and outcome of acute cerebellar ataxia. Ann Neurol. 1994;35:673–9. doi: 10.1002/ana.410350607. [DOI] [PubMed] [Google Scholar]
- [113].Dangond F, Engle E, Yessayan L, et al. Pre-eruptive varicella cerebellitis confirmed by PCR. Pediatr Neurol. 1993;9:491–3. doi: 10.1016/0887-8994(93)90032-8. [DOI] [PubMed] [Google Scholar]
- [114].Moses H, Nagel MA, Gilden DH. Acute cerebellar ataxia in a 41-year-old woman. Lancet Neurol. 2006;5:984–8. doi: 10.1016/S1474-4422(06)70601-9. [DOI] [PubMed] [Google Scholar]
- [115].Ratzka P, Schlachetzki JC, Bahr M, et al. Varicella zoster virus cerebellitis in a 66-year-old patient without herpes zoster. Lancet. 2006;367:182. doi: 10.1016/S0140-6736(06)67967-1. [DOI] [PubMed] [Google Scholar]
- [116].Funakawa I, Terao A, Koga M. A case of zoster sine herpete with involvement of the unilateral IX, X and XI cranial and upper cervical nerves. Rinsho Shinkeigaku. 1999;39:958–60. [PubMed] [Google Scholar]
- [117].Goon P, Wright M, Fink C. Ophthalmic zoster sine herpete. J R Soc Med. 2000;93:191–2. doi: 10.1177/014107680009300409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Hon C, Au WY, Cheng VC. Ophthalmic zoster sine herpete presenting as oculomotor palsy after marrow transplantation for acute myeloid leukemia. Haematologica. 2005;90:EIM04. [PubMed] [Google Scholar]
- [119].Noda Y, Nakazawa M, Takahashi D, et al. Retinal periphlebitis as zoster sine herpete. Arch Ophthalmol. 2001;119:1550–2. [PubMed] [Google Scholar]
- [120].Akpek EK, Gottsch JD. Herpes zoster sine herpete presenting with hyphema. Ocul Immunol Inflamm. 2000;8:115–8. [PubMed] [Google Scholar]
- [121].Yamamoto S, Tada R, Shimomura Y, et al. Detecting varicella-zoster virus DNA in iridocyclitis using polymerase chain reaction: a case of zoster sine herpete. Arch Ophthalmol. 1995;113:1358–9. doi: 10.1001/archopht.1995.01100110018009. [DOI] [PubMed] [Google Scholar]
- [122].Silverstein BE, Chandler D, Neger R, et al. Disciform keratitis: a case of herpes zoster sine herpete. Am J Ophthalmol. 1997;123:254–5. doi: 10.1016/s0002-9394(14)71044-x. [DOI] [PubMed] [Google Scholar]
- [123].Dueland AN, Devlin M, Martin JR, et al. Fatal varicella-zoster virus meningoradiculitis without skin involvement. Ann Neurol. 1991;29:569–72. doi: 10.1002/ana.410290520. [DOI] [PubMed] [Google Scholar]
- [124].Hevner R, Vilela M, Rostomily R, et al. An unusual cause of trigeminal-distribution pain and tumour. Lancet Neurol. 2003;2:567–71. doi: 10.1016/s1474-4422(03)00506-4. [DOI] [PubMed] [Google Scholar]
- [125].Takahashi M. Effectiveness oflive varicella vaccine. Expert Opin Biol Ther. 2004;4:199–216. doi: 10.1517/14712598.4.2.199. [DOI] [PubMed] [Google Scholar]
- [126].Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- [127].Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- [128].Honess RW, Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975;16:1308–26. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zhang YF, Wagner EK. The kinetics of expression of individual herpes simplex virus type 1 transcripts. Virus Genes. 1987;1:49–60. doi: 10.1007/BF00125685. [DOI] [PubMed] [Google Scholar]
- [130].Zhang YF, Devi-Rao GB, Rice M, et al. The effect of elevated levels of herpes simplex virus alpha-gene products on the expression ofmodel early and late genes in vivo. Virology. 1987;157:99–106. doi: 10.1016/0042-6822(87)90318-7. [DOI] [PubMed] [Google Scholar]
- [131].Cohrs RJ, Gilden DH, Kinchington PR, et al. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J Virol. 2003;77:6660–5. doi: 10.1128/JVI.77.12.6660-6665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kennedy PG, Grinfeld E, Craigon M, et al. Transcriptional analysis of varicella-zoster virus infection using long oligonucleotide-based microarrays. J Gen Virol. 2005;86:2673–84. doi: 10.1099/vir.0.80946-0. [DOI] [PubMed] [Google Scholar]
- [133].Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- [134].Straus SE, Hay J, Smith H, et al. Genome differences among varicella-zoster isolates. J Gen Virol. 1983;64:1031–41. doi: 10.1099/0022-1317-64-5-1031. [DOI] [PubMed] [Google Scholar]
- [135].Hyman RW, Ecker JR, Tenser RB. Varicella-zoster virus RNA in human trigeminal ganglia. Lancet. 1983;2:814–6. doi: 10.1016/s0140-6736(83)90736-5. [DOI] [PubMed] [Google Scholar]
- [136].Gilden DH, Rozemann Y, Murray R, et al. Detection of varicella-zoster virus nucleic acid in neurons of normal human thoracic ganglia. Ann Neurol. 1987;22:377–80. doi: 10.1002/ana.410220315. [DOI] [PubMed] [Google Scholar]
- [137].LaGuardia JJ, Cohrs RJ, Gilden DH. Prevalence of varicella-zoster virus DNA in dissociated human trigeminal ganglion neurons and nonneuronal cells. J Virol. 1999;73:8571–7. doi: 10.1128/jvi.73.10.8571-8577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Levin MJ, Cai GY, Manchak MD, et al. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J Virol. 2003;77:6979–87. doi: 10.1128/JVI.77.12.6979-6987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Clarke P, Beer T, Cohrs R, et al. Configuration of latent varicella-zoster virus DNA. J Virol. 1995;69:8151–4. doi: 10.1128/jvi.69.12.8151-8154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kennedy PG, Grinfeld E, Gow JW. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Nati Acad Sci USA. 1998;95:4658–62. doi: 10.1073/pnas.95.8.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Pevenstein SR, Williams RK, McChesney D, et al. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73:10514–8. doi: 10.1128/jvi.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Cohrs RJ, Randall J, Smith J, et al. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74:11464–71. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Cohrs RJ, LaGuardia JJ, Gilden D. Distribution of latent herpes simplex virus type-1 and varicella zoster virus DNA in human trigeminal ganglia. Virus Genes. 2005;31:223–7. doi: 10.1007/s11262-005-1799-5. [DOI] [PubMed] [Google Scholar]
- [144].Wang K, Lau TY, Morales M, et al. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J Virol. 2005;79:14079–87. doi: 10.1128/JVI.79.22.14079-14087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].De Jong MD, Weel JF, Schuurman T, et al. Quantitation of varicella-zoster virus DNA in whole blood, plasma and serum by PCR and electrochemiluminescence. J Clin Microbiol. 2000;38:2568–73. doi: 10.1128/jcm.38.7.2568-2573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Mainka C, Fuss B, Geiger H, et al. Characterization of viremia at different stages of varicella-zoster virus infection. J Med Virol. 1998;56:91–8. doi: 10.1002/(sici)1096-9071(199809)56:1<91::aid-jmv15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [147].Ljungman P, Lonnqvist B, Gahrton G, et al. Clinical and subclinical reactivations of varicella-zoster virus in immunocompromised patients. J Infect Dis. 1986;153:840–7. doi: 10.1093/infdis/153.5.840. [DOI] [PubMed] [Google Scholar]
- [148].Schunemann S, Mainka C, Wolff MH. Subclinical reactivation of varicella-zoster virus in immunocompromised and immunocompetent individuals. Intervirology. 1998;41:98–102. doi: 10.1159/000024920. [DOI] [PubMed] [Google Scholar]
- [149].Mehta SK, Cohrs RJ, Forghani B, et al. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–9. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- [150].Cohrs RJ, Srock K, Barbour MB, et al. Varicella-zoster virus (VZV) transcription during latency in human ganglia: construction of a cDNA library from latently infected human trigeminal ganglia and detection of a VZV transcript. J Virol. 1994;68:7900–8. doi: 10.1128/jvi.68.12.7900-7908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Cohrs RJ, Barbour MB, Mahalingam R, et al. Varicella-zoster virus (VZV) transcription during latency in human ganglia: prevalence of VZV gene 21 transcripts in latently infected human ganglia. J Virol. 1995;69:2674–8. doi: 10.1128/jvi.69.4.2674-2678.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Cohrs RJ, Gilden DH. Varicella zoster virus transcription in latently infected ganglia. Anticancer Res. 2003;23:2063–70. [PubMed] [Google Scholar]
- [153].Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J Virol. 2007;81:2950–6. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Mahalingam R, Wellish M, Cohrs R, et al. Expression of protein encoded by varicellazoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci USA. 1996;93:2122–4. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Theil D, Derfuss T, Paripovic I, et al. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol. 2003;163:2179–84. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Lungu O, Panagiotidis CA, Annunziato PW, et al. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA. 1998;95:7080–5. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Grinfeld E, Kennedy PG. Translation of varicella-zoster virus genes during human ganglionic latency. Virus Genes. 2004;29:317–9. doi: 10.1007/s11262-004-7434-z. [DOI] [PubMed] [Google Scholar]
- [158].Takahashi M, Okuno Y, Otsuka T, et al. Development of a live attenuated varicella vaccine. Biken J. 1975;18:25–33. [PubMed] [Google Scholar]
- [159].Myers MG, Duer HL, Hausler CK. Experimental infection of guinea pigs with varicellazoster virus. J Infect Dis. 1980;142:414–20. doi: 10.1093/infdis/142.3.414. [DOI] [PubMed] [Google Scholar]
- [160].Myers MG, Stanberry LR, Edmond BJ. Varicella-zoster virus infection of strain 2 guinea pigs. J Infect Dis. 1985;151:106–13. doi: 10.1093/infdis/151.1.106. [DOI] [PubMed] [Google Scholar]
- [161].Matsunaga Y, Yamanishi K, Takahashi M. Experimental infection and immune response of guinea pigs with varicella-zoster virus. Infect Immun. 1982;37:407–12. doi: 10.1128/iai.37.2.407-412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Wroblewska Z, Devlin M, Reilly K, et al. The production ofvaricella zoster virus antiserum in laboratory animals. Arch Virol. 1982;74:233–8. doi: 10.1007/BF01314717. [DOI] [PubMed] [Google Scholar]
- [163].Walz-Cicconi MA, Rose RM, Dammin GJ, et al. Inoculation of guinea pigs with varicellazoster virus via the respiratory route. Arch Virol. 1986;88:265–77. doi: 10.1007/BF01310880. [DOI] [PubMed] [Google Scholar]
- [164].Wroblewska Z, Valyi-Nagy T, Otte J, et al. A mouse model for varicella-zoster virus latency. Microb Pathog. 1993;15:141–51. doi: 10.1006/mpat.1993.1064. [DOI] [PubMed] [Google Scholar]
- [165].Myers MG, Connelly BL, Stanberry LR. Varicella in hairless guinea pigs. J Infect Dis. 1991;163:746–51. doi: 10.1093/infdis/163.4.746. [DOI] [PubMed] [Google Scholar]
- [166].Lowry PW, Sabella C, Koropchak CM, et al. Investigation of the pathogenesis ofvaricellazoster virus infection in guinea pigs by using polymerase chain reaction. J Infect Dis. 1993;167:78–83. doi: 10.1093/infdis/167.1.78. [DOI] [PubMed] [Google Scholar]
- [167].Tenser RB, Hyman RW. Latent herpesvirus infections of neurons in guinea pigs and humans. Yale J Biol Med. 1987;60:159–67. [PMC free article] [PubMed] [Google Scholar]
- [168].Sadzot-Delvaux C, Merville-Louis MP, Delree P, et al. An in vivo model of varicella-zoster virus latent infection of dorsal root ganglia. J Neurosci Res. 1990;26:83–9. doi: 10.1002/jnr.490260110. [DOI] [PubMed] [Google Scholar]
- [169].Debrus S, Sadzot-Delvaux C, Nikkels AF, et al. Varicella-zoster virus gene 63 encodes an immediate-early protein that is abundantly expressed during latency. J Virol. 1995;69:3240–5. doi: 10.1128/jvi.69.5.3240-3245.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Kennedy PG, Grinfeld E, Bontems S, et al. Varicella-zoster virus gene expression in latently infected rat dorsal root ganglia. Virology. 2001;289:218–23. doi: 10.1006/viro.2001.1173. [DOI] [PubMed] [Google Scholar]
- [171].Annunziato P, LaRussa P, Lee P, et al. Evidence of latent varicella-zoster virus in rat dorsal root ganglia. J Infect Dis. 1988;178(Suppl 1):S48–51. doi: 10.1086/514261. [DOI] [PubMed] [Google Scholar]
- [172].Moffat JF, Stein MD, Kaneshima H, et al. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–42. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Zerboni L, Hinchliffe S, Sommer MH, et al. Analysis of varicella zoster virus attenuation by evaluation of chimeric parent Oka/vaccine Oka recombinant viruses in skin xenografts in the SCIDhu mouse model. Virology. 2005;332:337–46. doi: 10.1016/j.virol.2004.10.047. [DOI] [PubMed] [Google Scholar]
- [174].Provost PJ, Keller PM, Banker FS, et al. Successful infection of the common marmoset (Callithrix jacchus) with human varicella-zoster virus. J Virol. 1987;61:2951–5. doi: 10.1128/jvi.61.10.2951-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Cohen JI, Moskal T, Shapiro M, et al. Varicella in chimpanzees. J Med Virol. 1996;50:289–92. doi: 10.1002/(SICI)1096-9071(199612)50:4<289::AID-JMV2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [176].Gray WL. Simian varicella: a model for human varicella-zoster virus infections. Rev Med Virol. 2004;14:363–81. doi: 10.1002/rmv.437. [DOI] [PubMed] [Google Scholar]
- [177].White TM, Mahalingam R, Traina-Dorge V, et al. Simian varicella virus DNA is present and transcribed months after experimental infection of adult African green monkeys. J Neurovirol. 2002;8:191–203. doi: 10.1080/13550280290049705. [DOI] [PubMed] [Google Scholar]
- [178].White TM, Mahalingam R, Traina-Dorge V, et al. Persistence of simian varicella virus DNA in CD4+ and CD8+ blood mononuclear cells for years after intratracheal inoculation of African green monkeys. Virology. 2002;303:192–8. doi: 10.1006/viro.2002.1664. [DOI] [PubMed] [Google Scholar]
- [179].Mahalingam R, Wellish M, Soike K, et al. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–42. doi: 10.1006/viro.2000.0700. [DOI] [PubMed] [Google Scholar]
- [180].Grinfeld E, Kennedy PG. The pattern of viral persistence in monkeys intra-tracheally infected with simian varicella virus. Virus Genes. 2007;35:289–92. doi: 10.1007/s11262-007-0077-0. [DOI] [PubMed] [Google Scholar]
- [181].Mahalingam R, Traina-Dorge V, Wellish M, et al. Naturally acquired simian varicella virus infection in African green monkeys. J Virol. 2002;76:8548–50. doi: 10.1128/JVI.76.17.8548-8550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Kennedy PG, Grinfeld E, Traina-Dorge V, et al. Neuronal localization of simian varicella virus DNA in ganglia of naturally infected African green monkeys. Virus Genes. 2004;28:273–6. doi: 10.1023/b:viru.0000025774.19557.39. [DOI] [PubMed] [Google Scholar]
- [183].Mahalingam R, Traina-Dorge V, Wellish M, et al. Simian varicella reactivation in cynomologous monkeys. Virology. 2007;368(1):50–9. doi: 10.1016/j.virol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- [184].Kolappaswamy K, Mahalingam R, Traina-Dorge V, et al. Disseminated simian varicella virus infection in an irradiated Rhesus macaque (Macaca mulatta) J Virol. 2007;81:411–5. doi: 10.1128/JVI.01825-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Mandal BK. Herpes zoster and the immunocompromised. J Infect. 1987;14:1–5. doi: 10.1016/s0163-4453(87)90652-9. [DOI] [PubMed] [Google Scholar]