Abstract
RNA interference (RNAi) is a powerful tool to induce loss-of-function phenotypes by inhibiting gene expression post-transcriptionally. Synthetic short interfering RNAs (siRNAs) as well as vector-based siRNA expression systems have been used successfully to silence gene expression in a variety of biological systems. We describe the development of an inducible siRNA expression system that is based on the tetracycline repressor and eukaryotic RNA polymerase III promoters (U6 and 7SK). For proof of concept we selectively inhibited expression of two catalytic subunits of the phosphatidylinositol 3-kinase (PI 3-kinase), p110α and p110β, by using vector-derived short hairpin RNAs (shRNAs). Stable pools of human prostate cancer cells (PC-3) exhibiting reduced levels of both PI 3-kinase catalytic subunits due to the expression of corresponding shRNAs in an inducible fashion were established and analyzed for their invasive potential in vitro as well as in an orthotopic metastatic mouse model. This inducible system for RNAi allows an unbiased and comparable analysis of loss-of-function phenotypes by comparing selected isogenic cell populations on the induced and non-induced level. In addition, conditional RNAi allows the study of essential and multifunctional genes involved in complex biological processes by preventing inhibitory and compensatory effects caused by constitutive knockdown.
INTRODUCTION
RNA interference (RNAi) was discovered in Caenorhabditis elegans as a gene silencing mechanism in response to injection of long double-stranded RNA (dsRNA) (1,2). In most mammalian cells the introduction of long dsRNA induces a cytotoxic response due to activation of the PKR system (3,4). This cellular defence response can be circumvented by use of synthetic short (21–22 nt) interfering RNAs (siRNAs) (5,6). Two different approaches have been described for expressing double-stranded siRNAs in mammalian cells. In the first case a single promoter directs the transcription of a stem–loop structure, whereas in the second case two promoters transcribe the sense and antisense strands separately. In most cases RNA polymerase III promoters such as H1 and U6 have been used to direct expression of functional short hairpin RNAs (shRNAs) from vectors (7–13). More recently, shRNA promoter expression cassettes have been incorporated into retroviral vectors, including lentivirus-based vectors, to allow for efficient, cell type-independent delivery (14–17). Nonetheless, the analysis of stable loss-of-function phenotypes requires selection for cells which continuously express shRNAs. This selection process may cause compensatory or even non-physiological responses masking the true biological consequence of a functional knockdown. Compensatory responses may be especially pronounced in experiments where loss-of-function interferes with proliferation or causes cell death. Therefore, the controlled expression of shRNA molecules in a temporal and spatial manner would be beneficial for studying loss-of-function phenotypes in the context of cellular development and differentiation.
Very recently, van de Wetering et al. reported a stable system for inducible expression of shRNAs. In their report, a Tet-regulated [Tet repressor (TetR)-responsive] variant of the RNA polymerase III-dependent H1 promoter was used for doxycycline (Dox)-induced shRNA expression and consequent knockdown of β-catenin in stably transfected colorectal cancer cell clones (18). In a similar approach, we aimed to develop our previously described shRNA expression system (13) into an inducible vector-based system to direct the conditional expression of shRNAs. We used the well-characterized phosphatidylinositol 3-kinase (PI 3-kinase) signaling pathway as a model system to measure biological effects of induced shRNA action. PI 3-kinase and its downstream effectors regulate a variety of cellular responses such as survival, proliferation and cell migration (19). In addition, chronic activation of PI 3-kinase signaling appears to contribute to invasive tumor cell growth (20). Due to this central role of PI 3-kinase signaling in regulating a variety of cellular functions, a more sophisticated gene inactivation approach such as the inducible expression of shRNAs is required. Here we have employed a new inducible shRNA expression system to describe conditional knockdown of two different catalytic subunits of PI 3-kinase, p110α and p110β. We analyzed the phenotypic consequences of the corresponding knockdown in a cell-based assay and, furthermore, we applied this system in a mouse model for metastatic human prostate cancer.
MATERIALS AND METHODS
Plasmid constructs
7SK and U6 RNA polymerase III promoter fragments were amplified from human genomic DNA [7SK primer derived from X05490, 5′-gaagagagaaagggcgaggaa-3′, 5′-ggtccttagatgggtaatgggtcaa-3′ (21); U6 primer derived from X07425, 5′-tcgggcaggaagagggcctatttc-3′, 5′-aaacagaaaaacaacctgatgtaa-3′] and subcloned into a promoter-less pcDNA3.1 (neomycin resistance)-derived vector (Invitrogen). One tetracycline operator (tetO) sequence was introduced between the TATA box and transcription start site by a PCR-based cloning approach leading to the core promoter sequences 7SKtetO (tataCTCCCTATCAGTGATAGAGATCgg) and U6tetO (tatatCTCCCTATCAGTGATAGAGATCgt) (tetO in capital letters).
Oligonucleotides coding for the p110α and p110β hairpin siRNAs were cloned into two non-palindromic BsmBI restriction sites and have been described previously (13). The TetR expression plasmid pcDNA™6/TR was obtained from Invitrogen.
Cell culture and generation of stable cell pools
Human prostate carcinoma PC-3 cells were grown in F-12K Kaighn’s modification medium (Gibco-BRL) containing 2 mM glutamine (Gibco-BRL), 20 mM HEPES (Biochrom) and 10% fetal bovine serum (Gibco-BRL). The shRNA expression plasmids and the TetR expression plasmid (pcDNA™6/TR) were transfected with Effectene transfection reagent (Qiagen). The cells were trypsinized 48 h after transfection and seeded into selective medium. PC-3 cells stably expressing siRNA were established by selection with medium containing geneticin (600 µg/ml; Invitrogen) alone or in the case of double transfection experiments with pcDNA™6/TR in combination with blasticidin (10 µg/ml). The medium was renewed every 3 days. After 2 weeks resistant colonies (a minimum of 200 individual clones) were trypsinized, combined in pools and cultured in selective medium. Transient transfection experiments with HeLa cells have been described in Czauderna et al. (13).
Northern blot analysis
Detection of the expressed shRNAs has been described previously (13). Total RNA was prepared from cells and tumor tissues using RNAzol (WAK Chemie, Germany). The RNA (20 µg) was denatured by DMSO/glyoxal treatment for 1 h at 50°C and subsequently separated in a sodium phosphate-buffered 3% Nusieve agarose (3:1) gel. During electrophoresis the buffer was recirculated. The RNA was transferred to nylon membranes (Nytran Supercharge; Schleicher & Schuell, Germany) and UV cross-linked. 5′-32P-end-labeled oligonucleotides (p110β, 5′-ttttttttttttaaattccagtggttcattcca-3′; p110α, 5′-ttttttttttttctccaaagcctcttgctcagt-3′; tRNAVal probe, 5′-gaacgtgataaccactacactacggaaac-3′) were used for hybridization. Blots were washed several times under stringent conditions and exposed to phosphorimager screens (Molecular Dynamics).
Immunoblot analysis
Cell lysates were prepared and aliquots of the cell extracts containing equal amounts of protein were analyzed by immunoblotting as described previously (13,22). The murine monoclonal anti-p110α antibody and monoclonal anti-p85 have been described (23). Rabbit polyclonal anti-phospho-Akt (S473) antibodies were obtained from Cell Signalling Technology and the polyclonal anti-p110β antibody was from Santa Cruz Biotechnology. The murine monoclonal anti-TetR was obtained from MoBiTec (Germany).
In vitro growth on matrigel matrix
Parental PC-3 cells or PC-3 pools stably transfected with shRNA expression plasmids were trypsinized, washed in medium and seeded into 24 wells (100 000 cells/well) pre-coated with 250 µl matrigel basement membrane matrix (Becton Dickinson). After incubation for 48–72 h photographs were taken at 5× magnification with an Axiocam camera attached to an Axiovert S100 microscope (Zeiss).
Orthotopic prostate tumor/metastasis model
Eight-week-old male Shoe:NMRI-nu/nu mice (DIMED, Germany) were inoculated with 2 × 106 stably transfected PC-3 cells resuspended in 30 µl PBS into the left dorsolateral lobe of the prostate gland under total body anesthesia (24). In the experiments with and without Dox treatment the animals were randomized into two groups (eight animals each) and cells were implanted alternately in both groups during surgery. Doxycycline hydrochloride (Sigma) was administered as a 0.1% solution via the drinking water with 3% sucrose dissolved in natural mineral water starting on the day of surgery. Animals were killed 56 days post-operation and tumors (prostate gland), regional metastases (caudal, lumbar and renal lymph node metastases) and distant metastases were measured in two dimensions by means of a pair of callipers and the volume was calculated according to V (mm3) = ab2/2 with b < a. All killed animals were completely dissected and photographically documented. The mouse experiments (mouse surgery, cell transplantation, measurement of tumor volume, etc.) were performed double blind and independently by two researchers (W.A. and F.L.).
RESULTS AND DISCUSSION
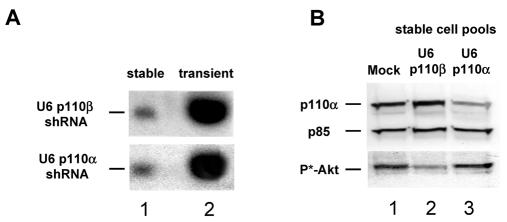
We have shown previously that transient expression of vector-derived shRNAs specific for the PI 3-kinase catalytic isoforms, p110α and p110β, mediate efficient gene silencing in HeLa cells (13). In a first step towards the development of a conditional RNAi system in mammalian cells we generated PC-3 cell pools stably expressing sufficiently high expression levels of shRNA molecules. For this purpose, we employed different RNA polymerase III promoter cassettes, namely the previously described H1 and U6 promoters, to drive vector-based shRNA expression (7–10,25). In addition, for further development of the conditional knockdown system we used the newly developed 7SK promoter cassette (21), which turned out to be suitable for generating high amounts of shRNA (Czauderna et al., unpublished). In selected PC-3 pools with stably integrated expression cassettes we observed a dramatic reduction in the amount of shRNA compared to transiently expressed shRNA (Fig. 1A). A very similar decrease in expression was observed for all three RNA polymerase III promoter (U6, H1 and 7SK) constructs after stable integration (data not shown). We and others have shown previously that the reduction in protein expression by RNAi is dependent on the intracellular amount of cytoplasmically localized or delivered siRNA effector molecules (13). To demonstrate that the lower level of shRNA expression in stable cell pools is still sufficient for gene silencing of the targets p110α and p110β, we performed immunoblot analyses. Figure 1B shows a reduction in p110α protein in PC-3 pools stably expressing shRNA generated with a U6 promoter-based p110α-shRNA expression construct. We and others have reported previously that knockdown of only one PI 3-kinase catalytic isoform, namely p110β, in PC-3 cells can be sufficient for a substantial reduction in phosphorylation of the PI 3-kinase downstream effector Akt, suggesting distinct signaling roles of the isoforms (13,22,26). Therefore, p110β knockdown was demonstrated indirectly by inhibition of Akt phosphorylation (Fig. 1B, lane 2).
Figure 1.
shRNA expression vectors specific for the two catalytic subunits of PI 3-kinase, p110α and p110β, are functional after stable integration in the genome of PC-3 cells. (A) Comparison of stable and transient U6 promoter-driven shRNA expression level by northern blot analysis. Aliquots of 20 µg of total RNA from pools of PC-3 cells stably expressing shRNA (lane 1) and 4 µg of total RNA (adjusted with 16 µg RNA from untreated cells; lane 2) from transiently transfected PC-3 cells (48 h post-transfection) were loaded and compared. (B) Stable expression of shRNA mediates inhibition of p110α and p110β protein expression in PC-3 cell pools. Cell lysates derived from PC-3 cells mock transfected (lane 1) or stably transfected with p110β-shRNA (lane 2) or p110α-shRNA (lane 3) were separated by SDS–PAGE and protein reduction was analyzed by immunoblotting using anti-p110α (upper row) and anti-phospho-Akt antibody (lower row) to monitor p110β inhibition indirectly through a reduction in phosphorylated Akt levels. The amount of p85, a regulatory subunit of PI 3-kinase, was used as a loading control (middle row).
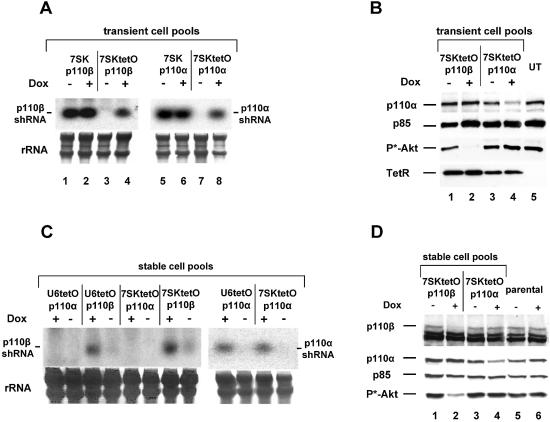
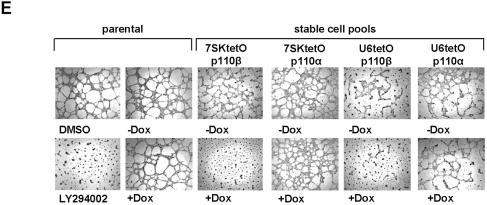
Having established that shRNA expression levels in stable PC-3 populations are sufficient to reduce protein expression, we set out to develop an inducible shRNA expression system for the generation of conditional loss-of-function phenotypes. A tetracycline-responsive derivative of the human U6 snRNA promoter has been described that allows inducible transcription of antisense RNA (27). Here we give an example of how TetR can be employed in combination with two different RNA polymerase III promoters, U6 and 7SK, for the inducible expression of shRNAs. We inserted into both promoters one tetO sequence immediately downstream of the TATA box (Fig. 2; see Materials and Methods). In the absence of its synthetic regulator, Dox, TetR binds to the operator and prevents expression of the shRNA transcripts by RNA polymerase III. In an initial step to assess inducible expression of p110α- and p110β-directed shRNAs, we performed transient co-transfection experiments with the TetR expression plasmid and the 7SK- or 7SKtetO-shRNA plasmids in HeLa cells. Northern blot analysis revealed Dox-dependent transcription of the p110α- and p110β-specific shRNA transcripts in a tetO-dependent manner (7SKtetO), since shRNA transcription was not affected by Dox in samples with control plasmids lacking the operator sequence (7SK) (Fig. 3A). Importantly, transient cell pools in the absence of Dox did not show any shRNA expression, demonstrating tight control of the system by TetR. With the U6-based constructs (U6 and U6tetO) a very similar regulation was observed (data not shown; see also Fig. 3C). Successful inhibition of p110α protein expression correlated with induced shRNA expression, as shown in Figure 3B (compare lanes 3 and 4). Dox-dependent p110β knockdown was again demonstrated indirectly by phospho-Akt inhibition (Fig. 3B, compare lanes 1 and 2), as shown in comparison to the non-induced control. Next, we addressed the question of whether the conditional shRNA expression system can achieve inducible suppression of gene expression after stable transfection. Pools of PC-3 prostate cancer cells co-transfected and selected for the TetR and shRNA expression plasmids were analyzed by northern blotting for Dox-dependent induction of shRNA transcription (Fig. 3C). Again, we observed a 50- to 100- fold lower level of stable expression when compared to transient expression (see also Fig. 1A; data not shown). However, Dox-dependent transcription could still be observed with both tetO-containing RNA polymerase III promoters (U6tetO and 7SKtetO) for each p110 isoform-specific shRNA. We did not observe significant differences in the expression level or induction of the two different RNA polymerase III promoters. Dox-induced shRNA transcription resulted in successful reductions in protein expression in different stable PC-3 pools analyzed by immunoblotting (Fig. 3D). In this experiment we also employed a p110β-specific antibody alongside the measurement of Akt phosphorylation to demonstrate functional knockdown of the target. Both p110β protein expression and phosphorylation of the downstream kinase Akt were reduced by Dox-mediated derepression of p110β shRNA expression (Fig. 3D). The reduction in p110α protein level was less prominent, but detectable, and had no functional consequences on downstream signaling, as shown previously (13,22). Having demonstrated inducible protein knockdown of the two PI 3-kinase catalytic subunits p110α and p110β in stable PC-3 cell pools we analyzed the phenotypic consequences in a cell-based assay. It has been shown by us and others that inhibition of PI 3-kinase activity leads to reduced growth of invasive cancer cells on basement membranes such as extracellular matrigel matrix (13,22,28). As a positive control for inhibition of cell growth we used the small molecule PI 3-kinase inhibitor LY294002 on parental PC-3 cells (Fig. 3E, left panel). The addition of Dox itself had no effect on PC-3 growth in this assay. However, Dox treatment of the PC-3 cell pools stably expressing 7SKtetOp110β and U6tetOp110β shRNA led to substantial inhibition of cell growth and a reduction in the formation of cellular network structures, similar to LY294002 treatment (Fig. 3E). Inducible expression of p110α-specific shRNAs had no inhibitory effect with either expression cassette. This result is consistent with the Dox-dependent inhibition of Akt phosphorylation and suggests that p110β is the more relevant catalytic isoform for growth on extracellular matrix in PC-3 cells.
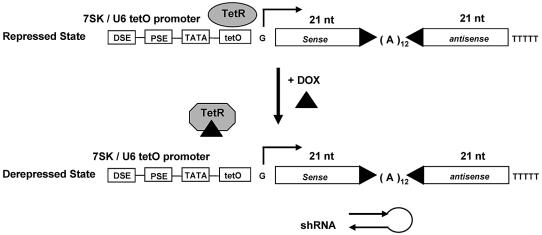
Figure 2.
Schematic illustration of the tetracycline (Dox)-regulated RNA polymerase III promoter (7SK, U6) shRNA expression system. Within the 7SK/U6-tetO-promoter, the tetO sequence is located immediately downstream of the TATA box. Transcription is repressed by binding of TetR in the absence of Dox. Addition of Dox results in dissociation of the repressor leading to derepression/activation of the transcriptional unit. The RNA polymerase III promoter elements are indicated: DSE, distal sequence element; PSE, proximal sequence element; TATA, TATA box. The G represents the transcription start site followed by the sequence encoding a 21 bp long shRNA molecule containing an (A)12 loop.
Figure 3.
Functional characterization of the Dox-inducible shRNA expression system. (A) Northern blot analysis to show inducible expression of p110β- (left panel, lanes 3 and 4) and p110α-specific (right panel, lanes 7 and 8) shRNA transcripts upon treatment with Dox (50 ng/ml, 72 h) after transient co-expression of TetR with corresponding 7SK-tetO-shRNA expression constructs (ratio 4:1) in HeLa cells. Non-inducible/constitutively active shRNA expression constructs 7SK-p110β (lanes 1 and 2) and 7SK-p110α (lanes 5 and 6) were used as controls. Equal loading of total RNA was assessed by detecting rRNA (lower panel). (B) Dox-induced inhibition of p110α and p110β protein expression in transiently transfected HeLa cells. Cell extracts from cell pools treated with (lanes 2 and 4) or without (lanes 1, 3 and 5) Dox (50 ng/ml, 72 h) were separated by SDS–PAGE and blotted and probed with anti-p110 (detecting p110α and p85), anti-phospho-Akt, anti-p85 and anti-TetR antibodies as indicated. TetR is only present in transfected cells. The signal for p85 was used as a loading control. (C) Dox-inducible expression of p110β-shRNA (left panel) and p110α-shRNA (right panel) in PC-3 cells stably co-transfected with TetR protein expressing plasmids and the indicated shRNA expression constructs (U6-shRNA and 7SK-shRNA). The rRNA served as a loading control. (D) Dox-induced reduction in p110β and p110α protein levels (lane 2 and 4) in doubly transfected stable PC-3 cell expressing TetR and the indicated shRNA expression constructs. Cell extracts from parental or stably transfected PC-3 cells treated with or without Dox (50 ng/ml, 48 h) were separated by SDS–PAGE and analyzed by western blotting using anti-p110 antibody (for p110α and p85), anti-phospho-Akt antibody, anti-p85 antibody and anti-p110β antibody. The p85 signal was used as a loading control. (E) Growth of corresponding PC-3 cell pools [taken from (D)] on extracellular matrix with or without Dox treatment. The different stable 7SktetO and U6tetO PC-3 cells were incubated for 72 h with or without 50 ng/ml Dox before seeding on matrigel. To rule out non-specific Dox effects untransfected PC-3 cells (parental) with or without Dox treatment were prepared in parallel. Parental cells were seeded on matrigel in the presence of 10 µM PI 3-kinase inhibitior (LY294002) or with the vehicle DMSO (left panels). After incubation for 24 h on matrigel, cells were photographed at 5× magnification.
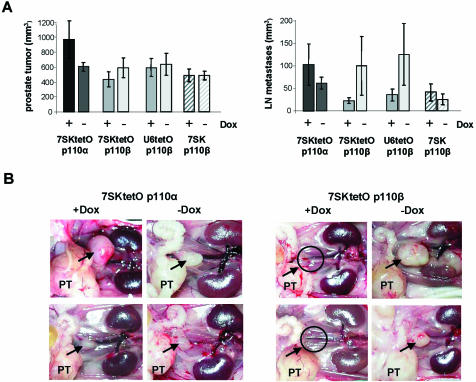
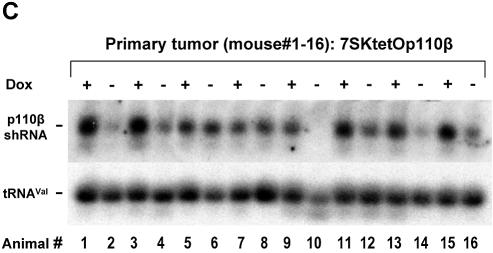
Tetracycline-dependent expression systems have been widely used in animal models for studying gene function (29). To assess whether inducible RNAi-mediated knockdown of the two PI 3-kinase isoforms shows distinct phenotypic consequences on tumorigenicity and/or the incidence of metastasis we implanted the above characterized stable PC-3 pools intraprostaticly into nude mice. In contrast to ectopic application, direct implantation of human PC-3 prostate cancer cells into the mouse prostate does permit expression of the metastatic potential of these cells and therefore represents an orthotopic metastatic model for human prostate cancer (24). Figure 4A summarizes the tumor volume in the prostate and the volume of lymph node metastases 56 days after implantation of the different inducible shRNA-expressing cell pools (for details see Materials and Methods). Stable PC-3 pools with inducible 7SKtetOp110β or U6tetOp110β expression cassettes showed a significant reduction in the formation of metastases but not in tumor size in mice treated with Dox. This inhibitory effect was not observed with cells stably transfected with the 7SKtetOp110α constructs. Cell pools constitutively expressing p110β shRNA (7SKp110β without tetO but co-selected with the TetR plasmid) did not show a difference in either tumor growth or metastases formation after Dox treatment. In Figure 4B representative in situ pictures of the abdominal cavity are shown visualizing the prostate tumor and the lumbar lymph node metastases (two mice per group are shown). The absence of lumbar lymph node metastases in Dox-treated mice implanted with 7SKtetOp110β transfectants, in particular, is striking, since the prostate tumor size itself is not significantly reduced. It can be speculated that p110β may mainly regulate more specific responses such as migration of prostate cancer cells from the primary tumor to other sites in the body. In order to demonstrate that Dox-dependent transcription is maintained during the 8 week time period of the in vivo experiment, we performed northern blots with total RNA derived from individual prostate tumors. Expression of p110β-specific shRNA in tumors of eight individual animals from the Dox-treated as well as untreated groups were analyzed and the existence of induced shRNA expression in mice treated with Dox was observed. Figure 4C clearly shows that p110β-shRNA expression is maintained and even increased in primary tumors derived from mice treated with Dox, in contrast to animals without Dox treatment. This result demonstrates that Dox treatment of the mice was effective and indicates that the conditional shRNA expression is stable and maintained for an extended time, here 56 days, in vivo. However, Dox-controlled shRNA expression in vivo did not appear to be as tightly regulated as observed for stable cell pools maintained in culture under selection pressure (see Fig. 3C). It is likely that during the tumor development period of several weeks in the absence of selection pressure, the applied cell pools partly lost TetR expression and consequently regulated shRNA expression. In addition, it is conceivable that the two promoter systems (polymerase III for shRNA expression and polymerase II for TetR expression) are affected differently by silencing mechanisms. To overcome the observed leakiness of the system in our mouse model, we are currently developing retrovirus-based systems in which TetR and shRNA expression are more tightly linked to ensure a more rigid control.
Figure 4.
Application of the inducible shRNA expression system in an orthotopic prostate cancer mouse model. (A) Prostate tumor volume and lymph node metastasis volume of orthotopically implanted PC-3 cells expressing inducible shRNAs specific for the two catalytic subunits of PI 3-kinase, p110α and p110β. The different shRNA expression constructs and the Dox treatment are indicated. Data represent mean values obtained from eight mice per group. (B) In situ pictures of male pelvic anatomy 56 days after implantation of PC-3 cells expressing stable and inducible shRNA into the prostate. Two representative mice treated with or without Dox are shown for the 7SKtetOp110α and 7SKtetOp110β groups. The prostate tumor is marked PT and the position of the caudal lymph node metastases are indicated by an arrow. (C) p110β-shRNA expression in tumors revealed by northern blot analysis with total RNA isolated from prostate tumors from 16 individual mice treated either with or without Dox for 56 days. Overall the p110β-shRNA transcript levels are increased in mice treated with Dox (for example for lanes 1, 3, 11, 13 and 15) compared to untreated control mice (lanes 2, 4, 14 and 16). tRNAVal was used to ensure equal loading.
Taken together, our data demonstrate that an inducible siRNA expression system can be applied to generate conditional loss-of-function phenotypes in vitro and in vivo allowing a more comprehensive functional analysis of genes. Surprisingly, the TetR-based system resulted in an extremely tight regulation of shRNA expression. In addition, regulated expression of shRNA allows direct analysis of loss-of-function phenotypes in a more controlled manner. For example, phenotypic consequences can be studied in single cell pools expressing shRNAs in direct comparison with the same pool but where shRNA expression is repressed. In future a combination of inducible shRNA expression systems with viral vector systems for effective delivery will enhance application of recombinant RNAi technology in functional gene analysis and for use as a potential therapeutic.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Manfred Gossen and Katharina Ahrens for critical reading of the manuscript. This study was supported by a grant from the Bundesministerium für Wirtschaft und Technologie (no. 1078/02).
REFERENCES
- 1.Fire A., Xu,S., Montgomery,M.K., Kostas,S.A., Driver,S.E. and Mello,C.C. (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature, 391, 806–811. [DOI] [PubMed] [Google Scholar]
- 2.Hannon G.J. (2002) RNA interference. Nature, 418, 244–251. [DOI] [PubMed] [Google Scholar]
- 3.Stark G.R., Kerr,I.M., Williams,B.R., Silverman,R.H. and Schreiber,R.D. (1998) How cells respond to interferons. Annu. Rev. Biochem., 67, 227–264. [DOI] [PubMed] [Google Scholar]
- 4.Gil J. and Esteban,M. (2000) Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis, 5, 107–114. [DOI] [PubMed] [Google Scholar]
- 5.Elbashir S.M., Harborth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498. [DOI] [PubMed] [Google Scholar]
- 6.Tuschl T. (2002) Expanding small RNA interference. Nat. Biotechnol., 20, 446–448. [DOI] [PubMed] [Google Scholar]
- 7.Brummelkamp T.R., Bernards,R. and Agami,R. (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science, 296, 550–553. [DOI] [PubMed] [Google Scholar]
- 8.Lee N.S., Dohjima,T., Bauer,G., Li,H., Li,M.J., Ehsani,A., Salvaterra,P. and Rossi,J. (2002) Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat. Biotechnol., 20, 500–505. [DOI] [PubMed] [Google Scholar]
- 9.Miyagishi M. and Taira,K. (2002) U6 promoter-driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat. Biotechnol., 20, 497–500. [DOI] [PubMed] [Google Scholar]
- 10.Paul C.P., Good,P.D., Winer,I. and Engelke,D.R. (2002) Effective expression of small interfering RNA in human cells. Nat. Biotechnol., 20, 505–508. [DOI] [PubMed] [Google Scholar]
- 11.Xia H., Mao,Q., Paulson,H.L. and Davidson,B.L. (2002) siRNA-mediated gene silencing in vitro and in vivo. Nat. Biotechnol., 20, 1006–1010. [DOI] [PubMed] [Google Scholar]
- 12.Yu J.Y., DeRuiter,S.L. and Turner,D.L. (2002) RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc. Natl Acad. Sci. USA, 99, 6047–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czauderna F., Fechtner,M., Aygun,H., Arnold,W., Klippel,A., Giese,K. and Kaufmann,J. (2003) Functional studies of the PI(3)-kinase signalling pathway employing synthetic and expressed siRNA. Nucleic Acids Res., 31, 670–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brummelkamp T.R., Bernards,R. and Agami,R. (2002) Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell, 2, 243–247. [DOI] [PubMed] [Google Scholar]
- 15.Barton G.M. and Medzhitov,R. (2002) Retroviral delivery of small interfering RNA into primary cells. Proc. Natl Acad. Sci. USA, 99, 14943–14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin X.F., An,D.S., Chen,I.S. and Baltimore,D. (2003) Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl Acad. Sci. USA, 100, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubinson D.A., Dillon,C.P., Kwiatkowski,A.V., Sievers,C., Yang,L., Kopinja,J., Zhang,M., McManus,M.T., Gertler,F.B., Scott,M.L. et al. (2003) A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nature Genet., 33, 401–406. [DOI] [PubMed] [Google Scholar]
- 18.Van De Wetering M., Oving,I., Muncan,V., Pon Fong,M.T., Brantjes,H., Van Leenen,D., Holstege,F.C., Brummelkamp,T.R., Agami,R. and Clevers,H. (2003) Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep., 4, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katso R., Okkenhaug,K., Ahmadi,K., White,S., Timms,J. and Waterfield,M.D. (2001) Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis and cancer. Annu. Rev. Cell. Dev. Biol., 17, 615–675. [DOI] [PubMed] [Google Scholar]
- 20.Roymans D. and Slegers,H. (2001) Phosphatidylinositol 3-kinases in tumor progression. Eur. J. Biochem., 268, 487–498. [DOI] [PubMed] [Google Scholar]
- 21.Boyd D.C., Greger,I.H. and Murphy,S. (2000) In vivo footprinting studies suggest a role for chromatin in transcription of the human 7SK gene. Gene, 247, 33–44. [DOI] [PubMed] [Google Scholar]
- 22.Sternberger M., Schmiedeknecht,A., Kretschmer,A., Gebhardt,F., Leenders,F., Czauderna,F., Von Carlowitz,I., Engle,M., Giese,K., Beigelman,L. et al. (2002) GeneBlocs are powerful tools to study and delineate signal transduction processes that regulate cell growth and transformation. Antisense Nucleic Acid Drug Dev., 12, 131–143. [DOI] [PubMed] [Google Scholar]
- 23.Klippel A., Escobedo,J.A., Hirano,M. and Williams,L.T. (1994) The interaction of small domains between the subunits of phosphatidylinositol 3-kinase determines enzyme activity. Mol. Cell. Biol., 14, 2675–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephenson R.A., Dinney,C.P., Gohji,K., Ordonez,N.G., Killion,J.J. and Fidler,I.J. (1992) Metastatic model for human prostate cancer using orthotopic implantation in nude mice. J. Natl Cancer Inst., 84, 951–957. [DOI] [PubMed] [Google Scholar]
- 25.Paddison P.J., Caudy,A.A. and Hannon,G.J. (2002) Stable suppression of gene expression by RNAi in mammalian cells. Proc. Natl Acad. Sci. USA, 99, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhaesebroeck B., Jones,G.E., Allen,W.E., Zicha,D., Hooshmand-Rad,R., Sawyer,C., Wells,C., Waterfield,M.D. and Ridley,A.J. (1999) Distinct PI(3)Ks mediate mitogenic signalling and cell migration in macrophages. Nature Cell Biol., 1, 69–71. [DOI] [PubMed] [Google Scholar]
- 27.Ohkawa J. and Taira,K. (2000) Control of the functional activity of an antisense RNA by a tetracycline-responsive derivative of the human U6 snRNA promoter. Hum. Gene Ther., 11, 577–585. [DOI] [PubMed] [Google Scholar]
- 28.Petersen O.W., Ronnov-Jessen,L., Howlett,A.R. and Bissell,M.J. (1992) Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl Acad. Sci. USA, 89, 9064–9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gossen M. and Bujard,H. (2002) Studying gene function in eukaryotes by conditional gene inactivation. Annu. Rev. Genet., 36, 153–173. [DOI] [PubMed] [Google Scholar]