Abstract
Magnetoencephalography (MEG) is a novel functional brain mapping technique capable of non-invasively measuring neurophysiological activity based on direct measures of the magnetic flux at the head surface associated with the synchronized electrical activity of neuronal populations. Among the most actively sought applications of MEG has been localization of language-specific cortex. This is in part due to its practical application for pre-surgical evaluation of patients with epilepsy or brain tumors. Until recently, comprehensive language mapping during surgical planning has relied on the application of invasive diagnostic methods, namely the Wada procedure and direct electrocortical stimulation mapping, often considered as the “gold standard” techniques for identifying language-specific cortex. In this review, we evaluate the utility of MEG as a tool for functional mapping of language in both clinical and normal populations. In particular, we provide a general description of MEG, with emphasis on facets of the technique related to language mapping. Additionally, we discuss the application of appropriate MEG language-mapping protocols developed to reliably generate spatiotemporal profiles of language activity, and address the validity of the technique against the “gold standards” of the Wada and electrocortical mapping procedures.
Keywords: Functional neuroimaging, Magnetoencephalography, Neurophysiology, Language, Lateralization
1 Introduction
Functional neuroimaging techniques have significantly advanced our understanding of the neurological basis of language. Among the most commonly applied of these methods for non-invasively mapping language on a whole-brain level are positron emission tomography (PET), functional magnetic resonance imaging (fMRI) and more recently, magnetoencephalography (MEG) [1]. Each of these techniques exploits different facets of the brain’s physiological response and varies in the degree to which they capture the spatial and temporal dynamics of neurophysiological activity. In the case of PET and fMRI, oxygen consumption associated with neuronal activity can be indexed by an increase in regional glucose metabolism or blood flow to the local vasculature (hemodynamic response), respectively. While these methods, in particular fMRI, can localize changes in brain activity with high spatial resolution, the metabolic responses they measure occur several seconds after the actual neurophysiological event has transpired, thus limiting the extent to which the temporal dynamics of the response can be captured. In contrast to hemodynamic imaging methods, MEG is a relatively novel technique which combines excellent temporal resolution with reasonable spatial accuracy, to provide information regarding the relative timing of activity in distinct anatomical structures.
MEG is sensitive to the change in magnetic flux (amount of magnetism) associated with intracellular electrical currents that arise from the rapid, increased activity in neuronal aggregates in response to external stimuli. Systematic variations in the strength of this magnetic flux, recorded at the scalp surface, are observed when regional neuronal activity exceeds baseline levels. In the context of linguistic processing, changes in magnetic flux can be consistently elicited using the appropriate stimuli, and their underlying sources estimated to obtain a spatiotemporal map of task-specific brain activity. Mapping of brain regions supporting linguistic functions using MEG has been particularly sought after due to the possibility that advanced knowledge of language-specific zones can facilitate surgical planning and reduce morbidity associated with resection of eloquent cortex, especially in cases of epilepsy surgery. Indeed, evidence supporting the validity of MEG as a tool for studying the neurophysiology of language has been obtained through direct comparisons with routine clinical invasive procedures, including the Wada procedure (intracarotid sodium amobarbital perfusion) [2] and direct electrocortical stimulation [3], recognized as the “gold standard” techniques for lateralizing and localizing brain activity associated with language function, respectively.
In the current article, we review the utility of MEG as a functional neuroimaging technique for mapping language-specific cortex. Specifically, we provide an overview of conventional invasive methods used to study the cortical representation of language, followed by a general description of MEG with emphasis on the facets of the technique related to functional mapping of language. In addition, we address the adequacy of MEG as an alternative to invasive language mapping procedures, in the context of validation studies undertaken against the Wada test and direct electrocortical stimulation mapping in clinical populations. Furthermore, we discuss the extended application of MEG language mapping protocols for the purposes of identifying mechanisms underlying more dynamic linguistic processes such as reading, and comment on their manifestation in individuals with reading disabilities.
2 Methods
2.1 Invasive Methods for Assessing Language Laterality
The Wada procedure [2] is the most commonly used method for assessing cerebral dominance for language during pre-surgical evaluation of patients with epilepsy [4, 5]. This technique in particular entails short-term unilateral hemispheric anesthetization by successively injecting the right and left internal carotid arteries with a barbiturate, usually sodium amobarbital, to temporarily suppress functioning in one hemisphere. Subsequently, this allows for the functional assessment of the un-anesthetized hemisphere, while the patient is engaged in performing a language task, eliciting either speech production or comprehension. It is worth noting that although the Wada procedure was originally developed for the purposes of determining hemispheric dominance for language, modifications to the technique have also made it possible to evaluate the relative contribution of the non-anesthetized hemisphere to memory[6]. The efficacy of the Wada procedure is widely acknowledged as a pre-operative tool for determining language lateralization in surgical candidates, though the technique has met with some concerns. For example, there is the risk of morbidity given the invasiveness of the procedure [7, 8], and variability in responses to sodium amobarbital, as well as arterial anatomy, exclude some individuals from the procedure [9]. In addition, the technique lacks the regional specificity necessary to determine the precise location and extent of language-specific cortex [10]. Moreover, the validity of the procedure has also been questioned due to potential inter-hemispheric cross-flow of arterial blood, and lack of verification by means of test-retest reliability studies [11].
2.2 Invasive Methods for Language Localization
Electrocortical stimulation is an invasive procedure which has been used to directly localize, with much success, cortical regions underlying both receptive and expressive language processing [3]. Using this technique, language localization is determined intra- or extra- operatively by having the patient perform a task relating to either speech comprehension or production, during which time transient interference with neurophysiological activity in language-specific cortical sites is induced via small electrical currents. To a large extent, this procedure has focused on the stimulation of inferior frontal and temporopatietal regions long-thought to distinguish the productive and receptive language centers of the brain, respectively [3]. Importantly, in a review of their pioneering work using electrocortical stimulation, Ojemann and colleagues [12] noted a high degree of inter-individual variability in the localization of language-specific cortex among neurosurgical cases, including a suggested functional overlap between inferior frontal and temporal perisylvian regions during speech production and comprehension, contrary to the traditional views maintaining distinct roles of these two regions in language processing. Clearly, the strength of electorcortical stimulation lay in its invasiveness and ability to address language organization on an individual basis, though the procedure is not without some limitations. In particular, it has been noted [13, 14] that the scope of electrocortical stimulation is determined by the area of the craniotomy, often confined to a small region of the cortical surface in one hemisphere, thus limiting the spatial extent to which language can be localized and hemispheric dominance is assessed. Furthermore, the fact that this procedure is only practiced in patients who are candidates for surgery excludes the possibility of it being applied to otherwise healthy individuals for large-scale normative studies of language mapping.
2.3 MEG: A Non-Invasive Alternative to Language Mapping
MEG represents the most novel of functional neuroimaging modalities capable of generating cortical activation maps in real-time using recordings of magnetic flux generated by intracellular electric currents in neuronal aggregates [15]. The rationale behind imaging cortical activity with MEG is based on fundamental electromagnetic properties of neural transmission. Brain activation in response to an external stimulus (either cognitive or sensory) results in regional increases in neuronal signaling, characterized by an elevated flow of intracellular ions (electrical currents) and their associated magnetic fields, with the latter being perpendicular to the direction of the current according to the right-hand rule [16]. Repetitive application of a stimulus continuously evokes these currents and fields which can be measured at the surface of the scalp in the form of evoked (electrical) potentials and their magnetic counterparts, the event-related magnetic fields (ERFs). Unlike evoked potentials however, which are significantly distorted by differences in conductivity between various layers of tissue (i.e., brain, skull, and scalp), magnetic signals penetrate the skull without impedance. Therefore, the distribution of ERFs over the scalp surface allows for fairly accurate estimates of the spatial extent (0.1–1 cm) and temporal dynamics (1 ms) of neuronal assemblies underlying cortical activity [17–20].
A salient property of neuromagnetic signals is their extremely weak nature, being several orders of magnitude smaller than the earth’s steady magnetic field, the associated surrounding environment (e.g. power lines and traffic) and myogenic activity (e.g. cardiac response). Therefore, measurement of minute changes in magnetic flux relies on sophisticated instrumentation capable of discriminating signals of interest from extraneous magnetic fields. Recording of the magnetic flux is performed using special sensors known as magnetometers, which consist of superconducting loops of wire positioned normal to the surface of the scalp, and immersed in cryogenic refrigerant, typically liquid helium. As variations in magnetic flux thread through the loop, they induce an electrical current, the strength of which is proportional to the density of the flux. Each magnetometer is coupled to an additional superconducting device known as a SQUID (Superconducting Quantum Interference Device), which acts as a high-gain amplifier that yields a voltage proportional to the current induced within the superconducting coil by the magnetic flux. In contrast to early single-channel recording units [17], modern day MEG systems are equipped with 150 to 300 magnetometers capable of providing whole-head coverage necessary for optimal spatial resolution, and housed inside shielded rooms constructed of materials with high magnetic permeability, capable of attenuating noise from the external environment.
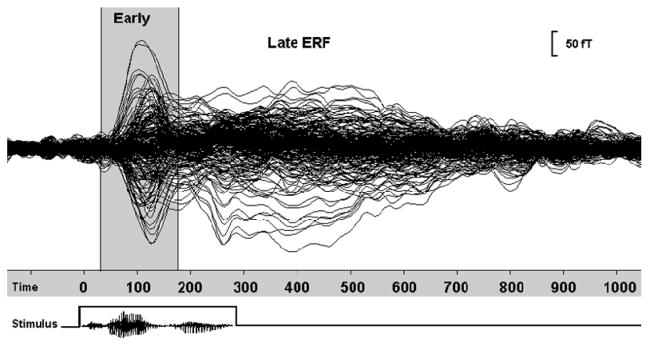
Similar to evoked potentials, ERFs are waveforms that represent systematic temporal variations in the strength of magnetic flux time-locked to the presentation of an external stimulus. In a typical MEG recording, a stimulus is presented repeatedly during which time the magnetic flux penetrating the surface of the head is sampled at regular intervals (typically every 4 ms for cognitive studies) at each magnetometer. For every repetition, an ERF segment in the form of a time series of magnetic flux measurements is recorded from each magnetometer sensor, beginning a few milliseconds before and continuing several hundred milliseconds after the onset of the stimulus. To enhance the profile of the time-varying magnetic activity, the ERF recordings made of each stimulus repetition are filtered to remove low and high frequency contaminants introduced by extraneous sources, and subsequently averaged together. A typical averaged ERF can be characterized by two segments (see Figure 1), namely an “early” component (50–200 ms pre-stimulus onset) reflecting activity in the sensory cortices specific to the modality of the stimulus (auditory or visual), and a “late” component (200–800 ms post-stimulus onset) corresponding to activity in association regions arising from neurophysiological processes underlying the execution of higher cognitive functions such as linguistic processing. At each time point the distribution of magnetic flux over the scalp surface can be displayed as an isofield contour map (Figure 2) and used to mathematically estimate the intracranial origin of the ERFs. While several algorithms exist for reconstructing intracranial activity sources [for detailed reviews see 21, 22], the most commonly applied model, and one subjected to external validation against direct electrocortical stimulation [10, 23], is the equivalent current dipole (ECD) [20, 24, 25]. Using this method, the most probable location and strength of intracranial activity sources are considered as equivalent to electric current dipoles, embedded in a spherical conductor approximating the skull curvature [24]. The location of each estimated dipolar source is determined with reference to a Cartesian coordinate system based on three fiducial points on the head (the nasion and external meatus of each ear), and subsequently approximated by co-registering these points to a high-resolution anatomical MRI. The procedure of establishing the spatiotemporal profile of evoked brain activity by combining the complementary information obtained from MEG and MRI is known as Magnetic Source Imaging (MSI).
Figure 1.
Averaged event-related field (ERF) consisting of early components (~ 50–200 ms post-stimulus onset) and late components (~200 – 800 ms post-stimulus onset), related to the time and pattern of the stimulus onset.
Figure 2.
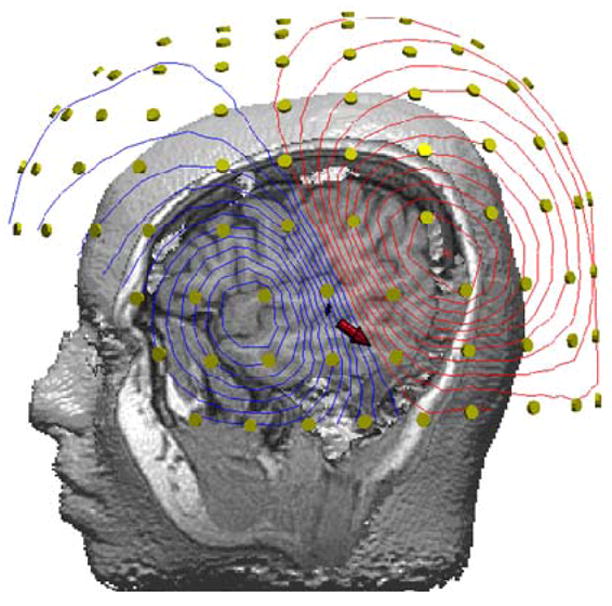
Contour map of the surface distribution of magnetic flux by an auditory evoked stimulus. The red arrow indicates the location and direction of the equivalent current dipole (ECD), in the half-space between the inward (blue lines) and outward (red) flowing magnetic fields relative to the multiple MEG sensors that encircle the whole head (yellow circles).
2.4 The Continuous Recognition Memory Paradigm: A Standardized Language Mapping Protocol
Over the past decade, a considerable amount of research has been undertaken in our lab to develop a reliable MEG language-mapping protocol, deemed valid against the “gold standard” invasive clinical procedures. Based on this body of work, mapping of language-specific cortex has most readily been achieved using a variant of the Sternberg task for short-term memory [26] known as the continuous recognition memory (CRM) paradigm [11, 27–32]. In effect, this paradigm is a test of verbal memory used to elicit activity in receptive language regions based on recognition judgments made about the occurrence of words, presented in either the auditory or visual modalities. The stimuli used in this paradigm are drawn from a set of 90 abstract English nouns, with scores of 3.0 or lower on the Paivio Concreteness scale [33] and word frequency ranging from “very frequent” (AA) to 9 occurrences per million for some, and used to create two subsets of words designated as targets and distractors. During both the auditory and visual versions of this task, participants are presented with a set of target words which they are instructed to study and accurately repeat immediately prior to the MEG recording session. Subsequently, these target words are mixed with the distractors and presented randomly, during which time participants are asked to make a response upon detection of a target stimulus, and ERFs time-locked to each word are recorded for a latency of about 150 ms pre- and 1000 ms post- stimulus onset.
Auditory language comprehension and reading are subserved by mechanisms that share many components [29]. Identifying both the similarities and the subtle differences between these mechanisms is possible because the same linguistic stimuli can be presented within identical time frames either acoustically or visually to participants, who are instructed to process the stimuli in an identical manner in both cases. Accordingly, the visual CRM task has also been applied to identify mechanisms involved in more dynamic linguistic processes such as single-word reading [13, 27, 34]. As a complement to this task, MEG studies in our lab have also addressed more elementary features of reading, such as phonological aspects of letter-to-sound decoding [11, 35, 36]. The protocol used to identify the neural correlates of phonological decoding has been an adaptation of the pseudoword rhyme-matching task [37]. During this task, participants are asked to make rhyming judgments about two orthographically dissimilar but pronounceable letter strings (e.g., gnume-noom), and ERFs are time-locked to the first stimulus of each pair to ensure that recorded brain activity only reflects phonological decoding operations as opposed to competing cognitive processes involved in matching of stimuli.
3 Mapping Language with MEG
3.1 Assessment of Language Laterality
A stable feature of MEG-derived cortical activation maps, in the context of the CRM paradigm, is a greater degree of activity in the left perisylvian region (number of ECDs computed during the late portion of the ERF), confirming the well-documented left hemisphere dominance for receptive language in the majority of neurologically intact individuals [38]. The efficacy, as well as the reliability, with which this protocol can be used to derive measures of hemispheric dominance for receptive language has been demonstrated in a series of studies of randomly selected normal adults and children [11, 27, 28, 30, 32]. Furthermore, the adequacy of MEG as a non-invasive alternative to the Wada procedure in assessing hemispheric dominance for language has also been addressed in several validation studies in clinical cohorts. Initial comparisons of the two modalities in epilepsy patients reported excellent concordance between hemispheric asymmetries in the degree of regional activity determined by MEG against laterality indices obtained from the Wada procedure in adults [11, 39, 40] and children [41]. In the largest systematic study to date, Papanicolaou et al. [42] reported a high degree of concordance (87%) between language laterality judgments obtained using MEG and the Wada procedure, independently, in 100 consecutive epilepsy cases ranging from 8 to 56 years in age. Additionally, this study also found that in 4% of the cases where findings between the two methodologies were discordant, MEG tended to indicate the possibility of bilateral representation (more activity in the non-dominant hemisphere) where the Wada procedure suggested unilateral representation, of receptive language. From a practical point of view, these divergent findings may have warranted the use of invasive mapping in only a small number of cases, indicating that MEG may provide a slightly more conservative assessment of hemispheric dominance for language, relative to the Wada procedure.
Clearly, non-invasive lateralization of receptive language using MEG has a number of advantages, including elimination of health risk, potential for test–retest reliability studies, and ability to use a number of different tasks of extended duration. Furthermore, the problems inherent in the Wada procedure, including potential over- or under-anesthetization and anomalous distribution of anesthetic due to cross-flow or atypical vascularization, are also eliminated. However, although independent reports of a high degree of concordance between MEG and the Wada procedure hold promise, several caveats must be addressed when considering substitution of the latter technique with the former. For example, language testing during the Wada procedure usually entails both expressive and receptive language tasks, where as the MEG protocol discussed here relies solely on the latter (reading and listening to single words). As pointed out by some [11, 43], this can have implications in cases where receptive and expressive language may dissociate during inter-hemispheric reorganization in individuals with epilepsy. Therefore, development of paradigms capable of exclusively recording and modeling brain activity in anterior speech regions are a necessary complement to existing protocols used to assess hemispheric dominance for receptive language. Moreover, whereas MEG may serve as an acceptable substitute for the purposes of determining receptive hemispheric dominance for language, the Wada procedure is also used to generate information regarding the relative contribution of each hemisphere to memory function. This may prove to be critical if the hemisphere contralateral to the epileptogenic zone cannot support memory, especially in patients undergoing anterior temporal lobectomy, who would be at particular risk for developing amnesic disorder [44]. Therefore, substitution of MEG for the Wada procedure may only be appropriate in cases where lateralization of memory is not critical to the surgical outcome.
3.2 Localization of Language-Specific Cortex
As well as providing an estimate of hemispheric dominance for receptive language functions, an invariable feature of MEG-based activation profiles obtained from the CRM paradigm is the localization of activity within the temporo-parietal cortex (including the posterior portions of the middle and superior temporal gyri; and the supramarginal and angular gyri), irrespective of the stimulus modality. This region, spatially coincident with Wernicke’s area, is defined as the patch of cortex which, when electrically stimulated, produces deficits in receptive language [10, 35]. The localization of late-onset active sources within the temporo-parietal cortex (e.g., Wernicke’s area) during receptive language processing using MEG has been previously documented in independent cohorts of normal controls [11, 27–29, 32, 35], using both the visual and auditory variations of the CRM task. More recently, a large-scale normative study by Papanicolaou et al. [32] employing a slightly different, more objective approach to ECD source modeling distinguished that the hemispheric dominance for receptive language is in fact driven by sustained activity in the left middle temporal gyrus, a finding which did not differ as a function of age, gender and stimulus modality, and one consistent with previous studies reporting a similar phenomenon [45, 46].
The reproducibility and spatial accuracy of MEG-derived profiles of brain activation during receptive language processing has also been addressed in studies of test-retest reliability in neurologically intact individuals. Initially, Breier et al. [30] reported a high degree of spatial overlap between late-activity sources in the region of Wernicke’s area across replications of the visual CRM task, in the same subjects. More recently, a systematic study by Simos et al. [47] demonstrated that during the auditory CRM task, intra-participant variability in the location of the geometric center of activity sources in Wernicke’s area, modeled as consecutive ECDs, ranged between 2–8 mm, clearly lower than the spatial resolution limit (~1cm) of direct electrocortical stimulation mapping. Furthermore, a finding common to both studies was the notable variability across subjects in the location of active sources in the region identified as Wernicke’s area, which highlights the sensitivity of MEG to inter-individual variability in the functional organization of the association cortex.
Studies of concurrent validity have attested to the adequacy of MEG as a potential substitute for electrocortical stimulation mapping for the purpose of localizing language-specific cortex. Based on our accumulated experience of over 40 consecutive patients undergoing pre-surgical functional mapping [10, 28, 48, 49], we have reported near-perfect agreement between MEG-derived maps of language-specific activity within the dominant hemisphere and the independent results of direct intra-/extra- operative electrocortical stimulation, in identifying brain regions critical to receptive language function. Notably, within these studies, perfect agreement between the two modalities was also demonstrated even in cases where patients were found to exhibit atypical language representation (outside the classically-defined borders of Wernicke’s area).
From a clinical point of view, the excellent agreement between MEG and direct electorcortical stimulation mapping have several practical implications, including reduced risk of morbidity, and facilitation of the craniotomy procedure using additional pre-operative information gathered about the precise location of language-specific cortex. However, as mentioned earlier, no definitive MEG activation protocol exists which readily allows for the mapping of expressive language cortex, equally critical to the preservation of function in surgical candidates [10]. A single case study by Castillo et al. [23] did find a concordance between the site of stimulation and MEG-derived activity sources localized to the inferior frontal cortex during a picture naming task, though no subsequent studies have confirmed this earlier report. Thus, in the current context, it appears that MEG is most valid for the purposes of localizing receptive-language specific cortex.
3.3 Neural Mechanisms of Reading
Normative brain activation profiles associated with reading have been successfully established in our lab by extension of the MEG protocols originally developed to map receptive language-specific cortex, and validated against the “gold standard” invasive brain mapping techniques. Specifically, the visual CRM protocol has provided the context for which brain mechanism underlying single-word reading have been identified in normally developing individuals. Furthermore, the neural correlates of more fundamental facets of reading such as phonological decoding have also been investigated in the context of the pseudoword rhyme-matching task. An important aspect of this work has also been the application of these protocols to studies of the neural correlates of reading disability in children with developmental dyslexia.
Over the course of several studies of non-reading impaired adults and children [11, 13, 27, 50, 51], we have established a generic spatiotemporal profile of cortical activity associated with both reading of real words and pseudowords, characterized by several distinct, relatively consistent features. Almost invariably during the early component of the ERF, bilateral activation of the occipital cortex, an area of the brain involved in basic visual perception, is followed by increased activity in the left occipitotemporal and basal temporal cortices, areas believed to be related to recognizing and decoding the orthographic word form. Following resolution of the early evoked response, a second stable feature associated with reading is increased activity in the temporo-parietal cortex (posterior superior temporal and supramarginal gyri), predominantly in the left hemisphere, accompanied by bilateral engagement of the posterior middle temporal gyrus. These temporal areas are believed to be involved in recognizing the phonological and semantic components of the word. In addition to temporal lobe regions, late activity sources are also found in the inferior frontal regions, primarily in the left hemisphere, a area believed to be involved in articulation of word sounds and learning. A distinguishing feature between activation profiles of real word and pseudoword reading is the evocation of greater posterior left middle temporal gyrus activity during the former task, whereas engagement in the latter task is associated with reduced activity in this region. In contrast, MEG-derived data has demonstrated that in fact phonological decoding, in the context of pseudoword reading, is mediated by the posterior left superior temporal gyrus, a finding concurrent with previous studies of direct electrocortical stimulation mapping [35], as well as other metabolic-based functional imaging studies [52–55].
Children with dyslexia exhibit a reduced ability to discriminate the phonological units of speech that contain fast auditory transients, such as syllables. Cortical responses to simple and more complex language units have been studied with MEG in children with dyslexia in our lab over a period of several years [29, 36, 56–58]. Specifically, we have used MEG to reliably differentiate children with dyslexia based on abnormal spatiotemporal profiles of activity during execution of reading tasks. As with the general pattern of activity generated by children without reading impairments, dyslexic children exhibit a generic pattern of activity marked by consistently reduced activation of the left posterior superior temporal/supramarginal gyri (primarily the posterior superior temporal region), accompanied by hyperactivation of the right homotopic regions and compensatory increases in prefrontal activity. Moreover, another salient feature of the atypical activation profile exhibited by dyslexic kids is the tendency for earlier prefrontal lobe activity, followed by a marked delay in the onset of activity within temporo-parietal regions (mainly left superior temporal gyrus) [36]. These anomalies are a notable departure from the temporal progression of late-onset brain activity characteristic of normal individuals during the reading of real-words, as well as phonological decoding. A noteworthy observation based on the studies of dyslexic children summarized here is that these individuals exhibit the normal pattern of hemispheric dominance for receptive language, as demonstrated by cortical activation profiles obtained during the during the auditory and visual CRM tasks, thus refuting the long-standing hypothesis [59] that the disorder is related to anomalous hemispheric dominance for language functions [29].
4 Conclusions
As a functional neuroimaging modality, MEG seems well-suited for efficiently mapping language in neurologically intact individuals. Furthermore, MEG-derived activation profiles appear to be reliable and valid indices of neural activity associated with receptive language function, given the high degree of concordance between MEG and the two “gold standard” techniques, namely the Wada procedure and direct electrocortical stimulation mapping. While certain limitations still preclude MEG from completely replacing invasive diagnostic methods in clinical practice, there is little doubt that the information obtained using this technique is an effective adjunct to existing pre-surgical routines. The development and validation of additional protocols, particularly those capable of assessing the relative contribution of each hemisphere to expressive language, as well as memory function, may further enhance the clinical utility of MEG during surgical planning. Moreover, as evident from the studies of reading in non-impaired children and those with dyslexia, MEG may have the potential to distinguish between normal and abnormal developmental conditions, particularly where impairment of language is believed to be the core deficit.
Acknowledgments
This paper was supported by NIH Grant NS046565 to Dr. Richard Frye.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Démonet JF, Thierry G, Cardebat D. Renewal of the neurophysiology of language: functional neuroimaging. Physiological Reviews. 2005;85:49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- 2.Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. Journal of Neurosurgery. 1960;107:1117–1133. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- 3.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 4.Snyder PJ, Novelly RA, Harris LJ. Mixed speech dominance in the Intracarotid Sodium Amytal Procedure: validity and criteria issues. Journal of Experimental Clinical Neuropsychology. 1990;12:629–643. doi: 10.1080/01688639008401007. [DOI] [PubMed] [Google Scholar]
- 5.Kurthen M, Helmstaedter C, Linke DB, Hufnagel A, Elger CE, Schramm J. Quantitative and qualitative evaluation of patterns of cerebral language dominance. An amobarbital study. Brain and Language. 1994;46:536–564. doi: 10.1006/brln.1994.1030. [DOI] [PubMed] [Google Scholar]
- 6.Milner B, Branch C, Rasmussen Y. Study of short-term memory after intacarotid injection of sodium amytal. Transactions of the American Neurological Association. 1962;87:224–226. [Google Scholar]
- 7.Dion JE, Gates PC, Fox AJ, Barnett HJ, Blom RJ. Clinical events following neuroangiography: a prospective study. Stroke. 1987;18:997–1004. doi: 10.1161/01.str.18.6.997. [DOI] [PubMed] [Google Scholar]
- 8.Akanuma N, Koutroumanidis M, Adachi N, Alarcón G, Binnie CD. Presurgical assessment of memory-related brain structures: the Wada test and functional neuroimaging. Seizure. 2003;12:346–358. doi: 10.1016/s1059-1311(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 9.Hietala SO, Silfvenius H, Aasly J, Olivecrona M, Jonsson L. Brain perfusion with intracarotid injection of 99mTc-HM-PAO in partial epilepsy during amobarbital testing. European Journal of Nuclear Medicine. 1990;16:683–687. doi: 10.1007/BF00998169. [DOI] [PubMed] [Google Scholar]
- 10.Simos PG, Papanicolaou AC, Breier JI, Wheless JW, Constantinou JE, Gormley WB, et al. Localization of language-specific cortex by using magnetic source imaging and electrical stimulation mapping. Journal of Neurosurgery. 1999;91:787–796. doi: 10.3171/jns.1999.91.5.0787. [DOI] [PubMed] [Google Scholar]
- 11.Breier JI, Simos PG, Zouridakis G, Wheless JW, Willmore LJ, Constantinou JE, et al. Language dominance determined by magnetic source imaging: a comparison with the Wada procedure. Neurology. 1999;53:938–945. doi: 10.1212/wnl.53.5.938. [DOI] [PubMed] [Google Scholar]
- 12.Ojemann GA. The neurobiology of language and verbal memory: observations from awake neurosurgery. International Journal of Psychophysiology. 2003;48:141–146. doi: 10.1016/s0167-8760(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 13.Simos PG, Breier JI, Zouridakis G, Papanicolaou AC. Identification of language-specific brain activity using magnetoencephalography. Journal of Clinical and Experimental Neuropsychology. 1998;20:706–722. doi: 10.1076/jcen.20.5.706.1127. [DOI] [PubMed] [Google Scholar]
- 14.Pouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. Journal of Neurosurgery. 2002;97:21–32. doi: 10.3171/jns.2002.97.1.0021. [DOI] [PubMed] [Google Scholar]
- 15.Papanicolaou AC, Tarkka IM. Magnetoencephalography. In: Bigler ED, editor. Neuroimaging I: Basic Science. Plenum; New York: 1996. [Google Scholar]
- 16.Lewine JD, Orrison WW., Jr Magnetic source imaging: basic principles and applications in neuroradiology. Academic Radiology. 1995;2:436–440. doi: 10.1016/s1076-6332(05)80351-4. [DOI] [PubMed] [Google Scholar]
- 17.Cohen D. Magnetoencephalography: detection of the brains electrical activity with a super conducting magnetometer. Science. 1972;175:664–666. doi: 10.1126/science.175.4022.664. [DOI] [PubMed] [Google Scholar]
- 18.Williamson SJ, Kaufman L. Analysis of neuromagnetic signals. In: Gevins AS, Remonds A, editors. Handbook of Electroencephalography in Clinical Neurophysiology. New York: Elsevier; 1987. pp. 405–448. [Google Scholar]
- 19.Rowley HA, Roberts TPL. Functional localization by magnetoencephalography. Neuroimaging Clinics of North America. 1995;5:695–710. [PubMed] [Google Scholar]
- 20.Papanicolaou AC. Fundamentals of Functional Brain Imaging. Lisse, The Netherlands: Swets & Zeitlinger; 1998. [Google Scholar]
- 21.Baillet S, Mosher JC, Leahy RM. Electromagnetic brain mapping. IEEE Signal Processing Magazine. 2001;18:14–30. [Google Scholar]
- 22.Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clinical Neurophysiology. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Castillo EM, Simos PG, Venkataraman V, Breier JI, Wheless JW, Papanicolaou AC. Mapping of expressive language cortex using magnetic source imaging. Neurocase. 2001;7:419–422. doi: 10.1076/neur.7.5.419.16249. [DOI] [PubMed] [Google Scholar]
- 24.Sarvas J. Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Physics in Medicine and Biology. 1987;32:11–22. doi: 10.1088/0031-9155/32/1/004. [DOI] [PubMed] [Google Scholar]
- 25.Hämäläinen MS. Magnetoencephalography: a tool for functional brain imaging. Brain Topography. 1992;5:95–102. doi: 10.1007/BF01129036. [DOI] [PubMed] [Google Scholar]
- 26.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 27.Zouridakis G, Simos PG, Breier JI, Papanicolaou AC. Functional hemispheric asymmetry assessment in a visual language task using MEG. Brain Topography. 1998;11:57–65. doi: 10.1023/a:1022270620396. [DOI] [PubMed] [Google Scholar]
- 28.Papanicolaou AC, Simos PG, Breier JI, Zouridakis G, Willmore LJ, Wheless JW, et al. Magnetoencephalographic mapping of the language-specific cortex. Journal of Neurosurgery. 1999;90:85–93. doi: 10.3171/jns.1999.90.1.0085. [DOI] [PubMed] [Google Scholar]
- 29.Papanicolaou AC, Simos PG, Breier JI, Fletcher JM, Foorman BR, Francis D, et al. Brain mechanisms for reading in children with and without dyslexia: a review of studies of normal development and plasticity. Developmental Neuropsychology. 2003;24:593–612. doi: 10.1080/87565641.2003.9651912. [DOI] [PubMed] [Google Scholar]
- 30.Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Lateralization of activity associated with language function using magnetoencephalography: a reliability study. Journal of Clinical Neurophysiology. 2000;17:503–510. doi: 10.1097/00004691-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Valaki CE, Maestu F, Simos PG, Zhang W, Fernandez A, Amo CM, et al. Cortical organization for receptive language functions in Chinese, English, and Spanish: a cross-linguistic MEG study. Neuropsychologia. 2004;42:967–979. doi: 10.1016/j.neuropsychologia.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Papanicolaou AC, Pazo-Alvarez P, Castillo EM, Billingsley-Marshall RL, Breier JI, Swank PR, et al. Functional neuroimaging with MEG: normative language profiles. Neuroimage. 2006;33:326–342. doi: 10.1016/j.neuroimage.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Paivio A, Yuille JC. Word abstractness and meaningfulness, and paired-associate learning in children. Journal of Experimental Child Psychology. 1966;4:81–89. doi: 10.1016/0022-0965(66)90052-x. [DOI] [PubMed] [Google Scholar]
- 34.Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Relative timing of neuronal activity in distinct temporal lobe areas during a recognition memory task for words. Journal of Clinical and Experimental Neuropsychology. 1998;20:782–790. doi: 10.1076/jcen.20.6.782.1116. [DOI] [PubMed] [Google Scholar]
- 35.Simos PG, Breier JI, Wheless JW, Maggio WW, Fletcher JM, Castillo EM, et al. Brain mechanisms for reading: the role of the superior temporal gyrus in word and pseudoword naming. Neuroreport. 2000;11:2443–2447. doi: 10.1097/00001756-200008030-00021. [DOI] [PubMed] [Google Scholar]
- 36.Simos PG, Breier JI, Fletcher JM, Foorman BR, Bergman E, Fishbeck K, et al. Brain activation profiles in dyslexic children during non-word reading: a magnetic source imaging study. Neuroscience Letters. 2000;290:61–65. doi: 10.1016/s0304-3940(00)01322-7. [DOI] [PubMed] [Google Scholar]
- 37.Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, et al. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- 38.Loring DW, Meador KJ, Lee GP, Flanigin HF, King DW, Smith JR. Crossed aphasia in a patient with complex partial seizures: evidence from intracarotid amobarbital testing, functional cortical mapping, and neuropsychological assessment. Journal of Clinical and Experimental Neuropsychology. 1990;12:340–354. doi: 10.1080/01688639008400979. [DOI] [PubMed] [Google Scholar]
- 39.Maestú F, Ortiz T, Fernandez A, Amo C, Martin P, Fernández S, et al. Spanish language mapping using MEG: a validation study. Neuroimage. 2002;17:1579–1586. doi: 10.1006/nimg.2002.1235. [DOI] [PubMed] [Google Scholar]
- 40.Szymanski MD, Perry DW, Gage NM, Rowley HA, Walker J, Berger MS, et al. Journal of Neurosurgery. 2001. Magnetic source imaging of late evoked field responses to vowels: toward an assessment of hemispheric dominance for language; pp. 445–453. [DOI] [PubMed] [Google Scholar]
- 41.Breier JI, Simos PG, Wheless JW, Constantinou JE, Baumgartner JE, Venkataraman V, et al. Language dominance in children as determined by magnetic source imaging and the intracarotid amobarbital procedure: a comparison. Journal of Child Neurology. 2001;16:124–130. doi: 10.1177/088307380101600211. [DOI] [PubMed] [Google Scholar]
- 42.Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Sarkari S, Pataraia E, et al. Magnetoencephalography: a noninvasive alternative to the Wada procedure. Journal of Neurosurgery. 2004;100:867–876. doi: 10.3171/jns.2004.100.5.0867. [DOI] [PubMed] [Google Scholar]
- 43.Helmstaedter C, Kurthen M, Linke DB, Elger CE. Right hemisphere restitution of language and memory functions in right hemisphere language-dominant patients with left temporal lobe epilepsy. Brain. 1994;117:729–737. doi: 10.1093/brain/117.4.729. [DOI] [PubMed] [Google Scholar]
- 44.Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Katz JS, Wright AA. The hippocampus and memory of verbal and pictorial material. Learning and Memory. 2002;9:99–104. doi: 10.1101/lm.44302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- 46.Poeppel D, Guillemin A, Thompson J, Fritz J, Bavelier D, Braun AR. Auditory lexical decision, categorical perception, and FM direction discrimination differentially engage left and right auditory cortex. Neuropsychologia. 2004;42:183–200. doi: 10.1016/j.neuropsychologia.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Simos PG, Sarkari S, Castillo EM, Billingsley-Marshall RL, Pataraia E, Clear T, et al. Reproducibility of measures of neurophysiological activity in Wernicke’s area: a magnetic source imaging study. Clinical Neurophysiology. 2005;116:2381–2391. doi: 10.1016/j.clinph.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Simos PG, Breier JI, Maggio WW, Gormley WB, Zouridakis G, Willmore LJ, et al. Atypical temporal lobe language representation: MEG and intraoperative stimulation mapping correlation. Neuroreport. 1999;10:139–142. doi: 10.1097/00001756-199901180-00026. [DOI] [PubMed] [Google Scholar]
- 49.Wheless JW, Castillo E, Maggio V, Kim HL, Breier JI, Simos PG, et al. Magnetoencephalography (MEG) and magnetic source imaging (MSI) Neurologist. 2004;10:138–153. doi: 10.1097/01.nrl.0000126589.21840.a1. [DOI] [PubMed] [Google Scholar]
- 50.Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Temporal course of regional brain activation associated with phonological decoding. Journal of Clinical and Experimental Neuropsychology. 1999;21:465–476. doi: 10.1076/jcen.21.4.465.883. [DOI] [PubMed] [Google Scholar]
- 51.Simos PG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC. Brain mechanisms for reading words and pseudowords: an integrated approach. Cerebral Cortex. 2002;12:297–305. doi: 10.1093/cercor/12.3.297. [DOI] [PubMed] [Google Scholar]
- 52.Démonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, et al. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 53.Burton MW, Small SL, Blumstein SE. The role of segmentation in phonological processing: an fMRI investigation. Journal of Cognitive Neuroscience. 2000;12:679–690. doi: 10.1162/089892900562309. [DOI] [PubMed] [Google Scholar]
- 54.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, et al. Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- 55.Vouloumanos A, Kiehl KA, Werker JF, Liddle PF. Detection of sounds in the auditory stream: event-related fMRI evidence for differential activation to speech and nonspeech. Journal of Cognitive Neuroscience. 2001;13:994–1005. doi: 10.1162/089892901753165890. [DOI] [PubMed] [Google Scholar]
- 56.Simos PG, Breier JI, Fletcher JM, Bergman E, Papanicolaou AC. Cerebral mechanisms involved in word reading in dyslexic children: a magnetic source imaging approach. Cerebral Cortex. 2000;10:809–816. doi: 10.1093/cercor/10.8.809. [DOI] [PubMed] [Google Scholar]
- 57.Sarkari S, Simos PG, Fletcher JM, Castillo EM, Breier JI, Papanicolaou AC. Contributions of magnetic source imaging to the understanding of dyslexia. Seminars in Pediatric Neurology. 2002;9:229–238. doi: 10.1053/spen.2002.35506. [DOI] [PubMed] [Google Scholar]
- 58.Breier JI, Simos PG, Fletcher JM, Castillo EM, Zhang W, Papanicolaou AC. Abnormal activation of temporoparietal language areas during phonetic analysis in children with dyslexia. Neuropsychology. 2003;17:610–621. doi: 10.1037/0894-4105.17.4.610. [DOI] [PubMed] [Google Scholar]
- 59.Orton S. Reading, writing, and speech problems in children. New York: Norton; 1937. [Google Scholar]