Abstract
Objective
Levator veli palatini muscles from normal palates of adult humans and goats are predominantly slow oxidative (type 1) fibers. However, 85% of levator veli palatini fibers from cleft palates of adult goats are physiologically fast (type 2). This fiber composition difference between cleft and normal palates may have implications in palatal function. For limb muscles, type 2 muscle fibers are more susceptible to lengthening contraction-induced injury than are type 1 fibers. We tested the hypothesis that, compared with single permeabilized levator veli palatini muscle fibers from normal palates of adult goats, those from cleft palates are more susceptible to lengthening contraction-induced injury.
Interventions
Congenital cleft palates were the result of chemically-induced decreased movement of the fetal head and tongue causing obstruction of palatal closure. Each muscle fiber was maximally activated and lengthened.
Outcome Measures
Fiber type was determined by contractile properties and gel electrophoresis. Susceptibility to injury was assessed by measuring the decrease in maximum force following the lengthening contraction, expressed as a percentage of the initial force.
Results
Compared with fibers from normal palates that were all type 1 and had force deficits of 23 ± 1%, fibers from cleft palates were all type 2 and sustained twofold greater deficits, 40 ± 1% (p = .001).
Conclusion
Levator veli palatini muscles from cleft palates of goats contain predominantly type 2 fibers that are highly susceptible to lengthening contraction-induced injury. This finding may have implications regarding palatal function and the incidence of velopharyngeal incompetence.
Keywords: lengthening contractions, levator veli palatini, velopharyngeal incompetence
The congenital cleft palate is the most common birth defect of the head, with rates as high as 7 per 10,000 live births worldwide (Elahi et al., 2004; Forrester and Merz, 2004; Centers for Disease Control and Prevention, 2006). For infants with cleft palates, middle ear disease and difficulties during feeding and breathing often develop (Randall and LaRossa, 1991). Regardless of the type of surgical repair of cleft palates, approximately 15% of patients still encounter problems achieving velopharyngeal closure, a condition known as velopharyngeal incompetence (VPI) (Marrinan et al., 1998). Severe impairment of the levator veli palatini (LVP) muscle, the skeletal muscle most critical for the achievement of velopharyngeal closure (Moon et al., 1994; Huang et al., 1998), can lead to VPI. A functional LVP muscle moves the soft palate superiorly and posteriorly to the back of the pharynx, effectively helping to close the velopharyngeal port (Moon et al., 1994; Huang et al., 1998).
For adults with normal palates, LVP muscles consist of a mixture of fiber types (Moon et al., 1998; Stal and Lindman, 2000). Fiber types can be distinguished by the velocity of force development for a given load, which is a function of their myosin heavy chain (MHC) isoforms (Talmadge and Roy, 1993; Moon et al., 1998; Stal and Lindman, 2000; Hanes et al., 2006). Fibers with the type 1 MHC, slow (type 1) fibers, contract slowly, whereas fast (type 2) fibers contain type 2 MHC and contract rapidly. In two reports of fibers from LVP muscles of normal palates of adults (Moon et al., 1998; Stal and Lindman, 2000), 70% were slow type 1 and 30% were fast type 2, but no fibers from cleft palates of age-matched patients were reported. The majority of LVP muscle fibers from cleft palates of infants are fast type 2 fibers (Lindman et al., 2001). For the LVP muscles of adult goats, approximately all of the fibers from normal palates were slow type 1, whereas approximately 85% of the fibers from cleft palates were fast type 2 (Hanes et al., 2006). The fast type 2 fibers were more prone to fatigue (Hanes et al., 2006), which suggests that the presence of a large population of fast type 2 fibers in cleft palates might contribute to impaired performance of LVP muscles and to VPI (Hanes et al., 2006).
Exposure of LVP muscle fibers from cleft palates to injurious events is another potential contributor to VPI. During daily activities, skeletal muscles are exposed to passive stretches and three different types of contractions: shortening, isometric, and lengthening (Friden et al., 1983; McCully and Faulkner, 1985). During activation, a muscle can be kept at a fixed length (isometric contraction) or can be shortened (shortening contraction) or lengthened (lengthening contraction) (Faulkner, 2003). When muscles of mice are administered a demanding protocol of shortening (McCully and Faulkner, 1985) or isometric contractions (McCully and Faulkner, 1985; Koh and Brooks, 2001) or passive stretches (McCully and Faulkner, 1985; Koh and Brooks, 2001), the force may decrease due to fatigue, but maximum force recovers completely within a few hours. In contrast, following a severe protocol of lengthening contractions (LCP), muscles of mice sustain an initial decrease in force due to the disruption of sarcomeres followed by a delayed secondary reduction in force due to the inflammatory response days later (McCully and Faulkner, 1985; Brooks and Faulkner, 1996; Koh and Brooks, 2001). For biopsies from muscles of humans following an LCP, initial sarcomere disruption (Friden et al., 1983; Newham et al., 1983; Gibala et al., 1995; Feasson et al., 2002) and the infiltration of inflammatory cells (Stupka et al., 2000; Feasson et al., 2002) were observed in some studies, although other reports lacked these findings (Yu et al., 2002, 2003, 2004; Crameri et al., 2004; Malm et al., 2004). The presence of contraction-induced injury and an inflammatory response following an LCP were supported by imaging whole muscles of humans (MacIntyre et al., 1996; Foley et al., 1999). The implication is that the lack of injury and inflammation reported in some studies (Yu et al., 2002, 2003, 2004; Crameri et al., 2004; Malm et al., 2004) may be the result of insufficient sampling owing to the limitations of biopsies rather than an accurate account of the events post-LCP.
For limb skeletal muscles, type 2 fibers, compared with type 1 fibers, are more severely injured by an LCP (Friden et al., 1983, 1988; Lieber et al., 1991; Macpherson et al., 1996). Whether type 2 fibers from LVP muscles of cleft palates are susceptible to contraction-induced injury is not known. To resolve this issue, we tested permeabilized single fibers from LVP muscles of normal and cleft palates of adult goats. Permeabilized muscle fibers are fibers that have had their plasma membranes breached severely by a “skinning” process (Brooks and Faulkner, 1996; Macpherson et al., 1996). The testing of permeabilized muscle fibers permitted the isolation of single fibers and investigation of properties intrinsic to each fiber in the absence of extrinsic influences such as neuronal or hormonal factors (Brooks and Faulkner, 1996; Macpherson et al., 1996). The hypothesis tested was that, compared with the population of predominantly slow type 1 fibers obtained from normal palates, those obtained from cleft palates are predominantly fast type 2 and, consequently, sustain a greater magnitude of contraction-induced injury, as evidenced by the decrease in maximum force.
METHODS
Experimental Design
To test the hypothesis, single muscle fibers from normal and cleft palates were permeabilized, isolated, and submerged in three solutions sequentially: the relaxation, preactivation, and activation solutions. While in the activation solution, each fiber was categorized based on the contractile properties (Hanes et al., 2006). The validity of fiber typing by assessment of contractile properties was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of MHC isoforms. The magnitude of contraction-induced injury following the LCP was determined by the force deficit, the decrease in maximum force expressed as a percentage of the initial maximum force (McCully and Faulkner, 1985; Brooks and Faulkner, 1996; Macpherson et al., 1996). All of these procedures, with the exceptions of the LCP and gel electrophoresis, have been described in detail in a previous report (Hanes et al., 2006).
Animal Model
Of six adult (14 to 15 months of age) female Spanish goats (25 to 56 kg), three goats had congenitally-induced cleft palates, whereas the remaining three goats had normal palates. The cleft palate was induced by gavaging pregnant goats with an extract of Nicotiana glauca plant slurry twice daily from day 32 to 41 of gestation, the critical developmental period when palatal shelf closure occurs (Weinzweig et al., 1999). As a consequence, the neck of the fetal goat remained hyperflexed and caused the tongue to obstruct the closure of the palatal shelves resulting in complete clefts of the secondary palates (Weinzweig et al., 1999). Each goat was sacrificed using an overdose of Beuthanasia (Schering-Plough Animal Health Corp., Kenilworth, NJ). The validity of the goat model was supported by the finding that the plant slurry extract did not alter the fiber type composition of the quadriceps muscles of 2-month-old goats. For each goat (n = 3 per group), one section of one fiber bundle (42 to 161 fibers per bundle) from the quadriceps muscles underwent fiber-type analysis following myosin ATPase staining (Brooke and Kaiser, 1970). Quadriceps muscles of goats with cleft palates were composed of 22 ± 5% type 1 and 78 ± 5% type 2 fibers, values not different from those for goats with normal palates, 30 ± 15% type 1 and 70 ± 15% type 2 fibers. The procedures were approved by the Harvard Medical Area Standing Committee on Animals and in accordance with the guidelines of the United States Public Health Service, National Institutes of Health Publication No. 85-23.
Permeabilization
Prior to sacrificing each goat, the LVP muscle was harvested. Bundles of 50 to 200 muscle fibers were transferred immediately into skinning solution composed of 125 mM potassium proprionate, 20 mM imidazole, 5 mM ethyleneglycol-bis (B-aminoethyl ether) tetra-acetic acid (EGTA), 2 mM MgCl2, and 2 mM ATP at 4°C and were separated into bundles in less than 20 minutes. Bundles were placed in skinning solution containing the detergent Brij 58 (0.5 w/v) for 30 minutes at 4°C to enhance permeabilization of the fibers. The bundles were then transferred into storage solution composed of skinning solution with glycerol substituted for 50% of the volume of water for 24 hours at 4°C. Afterward, the bundles were transferred to fresh storage solution and were kept at −20°C until the extraction of single fibers was necessary.
Extraction of Single Fibers in Relaxation Solution
For the extraction of single fibers, the bundles were thawed to 4°C and then were submerged in relaxation solution (pCa ~ 9.0) (pH 7.1) composed of 90 mM HEPES, 10.3 mM Mg (total), 1.0 mM Mg2+, 50 mM EGTA, 8.0 mM ATP, 10.0 mM CrP, 1.0 mM NaN3, 36 mM Na (total), 125 mM K (total), where individual fibers were extracted gently out of the bundle with fine forceps. Care was taken to prevent the stretching of individual fibers during extraction.
Determination of Fiber Length, Cross-sectional Area, and Mass in Relaxation Solution
Each muscle fiber was transferred to a chamber containing relaxation solution kept at 15°C. Two 10-0 monofilament nylon sutures were used to secure each end of the fiber, one end to a force transducer (Model 403A, Aurora Scientific Inc., Ontario, CA) and the other end to a servomotor (Model 322C, Aurora Scientific). Sarcomere length was set to 2.5 µm by projecting a laser beam through the fiber and referring to the resulting diffraction pattern on a calibrated target screen. This was the only occasion that the sarcomere length was measured and the length of the muscle fiber adjusted to achieve a specified sarcomere length. After this initial length adjustment, the resting fiber length (Lf) was kept constant throughout the experiment. To measure the Lf, the innermost tie at one end was centered in the crosshair of a microscope eyepiece. The entire apparatus was translated laterally with respect to the microscope using a micrometer drive with digital readout until the innermost tie at the other end of the fiber was centered. Fiber diameters were measured at Lf using two high-magnification digital images of the top and side views of the fiber to estimate the width and depth, respectively. A prism embedded in the side of the chamber was used to obtain the side view. Five pairs of measurements for width and depth were obtained at intervals, approximately 100 µm in length, along the midsection of each fiber. Cross-sectional areas were calculated for each pair of measurements of diameter, assuming an elliptical cross section. The overall fiber cross-sectional area (CSA) was estimated by calculating the mean of the five individual areas. Fiber mass was estimated from the product of Lf and CSA, assuming a density of 1 mg/mm3 (Macpherson et al., 1996).
Determination of Resting Force in Preactivation Solution
Each fiber was transferred to a chamber containing a low-[Ca2+] preactivation solution (pH 7.1) composed of 90 mM HEPES, 8.50 mM Mg (total), 1.0 mM Mg2+, 50 mM EGTA, 50 mM Ca2+ (total), 8.0 mM ATP, 10.0 mM CrP, 1.0 mM NaN3, 36 mM Na (total). The transfer to chambers of different solutions was accomplished using a system (Model 802A, Aurora Scientific) consisting of multiple separate glass-bottom chambers machined into a moveable, temperature-controlled stainless-steel plate. Movement of the plate with respect to the fiber was achieved by remote control of two stepper motors, one to lower and to raise the well array and the other to translate the fiber to a new chamber position. The fiber was allowed to equilibrate for 3 minutes in the preactivation solution. This solution was weakly-buffered for Ca2+, allowing for very rapid activation and force development when submerged in activation solution (Moisescu and Thieleczek, 1978). To determine the resting force (Prest) at the end of the 3-minute period, the fiber was exposed to a slack release for 30 milliseconds and returned to Lf.
Determination of Fiber Type and Specific Force in Activation Solution
A fiber was maximally activated when immersed in a chamber containing activation solution (pCa ~ 4.5) (pH 7.1). The activation solution was composed of 90 mM HEPES, 8.12 mM Mg (total), 1.0 mM Mg2+, 50 mM EGTA, 50 mM Ca2+ (total), 8.0 mM ATP, 10.0 mM CrP, 1.0 mM NaN3, 36 mM Na (total), 125 mM K (total). The value of Prest was subtracted from the force data obtained for a given fiber while submerged in the activation solution, so that the force reflected only the component of the force developed when maximally activated. To maintain structural stability throughout the period of activation, the fiber was cycled between an isometric contraction and short periods of isovelocity shortening near maximal velocity of shortening, followed by a rapid return to initial Lf (Sweeney et al., 1987).
After two cycles of isovelocity shortening, the fiber was exposed to a shortening step to momentarily unload the fiber and determine the rate constants of the subsequent exponential rise of force redevelopment (Fig. 1). The rate constants of force redevelopment are indicative of the MHC isoforms present (Burton et al., 2005) and, therefore, are indicative of fiber type (Hanes et al., 2006). For the description of force redevelopment, a double-exponential fit is superior to a single exponential fit (Burton et al., 2005). For this reason, the force redevelopment was fit to a double-exponential using analysis software (Signo, Alamedo Applied Sciences, San Leandro, CA) and a least-squares fit to the following equation was performed:
In this equation, P is force at time t, Pfmax is the maximal force for the fast component, krf is the rate constant of the fast component, Psmax is the maximal force for the slow component, krs is the rate constant of the slow component, and Pres represents the residual force present immediately after the length release and restretch maneuver. The value of krf or krs can be used to determine fiber type, and, for this study, each fiber was categorized using krf (Burton et al., 2005; Hanes et al., 2006). Values of krf equal to or less than 5 s−1 are indicative of slow type 1 fibers, whereas greater values are indicative of fast type 2 fibers (Hanes et al., 2006). The specific force (SPo; kN/m2) was determined by dividing the maximum isometric force (mN) by the CSA (mm2). Because fibers may be damaged during the isolation and mounting, data for a fiber were excluded when the SPo was less than 80 kN/m2. Five fibers from each goat had values of SPo greater than 80 kN/m2 and, therefore, their data were included in the analysis.
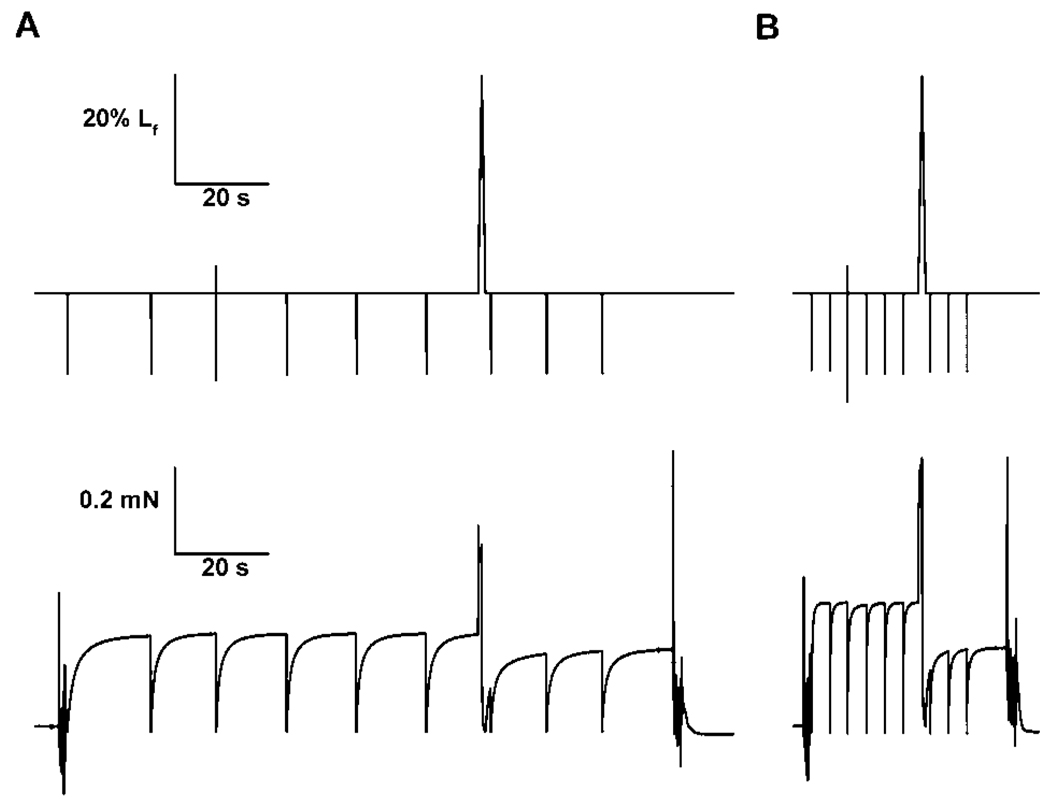
FIGURE 1.
Tracings of the length and force during a single lengthening contraction of two maximally activated permeabilized muscle fibers of goats: (A) a fiber from a normal palate and (B) a fiber from a congenitallyclefted palate. The krf for the fiber from the normal palate was 2.30 s−1 whereas the value was 16.98 s−1 for the fiber from the cleft palate. The force deficit following the lengthening contraction was 16% for the fiber from the normal palate and 39% for the fiber from the cleft palate.
Lengthening Contraction Protocol in Activation Solution
While remaining in the activation solution, the fiber was lengthened by 40% of Lf at a velocity of 0.5 Lf/s and returned to Lf at the same velocity (Fig. 1). The magnitude and direction of length change was selected so that differences in the susceptibility to injury would be clearly detectable between the groups. The protocol was not designed to test the type of contractions that typically occur during speech, namely shortening contractions (Ettema et al., 2002), because a protocol that caused a significant amount of injury for both groups of fibers was required for quantifying differences in susceptibility to injury. For permeabilized muscle fibers from the hind limbs of rats, injury-susceptible type 2 fibers had force deficits of 10% following a lengthening contraction of 10% Lf strain, whereas the injury-resistant type 1 fibers required a lengthening contraction of 40% Lf strain to produce force deficits of 10% (Macpherson et al., 1996). Therefore, the LCP selected ensured that even the most injury-resistant fibers would sustain some observable damage, which was important for comparison purposes. The forces during this protocol were normalized to CSA, and the mean force during the lengthening contraction (Pavg) and the force when the fiber was at 40% of Lf (Ppk) were recorded. The work done to lengthen the activated fiber was calculated by multiplying Pavg by the displacement. The work done (J) was normalized by the mass of the fiber (kg). Contraction-induced injury is a function of the amount of work done to lengthen activated fibers (Brooks and Faulkner, 1996). Consequently, determining this value for each fiber was critical for assessing whether fibers of normal and cleft palates were exposed to equivalent LCPs in terms of work done. The magnitude of the injury was assessed by the force deficit, the difference between the maximum isometric forces before and following the lengthening contraction expressed as a percentage of the maximum isometric force immediately prior to the lengthening contraction (Brooks and Faulkner, 1996; Macpherson et al., 1996). The fibers were stored at −20°C until gel electrophoresis analysis was performed.
Gel Electrophoresis for the Determination of MHC Isoforms
To verify that the values of krf correlated with the different fiber types, analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis was done (Talmadge and Roy, 1993). A subset of the muscle fibers were analyzed, 11 fibers from normal palates with krf values of 2.2 to 2.8 s−1 and 6 fibers from cleft palates with krf values of 13.6 to 17.4 s−1. Muscle fibers were assayed for protein concentration using the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA). Approximately 2.8 µg was loaded per well of a 4% stacking / 8% separating acrylamide gel for the analysis of slow type 1 and fast type 2 MHC isoforms. Molecular weight markers, Precision Plus Protein All Blue Standards (Bio-Rad Laboratories), also were added to distinguish MHC isoforms. The gel was run for 16 hours at 150 V and 4°C. Gels were then silver stained using Silver Stain Plus (Bio-Rad Laboratories).
Statistics
All data were expressed as mean ± one standard error of the mean. To account for the clustered structure of the data, wherein five fibers were measured within each goat, linear mixed models (LMMs) were fitted to the data (Verbeke and Molenberghs, 2000). Values of mass, CSA, and krf were modeled using the PROC MIXED command in SAS (Version 9.1, SAS Institute Inc., Carey, NC) due to the ability to fit LMMs with nonconstant error variance across the treatment groups. The remaining data were analyzed with LMMs using the MIXED command in SPSS (Version 13.0, SPSS Inc., Chicago, IL). The LMMs accounted for the fixed effect, the presence of a normal or cleft palate, and the random effect of the goats with the exception of the LMM for data regarding krf. For values of krf, marginal negative correlations were observed within data of individual goats. Consequently, the LMM was fitted excluding the random goat effects and using a compound symmetry covariance structure for the random errors. Significance for each statistical test was set a priori at p ≤ .05.
RESULTS
Characterization of Fiber Type
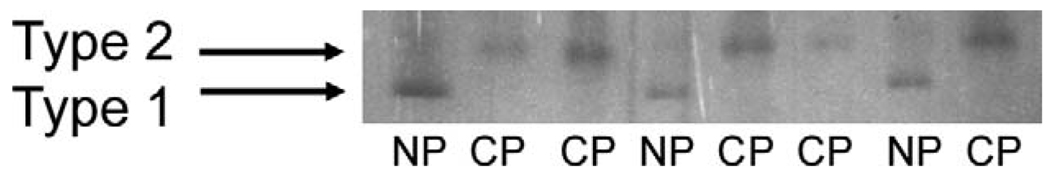
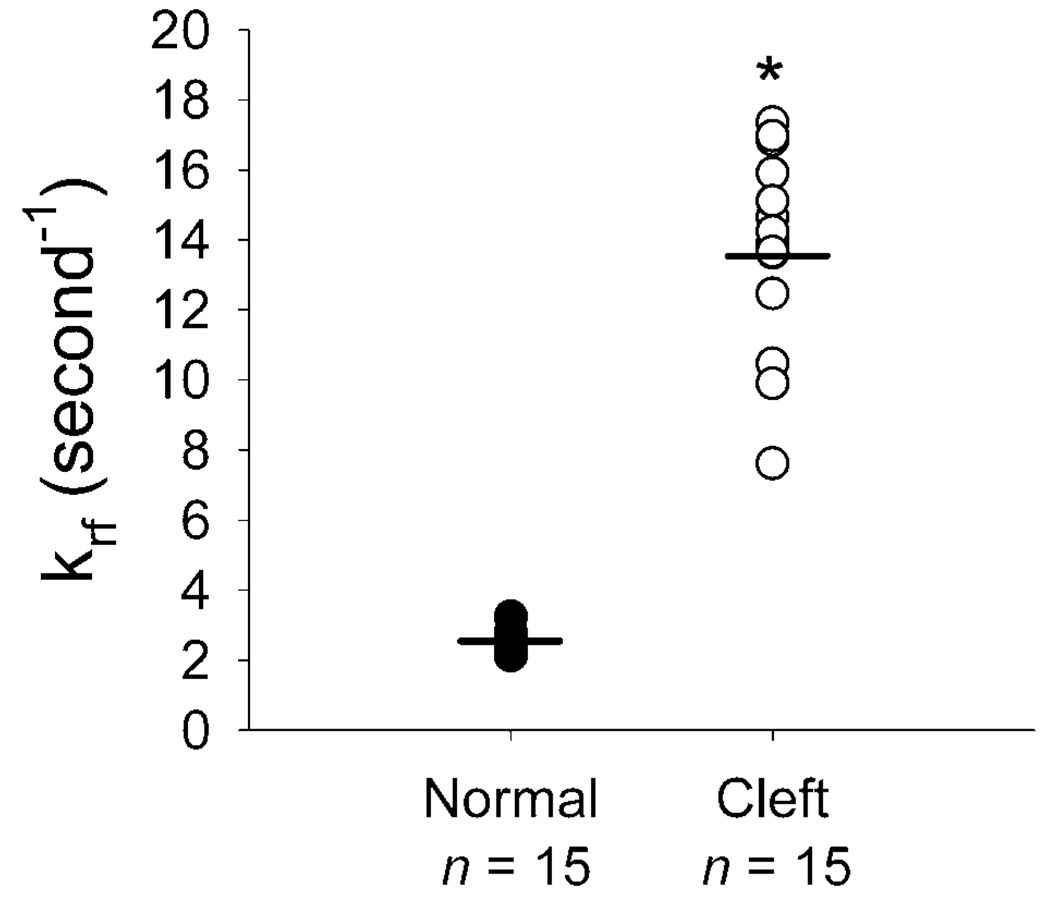
The correlation between the utilization of krf and the gel electrophoresis identification of fiber types was r2 = 1.00 (Fig. 2). Fibers with high krf values, 13.6 to 17.4 s−1, only contained type 2 MHCs and fibers with low krf values, 2.2 to 2.8 s−1, only contained type 1 MHCs (Fig. 2). The values of krf for the fibers from normal palates were all less than 5 s−1, the approximate cutoff value to distinguish fiber types (Hanes et al., 2006). Compared with the fibers from normal palates, fibers from cleft palates had values greater than 5 s−1 and a mean value that was sixfold greater (Fig. 3). This indicated that the fibers from normal palates were all slow type 1, whereas the fibers from cleft palates were all fast type 2.
FIGURE 2.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining for myosin heavy chains of single fibers (one fiber per lane) for normal (NP) and cleft (CP) palates. The muscle fibers of the normal palates were all slow type 1 whereas the fibers of the cleft palates were all fast type 2.
FIGURE 3.
Values of krf, for muscle fibers (n = 15 per group) from normal and cleft palates of goats before the lengthening contraction. Each circle represents one fiber and the bars denote the mean values. * The values for fibers from cleft palates, 13.8 ± 0.7 s−1 (mean ± SEM), were greater (p < .001) than those from normal palates, 2.5 ± 0.08 s−1.
Lf, CSA, mass, Prest, and SPo
For muscle fibers from normal and cleft palates, the values for Lf, CSA, mass, Prest, and SPo were not different (Table 2).
TABLE 2.
Contractile Parameters of Single Permeabilized Fibers From LVP Muscles of Normal and Congenitally-Clefted Goat Palates Before, During, and Following a Single Lengthening Contraction*
| Group | n | Lf, mm | CSA, µm2 | Mass, µg | Prest, kN/m2 | SPo, kN/m2 | Pavg, kN/m2 | Ppk, kN/m2 | Work, J/kg | Ppost, kN/m2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | 15 | 1.89 ± 0.07 | 2209 ± 129 | 4.2 ± 1.3 | 9 ± 1 | 107 ± 5 | 208 ± 7 | 224 ± 8 | 83 ± 3 | 83 ± 4 |
| Cleft | 15 | 1.84 ± 0.05 | 2459 ± 230 | 4.6 ± 2.0 | 13 ± 2 | 125 ± 7 | 243 ± 13 | 265 ± 14 | 97 ± 7 | 75 ± 4 |
Values are mean ± one standard error of the mean; n = number of fibers. No significant differences (p≤ .05) were observed between the data of the groups.
Lengthening Contraction Protocol
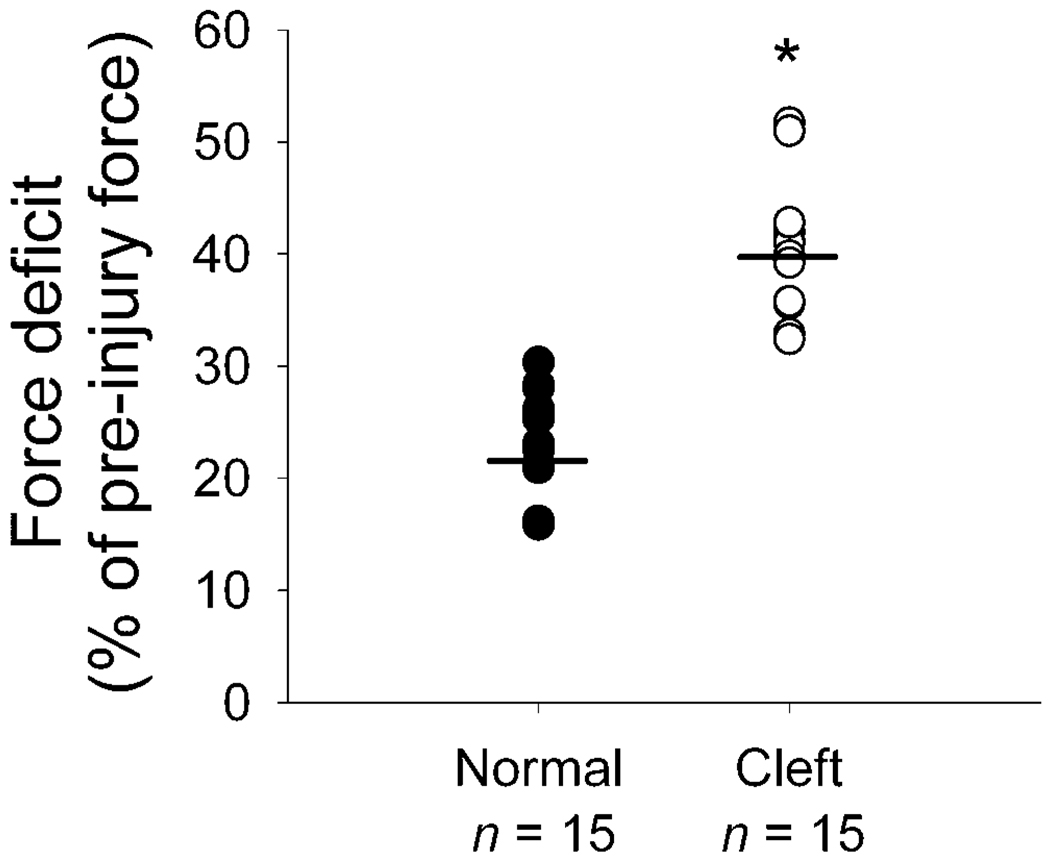
During the LCP, compared with fibers from normal palates, the values of Pavg, Ppk, and work done to stretch each fiber from cleft palates were not different (Table 2). Despite the lack of any difference during the LCP, following the LCP, the force deficits of fibers from cleft palates were twofold greater than those of normal palates (Fig. 4).
FIGURE 4.
Force deficits for the muscle fibers (n = 15 per group) from normal and cleft palates of goats following a lengthening contraction of 40% Lf strain. Each circle represents one fiber and the bars denote the mean values. * The values for fibers from cleft palates, 40 ± 1% (mean ± SEM), were greater (p = .001) than those from normal palates, 23 ± 1%.
DISCUSSION
The fibers from the LVP muscles of goats with congenital cleft palates were different from those with normal palates in fiber type composition and susceptibility to contraction-induced injury. Compared with permeabilized single muscle fibers from normal palates, those from cleft palates were composed exclusively of fast type 2 fibers and following the LCP, sustained twofold greater force deficits. Despite the high susceptibility to injury for the fibers from goats with cleft palates, the values of SPo were not different from those obtained from normal palates. The maintenance of SPo indicated that the muscle fibers from the cleft palates were undamaged and were functional prior to the LCP (McCully and Faulkner, 1985; Brooks and Faulkner, 1996; Macpherson et al., 1996). The conclusion is that muscle fibers of unrepaired cleft palates are rarely, if ever, exposed to lengthening contractions of sufficient magnitude to cause damage during daily activities. This finding was consistent with results from magnetic resonance imaging of LVP muscles in normal palates of humans that demonstrated that during speech, the muscle typically shortens rather than lengthens (Ettema et al., 2002). The results of the present study establish that factors intrinsic to the fibers of cleft palates predispose the fibers to injury from lengthening contractions. Whether other types of injurious events occur either during or following cleft palate repair and whether the same factors that render the fibers susceptible to lengthening contraction-induced injury also increase the susceptibility to these other injuries remain to be investigated.
The high degree of susceptibility to contraction-induced injury for fibers from cleft palates was potentially attributable to their fiber type (Friden et al., 1983, 1988; Lieber et al., 1991; Macpherson et al., 1996). The observation that LVP muscles of adult goats with normal palates are composed predominantly of slow type 1 fibers, whereas those of adult goats with cleft palates contain mostly fast type 2 fibers (present study, Hanes et al., 2006), is in good agreement with results for normal palates of human adults (Moon et al., 1998; Stal and Lindman, 2000) and for cleft palates of infants (Lindman et al., 2001). The fiber type distribution for cleft palates may have been the result of extrinsic factors such as loading (Wigmore and Evans, 2002). Under these circumstances, the fibers of the cleft palate might not have been exposed to the appropriate environmental cues to convert to or to maintain mature slow type 1 fibers. Loading abnormalities were possible during the induction of the cleft palate when the fetal neck was hyper-flexed and the tongue was wedged between the palatal shelves (Weinzweig et al., 1999). Following the induction of the cleft, atypical loading persisted in that the LVP muscle fibers were abnormally attached to the posterior edge of the palatal bone and, consequently, were exposed to forces along the posterior-anterior direction rather than the medial-lateral orientation of normal palatal LVP fibers (Weinzweig et al., 2002).
Compared with slow type 1 fibers, fast type 2 fibers within whole muscles of humans (Friden et al., 1983, 1988) and rabbits (Lieber et al., 1991) and single, permeabilized fast type 2 fibers of rats (Macpherson et al., 1996) are more susceptible to contraction-induced injury. The ultrastructure of sarcomeres are dependent on fiber type (Agarkova et al., 2004; Prado et al., 2005). Compared with type 1 fibers, type 2 fibers contain smaller isoforms of the sarcomeric proteins myomesin and nebulin (Agarkova et al., 2004; Prado et al., 2005). In addition, type 1 fibers contain a large, compliant isoform of the structural protein titin, whereas type 2 fibers contain a greater variation of titin isoforms with some being small and stiff (Prado et al., 2005). Further research is required to determine whether these fiber type differences in size and compliance of sarcomeric proteins affect the susceptibility to contraction-induced injury.
Although the present study did not test the effect of cleft palate repair and the rate of VPI in patients, the findings may have implications regarding VPI. The persistent rate of VPI, regardless of the type of surgical procedure performed to repair congenital cleft palates (Marrinan et al., 1998), may well reflect an underlying defect in muscle function. The results of the present study raise the possibility that this defect contributes to VPI when LVP fibers from cleft palates remain type 2 following repair. As more is learned about the extent of fiber type conversion following cleft palate repair surgery, alternative strategies for repair and precautionary measures can be introduced to those patients at increased risk, namely, those with a higher percentage of type 2 fibers. Determining the approximate fiber type composition prior to surgery could potentially identify these patients and may eventually allow surgeons to implement an intervention that reduces the incidence of VPI and the need for additional operations.
TABLE 1.
Abbreviations
| LVP | Levator veli palatini |
| VPI | Velopharyngeal incompetence |
| MHC | Myosin heavy chain |
| LCP | Lengthening contraction protocol |
| Lf | Resting fiber length |
| CSA | Cross-sectional area |
| Prest | Resting force |
| krf | Rate constant of force redevelopment |
| SPo | Specific force |
| Pavg | Mean force during the lengthening |
| Ppk | Force when fiber was maximally lengthened |
| LMM | Linear mixed model |
| Ppost | Force following lengthening |
Acknowledgments
The single muscle fiber preparation was funded by the Contractility Core of the Nathan Shock Center for Excellence in Basic Biology of Aging Grant P30 AG 13283. E. Rader, P. Cederna, and D. Yu were supported by additional fellowships: the NIH Regenerative Sciences Training Grant T90 DK-070071, the Academic Scholar of American Association of Plastic Surgeons, and the NIH Training Grant in Trauma, Burn, and Wound Healing Research Grant T32 GM008616-06A1, respectively.
Contributor Information
Dr. Erik P. Rader, Research Fellow, Department of Surgery, University of Michigan, Ann Arbor, Michigan.
Dr. Paul S. Cederna, Associate Chair, Department of Surgery; Associate Professor, Section of Plastic Surgery; and Assistant Research Scientist, Institute of Gerontology, University of Michigan, Ann Arbor, Michigan.
Dr. Jeffrey Weinzweig, Chairman, Department of Plastic and Reconstructive Surgery; Director Institute of Craniomaxillofacial Surgery, Lahey Clinic Medical Center, Burlington, Massachusetts.
Dr. Kip E. Panter, Research Animal Scientist, Poisonous Plant Research Laboratory, USDA Agricultural Research Service, Logan, Utah.
Dr. Deborah Yu, Research Fellow, Section of Plastic Surgery, University of Michigan, Ann Arbor, Michigan.
Dr. Steven R. Buchman, Professor, Section of Plastic Surgery; Chief, Pediatric Plastic Surgery; and Director, Craniofacial Anomalies Program, University of Michigan, Ann Arbor, Michigan.
Dr. Lisa M. Larkin, Assistant Research Professor, Institute of Gerontology; and Assistant Research Scientist, Department of Biomedical Engineering, University of Michigan, Ann Arbor, Michigan.
Dr. John A. Faulkner, Professor, Department of Biomedical Engineering; Professor, Department of Physiology; and Senior Research Scientist, Institute of Gerontology, University of Michigan, Ann Arbor, Michigan.
REFERENCES
- Agarkova I, Schoenauer R, Ehler E, Carlsson L, Carlsson E, Thornell LE, Perriard JC. The molecular composition of the sarcomeric M-band correlates with muscle fiber type. Eur J Cell Biol. 2004;83:193–204. doi: 10.1078/0171-9335-00383. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol. 1996;497:573–580. doi: 10.1113/jphysiol.1996.sp021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K, White H, Sleep J. Kinetics of muscle contraction and actomyosin NTP hydrolysis from rabbit using a series of metal-nucleotide substrates. J Physiol. 2005;563:689–711. doi: 10.1113/jphysiol.2004.078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Improved national prevalence estimates for 18 selected major birth defects—United States, 1999–2001. MMWR Morb Mortal Wkly Rep. 2006;54:1301–1305. [PubMed] [Google Scholar]
- Crameri RM, Langberg H, Magnusson P, Jensen CH, Schroder HD, Olesen JL, Suetta C, Teisner B, Kjaer M. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol. 2004;558:333–340. doi: 10.1113/jphysiol.2004.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi MM, Jackson IT, Elahi O, Khan AH, Mubarak F, Tariq GB, Mitra A. Epidemiology of cleft lip and cleft palate in Pakistan. Plast Reconstr Surg. 2004;113:1548–1555. doi: 10.1097/01.prs.0000117184.77459.2b. [DOI] [PubMed] [Google Scholar]
- Ettema SL, Kuehn DP, Perlman AL, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle during speech. Cleft Palate Craniofac J. 2002;39:130–144. doi: 10.1597/1545-1569_2002_039_0130_mriotl_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Faulkner JA. Terminology for contractions of muscles during shortening, while isometric, and during lengthening. J Appl Physiol. 2003;95:455–459. doi: 10.1152/japplphysiol.00280.2003. [DOI] [PubMed] [Google Scholar]
- Feasson L, Stockholm D, Freyssenet D, Richard I, Duguez S, Beckmann JS, Denis C. Molecular adaptations of neuromuscular disease-associated proteins in response to eccentric exercise in human skeletal muscle. J Physiol. 2002;543:297–306. doi: 10.1113/jphysiol.2002.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JM, Jayaraman RC, Prior BM, Pivarnik JM, Meyer RA. MR measurements of muscle damage and adaptation after eccentric exercise. J Appl Physiol. 1999;87:2311–2318. doi: 10.1152/jappl.1999.87.6.2311. [DOI] [PubMed] [Google Scholar]
- Forrester MB, Merz RD. Descriptive epidemiology of oral clefts in a multiethnic population, Hawaii, 1986–2000. Cleft Palate Craniofac J. 2004;41:622–628. doi: 10.1597/03-089.1. [DOI] [PubMed] [Google Scholar]
- Friden J, Sfakianos PN, Hargens AR, Akeson WH. Residual muscular swelling after repetitive eccentric contractions. J Orthop Res. 1988;6:493–498. doi: 10.1002/jor.1100060404. [DOI] [PubMed] [Google Scholar]
- Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4:170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, MacDougall JD, Tarnopolsky MA, Stauber WT, Elorriaga A. Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. J Appl Physiol. 1995;78:702–708. doi: 10.1152/jappl.1995.78.2.702. [DOI] [PubMed] [Google Scholar]
- Hanes M, Weinzweig J, Cederna P, Kuzon W, Panter K, Buchman S, Faulkner JA, Yu D, Larkin LM. Contractile properties of single permeabilized muscle fibers from congenitally-clefted and normal palates of Spanish goats. Plast Reconstr Surg. doi: 10.1097/01.prs.0000258832.84261.37. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MHS, Lee ST, Rajendran K. Anatomic basis of cleft palate and velopharyngeal surgery: implications from a fresh cadaveric study. Plast Reconstr Surg. 1998;101:613–627. doi: 10.1097/00006534-199803000-00007. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Brooks SV. Lengthening contractions are not required to induce protection from contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2001;281:R155–R161. doi: 10.1152/ajpregu.2001.281.1.R155. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Woodburn TM, Friden J. Muscle damage induced by eccentric contractions of 25-percent strain. J Appl Physiol. 1991;70:2498–2507. doi: 10.1152/jappl.1991.70.6.2498. [DOI] [PubMed] [Google Scholar]
- Lindman R, Paulin G, Stal PS. Morphological characterization of the levator veli palatini muscle in children born with cleft palates. Cleft Palate Craniofac J. 2001;38:438–448. doi: 10.1597/1545-1569_2001_038_0438_mcotlv_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- MacIntyre DL, Reid WD, Lyster DM, Szasz IJ, McKenzie DC. Presence of WBC, decreased strength, and delayed soreness in muscle after eccentric exercise. J Appl Physiol. 1996;80:1006–1013. doi: 10.1152/jappl.1996.80.3.1006. [DOI] [PubMed] [Google Scholar]
- Macpherson PC, Schork MA, Faulkner JA. Contraction-induced injury to single fiber segments from fast and slow muscles of rats by single stretches. Am J Physiol. 1996;271:C1438–C1446. doi: 10.1152/ajpcell.1996.271.5.C1438. [DOI] [PubMed] [Google Scholar]
- Malm C, Sjodin TL, Sjoberg B, Lenkei R, Renstrom P, Lundberg IE, Ekblom B. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J Physiol. 2004;556:983–1000. doi: 10.1113/jphysiol.2003.056598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrinan EM, Labrie RA, Mulliken JB. Velopharyngeal function in nonsyndromic cleft palate: relevance of surgical technique, age at repair, and cleft type. Cleft Palate Craniofac J. 1998;35:95–100. doi: 10.1597/1545-1569_1998_035_0095_vfincp_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- McCully KK, Faulkner JA. Injury to skeletal-muscle fibers of mice following lengthening contractions. J Appl Physiol. 1985;59:119–126. doi: 10.1152/jappl.1985.59.1.119. [DOI] [PubMed] [Google Scholar]
- Moisescu DG, Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in external bathing solution. J Physiol. 1978;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JB, Smith AE, Folkins JW, Lemke JH, Gartlan M. Coordination of velopharyngeal muscle activity during positioning of the soft palate. Cleft Palate Craniofac J. 1994;31:45–55. doi: 10.1597/1545-1569_1994_031_0045_covmad_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Moon JB, Thompson SA, Jaeckel E, Canady JW. Muscle fiber type distribution in the normal human levator veli palatini muscle. Cleft Palate Craniofac J. 1998;35:419–424. doi: 10.1597/1545-1569_1998_035_0419_mftdit_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Newham DJ, McPhail G, Mills KR, Edwards RH. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Prado LG, Makarenko I, Andresen C, Kruger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol. 2005;126:461–480. doi: 10.1085/jgp.200509364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall P, LaRossa D. Cleft palate. In: Smith JW, Aston SJ, editors. Plastic Surgery. Boston: Little, Brown; 1991. pp. 287–324. [Google Scholar]
- Stal PS, Lindman R. Characterisation of human soft palate muscles with respect to fibre types, myosins and capillary supply. J Anat. 2000;197:275–290. doi: 10.1046/j.1469-7580.2000.19720275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupka N, Lowther S, Chorneyko K, Bourgeois J, Hogben C, Tarnopolsky M. Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol. 2000;89:2325–2332. doi: 10.1152/jappl.2000.89.6.2325. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Corteselli SA, Kushmerick MJ. Measurements on permeabilized skeletal-muscle fibers during continuous activation. Am J Physiol. 1987;252:C575–C580. doi: 10.1152/ajpcell.1987.252.5.C575. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Pysiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag; 2000. [Google Scholar]
- Weinzweig J, Panter KE, Pantaloni M, Spangenberger A, Harper JS, Lui F, Gardner D, Wierenga TL, Edstrom LE. The fetal cleft palate: I. Characterization of a congenital model. Plast Reconstr Surg. 1999;103:419–428. doi: 10.1097/00006534-199902000-00009. [DOI] [PubMed] [Google Scholar]
- Weinzweig J, Panter KE, Spangenberger A, Harper JS, McRae R, Edstrom LE. The fetal cleft palate: III. Ultrastructural and functional analysis of palatal development following in utero repair of the congenital model. Plast Reconstr Surg. 2002;109:2355–2362. doi: 10.1097/00006534-200206000-00030. [DOI] [PubMed] [Google Scholar]
- Wigmore P, Evans D. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int Rev Cytol. 2002;216:175–232. doi: 10.1016/s0074-7696(02)16006-2. [DOI] [PubMed] [Google Scholar]
- Yu JG, Carlsson L, Thornell LE. Evidence for myofibril remodeling as opposed to myofibril damage in human muscles with DOMS: an ultrastructural and immunoelectron microscopic study. Histochem Cell Biol. 2004;121:219–227. doi: 10.1007/s00418-004-0625-9. [DOI] [PubMed] [Google Scholar]
- Yu JG, Furst DO, Thornell LE. The mode of myofibril remodelling in human skeletal muscle affected by DOMS induced by eccentric contractions. Histochem Cell Biol. 2003;119:383–393. doi: 10.1007/s00418-003-0522-7. [DOI] [PubMed] [Google Scholar]
- Yu JG, Malm C, Thornell LE. Eccentric contractions leading to DOMS do not cause loss of desmin nor fibre necrosis in human muscle. Histochem Cell Biol. 2002;118:29–34. doi: 10.1007/s00418-002-0423-1. [DOI] [PubMed] [Google Scholar]